Heteroatom-doped carbons: synthesis, chemistry and application in lithium/sulphur batteries
Sheng S.
Zhang
*
Electrochemistry Branch, RDRL-SED-C, Sensors and Electron Devices Directorate, U.S. Army Research Laboratory, Adelphi, MD 20783-1138, USA. E-mail: shengshui.zhang.civ@mail.mil; shengshui@gmail.com; Fax: +1-301-394-0273; Tel: +1-301-394-0981
First published on 6th October 2015
Abstract
Doping of heteroatoms into carbon not only changes the electronic distribution but also creates surface functional groups. These changes prove to enhance the chemical adsorption of carbon to sulphur species, and have been intensively investigated to sequestrate soluble lithium polysulphide in the cathode of lithium/sulphur (Li/S) batteries. The chemical adsorption varies with the type, valence state and content of heteroatoms in a complicated manner. In this review, the syntheses of heteroatom-doped carbons as well as their applications and mechanisms in the Li/S batteries are highlighted and discussed with a focus on oxygen, sulphur, nitrogen and their mixtures.
1. Introduction
The development of lithium/sulphur (Li/S) batteries faces great challenges in relation to the dissolution of lithium polysulphide (PS, Li2Sn with n > 2) in organic electrolytes. By its nature, PS dissolution is intrinsically unavoidable and essential for the effective utilisation and fast reaction kinetics of insulating sulphur species.1 The PS dissolution has been identified to be the source for a number of known problems, including low energy efficiency, fast self-discharge, severe Li corrosion, and poor safety of Li/S batteries. Aiming to solve these problems, researchers have focused on two directions of (1) trapping the soluble PS from diffusing out of the cathode and (2) protecting the Li anode from reacting with the dissolved PS.2,3 In efforts to trap the soluble PS within the cathode, a number of strategies have been attempted and investigated, including sulphur–carbon (S–C) composites, metal oxides and conducting polymers for advanced materials; dual-layer structured cathode (or called PS-absorbing interlayer) and PS diffusion blocking layer for improved cell configurations.4–8 Among these strategies, the S–C composites have been most intensively studied due to the wide variation and low cost of commercial and synthetic carbons. The trapping of PS by the S–C composites is typically a combined effect of physical absorption (PAB) and chemical adsorption (CAD). The PAB traps PS through weak van der Waals interactions and features temporary sequestration, whereas the CAD traps PS through strong chemical binding between the PS molecules and carbon surfaces featuring long-term sequestration, as schematically illustrated in Fig. 1. It is indicated that the PAB is able to increase the specific capacity of sulphur but fails to retain stable capacity because the weak absorption cannot effectively prevent out-diffusion of the negatively charged PS anions. In contrast, the CAD not only increases the specific capacity but also stabilises the capacity retention due to the strong sulphur sequestration.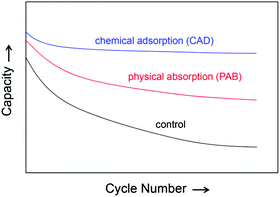 | ||
| Fig. 1 Schematic comparison of physical absorption (PAB) and chemical adsorption (CAD) in improving the cycling performance of Li/S batteries. | ||
The PAB is affected mainly by the physical properties of carbon such as the pore structure and porosity. In the past few years, research studies on the S–C composites have been overwhelmingly focused on the PAB strategy based on varieties of nanostructured porous carbon materials, for which the readers may refer to several review articles.2,4–7 In contrast, the CAD is much more complicated, and is affected not only by the electronic deficiency and hydrophilicity of the carbon surface but also by the type and content of the surface functional groups. A single adsorption system may involve one or more types of chemical interactions. Since surface functional groups are merely the form of heteroatoms (HAs) existing with carbon, this review highlights the syntheses of HA-doped carbons and their CAD mechanisms and applications in Li/S batteries with a focus on oxygen, sulphur, nitrogen and their mixtures.
2. Synthesis and basic properties of HA-doped carbons
The scope of this review is limited to oxygen, sulphur, nitrogen and their mixtures. These HAs are typically presented in the form of functional groups, which are briefly summarised in Table 1. In the process of preparing S–C composites, some functional groups are thermally eliminated, or substituted/reduced by elemental sulphur to form so-called sulphurised carbon (SC) that together with the remaining HAs affects the CAD of carbon. There are two general approaches for the synthesis of HA-doped carbons: (1) the treatment of commercial or synthetic carbons with the HA source and (2) the pyrolysis of HA-containing precursors. The former features the HAs doped on the surface of carbon with a low HA content (typically not higher than 5 wt%), and the latter features the HAs doped throughout the carbon bulk with a high HA content of up to more than 20 wt%, for example 21.6 wt% N for a N-doped carbon prepared by carbonising N-containing ionic liquids.9 The introduction of HAs into carbon changes the electronic distribution of the carbon conductive networks. The electronic conductivity of carbon will be dramatically decreased when the content of HAs exceeds a certain level. Therefore, the conductivity and doping dose must be balanced when one designs HA-doped carbons for use in the Li/S batteries. Since the CAD occurs on the solution–carbon interface, only those HAs doped on the carbon surface are responsible for the sulphur sequestration. Therefore, the capacity and ability of CAD are determined mainly by the concentration and type of HAs on the carbon surface, rather than the total HA content in the carbon bulk.| Heteroatom | Typical functional groups with carbon |
|---|---|
| O | –OH, >O, >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –C( O, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O–, –COOH O)O–, –COOH |
| S | –SnH, –Sn–, >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, –C( S, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)Sn–, –C( S)Sn–, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)SnH, –SO3H, –S( S)SnH, –SO3H, –S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2O– O)2O– |
| N | –NH2, >NH, >N– , ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, >N+<, –N N–, >N+<, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –NO2 O, –NO2 |
To be used in Li/S batteries, the HA-doped carbons can be either mixed with sulphur to form a S–C composite4,5 or coated as a PS-blocking layer onto the surface of sulphur cathodes2,10 or onto the separator facing the cathode.11,12 The S–C composites are prepared mostly through the sulphur melt diffusion method near the sulphur's critical temperature (159.4 °C) at which the sulphur melt has the lowest viscosity for diffusing into the pores of carbon.13 At such temperatures, in fact, significant amounts of the HAs and their functional groups will be thermally eliminated or substituted by sulphur to form SCs.14 Therefore, small amounts of S–C covalent bonds are an important characteristic of the S–C composites with the HA-doped carbon.
3. Heteroatom-doped carbons
3.1. Oxygen-doped carbons (ODCs)
Oxygens in carbon are present in the forms of hydroxyl (–OH), epoxy (>O), ketone (>C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), ester (–C(
O), ester (–C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O–), and carboxylic acid (–COOH), which can be introduced by mild oxidation of carbons or pyrolysis of oxygen-containing precursors. The mild oxidisation can be realised through a solid–gas reaction at high temperatures,15,16 or through a solid–liquid reaction at moderate temperatures.17 The oxygen source may be water or CO2 for the high temperature solid–gas reaction, and other relatively strong oxidising agents such as H2O2, KMnO4, Na2S2O8 and HNO3 for the moderate temperature solid–liquid reaction. For the pyrolysis approach, all organic compounds, natural or synthetic polymers can be used as the carbon precursor as long as they contain oxygen. At the temperatures of preparing S–C composites, the oxygen and oxygen-functionalised groups are easily eliminated, substituted, or reduced by sulphur to form S–C and S–O bonds. Therefore, the preparation of the S–C composites inevitably results in a considerable reduction in the oxygen content, and the S–C and S–O bonds are typically characteristic of the S–C composites.15,18,19 The role of the doped oxygen in enhancing the sulphur sequestration varies with the type of carbon–oxygen bonds in the ODC, which are highlighted below.
O)O–), and carboxylic acid (–COOH), which can be introduced by mild oxidation of carbons or pyrolysis of oxygen-containing precursors. The mild oxidisation can be realised through a solid–gas reaction at high temperatures,15,16 or through a solid–liquid reaction at moderate temperatures.17 The oxygen source may be water or CO2 for the high temperature solid–gas reaction, and other relatively strong oxidising agents such as H2O2, KMnO4, Na2S2O8 and HNO3 for the moderate temperature solid–liquid reaction. For the pyrolysis approach, all organic compounds, natural or synthetic polymers can be used as the carbon precursor as long as they contain oxygen. At the temperatures of preparing S–C composites, the oxygen and oxygen-functionalised groups are easily eliminated, substituted, or reduced by sulphur to form S–C and S–O bonds. Therefore, the preparation of the S–C composites inevitably results in a considerable reduction in the oxygen content, and the S–C and S–O bonds are typically characteristic of the S–C composites.15,18,19 The role of the doped oxygen in enhancing the sulphur sequestration varies with the type of carbon–oxygen bonds in the ODC, which are highlighted below.
