Single-molecule magnet involving strong exchange coupling in terbium(III) complex with 2,2′-bipyridin-6-yl tert-butyl nitroxide†
Takuya
Kanetomo
a,
Shunsuke
Yoshii
b,
Hiroyuki
Nojiri
b and
Takayuki
Ishida
*a
aDepartment of Engineering Science, The University of Electro-Communications, Tokyo 182-8585, Japan. E-mail: takayuki.ishida@uec.ac.jp; Fax: +81 42 443 5501; Tel: +81 42 443 5490
bInstitute of Materials Research, Tohoku University, Katahira, Sendai 980-8577, Japan
First published on 7th July 2015
Abstract
A novel terbium(III)-radical complex [TbIII(hfac)3(6bpyNO)] (Tb-6bpyNO; 6bpyNO = 2,2′-bipyridin-6-yl tert-butyl nitroxide) was synthesized. The intramolecular antiferromagnetic interaction in Tb-6bpyNO was confirmed by comparison with the magnetic properties of [TbIII(hfac)3(6bpyCO)] (Tb-6bpyCO; 6bpyCO = 2,2′-bipyridin-6-yl tert-butyl ketone) together with [YIII(hfac)3(6bpyNO)]. Tb-6bpyNO showed a hysteresis loop with negligible coersivity below 1.6 K. The dispersion of the magnetic susceptibility with an applied bias field of 2000 Oe gave the energy barrier Ea/kB = 21.1(8) K. In contrast, Tb-6bpyCO did not behave as a single-molecule magnet, despite having practically the same crystal field.
Introduction
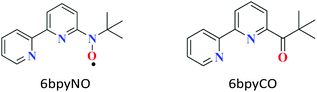
Molecule-based magnetic materials have the advantage of being able to arrange a variety of magnetic orbitals, which results in single-molecule magnets (SMMs) as well as interesting exchange-coupled systems.1 Recently, 4f-ion-based heterospin compounds have been intensively studied for the development of SMMs,2,3 because a strong magnetic anisotropy and large magnetic moment are available from lanthanoid (Ln) ions.4 When the spin centre of a paramagnetic ligand is bonded to a metal ion, the metal-radical complex shows strong exchange coupling via direct coordination. The 6bpyNO radical (6bpyNO = 2,2′-bipyridin-6-yl tert-butyl nitroxide; Scheme 1) has been synthesized for obtaining strong exchange coupling in transition metal-radical complexes.5 Recently, lanthanoid-radical complexes (4f–2p heterospin systems) have been the subject of intense research activity.6,7 We utilized 6bpyNO as a component of 4f–2p systems and found that [GdIII(hfac)3(6bpyNO)] (abbreviated as Gd-6bpyNO hereafter; Hhfac = 1,1,1,5,5,5-hexafluoropentane-2,4-dione) possesses the largest exchange coupling (2J/kB = –15.9(2) K) of all the Gd-nitroxide coordination compounds ever previously reported.8In the present work, we have synthesized the corresponding Tb analogue, Tb-6bpyNO, and investigated its magnetic properties to understand the role of the ligand spin when introduced to SMMs, which would be useful to create new heterospin compounds and SMMs. The present work also covers the empirical Δ(χmT) method9 using 2p-spin masked 6bpyCO (2,2′-bipyridin-6-yl tert-butyl ketone; Scheme 1) and clarifies the nature of intramolecular Tb–radical exchange coupling.
