Supported gold-catalyzed and ammonia-promoted selective synthesis of quinazolines in aqueous media†
Lin
Tang‡
,
Yu
Yang‡
,
Lixian
Wen
,
Sheng
Zhang
,
Zhenggen
Zha
and
Zhiyong
Wang
*
Hefei National Laboratory for Physical Sciences at Microscale, CAS Key Laboratory of Soft Matter Chemistry & Collaborative Innovation Center of Suzhou Nano Science and Technology, University of Science and Technology of China, Hefei, 230026, P. R. China. E-mail: zwang3@ustc.edu.cn; Fax: +86 551-360-3185
First published on 15th December 2014
Abstract
A highly efficient and selective nitrogen source-promoted reaction for the synthesis of 2,4-disubstituted quinazolines from o-nitroacetophenones and alcohols catalyzed by Au/TiO2 has been developed via a hydrogen-transfer strategy. This reaction has good tolerance to air and water, a wide substrate scope, and represents a new avenue for practical multiple C–N bond formation. More importantly, no additional additive, oxidant and reductant are required in the reaction and the catalyst can be recovered and reused readily.
Quinazolines and its derivatives are important compounds due to their widespread applications in organic synthesis and medicinal chemistry.1 Usually, the traditional synthesis of quinazolines involves reactions of Bischler cyclization, dicarbonyl compounds with diamines and reactions from 2-aminobenzonitriles or anthranilic acids as well as N-arylbenzamides.2 Recently, copper-catalyzed cascade coupling of o-halobenzaldehyde with acetamidine hydrochloride (or benzaldehyde) to synthesize quinazolines was developed.3 Also, the reactions of o-aminoacetophenones with benzaldehydes were reported for the synthesis of 2-phenylquinazolines in the presence of a nitrogen source.4 Very recently, new approaches for the synthesis of 2-phenylquinazolines were developed by using o-aminoacetophenones and benzylamines (or DMF) as the starting materials under the catalysis of I2,5 NIS,6 nano-CuO7 and 4-hydroxy-TEMPO8 respectively, by our group and Han et al. Nevertheless, these methods generally involved large excess of oxidants and suffered from limitations of substrate generality and availability of starting material.
Because of the advantages of high catalytic efficiency and easy recycling, heterogeneous catalysts have been receiving more and more attention.9 Metal nanoparticles supported on different templates have been widely used as heterogeneous catalysts in recent years.10,11 In particular, various heterogeneous catalytic systems for direct C–N bond formation from nitroarenes (or amines) and alcohols were successfully developed by Cao,12 Shi,13 our group,14 and others.15 As an ongoing interest in the synthesis of quinazolines and the construction of a C–N bond, we herein developed a one-pot direct synthesis of 2,4-disubstituted quinazolines using o-nitroacetophenones and alcohols as the starting materials in the presence of a nitrogen source catalyzed by gold nanoparticles supported on titanium dioxide (Au/TiO2). No additional additive, oxidant and reductant were required in this reaction. Various transformations, including dehydrogenative oxidation of alcohol and C–N bond, reduction of the nitro group and condensation of aldehyde with amine, can be achieved in this cascade reaction.