Due to the high surface area and high oxygen concentration of graphene oxide (GO), the two-dimensional structured GO was first proposed to anchor PS by Ji et al.20 who prepared a S–GO composite by first depositing sulphur onto GO via a solution method and then heating the mixture at 155 °C for 12 h. In this process, the >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the GO are reduced by sulphur and sulphur itself is oxidised to form sulphur–oxygen bonds while the epoxy and hydroxyl oxygens are substituted by sulphur to form C–S bonds. Therefore, the resulting S–GO composites contain significant amounts of S–O and C–S bonds as identified by the K-edge X-ray absorption spectroscopy (XAS) spectra,18,20 which contribute to the sulphur sequestration by serving as a bridge to covalently immobilise sulphur species on the carbon surface. As a result, a Li/S cell with the S–GO composite exhibited a reversible capacity of 950–1400 mA h−1 g−1 and cycled stably at 0.1 C for more than 50 cycles.20 This concept has been further studied and better understood by using either GO or reduced-GO (rGO) as the starting material.18,19,21,22 Owing to the reduction by sulphur, the ultimate S–C composites prepared from the GO and rGO turn out to have very similar chemistry. Besides the covalent S–O and C–S bonds, there is strong evidence for the >O⋯Li+ binding between the epoxy oxygen and PS cluster.19 Using first-principle molecular dynamics simulations and density functional theory (DFT) calculations, Wang et al.23 showed that the oxygens on the graphene basal plane have adsorption energies of 1.1–1.5 eV with the Li2S8 cluster, providing an excellent theoretical support for the enhancement of CAD by the oxygen HAs. A similar enhancement was reported by Yan et al.24 who treated carbon nanotubes (CNTs) with a HNO3–H2SO4 mixed acid and H2O2, respectively, and then loaded sulphur at 300 °C for 5 h. The S–O and C–S bonds are formed in the S–CNT composite as suggested by two new absorption bands at 1050 cm−1 and 925 cm−1, respectively, in Fourier transform infrared (FTIR) spectroscopy. As a result, the S–CNT composite with a sulphur content of 68 wt% showed an initial capacity of 1180 mA h−1 g−1 and remained 799 mA h−1 g−1 after 300 cycles at 0.25 C. It should be noted that although detected spectroscopically, the S–O single bond is thermodynamically unstable with a trend being oxidised to a more stable S
O bonds in the GO are reduced by sulphur and sulphur itself is oxidised to form sulphur–oxygen bonds while the epoxy and hydroxyl oxygens are substituted by sulphur to form C–S bonds. Therefore, the resulting S–GO composites contain significant amounts of S–O and C–S bonds as identified by the K-edge X-ray absorption spectroscopy (XAS) spectra,18,20 which contribute to the sulphur sequestration by serving as a bridge to covalently immobilise sulphur species on the carbon surface. As a result, a Li/S cell with the S–GO composite exhibited a reversible capacity of 950–1400 mA h−1 g−1 and cycled stably at 0.1 C for more than 50 cycles.20 This concept has been further studied and better understood by using either GO or reduced-GO (rGO) as the starting material.18,19,21,22 Owing to the reduction by sulphur, the ultimate S–C composites prepared from the GO and rGO turn out to have very similar chemistry. Besides the covalent S–O and C–S bonds, there is strong evidence for the >O⋯Li+ binding between the epoxy oxygen and PS cluster.19 Using first-principle molecular dynamics simulations and density functional theory (DFT) calculations, Wang et al.23 showed that the oxygens on the graphene basal plane have adsorption energies of 1.1–1.5 eV with the Li2S8 cluster, providing an excellent theoretical support for the enhancement of CAD by the oxygen HAs. A similar enhancement was reported by Yan et al.24 who treated carbon nanotubes (CNTs) with a HNO3–H2SO4 mixed acid and H2O2, respectively, and then loaded sulphur at 300 °C for 5 h. The S–O and C–S bonds are formed in the S–CNT composite as suggested by two new absorption bands at 1050 cm−1 and 925 cm−1, respectively, in Fourier transform infrared (FTIR) spectroscopy. As a result, the S–CNT composite with a sulphur content of 68 wt% showed an initial capacity of 1180 mA h−1 g−1 and remained 799 mA h−1 g−1 after 300 cycles at 0.25 C. It should be noted that although detected spectroscopically, the S–O single bond is thermodynamically unstable with a trend being oxidised to a more stable S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond or broken into S˙ and O˙ radicals. The S–O single bond can be rarely found from small organic compounds except for those contained in the –SO3R groups (R = H, metal ion, or alkyl groups). Therefore, the contribution of the S–O bond to the sulphur sequestration may be very limited.
O double bond or broken into S˙ and O˙ radicals. The S–O single bond can be rarely found from small organic compounds except for those contained in the –SO3R groups (R = H, metal ion, or alkyl groups). Therefore, the contribution of the S–O bond to the sulphur sequestration may be very limited.
Mild oxidation of carbons is a facile and viable method for doping of oxygen into carbon. As an example, Xiao et al.15 treated porous CNTs with water steam at 850 °C and then prepared a S–C composite by mixing the treated carbon with sulphur and heating at 160 °C for 12 h and at 180 °C for another 12 h. High-resolution X-ray photoelectron spectroscopy (XPS) spectra of the resultant S–C composite show distinct characteristics of the S–O and C–S bonds. As a result, a Li/S cell with such a cathode material exhibited an initial capacity of 1165 mA h−1 g−1 and remained 792 mA h−1 g−1 after 200 cycles at 0.2 C even when the sulphur content in the S–C composite reached as high as 89 wt%. The XPS analyses verify that the excellent capacity and capacity retention are due to the enhanced CAD of the carbon surface to sulphur species via the C–S and S–O bonds. However, discrepant results were reported by Li et al.16 who introduced oxygens into carbon by treating carbon with CO2 at 1050 °C for several minutes and prepared a S–C composite by heating the sulphur–carbon mixture at 150 °C for 9 h and at 300 °C for an additional 3 h. The cathode made by such a composite showed inferior performance, which these authors attributed to the reduced conductivity and possible side reactions occurring between sulphur and surface oxygens.
Provided that the oxygen-functionalised groups remain in the S–C composites, the >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯Li(SnLi), >O⋯Li(SnLi), and Li2Sn⋯HO– bindings are possible to exist between the ODC and polysulphide species. The ab initio simulations on several common binders of the sulphur cathode by Seh et al.25 reveal that strong >C
O⋯Li(SnLi), >O⋯Li(SnLi), and Li2Sn⋯HO– bindings are possible to exist between the ODC and polysulphide species. The ab initio simulations on several common binders of the sulphur cathode by Seh et al.25 reveal that strong >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯Li(SnLi) binding exists between the double-bonded oxygens in the carbonyl groups and the Li+ ions in Li2S and PS molecules with typical binding energies in the range of 1.20–1.26 eV. Based on this finding, Park et al.26 made a significant improvement in the sulphur sequestration by coating CNTs with an ester-based polymer. The >O⋯Li(SnLi) binding energies computed by the DTF calculations, suggest that the epoxide and hydroxyl oxygens on the graphene surface do not bind the neutral S3 cluster, instead they increase the binding between the graphene and negatively charged S3− and S32− clusters indirectly through the O⋯Li+ complexing bond as the binding bridge.19 Sulphur sequestration by the Li2Sn⋯HO– hydrogen-bond was first proposed and experimentally verified by Zhang et al.27 who coated a gelable poly(acrylic acid) polymer onto the surface of a conventional sulphur cathode and observed a significant reduction in the out-diffusion of PS. In addition to enhancing the CAD, the oxygen HAs are also shown to improve the hydrophilicity of the carbon surface and facilitate the electrochemical redox of PS species on the carbon surface. The former improves the distribution of PS species and the liquid electrolyte on the cathode, and the latter facilitates the deposition of insoluble sulphur reduction products (Li2S2 and Li2S) in discharge.28,29
O⋯Li(SnLi) binding exists between the double-bonded oxygens in the carbonyl groups and the Li+ ions in Li2S and PS molecules with typical binding energies in the range of 1.20–1.26 eV. Based on this finding, Park et al.26 made a significant improvement in the sulphur sequestration by coating CNTs with an ester-based polymer. The >O⋯Li(SnLi) binding energies computed by the DTF calculations, suggest that the epoxide and hydroxyl oxygens on the graphene surface do not bind the neutral S3 cluster, instead they increase the binding between the graphene and negatively charged S3− and S32− clusters indirectly through the O⋯Li+ complexing bond as the binding bridge.19 Sulphur sequestration by the Li2Sn⋯HO– hydrogen-bond was first proposed and experimentally verified by Zhang et al.27 who coated a gelable poly(acrylic acid) polymer onto the surface of a conventional sulphur cathode and observed a significant reduction in the out-diffusion of PS. In addition to enhancing the CAD, the oxygen HAs are also shown to improve the hydrophilicity of the carbon surface and facilitate the electrochemical redox of PS species on the carbon surface. The former improves the distribution of PS species and the liquid electrolyte on the cathode, and the latter facilitates the deposition of insoluble sulphur reduction products (Li2S2 and Li2S) in discharge.28,29
Based on the above results, the roles of ODCs in enhancing the sulphur sequestration can be summarised as: (1) covalently immobilising PS anions onto carbon surfaces via S–C and S–O covalent bonds,15,30 (2) complexing PS molecules with carbon surfaces indirectly through >O⋯Li+ and >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯Li+(SnLi)− bindings,23,25 (3) forming Li2Sn⋯HO– hydrogen-bond between the PS anion and –OH group,27 and (4) increasing the hydrophilicity of carbon surfaces to facilitate the redox of sulphur species and the deposition of Li2S2 and Li2S.28,29 The CAD in a single system may involve one or more of the chemical interactions listed above, depending on the type and concentration of the oxygen-functionalised groups on the surface of the ODC.