Results and discussion
Synthesis and description of the crystal structure
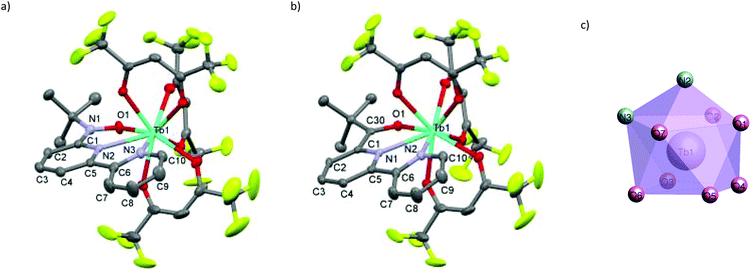
We synthesized RE-6bpyNO (RE = Tb, Y) according to the method developed for the RE = Gd analogue with a slight modification.8 The Y3+ ion has often been used as a diamagnetic metal centre because the ionic radius is close to those of the heavy lanthanide ions (0.90 Å for Y3+vs. 0.94 Å for Gd3+ and 0.92 Å for Tb3+).10 The products are stable at room temperature under air. Fine crystals were obtained and subjected without further purification to spectroscopic, X-ray crystallographic and magnetic analyses. The crystallography results are given in Table 1. RE-6bpyNO (RE = Tb, Y) crystallizes in a monoclinic P21/n space group, being completely isomorphous to Gd-6bpyNO (Fig. 1a).| Compound | Gd-6bpyNO | Tb-6bpyNO | Y-6bpyNO | Tb-6bpyCO | |
|---|---|---|---|---|---|
| a Ref. 8. b R = ∑||Fo| − |Fc||/∑|Fo|. c R w = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]1/2. | |||||
| Formula | C29H19F18N3O7Gd | C29H19F18N3O7Tb | C29H19F18N3O7Dy | C30H19F18N2O7Tb | |
| Formula weight | 1020.71 | 1022.38 | 952.36 | 1020.39 | |
| T/K | 100 | 100 | 100 | 100 | |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Monoclinic | |
| Space group | P21/n | P21/n | P21/n | P21/n | |
| a/Å | 13.467(3) | 13.433(2) | 13.392(2) | 13.456(3) | |
| b/Å | 17.045(4) | 17.068(3) | 17.074(3) | 17.130(3) | |
| c/Å | 16.692(4) | 16.695(2) | 16.648(2) | 16.654(3) | |
| β/° | 105.43(1) | 105.208(7) | 105.114(7) | 105.460(8) | |
| V/Å3 | 3694(2) | 3693.8(9) | 3675.0(9) | 3700(1) | |
| Z | 4 | 4 | 4 | 4 | |
| D calc/g cm−3 | 1.835 | 1.838 | 1.721 | 1.832 | |
| μ (MoKα)/mm−1 | 1.939 | 2.049 | 1.728 | 2.045 | |
| R(F)b (I > 2σ(I)) | 0.0463 | 0.0424 | 0.0633 | 0.0340 | |
| R w (F2)c (all data) | 0.0482 | 0.0530 | 0.0855 | 0.0434 | |
| Goodness-of-fit | 1.148 | 1.098 | 1.037 | 1.077 | |
| RE1–O1/Å | 2.373(4) | 2.365(4) | 2.333(3) | Tb1–O1/Å | 2.396(3) |
| RE1–N2/Å | 2.560(4) | 2.550(3) | 2.539(3) | Tb1–N1/Å | 2.569(3) |
| RE1–N3/Å | 2.568(4) | 2.547(4) | 2.539(4) | Tb1–N2/Å | 2.559(3) |
| RE1–O1–N1/° | 126.2(3) | 126.3(3) | 126.1(3) | Tb1–O1–C30/° | 127.8(2) |
| RE1–O1–N1–C1/° | –16.5(5) | –16.6(5) | –15.4(5) | Tb1–O1–C30–C1/° | –13.1(4) |
The RE3+ centre is nine-coordinate including two RE–N and one RE–O bond from the chelate ligand 6bpyNO. The other six bonds involve oxygen atoms from three hfac coligands. The coordination polyhedron can be best described as a capped square antiprism (CSAPR) with N2 as a cap (Fig. 1c). We further confirmed the CSAPR structure around the Tb and Y ions‡ using the SHAPE program.11 A magnetic axial anisotropy is expected in the CSAPR structure. Important geometrical parameters possibly relating to the intramolecular 4f–2p exchange coupling, such as bond lengths (RE–O1, RE–N1 and RE–N2), bond angles (RE1–O1–N1) and torsion angles (RE1–O1–N1–C1), are summarized in Table 1.
We also prepared and characterized Tb-6bpyCO (Fig. 1b) as a radical-spin silent reference. Crystallographic analysis clarified that it is isostructural to the 6bpyNO analogues (Table 1). A carbonyl group (C30–O1 in Fig. 1b) provides a ligating oxygen atom in place of the nitroxide group (N1–O1 in Fig. 1a). The Tb ion in Tb-6bpyCO also has a CSAPR structure. Furthermore the shape measurement results for Tb-6bpyNO and Tb-6bpyCO are quite close to each other,‡ in particular 0.241 and 0.249, respectively, for the CSAPR, according to the SHAPE program.11 This finding suggests that Tb-6bpyCO is a suitable model for reproducing the crystal field around the Tb ion in Tb-6bpyNO.
