Assembly, structures, photophysical properties and photocatalytic activities of a series of coordination polymers constructed from semi-rigid bis-pyridyl-bis-amide and benzenetricarboxylic acid†
Xiuli
Wang
*,
Mao
Le
,
Hong-Yan
Lin
,
Jian
Luan
,
Guo-Cheng
Liu
,
Fang-Fang
Sui
and
Zhi-Han
Chang
Department of Chemistry, Bohai University, Jinzhou, 121000, P. R. China. E-mail: wangxiuli@bhu.edu.cn; Fax: +86-416-3400158; Tel: +86-416-3400158
First published on 2nd February 2015
Abstract
A series of new metal(II)–organic coordination polymers, namely [Co(3-DPNA)(1,3,5-HBTC)]·2H2O (1), [Ni(3-DPNA)1.5(1,3,5-HBTC)(H2O)2] (2), [Cu(3-DPNA)(1,3,5-HBTC)]·H2O (3), [Zn(3-DPNA)(1,3,5-HBTC)]·2H2O (4), [Cd(3-DPNA)(1,3,5-HBTC)(H2O)]·2H2O (5), [Co(4-DPNA)(1,3,5-HBTC)]·4H2O (6), [Ni(4-DPNA)(1,3,5-BTC)2/3] (7), [Cu(4-DPNA)(1,3,5-BTC)2/3] (8), [Zn(4-HDPNA)(1,3,5-BTC)] (9), [Cd(4-DPNA)(1,3,5-BTC)1/3Cl] (10), have been purposefully synthesized under hydrothermal conditions [N1,N4-di(pyridin-3-yl)naphthalene-1,4-dicarboxamide (3-DPNA) and N1,N4-di(pyridin-4-yl)naphthalene-1,4-dicarboxamide (4-DPNA), 1,3,5-H3BTC = 1,3,5-benzenetricarboxylic acid]. Complexes 1 and 4 exhibit similar 1D belt-like chains constructed from 1D left- and right-hand [Co-(3-DPNA)]n helical chains and [Co-(1,3,5-HBTC)]n linear chains. Complex 2 furnishes a 1D [Ni-(3-DPNA)1.5]n “loop + line” chain with 1,3,5-HBTC anions hanging on both sides. Complex 3 features a 2D sql network based on [Cu-(3-DPNA)]nmeso-helical chains and [Cu-(1,3,5-HBTC)]n linear chains. Complex 5 shows a 1D ladder-like chain constructed from [Cd-1,3,5-HBTC]n linear chains and [Cd2(3-DPNA)2] loops. Complex 6 displays a 2D double sheet containing (H2O)8 clusters. Complexes 7 and 8 possess similar 3D (3,4)-connected frameworks with the (63)2(64·8·10)3 topology containing a dodecagon-shape cavity and a hexagonal cavity, respectively. Complex 9 shows a 2D network, in which one pyridine nitrogen atom of 4-DPNA was protonated. Complex 10 is a 3D (3,5)-connected framework with (3·44·63·82)3(63) topology. All the 1D chains and 2D networks are further extended into 2D supramolecular sheets or 3D supramolecular frameworks through hydrogen-bonding interactions. The influence of metal(II) ions and N-donor ligands on the structures of the title compounds was investigated. The results suggest that the central metals with various coordination nature and the semi-rigid bis-pyridyl-bis-amide ligands with different coordination sites show a great effect on the structures of the title compounds. In addition, the fluorescence sensing properties of 4, 5, 9, and 10 and the photocatalytic activities of complexes 1–10 have been investigated in detail, and the results show that 5 possesses good fluorescence selectivity for different solvent molecules.