O⋯Li+(SnLi)− bindings,23,25 (3) forming Li2Sn⋯HO– hydrogen-bond between the PS anion and –OH group,27 and (4) increasing the hydrophilicity of carbon surfaces to facilitate the redox of sulphur species and the deposition of Li2S2 and Li2S.28,29 The CAD in a single system may involve one or more of the chemical interactions listed above, depending on the type and concentration of the oxygen-functionalised groups on the surface of the ODC.
3.2. Sulphur-doped carbons (SDCs)
SDCs are typically made through three approaches: (1) sulphurising of commercial or synthetic carbons with a sulphur source, (2) pyrolysing of sulphur-containing precursors, and (3) heating of mixtures consisting of a carbon precursor and a sulphur source.31 In these approaches, the sulphurisation is based on the reactions of surface functional groups on carbon with a sulphur source, in which the surface functional groups may be any species that can be thermally eliminated or substituted by sulphur such as C–H, –OH, >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –C(
O, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)OH and >C
O)OH and >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C<. The sulphur source and precursor may be any type of compounds containing sulphur, such as elemental sulphur, H2S, SO2, CS2, sulphur-containing organic compounds and polymers. Of particular interest are sulphur-containing precursors in which the covalent C–S bonds are already present, providing a facile approach for making the SDC with a relatively high sulphur content. In the SDC, sulphurs are present in multiple forms of functional groups, such as –SnH, –Sn–, >C
C<. The sulphur source and precursor may be any type of compounds containing sulphur, such as elemental sulphur, H2S, SO2, CS2, sulphur-containing organic compounds and polymers. Of particular interest are sulphur-containing precursors in which the covalent C–S bonds are already present, providing a facile approach for making the SDC with a relatively high sulphur content. In the SDC, sulphurs are present in multiple forms of functional groups, such as –SnH, –Sn–, >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S, –C(
S, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)Sn–, –C(
S)Sn–, –C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)SnH, –S(
S)SnH, –S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2OH, and –S(
O)2OH, and –S(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)2O–. However, these functional groups are eventually converted to the most stable monosulphur (–S–) through the elimination or carbothermal reaction at elevated temperatures. With an increase in the sulphur content, two or more sulphur atoms tend to be combined into short polysulphide chains (–Sn–) to form SCs, which have long been studied as the efficient sorbent for heavy metal ion removal in environmental pollution treatment32 and as a catalyst or catalyst support.31 The temperature and pressure are two major factors affecting the sulphur content and the length of the polysulphide chains. In general, high temperature leads to short polysulphide chains and low sulphur contents.33,34 As such, the SDCs are among the SC materials with low sulphur contents and short polysulphide chains. Based on the results of elemental analysis and thermogravimetric analysis (TGA), a calculation on the SC made by heating a 1
O)2O–. However, these functional groups are eventually converted to the most stable monosulphur (–S–) through the elimination or carbothermal reaction at elevated temperatures. With an increase in the sulphur content, two or more sulphur atoms tend to be combined into short polysulphide chains (–Sn–) to form SCs, which have long been studied as the efficient sorbent for heavy metal ion removal in environmental pollution treatment32 and as a catalyst or catalyst support.31 The temperature and pressure are two major factors affecting the sulphur content and the length of the polysulphide chains. In general, high temperature leads to short polysulphide chains and low sulphur contents.33,34 As such, the SDCs are among the SC materials with low sulphur contents and short polysulphide chains. Based on the results of elemental analysis and thermogravimetric analysis (TGA), a calculation on the SC made by heating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (wt) mixture of polyacrylonitrile and sulphur at 300 °C for 4 h shows that sulphurs are present in the form of short polysulphide chains with an averaged n value of 3.37 in the –Sn– chains even if the sulphur content reaches as high as 49.1 wt%.35 Since the SCs themselves are a class of high capacity cathode materials, previous efforts on the subject have been centred on the SCs with the sulphur content of up to 50 wt%.14
4 (wt) mixture of polyacrylonitrile and sulphur at 300 °C for 4 h shows that sulphurs are present in the form of short polysulphide chains with an averaged n value of 3.37 in the –Sn– chains even if the sulphur content reaches as high as 49.1 wt%.35 Since the SCs themselves are a class of high capacity cathode materials, previous efforts on the subject have been centred on the SCs with the sulphur content of up to 50 wt%.14
Since sulphurs in the SCs are covalently bound to carbon, they are neither removed by the conventional thermal vaporisation near the sulphur's melting point (293 °C) nor extracted out by the solvent such as CS2, toluene, and electrolyte solvents.14 Therefore, the redox of sulphur species in a Li/SC cell can only occur on the solid–solid two-phase interface (i.e. the SC and Li2S phases), leading to unique electrochemical behaviours compared with the conventional S–C composites, as illustrated in Fig. 2. Firstly, the Li/SC cell shows only a slightly sloping discharge voltage plateau at ∼1.8 V due to the single solid–solid phase transition (Fig. 2a). Secondly, the first discharge of the Li/SC cell suffers significant voltage hysteresis and irreversible capacity loss due to the large grain boundary resistance (GBR) of fresh SC particles and irreversible formation of the solid electrolyte interphase (SEI) on the SC surface (Fig. 2a). Thirdly, the Li/SC cell prefers a carbonate-based electrolyte due to the insolubility of SC and its reduction intermediates/products, which substantially mitigates the reactivity of sulphur species with carbonate solvents (Fig. 2b). Other unique features of the Li/SC cells are near 100% coulombic efficiency, extremely low self-discharge, and excellent safety.14
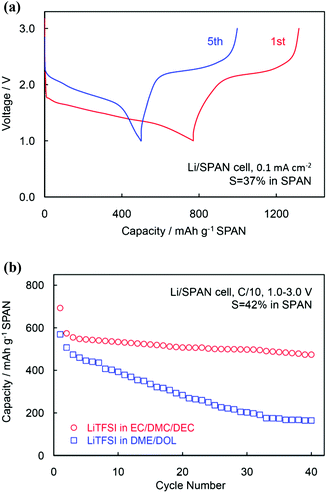 | ||
| Fig. 2 (a) Voltage profile and (b) electrolyte preference of the Li/SC cells. The figure is reprinted from ref. 35, an open access without Copyright. | ||
The SDCs sequestrate soluble PS through sulphur–sulphur chemical interactions as indicated by eqn (1) and eqn (2), and the sequestrating ability increases with a decrease in the length of polysulphide chains (i.e., the n value in the –Sn– chain). In solutions, it is possible for sulphur to covalently insert into the short –Sn– chains, forming longer chains, as indicated by eqn (3).
| –Sn– + Li2Sm ↔ >Sn⋯SmLi2 | (1) |
| >Sn⋯SmLi2 ↔ –Sn+xLi + –Sm−xLi | (2) |
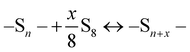 | (3) |
The S–C composites made of the SDC share many similarities with the SC materials, and hence the SC materials can be used to understand the role of the S-doped carbons in sulphur sequestration.