Magnetic properties
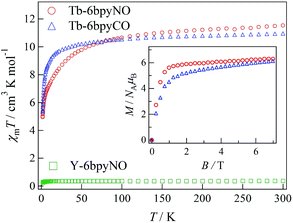
The direct current (dc) magnetic susceptibilities of Tb-6bpyNO, measured at an applied magnetic field of 5000 Oe in a temperature range of 1.8–300 K, are displayed in Fig. 2. The specimen was fixed with a small amount of mineral oil. At 300 K, the χmT value was 11.5 cm3 K mol−1 for Tb-6bpyNO. This is close to the theoretical value at the high-temperature limit (12.2 cm3 K mol−1 as the sum from Jz = 6, gJ = 3/2 for a free Tb3+ ion and S = 1/2, g = 2 for an organic radical). On cooling, the χmT value monotonically decreased to 5.17 cm3 K mol−1 at 2 K. Since we used powder samples, the major part of the reduction is caused by the anisotropy of the Ln ions. It should be noted that the χmT value of a radical spin is as small as 0.38 cm3 K mol−1 and is minor compared to the large reduction.We tried to obtain a clue for the nature of the magnetic coupling using a conventional approach. For 4f–3d heterospin systems there has been an empirical method known, where the χmT difference between the heterospin compound and 3d-spin masked reference is evaluated.9 On the other hand, corresponding researches for 4f–2p systems are somewhat rare.12 Some “nitronyl nitroxide” complexes have been modelled with nitrone complexes12,13 (“nitronyl nitroxide” stands for 4,4,5,5-tetramethylimidazolin-1-oxyl 3-oxide). Alternatively, a succinimide has also been proposed as a diamagnetic model.14 In the present case, 6bpyCO is rationally assumed as a diamagnetic reference for 6bpyNO.
Actually, Tb-6bpyCO was found to be isomorphous to Tb-6bpyNO (see above). The results of magnetic susceptibility for the two model compounds are superimposed in Fig. 2. The data on Tb-6bpyCO involve only the contribution of Tb3+ with the single-ion magnetic anisotropy regulated by the crystal field, and the data on Y-6bpyNO contain only the contribution of the radical spin affected by the radical–radical intermolecular interactions. Hence, the difference Δ(χmT) = (χmT)Tb-6bpyNO − (χmT)Y-6bpyNO − (χmT)Tb-6bpyCO implies the nature of the overall exchange interactions between Tb3+ and the radical spins. Positive and negative values of Δ(χmT) are directly related to ferro- and antiferromagnetic interactions, respectively. The temperature dependence of Δ(χmT) is displayed in Fig. 3. The Δ(χmT) shows a large negative value in the wide range below ca. 250 K. At the lowest temperature region below 10 K, an upturn appeared, possibly ascribed to the effect of intermolecular interactions or slight differences of the crystal fields between Tb-6bpyNO and Tb-6bpyCO.
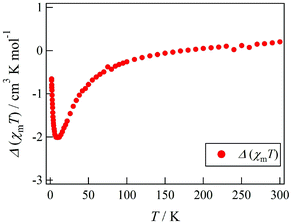 | ||
| Fig. 3 Temperature dependence of Δ(χmT) as defined by Δ(χmT) = (χmT)Tb-6bpyNO − (χmT)Y-6bpyNO − (χmT)Tb-6bpyCO. | ||
The field dependence of the magnetizations measured using SQUID at 1.8 K is shown in the inset of Fig. 2. The specimens were not fixed, so that the microcrystalline specimens would be aligned in the field direction. The magnetizations tend to saturate in higher fields and the value was 6.28μB at 7 T for Tb-6bpyNO. The expected value is 8 μB from the subtraction of those values for a free Tb3+ ion and 6bpyNO radical. There are two molecular directions in a unit cell, and the CSAPR principal axes are canted by 43° to each other, which was estimated using the Tb1–N2 bond directions. Thus, we have to reduce the saturation moments by about 7% from those of the single site values, giving 7.4μB. The reduction in the measured magnetization may be caused by the imperfect field-alignment. The magnetization of Tb-6bpyCO also shows gradual saturation and the value was 6.12μB at 7 T. The larger slope found around 7 T is compatible with the uncompensated magnetization of the Tb3+ ions. However, it is difficult to distinguish between the effect of intramolecular exchange coupling and that of the Tb3+ single ion anisotropy from these magnetization curves.