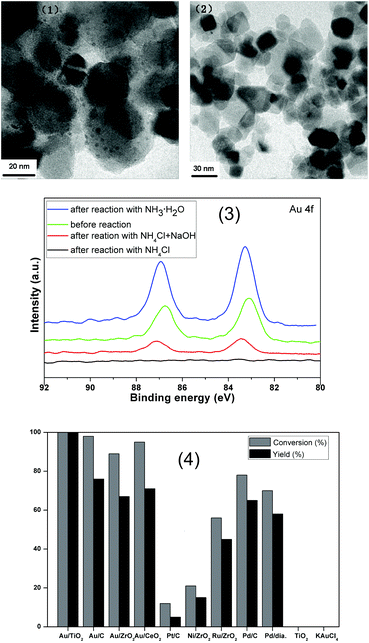
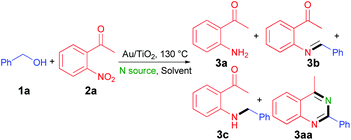
We began our study with the reaction of benzyl alcohol (1a) with o-nitroacetophenone (2a) under catalysis of Au/TiO2 (Table 1).16 Initial experiments were performed to investigate the influence of nitrogen sources on the reaction. Moderate yields of 3aa were obtained with NH4OAc, NH4HCO3 or urea as the nitrogen source, whereas the reaction could hardly take place in the presence of NH4HSO4 or NH4Cl (Table 1, entries 1–5). 3aa was selectively obtained in an excellent yield of 92% when NH3·H2O was employed as the nitrogen source (Table 1, entry 6). However, when NaOH was added to the reaction with NH4Cl as the nitrogen source, 75% 3aa was obtained (Table 1, entry 7). Some analyses were conducted to explain this phenomenon. No gold nanoparticle was observed in the TEM analysis and XPS test could not detect the element of gold on the surface of titanium dioxide after reaction with acidic NH4Cl as the nitrogen source. However, a typical doublet of 4f core level bands could be observed when we added NaOH in the reaction or alkaline NH3·H2O was used as the nitrogen source (Fig. 1(2) and (3)). These results indicated that the catalyst could be maintained well under alkaline conditions, whereas under acidic conditions gold nanoparticles would detach from the surface of titanium dioxide. This is why an alkaline nitrogen source like NH3·H2O could promote the reaction, but an acidic nitrogen source was unfavorable to the reaction. 3aa was not detected in the absence of the nitrogen source (Table 1, entry 8). 15N-labeling experiment unambiguously established that the additional nitrogen atom of 3aa was derived from NH3·H2O.17 Subsequently, various solvents were tested in the reaction (Table 1 entries 9–15). To our delight, when toluene–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was employed as the mixture solvent, 99% isolated yield was obtained (Table 1, entry 14).
1) was employed as the mixture solvent, 99% isolated yield was obtained (Table 1, entry 14).
| Entry | N source | Solvent | Yieldb (%) | |||
|---|---|---|---|---|---|---|
| 3a | 3b | 3c | 3aa | |||
| a The reactions were carried out with 1a (1.5 equiv., 0.75 mmol), 2a (1.0 equiv., 0.25 mmol), nitrogen source (3.0 equiv., 0.75 mmol), solvent (1.0 ml) and catalyst (Au/TiO2, metal: 0.8 mol%) for 20 h under a N2 atmosphere. b GC yield of the product is based on 2a, n.d. = not detected, isolated yield in parentheses. c NaOH (0.75 mmol) was added. | ||||||
| 1 | NH4HSO4 | Toluene | <1 | <1 | n.d. | n.d. |
| 2 | NH4Cl | Toluene | <1 | <1 | n.d. | n.d. |
| 3 | NH4OAc | Toluene | 35 | 10 | 3 | 51 |
| 4 | NH4HCO3 | Toluene | 25 | 5 | 2 | 62 |
| 5 | Urea | Toluene | 10 | 4 | 2 | 57 |
| 6 | NH3·H2O | Toluene | 8 | <1 | <1 | 92 |
| 7c | NH4Cl | Toluene | 6 | 2 | 3 | 75 |
| 8 | None | Toluene | 51 | 25 | 8 | n.d. |
| 9 | NH3·H2O | 1,4-Xylene | 13 | <1 | <1 | 85 |
| 10 | NH3·H2O | NMP | 46 | 4 | <1 | 45 |
| 11 | NH3·H2O | DMF | 91 | 5 | <1 | <1 |
| 12 | NH3·H2O | Water | 2 | 3 | 8 | 86 |
| 13 | NH3·H2O | Toluene–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3 | 2 | <1 | 93 |
| 14 | NH3·H2O | Toluene–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
<1 | <1 | <1 | >99 (99) |
| 15 | NH3·H2O | Toluene–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
4 | 3 | 4 | 88 |
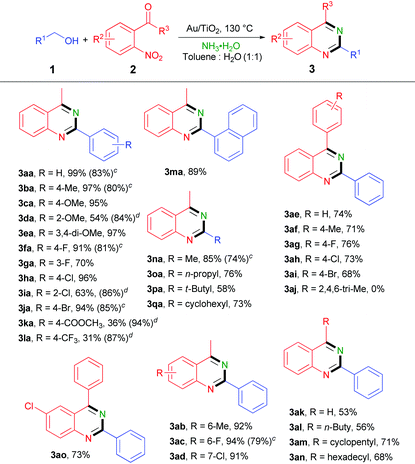
With the optimized conditions in hand, we then studied the activities of various catalysts (Fig. 1(4)). When other supported gold catalysts, such as Au/C, Au/ZrO2 and Au/CeO2, were employed in this reaction, relatively low activity and selectivity were obtained. The comparison of Au/TiO2 with other supported gold catalysts showed that the nature of the support had a strong influence on the activity of the Au catalysts and TiO2 was the best support for this reaction. When catalysts of Pd/C and Pd/diatomite were employed in this reaction, low conversion but good selectivity were achieved to afford 3aa in moderate yields. However, other supported metal catalysts including Pt/C, Ni/ZrO2 and Ru/ZrO2 presented very low activity in this reaction. No product was obtained only in the presence of TiO2 or KAuCl4. After catalyst screening, Au/TiO2 showed the best catalytic efficiency and selectivity for this reaction.