Synthetic carbons contain more or less surface functional groups. This feature has been widely adopted to produce SC materials on a large scale. In this effort, Chang36 synthesised three SCs with sulphur contents of 24.8, 26.0, and 38.1 wt%, respectively, by reacting carbon with SO2 at 600 °C for several hours, and showed that the Li/SC cells had an ∼1.7 V discharge voltage plateau with a 96% sulphur utilisation. A similar concept was reported by Kim et al.37 who heated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (wt) mixture of mesoporous hard carbon spheres and sulphur at 150 °C for 7 h and held the mixture at 300 °C for another 2 h. They observed that the stable SC could be formed only when the sulphur content was not more than 20 wt%, beyond which extra sulphurs combine into elemental sulphur. On the other hand, based on the carbothermal reaction of carbon, Ning et al.38 synthesised a SDC with the sulphur content of up to 26.8 wt% by reacting MgSO4 as the solid sulphur source with carbon at 700 °C for 30 minutes. They found that S-doping considerably enhanced the coulombic efficiency in the initial SEI formation of a carbon anode material. In the same principle, Li et al.39 prepared a pre-lithiated SC by reacting Li2SO4 and graphene nanoplatelet aggregates as indicated by eqn (4), and found that the obtained product had excellent electrochemical behaviour in the Li/S batteries.
5 (wt) mixture of mesoporous hard carbon spheres and sulphur at 150 °C for 7 h and held the mixture at 300 °C for another 2 h. They observed that the stable SC could be formed only when the sulphur content was not more than 20 wt%, beyond which extra sulphurs combine into elemental sulphur. On the other hand, based on the carbothermal reaction of carbon, Ning et al.38 synthesised a SDC with the sulphur content of up to 26.8 wt% by reacting MgSO4 as the solid sulphur source with carbon at 700 °C for 30 minutes. They found that S-doping considerably enhanced the coulombic efficiency in the initial SEI formation of a carbon anode material. In the same principle, Li et al.39 prepared a pre-lithiated SC by reacting Li2SO4 and graphene nanoplatelet aggregates as indicated by eqn (4), and found that the obtained product had excellent electrochemical behaviour in the Li/S batteries.
| Li2SO4 + 2C → Li2S + 2CO2 | (4) |
While numerous organic sulphides, sulphones, sulphonic acids, and thiophene-based compounds/polymers are available for the precursors of the SDC,31 natural gas, liquefied natural gas and sulphurised rubbers (e.g. wasted tires) provide a vast resource of cheap and abundant precursors for large-scale production of the SDC by the pyrolysis of S-containing precursors. Owing to the limited sulphur content in these precursors and the inevitable loss of small S-containing molecular moieties in the pyrolysis, the sulphur contents in such-obtained SDCs generally do not exceed 5 wt%. Though such materials have been widely used for the removal of heavy metal ions in the environmental pollution treatment, there are no publications available for their applications in the Li/S batteries.
Heating of the mixture of a carbon precursor (preferably a non-volatile polymer) and elemental sulphur provides a facile approach for the preparation of SC materials with a high sulphur content. In heating, elemental sulphur not only serves as the sulphur source but also acts as the dehydrogenator to promote the generation of >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C< double bonds and resultant vulcanisation. It was reported that sulphurised polyethylene could be made simply by heating a mixture of polyethylene (PE) and sulphur at 160–365 °C.40 This process typically produced sulphurised polyethylene with a sulphur content of 20–80 wt% and a conductivity of 4.4 × 10−14–2.0 × 10−9 S cm−1, depending on the PE/S ratio, heating temperature and time. Interestingly, the conductivity increases to the range of 2.0 × 10−7–4.9 × 10−6 S cm−1 when doped with iodine, indicating that the well-conjugated >C
C< double bonds and resultant vulcanisation. It was reported that sulphurised polyethylene could be made simply by heating a mixture of polyethylene (PE) and sulphur at 160–365 °C.40 This process typically produced sulphurised polyethylene with a sulphur content of 20–80 wt% and a conductivity of 4.4 × 10−14–2.0 × 10−9 S cm−1, depending on the PE/S ratio, heating temperature and time. Interestingly, the conductivity increases to the range of 2.0 × 10−7–4.9 × 10−6 S cm−1 when doped with iodine, indicating that the well-conjugated >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C< double bonds are present in the sulphurised polyethylene.
C< double bonds are present in the sulphurised polyethylene.
Sulphurisation of polyacrylonitrile (PAN) was first reported by Wang et al.41 who heated a mixture of PAN and sulphur at 280 to 300 °C in argon for 6 h, and followed by intensive investigation to optimise the sulphurisation conditions and understand the electrochemistry of sulphurised polyacrylonitrile (SPAN).42–44 Though still poorly understood and debated, the PAN sulphurisation can be generally described by Scheme 1. In heating, elemental sulphur dehydrogenates PAN to produce >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C< double bonds by loss of small H2S molecules and the –CN group promotes cyclisation of conjugated six-member aromatic rings. Meanwhile, sulphur vulcanises the resultant >C
C< double bonds by loss of small H2S molecules and the –CN group promotes cyclisation of conjugated six-member aromatic rings. Meanwhile, sulphur vulcanises the resultant >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C< double bonds to form C–S bonds, through which the short polysulphide chains are covalently bound to the cyclised, partially dehydrogenated, and ribbon-like PAN backbones.35,45
C< double bonds to form C–S bonds, through which the short polysulphide chains are covalently bound to the cyclised, partially dehydrogenated, and ribbon-like PAN backbones.35,45
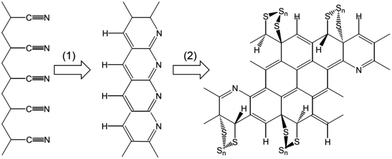 | ||
| Scheme 1 Reactions involved in the PAN sulphurisation and possible chemical structure of SPAN. (1) Sulphur-assisted cyclisation by release of H2S. (2) Vulcanisation. | ||
A suitable temperature for the PAN sulphurisation is in the range of 280 to 550 °C, which typically generates SPAN with a sulphur content of 30–55 wt%. In general, high temperature leads to low sulphur contents and short polysulphide chains with a relatively high electronic conductivity, which corresponds to low specific capacity but stable capacity retention; low temperature can neither break down the S8 ring into small Sn molecules nor dehydrogenate polymer effectively, resulting in high sulphur contents and long polysulphide chains with relatively low electronic conductivity, which corresponds to high specific capacity but fast capacity fading.46,47 Since the short polysulphide chains are covalently bound to the polymer backbone, the SPANs are thermally stable until 450 °C and do not form soluble PS in discharge, exhibiting very stable capacity retention, extremely low self-discharge, and excellent safety.14 By optimising the PAN/S ratio, sulphurisation temperature and time, Wang et al.43 synthesised SPAN with 42 wt% S, showing that the Li/SC cell had a specific capacity of 811 mA h−1 g−1 with respect to the mass of entire SPAN in the second discharge and stabilised 795 mA h−1 g−1 after 50 cycles. These numbers are even higher than the theoretical capacity (1675 mA h−1 g−1) of elemental sulphur if normalised to the mass of active sulphur in the SPAN, because of the extra contribution of conjugated SPAN polymeric backbones to the overall capacity.14,35,45 Inspired by the knowledge of rubber vulcanisation, on the other hand, Chen et al.48 added ∼5 wt% 2-mercaptobenzothiazole (a common vulcanisation accelerator for rubber vulcanisation) into a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PAN/S mixture, which led the SPAN to an ∼8 wt% increase in the sulphur content and an ∼120 mA h−1 g−1 increase in the specific capacity compared with the control. This finding reveals that the vulcanisation accelerator effectively enhances the efficiency of sulphurisation, offering a facile method for the preparation of SPAN and other SCs.
1 PAN/S mixture, which led the SPAN to an ∼8 wt% increase in the sulphur content and an ∼120 mA h−1 g−1 increase in the specific capacity compared with the control. This finding reveals that the vulcanisation accelerator effectively enhances the efficiency of sulphurisation, offering a facile method for the preparation of SPAN and other SCs.