Let us compare the strong antiferromagnetic coupling found in the present system with the results of a similar system. Another 4f–2p heterospin system, [REIII(hfac)3(2pyNO)] (RE-2pyNO), has been developed through the use of tert-butyl 2-pyridyl nitroxide (2pyNO).15,16 Relatively strong antiferromagnetic 4f–2p exchange coupling was characterized in Gd-2pyNO15 like Gd-6bpyNO.8 The antiferromagnetic coupling in Tb-2pyNO was precisely evaluated by means of inelastic neutron scattering and high-frequency electron paramagnetic reasonance.17 In the 6bpyNO system, although conventional experimental methods were utilized, Gd- and Tb-6bpyNO were both clarified to have intramolecular antiferromagnetic coupling. It is likely that the antiferromagnetic coupling is considerable in Tb-6bpyNO from the notably negative Δ(χmT) even around 100 K. This notion is consistent with the empirical rule that the sign of exchange coupling is common among the heterospin molecules of Gd3+ and heavy lanthanide ions coupled with 3d transition metal ions18 and radicals.17 Unfortunately, the Δ(χmT) method could not afford any further quantitative information.
Dynamic magnetic properties
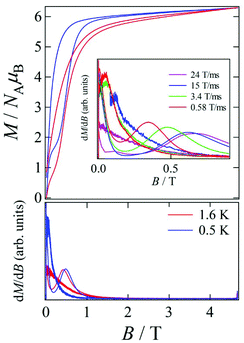
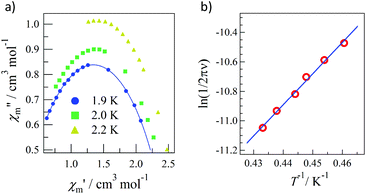
Pulsed-field magnetization experiments19 on Tb-6bpyNO were carried out using a 3He cryostat (Fig. 4). Hysteresis and magnetization jumps were found at 0.5 and 1.6 K, and the derivative of the magnetization clarifies the apparent coercive field of about 0.5 T. The field position depends slightly on temperature and field-sweeping rate, which shows the role of thermal relaxation.As the applied field was removed, the magnetization quickly disappeared (Fig. 4); namely, the coersivity does not appear from a bulk origin. It should be noted that the downward sweep is much slower than the upward sweep for the non-symmetric pulse shape. To investigate the dynamics of the magnetization reversal, we measured the alternating current (ac) magnetic susceptibility. Fig. 5a shows the in-phase and out-of-phase portions of the ac magnetic susceptibility (χ′m and χ′′m, respectively) for Tb-6bpyNO, measured without any applied dc field. No appreciable χ′′m was recorded, consistent with the fast relaxation of magnetization found in the pulsed-field experiments. On the other hand, when a direct current (dc) bias field20 of 2000 Oe was applied, a decrease in χ′m and concomitant increase in χ′′m were recorded (Fig. 5b). Frequency dependence was also observed. When we plotted the χ′′m against χ′m for various temperatures according to Cole–Cole analysis,21 a semicircle was drawn for 1.9 K with α = 0.236(2) (Fig. 6a). The α value in the Debye model is relatively small in χ(ω) = χS + (χT − χS)/(1 + (1ωτ)1−α), where χT and χS are the isothermal and adiabatic susceptibilities, respectively.22 This finding guarantees a single relaxation process in each molecule. The Arrhenius plot for Tb-6bpyNO (Fig. 6b) shows a straight line for the data of the χ′′ peak in Fig. 5b, and the activation energy (Ea) for the magnetization reversal was estimated as Ea/kB = 21.1(8) K with τ0 = 1.7(6) × 10−9 s, where τ0 stands for the pre-exponential factor in the Arrhenius equation, ln(2πν) = ln(τ0) + Ea/kB.23
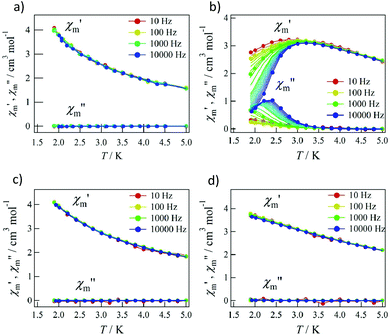 | ||
| Fig. 5 The ac magnetic susceptibilities for Tb-6bpyNO at applied dc bias fields of (a) 0 Oe and (b) 2000 Oe and for Tb-6bpyCO at applied dc bias fields of (c) 0 Oe and (d) 2000 Oe. | ||
In the magnetic field, splitting of the lowest doublet causes a Zeeman energy gap. For 7.4μB per molecule and 2000 Oe the gap is about 2 K. This gap is minor compared to the energy gap obtained using the Arrhenius plot, and the main cause of the activation energy is the anisotropy of the Tb-6bpyNO molecule as a SMM.