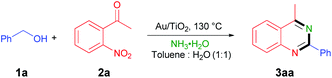
After the confirmation of Au/TiO2 as the most efficient catalyst, the substrate scope of the reaction was extended under the optimized reaction conditions (Table 2). Firstly, the electronic properties of a series of aromatic alcohols were found to have little influence on the reaction. The reactions could be carried out effectively to afford the desired products (3aa–3ca, 3ea–3ha, 3ja) with good yields regardless of the electron-donating groups or electron-withdrawing groups at the benzene ring of benzylic alcohols. However, the steric hindrance of the substituents had a negative influence on the reaction. Relatively low yields of the products (3da, 3ia) were obtained when the substituent groups appeared at the ortho-position of the benzene ring. Besides, low yields of the products (3ka, 3la) were obtained in the presence of an ester group and trifluoromethyl. This negative effect could be compensated by elevating the reaction temperature. Product 3ma was obtained readily in 89% yield when 1-naphthalenemethanol was employed. It was worth noting that aliphatic alcohols were also good substrates to afford the corresponding products (3na–3qa) in moderate to good yields. In contrast, the previous methods were limited to only 2-arylquinazolines.4a,c,5,7,8 Excellent yields of 3ab–3ad were achieved when R2 was methyl, fluoro, or chloro. When R3 was an aromatic substituent, the reaction could also give the desired products of 3ae–3ai in good yields. Nevertheless, 3aj could not be obtained, perhaps due to the steric hindrance. Moreover, 3ak–3an could also be obtained when R3 was replaced by other aliphatic groups. These results indicated that this reaction had a broad substrate scope and a variety of quinazolines could be synthesized by virtue of this developed reaction. When these reactions were performed under an air atmosphere, 3aa, 3ba, 3fa, 3ja, 3na and 3ac could still be achieved in good yields, which delivered that this reaction had good tolerance to air.
The recovery and reuse of the Au/TiO2 catalyst in the reaction could be achieved by a simple phase separation. A little loss of the catalytic activity was observed after the seventh round, as shown in Table 3. When we added some additional catalyst to keep Au loading of 0.8 mol% (Run 8), we were pleased to find that an excellent yield of 99% was obtained again. The result showed that the catalyst could be reused at least 8 times without loss of its activity.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Run | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8c |
|---|---|---|---|---|---|---|---|---|
| a The reactions were carried out with 1a (1.5 equiv., 0.75 mmol), 2a (1.0 equiv., 0.25 mmol), ammonia (25% in H2O, 3.0 equiv.), 1.0 ml of the solvent and initial catalyst (Au/TiO2, Au: 0.8 mol%) for 20 h under a N2 atmosphere. b Yield of the isolated product is based on 2a. c Catalyst (Au/TiO2, Au: 0.8 mol%). | ||||||||
| Yield (%)b | 99 | 99 | 99 | 99 | 98 | 96 | 95 | 99 |
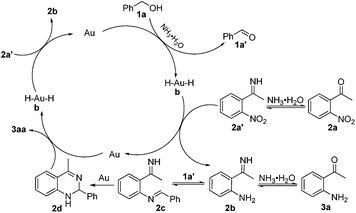
Based on control experiments18 and previous reports,12,13,14,15c the possible mechanism is depicted in Scheme 1. First of all, dehydrogenative oxidation of the alcohol (1a) into the corresponding carbonyl compound (1a′) promoted by ammonia, generates the gold-hydride species (b)12b,c,13c,e,19 and 2a′ is reduced into 2bin situ by b. This is the first hydrogen-transfer process and also the rate-limiting step of the reaction. Then, 2b can readily react with 1a′ to afford the corresponding imine 2c. 2c is converted to the intermediate 2d under the catalysis of gold. Subsequently, 2d can be rapidly oxidized to the product of 3aa in the presence of the gold catalyst accompanied with the generation of b. And 2a′ is again reduced into 2b by b. This is the second hydrogen-transfer process. In the whole catalytic cycle, the alcohol 1a and the intermediate 2d are used as the reductant (hydrogen donor) once and the nitrocompound 2a′ is used as the oxidant (hydrogen acceptor) twice.