Introduction
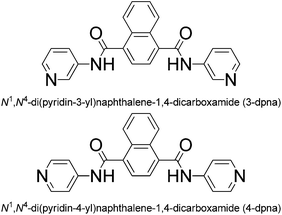
The coordination behaviours of the pyridyl-amide-based derivatives and the corresponding metal–organic coordination polymers (MOCPs) represent a research branch that has been under rapid expansion in recent years,1 mainly owing to their structural diversity and potential applications in gas storage and separation, heterogeneous catalysis, chemical sensing, magnetism, and electrochemistry.2 Usually, pyridyl-amide-based MOCPs exhibit a variety of open frameworks such as layered and microporous structures, which could be controlled by adjusting the geometry of organic ligands and the coordination modes of metal centers.3 Many studies have been devoted to the exploration of pyridyl-amide-based MOCP materials with multiple properties.4 For example, Zhou's group has obtained a multi-functional MOCP based on a mono-pyridyl-bis-amide ligand (5,5-((pyridine-3,5-dicarbonyl)bis(azanediyl))diisophthalate), which shows selective adsorption of CO2 over CH4 and excellent catalytic activity in a tandem one-pot deacetalization–Knoevenagel condensation reaction as a cooperative catalyst.5 Lu and co-workers have reported a porous MOCP {[Zn4(BDC)4(BPDA)4]·5DMF·3H2O}n (BDC = 1,4-benzyl dicarboxylate, BPDA = N,N′-bis(4-pyridinyl)-1,4-benzenedicarboxamide), formed from 44-sql [Zn4(BDC)4] layers and a long pillar of BPDA, which exhibits a significant increase with CO2 uptake.6 However, only a few examples have been reported on the exploration of pyridyl-amide-based MOCPs materials with fluorescence sensing activities and photocatalytic properties.2cTaking inspiration from the above viewpoints, we synthesized two semi-rigid bis-pyridyl-bis-amide ligands, N1,N4-di(pyridin-3-yl)naphthalene-1,4-dicarboxamide (3-DPNA) and N1,N4-di(pyridin-4-yl)naphthalene-1,4-dicarboxamide (4-DPNA) (Scheme 1), based on the following considerations: (i) the rigid nature of naphthalene spacer allows the ligand to possess a large conjugated system when coordinating to metal centres so as to obtain fluorescent materials; (ii) the rigid and symmetrical ligands may conduce to constructing high-dimensional frameworks with large pores, which may have a tendency to accommodate discrete water clusters or other guest molecules; (iii) pyridyl-amide groups may supply the charge transfer excited state to oxygenate water molecules and generate the ˙OH radicals,7 which have potential photocatalytic activity to degrade some organic dyes. Thus, in this work, two semi-rigid bis-pyridyl-bis-amides (3-DPNA and 4-DPNA) and 1,3,5-benzenetricarboxylic acid (1,3,5-H3BTC) were selected as the mixed ligands to assemble with five kinds of Co(II)/Ni(II)/Cu(II)/Zn(II)/Cd(II) ions under hydrothermal conditions, aiming at constructing the target MOCPs with the fluorescence sensing properties and photocatalytic activities and investigating their effect on the final structures. To the best of our knowledge, only rare MOCPs based on rigid naphthalene-based bis-pyridyl-bis-amide ligands have been reported so far.8
As a result, ten MOCPs, namely [Co(3-DPNA)(1,3,5-HBTC)]·2H2O (1), [Ni(3-DPNA)1.5(1,3,5-HBTC)(H2O)2] (2), [Cu(3-DPNA)(1,3,5-HBTC)]·H2O (3), [Zn(3-DPNA)(1,3,5-HBTC)]·2H2O (4), [Cd(3-DPNA)(1,3,5-HBTC)(H2O)]·2H2O (5), [Co(4-DPNA)(1,3,5-HBTC)]·4H2O (6), [Ni(4-DPNA)(1,3,5-BTC)2/3] (7), [Cu(4-DPNA)(1,3,5-BTC)2/3] (8), [Zn(4-HDPNA)(1,3,5-BTC)] (9), and [Cd(4-DPNA)(1,3,5-BTC)1/3Cl] (10), have been designed and synthesized. Although there are no lattice water molecules in complex 9, the 2D network of 9 is the same as the complex [Zn(4-Hdpna)(1,3,5-BTC)]·0.5H2O recently reported by Luo and co-workers, which was synthesized using DMF and H2O as solvents at 115 °C for 72 h.8a So, in this work, 9 is used as the reference for comparison in the discussion parts. All the complexes were characterized by single crystal X-ray crystallography, IR spectroscopy, powder X-ray diffraction (PXRD), and thermogravimetric (TG) analysis. Moreover, the fluorescence sensing properties of 4, 5, 9, and 10, the electrochemical properties of 1–3 and 6–8, as well as the photocatalytic activities of complexes 1–10 have been studied.