A square antiprismatic (SAPR) coordination polyhedron seems to be suitable from the strong axial anisotropy and the energy gap is quite large between the ground state (Jz = 6) and lowest-excited state (Jz = 5) for a Tb3+ ion.4,24,25 It has already been clarified that SAPR-configured Tb-2pyNO was a SMM.16 In the present work, the coordination structure of Tb-6bpyNO belongs to CSAPR, which is similar to SAPR. It is natural that both Tb-6bpyNO and Tb-2pyNO behave as SMMs. However, the crystal field effect is not a decisive factor, as follows.
Tb-6bpyNO affords one of the best opportunities where we can assess the role of paramagnetic ligands in SMMs through comparing with a reference compound with the 2p-spin masked. The ac magnetic susceptibility measurements on Tb-6bpyCO displayed no χ′′m at 0 Oe or even at a 2000 Oe dc bias field (Fig. 5c and d). This remarkable difference is hard to understand because the energy barrier caused by the Tb3+ anisotropy should be nearly identical between Tb-6bpyNO and Tb-6bpyCO. Long et al. have noted that the bistability is not guaranteed for a non-Kramers Tb3+ ion and accordingly a suitable crystal field is required so that Tb3+ can acquire axial magnetic anisotropy.24 If strong axial magnetic anisotropy is present, both Tb-6bpyNO and Tb-6bpyCO should behave as SMMs, which contradicts the present experimental results. At this point it is noticed that the even/odd components of the total magnetic moment are different between the two species, which is related to the Kramers theorem. The ground total spin of Tb-6bpyNO is 5/2 (a Kramers molecule) whilst that of Tb-6bpyCO is 3 (a non-Kramers molecule), assuming that the spin–spin coupling is strong enough, compared with cryogenic temperatures.
The presence of a 2p spin is critical for SMM performance in the present study. One may be reminded of the studies on [Tb(pc)2]0 and [Tb(pc)2]− (pc = phthalocyaninate).4,26 Only the former has a delocalized π spin on the (pc)2 moiety. The SMM performance of the former was found to be better,26 which can be also interpreted from an odd/even nature of the ground state moments. Another example is found in the fact that SMM behaviour of non-Kramers Dy-2pyNO or Dy-6bpyNO has never been reported.16 In fact, no meaningful χ′′m was observed for Dy-2pyNO or Dy-6bpyNO (Fig. S1 and S2, ESI†). Establishment of the spin-parity effect27 may need further theoretical clarification, but control of the relaxation by radical spin modification would lead to a new functionality of molecule-based magnets.
Finally we comment on the comparison with Tb-2pyNO, showing large coercivity and slow magnetization reversal in the ac magnetic susceptibility.16 It is known that magnetization reversal is sensitive to the relaxations caused by hyperfine couplings,28 dipolar interactions in Ln ions,29 and weak f–d and f–p exchange couplings.30,31 It is noticed that the CSAPR principal axes of monoclinic Tb-6bpyNO are canted by 43° between neighbouring molecules. On the other hand, Tb-2pyNO crystallizing in a triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group16 exhibits a cant angle of only ca. 5° between two independent molecules. This difference may affect the relaxation along with the dipolar interactions and the intramoleculer interactions. This is because the transverse components of fluctuating magnetic fields dominating the relaxation are enhanced by the tilting of the principle axes.
space group16 exhibits a cant angle of only ca. 5° between two independent molecules. This difference may affect the relaxation along with the dipolar interactions and the intramoleculer interactions. This is because the transverse components of fluctuating magnetic fields dominating the relaxation are enhanced by the tilting of the principle axes.