In summary, we have developed a highly efficient protocol for one-pot synthesis of 2,4-disubstituted quinazolines by taking advantage of the hydrogen-transfer strategy. To the best of our knowledge, this is the first example to prepare quinazolines under catalysis of heterogeneous gold nanoparticles. By virtue of this catalytic system, the scope of alcohols and nitro compounds was extended widely to give a variety of quinazolines. It is noteworthy that no additional additive, oxidant and reductant were involved in this reaction. More importantly, all the transformations could be carried out smoothly in aqueous media and reuse of the catalyst has also been accomplished by using a simple phase separation process. Detailed mechanistic studies and other applications in organic reactions of this catalyst are in progress in our laboratory.
We are grateful to National Nature Science Foundation of China (21432009, 2127222, 91213303, 21172205).
Notes and references
- (a) P. A. Ple, T. P. Green, L. F. Hennequin, J. Curwen, M. Fennell, J. Allen, C. Lambertvan der Brempt and G. Costello, J. Med. Chem., 2004, 47, 871 CrossRef CAS PubMed; (b) L. A. Doyle and D. D. Ross, Oncogene, 2003, 22, 7340 CrossRef PubMed; (c) K. Waisser, J. Gregor, H. Dostal, J. Kunes, L. Kubicova, V. Klimesova and J. Kaustova, Farmaco, 2001, 56, 803 CrossRef CAS; (d) J. P. Michael, Nat. Prod. Rep., 2008, 25, 166 RSC; (e) V. Colotta, D. Catarzi, F. Varano, O. Lenzi, G. Filacchioni, C. Costagli, A. Galli, C. Ghelardini, N. Galeotti, P. Gratteri, J. Sgrignani, F. Deflorian and S. Moro, J. Med. Chem., 2006, 49, 6015 CrossRef CAS PubMed.
- (a) A. Witt and J. Bergman, Curr. Org. Chem., 2003, 7, 659 CrossRef CAS; (b) D. J. Connolly, D. Cusack, T. P. O'Sullivan and P. J. Guiry, Tetrahedron, 2005, 61, 10153 CrossRef CAS PubMed; (c) J. Li, X. Chen, D. Shi, S. Ma, Q. Li, Q. Zhang and J. Tang, Org. Lett., 2009, 11, 1193 CrossRef CAS PubMed; (d) P. R. Marsham, A. L. Jackman, A. J. Barker, F. T. Boyle, S. J. Pegg, J. M. Wardleworth, R. Kimbell, B. M. O'Connor, A. H. Calvert and L. R. Hughes, J. Med. Chem., 1995, 38, 994 CrossRef CAS.
- (a) C. Huang, Y. Fu, H. Fu, Y. Jiang and Y. Zhao, Chem. Commun., 2008, 6333 RSC; (b) X. Liu, H. Fu, Y. Jiang and Y. Zhao, Angew. Chem., Int. Ed., 2009, 48, 348 CrossRef CAS PubMed; (c) X. Yang, H. Liu, H. Fu, R. Qiao, Y. Jiang and Y. Zhao, Synlett, 2010, 101 Search PubMed; (d) V. L. Truong and M. Morrow, Tetrahedron Lett., 2010, 51, 758 CrossRef CAS PubMed; (e) J. Ju, R. Hua and J. Su, Tetrahedron, 2012, 68, 9364 CrossRef CAS PubMed.