On the other hand, See et al.49 modified mesoporous carbon by in situ polymerising thiophene onto the carbon surface and then pyrolysing at 800 °C under a 5% H2 flow for 4 h. The XPS analysis verified that sulphurs in SDCs were covalently bound to carbon, and a cell cycling test showed that a 5.5 wt% S doping led to as high as a 50% increase in the capacity of the S–C composite with much stable retention, as compared with the pristine carbon, although they found these sulphurs did not provide any capacities. Suggested by the relatively low heat observed when PS species were titrated into SDCs, they considered that the achieved improvement in sulphur sequestration was due to the enhanced affinity between the strongly polar PS anions and non-polar carbon surfaces as a result of S-doping converting the carbon surface from being hydrophobic to hydrophilic. In fact, this explanation is in principle not contradictory with eqn (1), predicting that the S⋯S complex binding between the PS anions and SDCs contributes to the sulphur sequestration of SDCs. In addition, it should be pointed out that the Li/S cells by See et al. did not behave like a Li/SC battery, instead exhibited two distinct voltage plateaus at ∼2.3 V and ∼2 V, respectively, in the first discharge.49 This is because (1) the S–C composite was prepared at a relatively low temperature (155 °C for 2 h), which could not enable effective sulphurisation, and (2) ethylmethyl sulfone was used as the electrolyte solvent, in which PS has high solubility.
In brief, the SDC sequestrate sulphur species through the C–S bonds that anchor short polysulphide anions to carbon via either S⋯S complex binding (eqn (1)) or S–S covalent binding (eqn (2)). In solutions, it is possible for sulphur to covalently insert into the short polysulphide chains in the SDC, forming longer polysulphide chains (eqn (3)). The sulphur content and the polysulphide chain length are two important factors determining the sequestrating efficiency. When the sulphur content is low, the S–C composites with SDCs behave in very similar manners as the SCs that show only a single discharge voltage plateau at ∼1.8 V and prefer a carbonate-based electrolyte for cycling.
3.3 Nitrogen-doped carbons (NDCs)
In order to distinguish nitrogen-rich compounds such as graphitic carbon nitride (C3N4), NDCs are referred to as these carbons with the N/C atomic ratio less than 1. The NDCs are prepared either by post-treatment of carbons with a nitrogen source such as NH3 and CH3CN or by carbonisation of nitrogen-containing precursors.9 The post-treatment generally dopes nitrogen on the surface with the nitrogen content lower than 5 wt% whereas the carbonisation dopes nitrogen throughout the bulk with the nitrogen content of up to more than 20 wt%. The nitrogen content in NDCs greatly depends on the precursor and preparation conditions with a trend that high reaction temperature leads to a low nitrogen content.9,50 In order to obtain a high nitrogen content, non-volatile ionic liquids and polymers are preferable precursors. However, the upper limits of the nitrogen content are 14.32 wt% for those made at 1000 °C and 21.66 wt% for those made at 900 °C, irrespective of the precursor or preparation conditions.9Identified by three distinguishable peaks in the binding energy range of 398–404 eV in the high resolution XPS spectra,51–53 nitrogens in NDCs are present in three main forms of pyridinic N, pyrrolic N, and quaternary N, as indicated in Fig. 3. The ab initio calculations indicate that the first two types of nitrogens are more effective in forming LiSnLi+⋯N binding and dominate the sequestration of Li2Sn.52 Other nitrogen-functionalised groups such as –NH2, –CN, –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –NO2 groups are rarely present because –NH2 is either eliminated in pyrolysis or substituted by sulphur in the process of making S–C composites; –CN is easily eliminated or converted to more stable pyridinic or pyrrolic nitrogens by forming conjugated aromatic rings in pyrolysis; –N
O and –NO2 groups are rarely present because –NH2 is either eliminated in pyrolysis or substituted by sulphur in the process of making S–C composites; –CN is easily eliminated or converted to more stable pyridinic or pyrrolic nitrogens by forming conjugated aromatic rings in pyrolysis; –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –NO2 are carbothermally reduced in pyrolysis. Sulphur sequestration of NDCs is mainly through the chemical binding between the Li+ ion in Li2Sn and the strong electron-donating lone-pair electrons in nitrogen, namely the LiSnLi+⋯N binding. The similar principle is well-known in solution chemistry and has long been used for the syntheses of polysulphide clusters of alkaline earth metals and transition metals, in which N-containing solvents were frequently used to promote the dissolution of polysulphide clusters through strong solvation between the metal ion and the lone-pair electrons of nitrogen.54–56 Besides the LiSnLi+⋯N binding, the C–S bond makes additional contribution to the sulphur sequestration because the synthetic carbons contain more or less hydrogens that are dehydrogenated by sulphur and subsequently vulcanised to form C–S bonds in the process of making S–C composites.
O and –NO2 are carbothermally reduced in pyrolysis. Sulphur sequestration of NDCs is mainly through the chemical binding between the Li+ ion in Li2Sn and the strong electron-donating lone-pair electrons in nitrogen, namely the LiSnLi+⋯N binding. The similar principle is well-known in solution chemistry and has long been used for the syntheses of polysulphide clusters of alkaline earth metals and transition metals, in which N-containing solvents were frequently used to promote the dissolution of polysulphide clusters through strong solvation between the metal ion and the lone-pair electrons of nitrogen.54–56 Besides the LiSnLi+⋯N binding, the C–S bond makes additional contribution to the sulphur sequestration because the synthetic carbons contain more or less hydrogens that are dehydrogenated by sulphur and subsequently vulcanised to form C–S bonds in the process of making S–C composites.
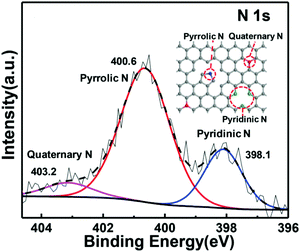 | ||
| Fig. 3 XPS spectrum of NDCs and three main forms of nitrogens in NDCs. The figure is reprinted with permission from ref. 53. Copyright (2015) John Wiley and Sons. | ||
Using the post-treatment method, Qiu et al.52 prepared N-doped graphene by treating GO sheets under a NH3 atmosphere at 750 °C for 30 minutes. It was shown that the treatment not only reduced GO but also introduced nitrogens into the graphene sheets, resulting in N-doped graphene with an N/C atomic ratio of ∼3.9%. The cathode with a 60 wt% S versus the overall electrode delivered initial capacities of ∼1167 mA h−1 g−1 at 0.2 C and ∼802 mA h−1 g−1 at 2 C, and cycled for 2000 cycles with an averaged capacity fading rate as low as 0.028% per cycle and an averaged coulombic efficiency of above 97%. The XPS analysis and ab initio calculation indicate that the excellent performances are due to the strong LiSnLi+⋯N binding that effectively hinders the dissolved PS from diffusing out of the cathode. Using the same principle, Li et al.16 treated a commercial carbon black with NH3 at 1050 °C for 3–5 minutes, which led to a NDC with 1.5 wt% N. Using such an NDC, a S–C composite with a 60 wt% S was prepared and shown to have significantly improved capacity, capacity retention and rate capability compared with the control. In particular, the composite delivered a capacity of about 1490 mA h−1 g−1 in the first discharge and retained 1020 mA h−1 g−1 after 40 cycles with a coulombic efficiency of 93%. The XPS analysis indicates that the defect sites of NDCs are favourable for the deposition of discharge products, leading to high utilisation and reversibility of sulphur cathodes. This observation agrees with the fact that the N atoms chemically adsorb PS molecules through the LiSnLi+⋯N binding and consequently facilitate the electrochemical redox of PS on the carbon surface.
Carbonisation of N-containing precursors has been one of the most studied approaches for the preparation of NDCs. In this practice, Xu et al.57 synthesised a NDC by pyrolysing a polydopamine at 800 °C for 1 h, and used it to prepare a S–NDC composite. The results showed that the composite retained a reversible capacity of ∼605 mA h−1 g−1 after 500 cycles at 2 C with a low capacity fading rate of 0.030% per cycle. In addition to the highly porous structure of NDCs, the strong chemical interactions of the electron-donating N and O atoms with the Li+ ions in PS molecules are responsible for this excellent cyclability. Aiming to enhance the efficiency of sulphur sequestration, Sun et al.58 developed a colloidal silica assisted sol–gel process for the preparation of mesoporous NDCs. In their process, a mixture of phenol, melamine and formaldehyde was condensed in a solution containing SiO2 sol. The resulting polymer resin was dried and carbonised at 800 °C for 3 h, followed by etching SiO2 away to generate a mesoporous structure. They found that N-doping assisted mesoporous carbon to sequestrate sulphur through enhanced surface interactions between the basic nitrogen functionalities and polysulphide species, but meanwhile adversely affected the electronic conductivity of the carbon matrix. Only when the nitrogen content is in the range of 4–8 wt%, the NDCs are able to improve the performance of Li/S batteries. An optimal nitrogen content was determined to be 8.1 wt%, which resulted in the S–C composite demonstrating a reversible capacity of 758 mA h−1 g−1 at 0.2 C and 620 mA h−1 g−1 at 1 C after 100 cycles.