Conclusions
We have successfully prepared Tb-6bpyNO as an exchange-involving SMM or single-ion magnet. Intramolecular antiferromagnetic coupling was indicated using the empirical Δ(χmT) method after a corresponding carbonyl compound was proposed as a 2p-spin-masked reference. Tb-6bpyNO behaved as a SMM with Ea/kB = 21.1(8) K and τ0 = 1.7(6) × 10−9 s at the external dc bias of 2000 Oe. The 4f–2p heterospin approach seems to be one of the promising strategies toward novel SMMs. Relaxation by the parity effect is also discussed in terms of the marked differences of the SMM characteristics with and without a radical spin.Experimental section
Synthesis
6bpyNO was synthesized using the method reported in the literature.5 The complexes RE-6bpyNO (RE = Tb, Y) were prepared from [REIII(hfac)3(H2O)2] (RE = Tb, Y)32 and 6bpyNO in a dichloromethane–n-heptane mixed solvent, according to the procedure known for the Gd analogue.8X-ray crystallography
X-ray diffraction data of RE-6bpyNO (RE = Tb, Y) and Tb-6bpyCO were collected on a Rigaku Saturn70 CCD diffractometer with graphite monochromated MoKα radiation (λ = 0.71073 Å). The structures were directly solved using a heavy-atom method and expanded using Fourier techniques in the CrystalStructure program package.34 Numerical absorption correction was used. All of the hydrogen atoms were located at calculated positions and the parameters were refined as “riding”. The thermal displacement parameters of the non-hydrogen atoms were refined anisotropically. Selected crystallographic data and geometrical parameters are listed in Table 1. CCDC numbers 1011564, 1404995 and 1404996.Magnetic measurements
The dc magnetic susceptibilities and magnetizations of polycrystalline specimens of RE-6bpyNO (RE = Tb, Y) and Tb-6bpyCO were measured on a Quantum Design MPMS-XL SQUID magnetometer equipped with a 7 T coil in the temperature range 1.8–300 K. The magnetic data were corrected using diamagnetic blank data of the sample holder measured separately. The diamagnetic contribution of the sample itself was estimated from Pascal's constants. The ac magnetic susceptibilities were recorded on a Quantum Design PPMS ac magnetometer.Low-temperature magnetization was measured using a conventional inductive probe in pulsed-magnetic fields, and a temperature as low as 0.5 K was reached using a 3He cryostat.19 Polycrystalline specimens were mounted in a capillary made of polyimide. The samples were not fixed within the sample tube and they aligned in the magnetic field direction. After we applied the magnetic field several times, the orientation effect was saturated, and the magnetization curves obtained in further shots were found to be identical. The magnetization was calibrated using independent measurements on a SQUID magnetometer.
Acknowledgements
T. K. was supported by the JSPS program for Research Fellowships for Young Scientist (no. 15J11497).Notes and references
- O. Kahn, Molecular Magnetism, VCH Publications, New York, 1993 Search PubMed; J. S. Miller and M. Eds, Magnetism Molecules to Materials I–V, Wiley-VCH, Weinheim, Germany, 1999–2005 Search PubMed; D. Gatteschi, R. Sessoli and J. Villan, Molecular Nanomagnets, Oxford University Press, New York, 2006 Search PubMed; D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 2003 Search PubMed.
- S. Osa, T. Kido, N. Matsumoto, N. Re, A. Pochaba and J. Mrozinski, J. Am. Chem. Soc., 2004, 126, 420 CrossRef CAS PubMed; F. Pointillart, K. Bernot, R. Sessoli and D. Gatteschi, Chem. – Eur. J., 2007, 13, 1602 CrossRef PubMed.
- F. Mori, T. Ishida and T. Nogami, Polyhedron, 2005, 24, 2588 CrossRef CAS PubMed; F. Mori, T. Nyui, T. Ishida, T. Nogami, K.-Y. Choi and H. Nojiri, J. Am. Chem. Soc., 2006, 128, 1440 CrossRef PubMed; S. Ueki, T. Ishida, T. Nogami, K.-Y. Choi and H. Nojiri, Chem. Phys. Lett., 2007, 440, 263 CrossRef PubMed.
- N. Ishikawa, M. Sugita, T. Ishikawa, S.-Y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694 CrossRef CAS PubMed.