- (a) R. Sarma and D. Prajapati, Green Chem., 2011, 13, 718 RSC; (b) Z.-H. Zhang, X.-N. Zhang, L.-P. Mo, Y.-X. Li and F.-P. Ma, Green Chem., 2012, 14, 1502 RSC; (c) Z. Chen, J. Chen, M. Liu, J. Ding, W. Gao, X. Huang and H. Wu, J. Org. Chem., 2013, 78, 11342 CrossRef CAS PubMed.
- (a) J. Zhang, D. Zhu, C. Yu, C. Wan and Z. Wang, Org. Lett., 2010, 12, 2841 CrossRef CAS PubMed; (b) Y. Yan and Z. Wang, Chem. Commun., 2011, 47, 9513 RSC.
- Y. Yan, Y. Zhang, C. Feng, Z. Zha and Z. Wang, Angew. Chem., Int. Ed., 2012, 51, 8077 CrossRef CAS PubMed.
- J. Zhang, C. Yu, S. Wang, C. Wan and Z. Wang, Chem. Commun., 2010, 46, 5244 RSC.
- B. Han, C. Wang, R.-F. Han, W. Yu, X.-Y. Duan, R. Fan and X.-L. Yang, Chem. Commun., 2011, 47, 7818 RSC.
- For reviews on heterogeneous catalysts, see: (a) N. Mizuno and M. Misono, Chem. Rev., 1998, 98, 199 CrossRef CAS PubMed; (b) R. Akiyama and S. Kobayashi, Chem. Rev., 2009, 109, 594 CrossRef CAS PubMed; (c) S. Ikegami and H. Hamamoto, Chem. Rev., 2009, 109, 583 CrossRef CAS PubMed; (d) M. J. Climent, A. Corma and S. Iborra, Chem. Rev., 2011, 111, 1072 CrossRef CAS PubMed , and references therein.
- For reviews on supported metal catalysts in nanoscale, see: (a) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS PubMed; (b) J. M. Campelo, D. Luna, R. Luque, J. M. Marinas and A. A. Romero, ChemSusChem, 2009, 2, 18 CrossRef CAS PubMed; (c) J. Lu and P. H. Toy, Chem. Rev., 2009, 109, 815 CrossRef CAS PubMed; (d) R. J. White, R. Luque, V. L. Budarin, J. H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 2009, 38, 481 RSC , and references therein.
- For reviews on supported gold catalysts in nanoscale, see: (a) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef PubMed; (b) T. V. W. Janssens, B. S. Clausen, B. Hvolbaek, H. Falsig, C. H. Christensen, T. Bligaard and J. K. Norskov, Top. Catal., 2007, 44, 15 CrossRef CAS PubMed; (c) B. K. Min and C. M. Friend, Chem. Rev., 2007, 107, 2709 CrossRef CAS PubMed; (d) A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096 RSC; (e) G. J. Hutchings, Chem. Commun., 2008, 1148 RSC; (f) A. Corma, A. Leyva-Pe'rez and M. J. Sabater, Chem. Rev., 2011, 111, 1657 CrossRef CAS PubMed, and references therein; For selected examples of supported gold heterogeneous catalysts: (g) A. Corma and P. Serna, Science, 2006, 313, 332 CrossRef CAS PubMed; (h) H. Miyamura, R. Matsubara, Y. Miyazaki and S. Kobayashi, Angew. Chem., Int. Ed., 2007, 46, 4151 CrossRef CAS PubMed; (i) A. Grirrane, A. Corma and H. Garcia, Science, 2008, 322, 1661 CrossRef CAS PubMed; (j) T. Mitsudome, A. Noujima, T. Mizugaki, K. Jitsukawa and K. Kaneda, Green Chem., 2009, 11, 793 RSC; (k) S. Kegnæs, J. Mielby, U. V. Mentzel, C. H. Christensen and A. Riisager, Green Chem., 2010, 12, 1437 RSC.