Conducting polymers such as polyaniline and polypyrrole have been proven to be excellent precursors for NDCs with a novel pore structure because of their flexibility and easiness to be synthesised into desirable nanopore structures. In this effort, Xiao et al.59 synthesised polyaniline nanotubes, followed by treating them with sulphur at 280 °C to obtain an S–NDC composite. As a result of the in situ vulcanisation, the obtained composite is of a three-dimensional structure with covalently crosslinked polymeric frameworks that provide strongly physical and chemical sequestration to sulphur. After tens of activation cycles, which were due to the poor penetration of the liquid electrolyte into the nanostructured pores, the S–NDC composite exhibited superior cycling stability and rate capability with an initial capacity of 755 mA h−1 g−1 at 0.1 C, which stabilised 837 mA h−1 g−1 after 100 cycles. When cycled at 1 C, the cell was able to retain a capacity of 432 mA h−1 g−1 with a coulombic efficiency of over 90% even after 500 cycles. The observed improvement is attributed to not only the novel nanostructured pores but also the enhanced chemical binding between the electron-donating N atoms and polysulphide species.
Pyrolysed PAN is of particular interest in the understanding of the sulphur sequestration mechanism of NDCs. In order to confirm the LiSnLi+⋯N interactions, two composites of the pyrolysed PAN with elemental sulphur and lithium polysulphide (Li2S3), respectively, were prepared by the procedures described in Table 2 and compared in Fig. 4. It can be observed from Fig. 4a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 that the S–NDC composite without Li+ ions in its initial composition shows only a single discharge voltage plateau at ∼1.8 V, indicative of a SC in which the sulphur species are sequestrated through the C–S covalent bond formed in the process of making the S–NDC composite through the vulcanisation of >C
60 that the S–NDC composite without Li+ ions in its initial composition shows only a single discharge voltage plateau at ∼1.8 V, indicative of a SC in which the sulphur species are sequestrated through the C–S covalent bond formed in the process of making the S–NDC composite through the vulcanisation of >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C< double bonds and the substitution of sulphur for remaining hydrogens and removable nitrogen-functionalised groups such as –NH2. In contrast, the Li2S3–NDC composite with Li+ ions in its initial composition exhibits two discharge voltage plateaus at ∼2.3 V and ∼2 V, as shown in Fig. 4b,61 with significantly improved capacity and capacity retention compared with the conventional Li2S3–C composite. A remarkable difference in the cells’ voltage profile between the S–NDC and Li2S3–NDC composites strongly supports the role of LiSnLi+⋯N binding in sulphur sequestration.
C< double bonds and the substitution of sulphur for remaining hydrogens and removable nitrogen-functionalised groups such as –NH2. In contrast, the Li2S3–NDC composite with Li+ ions in its initial composition exhibits two discharge voltage plateaus at ∼2.3 V and ∼2 V, as shown in Fig. 4b,61 with significantly improved capacity and capacity retention compared with the conventional Li2S3–C composite. A remarkable difference in the cells’ voltage profile between the S–NDC and Li2S3–NDC composites strongly supports the role of LiSnLi+⋯N binding in sulphur sequestration.
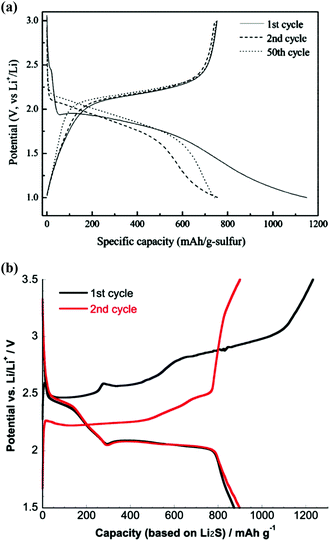 | ||
| Fig. 4 Voltage profile of (a) S–NDC composite and (b) Li2S3–NDC composite. The figures are reprinted with permission from ref. 60 and 61. Copyright (2009 and 2013) American Chemical Society. | ||
| S–NDC composite | Li2S3–NDC composite | |
|---|---|---|
| Note: DMF = N,N-dimethyl formamide, PC = propylene carbonate, EC = ethylene carbonate, DEC = diethylene carbonate, LiTFSI = lithium bis(trifluoromethanesulfonyl)imide, TG = tetraethylene glycol dimethyl ether, and PYR14TFSI = N-methyl-(N-butyl) pyrrolidinium bis(trifluoromethanesulfonyl)imide. | ||
| Preparation | PAN and Na2CO3 were mixed using DMF solvent, followed by pyrolysing at 750 °C for 2 h; a composite with 57 wt% S was prepared by heating a S–NDC mixture at 300 °C for 3 h | PAN was dissolved in a Li2S3 DMF solution, followed by drying and pyrolysing at 300 °C for 2 h and at 600 °C for another 30 min. |
| Electrolyte | 1 M LiPF6 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (vol.) PC/EC/DEC 5 (vol.) PC/EC/DEC |
1 M LiTFSI 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (vol.) TG/PYR14TFSI 1 (vol.) TG/PYR14TFSI |
| Results | Significant capacity loss in the 1st discharge; a single discharge voltage plateau at ∼1.8 V | Significant capacity loss in the 1st charge; two discharge voltage plateaus at ∼2.3 V and ∼2 V, respectively |
| Comment | Behave as a sulphurised carbon; sequestrate sulphur via C–S bonds | Behave as a normal Li/S cell; sequestrate sulphur via LiSnLi+⋯N binding |
| Reference | 60 | 61 |
3.4. Multiple HA codoped carbons
As highlighted above, the oxygen, sulphur, and nitrogen HAs enhance the sulphur sequestration in different mechanisms. Thus, there is a need to know whether codoping of these HAs can lead to a synergistic improvement in the sulphur sequestration. In this effort, Wang's group intensively investigated O, N-codoped mesoporous carbon that was synthesised by heating a mixture of poly(melamine-co-formaldehyde) resin as the carbon precursor and colloidal silica nanoparticles as the pore generator at 900 °C for 2 h, followed by the removal of silica using hydrofluoric acid solution.62,63 The resultant mesoporous carbon, containing the oxygen- and nitrogen-functionalised groups, was mixed with sulphur and heated at 155 °C for 10 h to form a C–S nanocomposite. While obtaining much improved capacity and capacity retention, they analysed K-edge X-ray absorption near edge structure (XANES) spectra of the O, N and S atoms, finding that the C and N spectra were barely changed before and after sulphur loading because there were no Li+ ions in the C–S composite. This observation coincides with the conclusion that the doped N atoms adsorb PS species through Li+ ions, namely the LiSnLi+⋯N binding as reported elsewhere,51–53 other than directly through the sulphur atoms. However, the O K-edge XANES spectrum was subject to a considerable change after sulphur loading as shown in Fig. 5a, whereas the similar change was not observed from the counterpart carbon without N-doping. Therefore, these authors attributed the improved sulphur sequestration to the enhanced chemical adsorption between sulphur species and oxygen-functionalised groups on the carbon surface, which was promoted by N-doping. Based on the DFT calculations, these authors proposed chemical adsorption of sulphur species through the O–S bonds, as schematically illustrated in Fig. 5b.63 However, these authors did not explain how the –OSO2– group and –OSO3− anion with the S valence of +6 were formed in the strongly reductive environment where the S–C composite was prepared.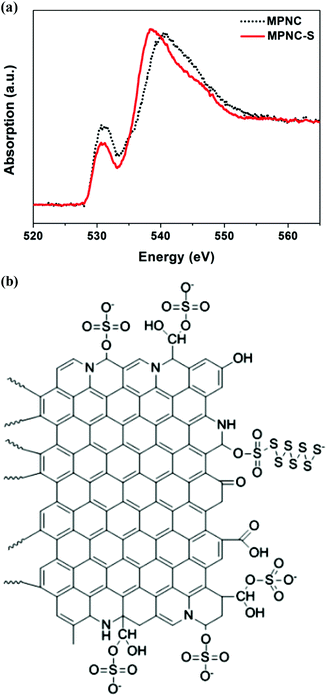 | ||
| Fig. 5 (a) Oxygen K-edge XANES spectra before and after sulphur loading,62 (b) proposed chemical binding between sulphur species and oxygen-functionalised groups.63 The figures are reprinted with permission from ref. 62 and 63. Copyright (2014) John Wiley and Sons and Copyright (2014) American Chemical Society. | ||
On the other hand, Zhou et al.64 investigated the synergistic effect of N,S-codoping on the sulphur sequestration. They prepared N,S-codoped graphene sponge by the hydrothermal reaction of GO sponge and thiourea at 180 °C for 12 h, and compared it with the single S-doped and N-doped counterparts. The S 2p XPS spectrum of N,S-codoped graphene shows distinct binding energy peaks of S–O bonds at 165.9 eV and S–S/S–C bonds at 164.9 and 163.7 eV; and the N 1s XPS spectrum shows peaks of quaternary N at 401.2 eV, pyrrolic N at 399.7 eV, and pyridinic N at 398.6 eV. Using graphene sponge as the cathode current collector and a 1.0 M Li2S6 solution as the catholyte, Li/dissolved polysulphide cells were assembled and tested. A long-term cycling test indicated that N,S-codoping remarkably enhanced the specific capacity and rate capability compared with the single HA-doping in an order of N,S-codoped graphene ≫ N-doped graphene > S-doped graphene > O-doped graphene (i.e. rGO). In particular, the N,S-codoped graphene cell exhibited an initial capacity of 925 mA h−1 g−1 and stabilised ∼670 mA h−1 g−1 after 200 cycles. The DFT calculations on several small model compounds reveal that the remarkable superiority is attributed to the synergistic enhancement of N,S-codoping in the chemical binding between the sulphur/nitrogen heteroatoms and lithium polysulphide/Li2S, which significantly reduces the loss of sulphur active materials and consequently suppresses the redox shuttle effect.