- K. Osanai, A. Okazawa, T. Nogami and T. Ishida, J. Am. Chem. Soc., 2006, 128, 14008 CrossRef CAS PubMed.
- S. Demir, L.-R. Jeon, J. R. Long and T. D. Harris, Coord. Chem. Rev., 2015, 289–290, 149 CrossRef CAS PubMed.
- J. D. Rinehart, M. Fang, W. J. Evans and J. R. Long, Nat. Chem., 2011, 3, 538 CrossRef CAS PubMed; N. G. R. Hearns, K. E. Preuss, J. F. Richardson and S. Bin-Salamon, J. Am. Chem. Soc., 2004, 126, 9942 CrossRef PubMed; A. Caneschi, A. Dei, D. Gatteschi, L. Sorace and K. Vostrikova, Angew. Chem., Int. Ed., 2000, 39, 246 CrossRef; C. Benelli, A. Caneschi, D. Gatteschi, L. Pardi and P. Rey, Inorg. Chem., 1989, 28, 275 CrossRef.
- T. Kanetomo and T. Ishida, Inorg. Chem., 2014, 53, 10794 CrossRef CAS PubMed.
- J.-P. Costes, F. Dahan, A. Dupuis and J.-P. Laurent, Chem. – Eur. J., 1998, 9, 1616 CrossRef CAS; M. L. Kahn, C. Mathoniére and O. Kahn, Inorg. Chem., 1999, 38, 3692 CrossRef PubMed; M. Towatari, K. Nishi, T. Fujinami, N. Matsumoto, Y. Sunatsuki, M. Kojima, N. Mochida, T. Ishida, N. Re and J. Mrozinski, Inorg. Chem., 2013, 52, 6160 CrossRef PubMed; A. Okazawa, T. Shimada, N. Kojima, S. Yoshii, H. Nojiri and T. Ishida, Inorg. Chem., 2013, 52, 13351 CrossRef PubMed.
- R. D. Shannon and C. T. Prewitt, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1969, 25, 925 CrossRef CAS; R. D. Shannon, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Cryst., 1976, 32, 751 CrossRef.
- M. Lluncll, D. Casanova, J. Circra, J. M. Bofill, P. Alcmany, S. Alvarez, M. Pinsky and D. Avnir, SHAPE, v2.1, University of Barcelona and The Hebrew University of Jerusalem, Barcelona, 2005 Search PubMed.
- J.-P. Sutter, M. L. Kahn and O. Kahn, Adv. Mater., 1999, 11, 863 CrossRef CAS; M. L. Kahn, J.-P. Sutter, S. Golhen, P. Guionneau, L. Ouahab, O. Kahn and D. Chasseau, J. Am. Chem. Soc., 2000, 122, 3413 CrossRef.
- T. Mochizuki, T. Nogami and T. Ishida, Inorg. Chem., 2009, 48, 2254 CrossRef CAS PubMed; F. A. Villamena, M. H. Dickman and D. R. Crist, Inorg. Chem., 1998, 37, 1446 CrossRef.
- W. Wong and S. F. Watkins, J. Chem. Soc., Chem. Commun., 1973, 888 RSC.
- T. Ishida, R. Murakami, T. Kanetomo and H. Nojiri, Polyhedron, 2013, 66, 183 CrossRef CAS PubMed.
- R. Murakami, T. Ishida, S. Yoshii and H. Nojiri, Dalton Trans., 2013, 42, 1396 RSC.
- M. L. Baker, T. Tanaka, R. Murakami, S. Ohira-Kawamura, K. Nakajima, T. Ishida and H. Nojiri, Inorg. Chem., 2015, 54, 5732 CrossRef CAS PubMed.
- T. Ishida, R. Watanabe, K. Fujiwara, A. Okazawa, N. Kojima, G. Tanaka, S. Yoshii and H. Nojiri, Dalton Trans., 2012, 41, 13609 RSC; R. Watanabe, K. Fujiwara, A. Okazawa, G. Tanaka, S. Yoshii, H. Nojiri and T. Ishida, Chem. Commun., 2011, 47, 2110 RSC; T. Shimada, A. Okazawa, N. Kojima, S. Yoshii, H. Nojiri and T. Ishida, Inorg. Chem., 2011, 50, 10555 CrossRef CAS PubMed; A. Okazawa, T. Shimada, N. Kojima, S. Yoshii, H. Nojiri and T. Ishida, Inorg. Chem., 2013, 52, 13351 CrossRef PubMed.