- (a) H. Sun, F. Z. Su, J. Ni, Y. Cao, H. Y. He and K. N. Fan, Angew. Chem., Int. Ed., 2009, 48, 4390 CrossRef CAS PubMed; (b) L. He, X. B. Lou, J. Ni, Y. M. Liu, Y. Cao, H. Y. He and K. N. Fan, Chem. – Eur. J., 2010, 16, 13965 CrossRef CAS PubMed; (c) C. H. Tang, L. He, Y. M. Liu, Y. Cao, H. Y. He and K. N. Fan, Chem. – Eur. J., 2011, 17, 7172 CrossRef CAS PubMed; (d) J. Huang, L. Yu, L. He, Y. M. Liu, Y. Cao and K. N. Fan, Green Chem., 2011, 13, 2672 RSC; (e) H. Huang, J. Huang, Y. M. Liu, H. Y. He, Y. Cao and K. N. Fan, Green Chem., 2012, 14, 930 RSC.
- (a) F. Shi, Q. Zhang, Y. Ma, Y. He and Y. Deng, J. Am. Chem. Soc., 2005, 127, 4182 CrossRef CAS PubMed; (b) X. Cui, F. Shi and Y. Deng, Chem. Commun., 2012, 48, 7586 RSC; (c) Q. Peng, Y. Zhang, F. Shi and Y. Deng, Chem. Commun., 2011, 47, 6476 RSC; (d) X. Cui, X. Dai, Y. Zhang, Y. Deng and F. Shi, Chem. Sci., 2014, 5, 649 RSC; (e) X. Cui, C. Zhang, F. Shi and Y. Deng, Chem. Commun., 2012, 48, 9391 RSC; (f) X. Cui, Y. Zhang, F. Shi and Y. Deng, Chem. – Eur. J., 2011, 17, 2587 CrossRef CAS PubMed.
- (a) Y. Wang, D. Zhu, L. Tang, S. Wang and Z. Wang, Angew. Chem., Int. Ed., 2011, 50, 8917 CrossRef CAS PubMed; (b) L. Tang, H. Sun, Y. Li, Z. Zha and Z. Wang, Green Chem., 2012, 14, 3423 RSC; (c) L. Tang, X. Guo, Y. Li, S. Zhang, Z. Zha and Z. Wang, Chem. Commun., 2013, 49, 5213 RSC; (d) X. Guo, L. Tang, Y. Yang, Z. Zha and Z. Wang, Green Chem., 2014, 16, 2443 RSC; (e) L. Tang, X. Guo, Y. Yang, Z. Zha and Z. Wang, Chem. Commun., 2014, 50, 6145 RSC.
- (a) F. Gelman, J. Blum and D. Avnir, New J. Chem., 2003, 27, 205 RSC; (b) C. Gonzalez-Arellano, K. Yoshida, R. Luque and P. L. Gai, Green Chem., 2010, 12, 1281 RSC; (c) A. Corma, T. Ródenas and M. J. Sabater, Chem. – Eur. J., 2010, 16, 254 CrossRef CAS PubMed; (d) C.-C. Lee and S.-T. Liu, Chem. Commun., 2011, 47, 6981 RSC; (e) K. Shimizu, K. Shimura, M. Nishimura and A. Satsuma, ChemCatChem, 2011, 3, 1755 CrossRef CAS; (f) R. Cano, D. J. Ramón and M. Yus, J. Org. Chem., 2011, 76, 5547 CrossRef CAS PubMed; (g) Y. Shiraishi, M. Ikeda, D. Tsukamoto, S. Tanaka and T. Hiraiai, Chem. Commun., 2011, 47, 4811 RSC; (h) J.-F. Soulé, H. Miyamura and S. Kobayashi, J. Am. Chem. Soc., 2011, 133, 18550 CrossRef PubMed; (i) W.-J. Yoo, H. Yuan, H. Miyamura and S. Kobayashi, Adv. Synth. Catal., 2011, 353, 3085 CrossRef CAS.
- See ESI† for detailed experiments and characterization of metal nanoparticles supported on different templates.
- For detailed 15N-labeling experiment and NMR data, see ESI.†.
- See control experiments in the ESI.†.
- (a) A. Abad, C. Almela, A. Corma and H. Garcia, Chem. Commun., 2006, 3178 RSC; (b) F. Z. Su, L. He, J. Ni, Y. Cao, H. Y. He and K. N. Fan, Chem. Commun., 2008, 3531 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details, characterization of catalysts and characterization of products. See DOI: 10.1039/c4qo00278d |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2015 |