4. Conclusions and outlook
Porous carbons sequestrate sulphur species through PAB and CAD. The PAB depends on the pore structure and porosity of carbons, whereas the CAD relies on the specific surface area and surface functional groups. The CAD not only sequestrates sulphur species but also facilitates the redox and deposition of sulphur species on the carbon surface, leading to high specific capacity and stable capacity retention. The CAD capacity of HA-doped carbons to sulphur species increases with the concentration of HAs (i.e. the adsorbing sites), which on the other hand adversely affects the electronic conductivity of the carbon matrix, leading to an optimal concentration for the performance of Li/S batteries. The oxygen, sulphur, and nitrogen HAs enhance the CAD by different mechanisms. Oxygen-functionalised groups are either removable or oxidative, which are easily eliminated or reacted with sulphur to form C–S bonds in the process of preparing C–S composites. In addition to the C–S bonds that covalently anchor short polysulphide chains to the carbon surface, remaining oxygens are also responsible for sulphur sequestration through the >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯Li+SnLi, >O⋯Li+SnLi, and –OH⋯Li+SnLi bindings. In the S-doped carbons, sulphurs are able to exist up to 50 wt% in the form of short polysulphide (–Sn–) chains, which are covalently bound to the surface or/and frameworks of carbon. Therefore, the S-doped carbons sequestrate sulphur mainly through the C–S bond. Nitrogen-functionalised groups are strongly bound to the graphitic plane in three main forms of pyridinic, pyrrolic, and quaternary nitrogens, among which the first two types of nitrogens are more effective in forming LiSnLi+⋯N binding via the N lone-pair electrons. Unless formed during the pyrolysis of the mixture of nitrogen-containing precursors and elemental sulphur, the C–S bonds are barely present in the N-doped carbons. Therefore, the N-doped carbons sequestrate sulphur mainly through the LiSnLi+⋯N binding, a similar principle of the metal ion solvation in solution chemistry. Since hydrogens inevitably exist in the synthetic carbons and sulphur is an excellent dehydrogenator, the process of preparing S–C composites near or above the sulphur's critical temperature (159.4 °C) results in the formation of more or less C–S bonds, which makes additional contribution to the sulphur sequestration. Codoping of two or more HAs may lead to synergistic enhancement in the sulphur sequestration, which could be a promising direction for future development of porous carbon materials to be used in practically viable Li/S batteries.
O⋯Li+SnLi, >O⋯Li+SnLi, and –OH⋯Li+SnLi bindings. In the S-doped carbons, sulphurs are able to exist up to 50 wt% in the form of short polysulphide (–Sn–) chains, which are covalently bound to the surface or/and frameworks of carbon. Therefore, the S-doped carbons sequestrate sulphur mainly through the C–S bond. Nitrogen-functionalised groups are strongly bound to the graphitic plane in three main forms of pyridinic, pyrrolic, and quaternary nitrogens, among which the first two types of nitrogens are more effective in forming LiSnLi+⋯N binding via the N lone-pair electrons. Unless formed during the pyrolysis of the mixture of nitrogen-containing precursors and elemental sulphur, the C–S bonds are barely present in the N-doped carbons. Therefore, the N-doped carbons sequestrate sulphur mainly through the LiSnLi+⋯N binding, a similar principle of the metal ion solvation in solution chemistry. Since hydrogens inevitably exist in the synthetic carbons and sulphur is an excellent dehydrogenator, the process of preparing S–C composites near or above the sulphur's critical temperature (159.4 °C) results in the formation of more or less C–S bonds, which makes additional contribution to the sulphur sequestration. Codoping of two or more HAs may lead to synergistic enhancement in the sulphur sequestration, which could be a promising direction for future development of porous carbon materials to be used in practically viable Li/S batteries.
Acknowledgements
The author thanks Dr C. Lundgren for her critical reading of the manuscript and valuable suggestions.Notes and references
- S. S. Zhang, J. Power Sources, 2013, 231, 153–162 CrossRef CAS PubMed.
- A. Manthiram, Y. Fu, S. H. Chung, C. Zu and Y. S. Su, Chem. Rev., 2014, 114, 11751–11787 CrossRef CAS PubMed.
- S. Urbonaite, T. Poux and P. Novák, Adv. Energy Mater., 2015, 5 CAS , 1500118.
- D. W. Wang, Q. Zeng, G. Zhou, L. Yin, F. Li, H. M. Cheng, I. R. Gentle and G. Q. M. Lu, J. Mater. Chem. A, 2013, 1, 9382–9394 CAS.
- Y. Yang, G. Zheng and Y. Cui, Chem. Soc. Rev., 2013, 42, 3018–3032 RSC.
- S. Evers and L. F. Nazar, Acc. Chem. Res., 2013, 46, 1135–1143 CrossRef CAS PubMed.
- L. Ma, K. E. Hendrickson, S. Wei and L. A. Archer, Nano Today, 2015, 10, 315–338 CrossRef CAS PubMed.
- J. Wang, Y. S. He and J. Yang, Adv. Mater., 2015, 27, 569–575 CrossRef CAS PubMed.
- S. Zhang, S. Tsuzuki, K. Ueno, K. Dokko and M. Watanabe, Angew. Chem., Int. Ed., 2015, 54, 1302–1306 CrossRef CAS PubMed.
- Q. Zeng, X. Leng, K. H. Wu, I. R. Gentle and D. W. Wang, Carbon, 2015, 93, 611–619 CrossRef CAS PubMed.
- G. Zhou, L. Li, D. W. Wang, X. Y. Shan, S. Pei, F. Li and H. M. Cheng, Adv. Mater., 2015, 27, 641–647 CrossRef CAS PubMed.
- Z. Yunbo, M. Lixiao, N. Jing, X. Zhichang, H. Long, W. Bin and Z. Linjie, 2D Mater., 2015, 2, 024013, DOI:10.1088/2053-1583/2/2/024013.
- B. Meyer, Chem. Rev., 1976, 76, 367–388 CrossRef CAS.
- S. S. Zhang, Front. Energy Res., 2013, 1, 10, DOI:10.3389/fenrg.2013.00010.
- Z. Xiao, Z. Yang, H. Nie, Y. Lu, K. Yang and S. Huang, J. Mater. Chem. A, 2014, 2, 8683–8689 CAS.
- X. Li, X. Li, M. N. Banis, B. Wang, A. Lushington, X. Cui, R. Li, T. K. Sham and X. Sun, J. Mater. Chem. A, 2014, 2, 12866–12872 CAS.
- W. Zhou, S. Sasaki and A. Kawasaki, Carbon, 2014, 78, 121–129 CrossRef CAS PubMed.
- L. Zhang, L. Ji, P. A. Glans, Y. Zhang, J. Zhu and J. Guo, Phys. Chem. Chem. Phys., 2012, 14, 13670–13675 RSC.
- G. Zhou, L. C. Yin, D. W. Wang, L. Li, S. Pei, I. R. Gentle, F. Li and H. M. Cheng, ACS Nano, 2013, 7, 5367–5375 CrossRef CAS PubMed.
- L. Ji, M. Rao, H. Zheng, L. Zhang, Y. Li, W. Duan, J. Guo, E. J. Cairns and Y. Zhang, J. Am. Chem. Soc., 2011, 133, 18522–18525 CrossRef CAS PubMed.