- H. Nojiri, K.-Y. Choi and N. Kitamura, J. Magn. Magn. Mater., 2007, 310, 1468 CrossRef CAS PubMed.
- S. L. Castro, Z. Sun, C. M. Grant, J. C. Bollinger, D. N. Hendrickson and G. Christou, J. Am. Chem. Soc., 1998, 120, 2365 CrossRef CAS; M. Sugita, N. Ishikawa, T. Ishikawa, S.-Y. Koshihara and Y. Kaizu, Inorg. Chem., 2006, 45, 1299 CrossRef PubMed.
- K. S. Cole and H. R. Cole, J. Chem. Phys., 1941, 9, 341 CrossRef CAS PubMed.
- R. Sessoli and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2328 CrossRef CAS PubMed.
- R. Sessoli, H.-L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef CAS.
- J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078 RSC.
- M. A. AlDamen, S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Arino, C. Marti-Gastaldo, F. Luis and O. Montero, Inorg. Chem., 2009, 48, 3467 CrossRef CAS PubMed; J. J. Baldovi, S. Cardona-Serra, J. M. Clemente-Juan, E. Coronado, A. Gaita-Arino and A. Palii, Inorg. Chem., 2012, 51, 12565 CrossRef PubMed; G. F. Xu, Q. L. Wang, P. Gamez, Y. Ma, R. Clérac, J. Tang, S. P. Yan, P. Cheng and D. Z. Liao, Chem. Commun., 2010, 46, 1506 RSC; X.-L. Wang, L.-C. Li and D.-Z. Liao, Inorg. Chem., 2010, 49, 4735 CrossRef PubMed; V. Chandrasekhar, P. Bag, M. Speldrich, J. van Leusen and P. Kögerler, Inorg. Chem., 2013, 52, 5035 CrossRef PubMed.
- N. Ishikawa, M. Sugita, N. Tanaka, T. Ishikawa, S.-Y. Koshihara and Y. Kaizu, Inorg. Chem., 2004, 43, 5498 CrossRef CAS PubMed.
- W. Wernsdorfer, N. E. Chakov and G. Christou, Phys. Rev. Lett., 2005, 95 Search PubMed , 037203 M.-E. Boulon, G. Cucinotta, J. Luzon, C. Degl'Innocenti, N. Perfetti, K. Bernot, G. Calvez, A. Caneschi and R. Sessoli, Angew. Chem. Int. Ed., 2013, 52, 350 CrossRef CAS PubMed.
- N. Ishikawa, M. Sugita and W. Wernsdorfer, Angew. Chem., Int. Ed., 2005, 44, 2931 CrossRef CAS PubMed.
- T. Fukuda, K. Matsumura and N. Ishikawa, J. Phys. Chem. A, 2013, 117, 10447 CrossRef CAS PubMed.
- E. M. Pineda, N. F. Chilton, F. Tuna, R. E. P. Winpenny and E. J. L. McInnes, Inorg. Chem., 2015, 54, 5930 CrossRef PubMed.
- M. Gonidec, E. S. Davies, J. McMaster, D. B. Amabilino and J. Veciana, J. Am. Chem. Soc., 2010, 132, 1756 CrossRef CAS PubMed.
- M. F. Richardson, W. F. Wagner and D. E. Sands, J. Inorg. Nucl. Chem., 1968, 30, 1275 CrossRef CAS.
- S. E. Denmark, Y. Fan and M. D. Eastgate, J. Org. Chem., 2005, 70, 5235 CrossRef CAS PubMed.
- CrystalStructure version 4.0, Rigaku Corp., Tokyo, Japan, 2010 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1011564 (Tb-6bpyNO), 1404995 (Y-6bpyNO) and 1404996 (Tb-6bpyCO). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qi00098j |
| ‡ Shape measurements were 0.241 (CSAPR) and 1.053 (TCTPR; tricapped trigonal prism) for Tb-6bpyNO, 0.235 (CSAPR) and 1.023 (TCTPR) for Y-6bpyNO, and 0.249 (CSAPR) and 1.019 (TCTPR) for Tb-6bpyCO. |
| This journal is © the Partner Organisations 2015 |