- J. W. Kim, J. D. Ocon, D. W. Park and J. Lee, J. Energy Chem., 2013, 22, 336–340 CrossRef CAS.
- X. Feng, M. K. Song, W. C. Stolte, D. Gardenghi, D. Zhang, X. Sun, J. Zhu, E. J. Cairns and J. Guo, Phys. Chem. Chem. Phys., 2014, 16, 16931–16940 RSC.
- B. Wang, S. M. Alhassan and S. T. Pantelides, Phys. Rev. Appl., 2014, 2, 034004, DOI:10.1103/PhysRevApplied.2.034004.
- J. Yan, X. Liu, X. Wang and B. Li, J. Mater. Chem. A, 2015, 3, 10127–10133 CAS.
- Z. W. Seh, Q. Zhang, W. Li, G. Zheng, H. Yao and Y. Cui, Chem. Sci., 2013, 4, 3673–3677 RSC.
- K. Park, J. H. Cho, J. H. Jang, B. C. Yu, A. T. De La Hoz, K. M. Miller, C. J. Ellison and J. B. Goodenough, Energy Environ. Sci., 2015, 8, 2389–2395 CAS.
- S. S. Zhang, D. T. Tran and Z. Zhang, J. Mater. Chem. A, 2014, 2, 18288–18292 CAS.
- X. Feng, M. K. Song, W. C. Stolte, D. Gardenghi, D. Zhang, X. Sun, J. Zhu, E. J. Cairns and J. Guo, Phys. Chem. Chem. Phys., 2014, 16, 16931–16940 RSC.
- J. H. Kim, K. Fu, J. Choi, S. Sun, J. Kim, L. Hu and U. Paik, Chem. Commun., 2015, 51, 13682–13685 RSC.
- J. W. Kim, J. D. Ocon, D. W. Park and J. Lee, J. Energy Chem., 2013, 22, 336–340 CrossRef CAS.
- W. Kiciński, M. Szala and M. Bystrzejewski, Carbon, 2014, 68, 1–32 CrossRef PubMed.
- K. A. Krishnan and T. S. Anirudhan, Ind. Eng. Chem. Res., 2002, 41, 5085–5093 CrossRef CAS.
- J. A. Korpiel and R. D. Vidic, Environ. Sci. Technol., 1997, 31, 2319–2325 CrossRef CAS.
- S. Kwon and R. D. Vidic, Environ. Eng. Sci., 2000, 17, 303–313 CrossRef CAS.
- S. S. Zhang, Energies, 2014, 7, 4588–4600 CrossRef PubMed.
- C. H. Chang, Carbon-sulphur compounds as cathodes for lithium high energy secondary cells, in Proceedings of 29th Power Sources Conference, The Electrochemical Society, Inc., Pennington, NJ, 1980, pp. 208–211 Search PubMed.
- J. Kim, D. J. Lee, H. G. Jung, Y. K. Sun, J. Hassoun and B. Scrosati, Adv. Funct. Mater., 2013, 23, 1076–1080 CrossRef CAS PubMed.
- G. Ning, X. Ma, X. Zhu, Y. Cao, Y. Sun, C. Qi, Z. Fan, Y. Li, X. Zhang, X. Lan and J. Gao, ACS Appl. Mater. Interfaces, 2014, 6, 15950–15958 CAS.
- Z. Li, S. Zhang, C. Zhang, K. Ueno, T. Yasuda, R. Tatara, K. Dokko and M. Watanabe, Nanoscale, 2015, 7, 14385–14392 RSC.
- B. A. Trofimov, T. A. Skotheim, A. G. Mal'kina, L. V. Sokolyanskaya, G. F. Myachina, S. A. Korzhova, E. S. Stoyanov and I. P. Kovalev, Russ. Chem. Bull., 2000, 49, 863–869 CrossRef CAS.
- J. Wang, J. Yang, J. Xie and N. Xu, Adv. Mater., 2002, 14, 963–965 CrossRef CAS.
- X. He, Q. Shi, X. Zhou, C. Wan and C. Jiang, Electrochim. Acta, 2005, 51, 1069–1075 CrossRef CAS PubMed.
- L. Wang, X. He, J. Li, J. Gao, J. Guo, C. Jiang and C. Wan, J. Mater. Chem., 2012, 22, 22077–22081 RSC.
- L. Wang, X. He, J. Li, M. Chen, J. Gao and C. Jiang, Electrochim. Acta, 2012, 72, 114–119 CrossRef CAS PubMed.
- J. Fanous, M. Wegner, J. Grimminger, Ä. Andresen and M. R. Buchmeiser, Chem. Mater., 2011, 23, 5024–5028 CrossRef CAS.
- X. Yu, J. Xie, Y. Li, H. Huang, C. Lai and K. Wang, J. Power Sources, 2005, 146, 335–339 CrossRef CAS PubMed.
- J. Fanous, M. Wegner, J. Grimminger, M. Rolff, M. B. M. Spera, M. Tenzer and M. R. Buchmeiser, J. Mater. Chem., 2012, 22, 23240–23245 RSC.
- H. Chen, C. Wang, C. Hu, J. Zhang, S. Gao, W. Lu and L. Chen, J. Mater. Chem. A, 2015, 3, 1392–1395 CAS.
- K. A. See, Y. S. Jun, J. A. Gerbec, J. K. Sprafke, F. Wudl, G. D. Stucky and R. Seshadri, ACS Appl. Mater. Interfaces, 2014, 6, 10908–10916 CAS.
- J. P. Paraknowitsch, J. Zhang, D. Su, A. Thomas and M. Antonietti, Adv. Mater., 2010, 22, 87–92 CrossRef CAS PubMed.
- Y. Wang, Y. Shao, D. W. Matson, J. Li and Y. Lin, ACS Nano, 2010, 4, 1790–1798 CrossRef CAS PubMed.
- Y. Qiu, W. Li, W. Zhao, G. Li, Y. Hou, M. Liu, L. Zhou, F. Ye, H. Li, Z. Wei, S. Yang, W. Duan, Y. Ye, J. Guo and Y. Zhang, Nano Lett., 2014, 14, 4821–4827 CrossRef CAS PubMed.
- G. Zhou, Y. Zhao and A. Manthiram, Adv. Energy Mater., 2015, 5, 1402263, DOI:10.1002/aenm.201402263.
- E. Ramli, T. B. Rauchfuss and C. L. Stern, J. Am. Chem. Soc., 1990, 112, 4043–4044 CrossRef CAS.
- S. Dev, E. Ramli, T. B. Rauchfuss and S. R. Wilson, Inorg. Chem., 1991, 30, 2514–2519 CrossRef CAS.
- A. K. Verma, T. B. Rauchfuss and S. R. Wilson, Inorg. Chem., 1995, 34, 3072–3078 CrossRef CAS.
- H. Xu, Y. Deng, Z. Zhao, H. Xu, X. Qin and G. Chen, Chem. Commun., 2014, 50, 10468–10470 RSC.
- F. Sun, J. Wang, H. Chen, W. Li, W. Qiao, D. Long and L. Ling, ACS Appl. Mater. Interfaces, 2013, 5, 5630–5638 CAS.
- L. Xiao, Y. Cao, J. Xiao, B. Schwenzer, M. H. Engelhard, L. V. Saraf, Z. Nie, G. J. Exarhos and J. Liu, Adv. Mater., 2012, 24, 1176–1181 CrossRef CAS PubMed.
- C. Lai, X. P. Gao, B. Zhang, T. Y. Yan and Z. Zhou, J. Phys. Chem. C, 2009, 113, 4712–4716 CAS.
- J. Guo, Z. Yang, Y. Yu, H. D. Abruña and L. A. Archer, J. Am. Chem. Soc., 2013, 135, 763–767 CrossRef CAS PubMed.
- J. Song, T. Xu, M. L. Gordin, P. Zhu, D. Lv, Y. B. Jiang, Y. Chen, Y. Duan and D. Wang, Adv. Funct. Mater., 2014, 24, 1243–1250 CrossRef CAS PubMed.
- P. Zhu, J. Song, D. Lv, D. Wang, C. Jaye, D. A. Fischer, T. Wu and Y. Chen, J. Phys. Chem. C, 2014, 118, 7765–7771 CAS.
- G. Zhou, E. Paek, G. S. Hwang and A. Manthiram, Nat. Commun, 2015, 6, 7760, DOI:10.1038/ncomms8760.
| This journal is © the Partner Organisations 2015 |