Experimental
General
The ligands 3-DPNA and 4-DPNA were prepared according to a literature method.9 All chemical reagents and solvents for syntheses were purchased from commercial sources and used without any further purification. Infrared spectra were recorded using a Varian 640-IR spectrometer with KBr pellets in the range of 4000–500 cm−1. Powder X-ray diffraction (PXRD) investigations were carried out with an Ultima IV with D/teX Ultra diffractometer at 40 kV, 40 mA with Cu Kα (λ = 1.5406 Å) radiation. Thermogravimetric analyses were completed on a Pyris Diamond thermal analyzer at a heating rate of 10 °C min−1 under a N2 atmosphere. Fluorescence spectra were recorded in the solid state at room temperature on a Hitachi F-4500 fluorescence/phosphorescence spectrophotometer. A CHI 440 electrochemical workstation was used for the control of the electrochemical measurements and for data collection. A conventional three-electrode system was used with an SCE as the reference electrode, a platinum wire as the auxiliary electrode and the carbon paste electrodes (CPEs) bulk-modified with the title complexes as the working electrodes, respectively. The UV-Vis absorption spectra were obtained using an SP-1901 UV-Vis spectrophotometer.X-ray crystallography
The single-crystal diffraction data for complexes 1–10 were collected on a Bruker SMART APEX II with Mo Kα (λ = 0.71073 Å) by ω and θ scan mode at room temperature. All the structures were solved by direct methods using the SHELXS program of the SHELXTL package.10 C24, C25 and C26 of complex 2 and N2, C7, C8 and C9 of complex 8 were disposed by the disorder method. C2, C10 and C11 were restricted by the ‘isor’ command in complex 8. In addition, C6 of complex 8 was disposed by the half-occupancy method. The crystal data and structure refinement results are summarized in Tables 1 and 2. Selected bond lengths and angles are listed in Tables S1–10.† Hydrogen bonding geometries of complexes 1–3, 5, 6 and 9 are summarized in Table S11.† CCDC 1005163–1005172 for complexes 1–10 contain the supplementary crystallographic data of this paper.| Complex | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|. b wR2 = ∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]1/2. | |||||
| Empirical formula | C31H24CoN4O10 | C42H32NiN6O11 | C31H22CuN4O9 | C31H24ZnN4O10 | C31H26CdN4O11 |
| Formula weight | 671.47 | 855.45 | 658.07 | 677.91 | 742.96 |
| Crystal system | Monoclinic | Triclinic | Monoclinic | Monoclinic | Triclinic |
| Space group | C2/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
C2/c | C2/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 27.913(2) | 9.4576(4) | 27.413(4) | 27.781(2) | 10.0186(6) |
| b (Å) | 9.7024(7) | 11.7570(6) | 10.0478(16) | 10.0395(8) | 10.2419(6) |
| c (Å) | 22.8070(16) | 17.2174(8) | 20.669(3) | 22.2588(17) | 14.6114(8) |
| α (°) | 90 | 83.1940(10) | 90 | 90 | 90.7530(10) |
| β (°) | 109.5180(10) | 80.7120(10) | 105.821(3) | 109.2150(10) | 93.9340(10) |
| γ (°) | 90 | 77.6280(10) | 90 | 90 | 90.7470(10) |
| V (Å3) | 5821.7(7) | 1838.47(15) | 5477.4(15) | 5862.4(8) | 1495.46(15) |
| Z | 8 | 2 | 8 | 8 | 2 |
| D c (g cm−3) | 1.532 | 1.5045 | 1.596 | 1.536 | 1.650 |
| μ (mm−1) | 0.658 | 0.604 | 0.865 | 0.905 | 0.801 |
| F (000) | 2760 | 884 | 2696 | 2784 | 752 |
| Reflection collected | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220 220 |
9359 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 469 469 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524 524 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 175 175 |
| Unique reflections | 5137 | 6402 | 4774 | 5161 | 7471 |
| Parameters | 415 | 569 | 406 | 415 | 424 |
| R int | 0.0205 | 0.0114 | 0.0304 | 0.0220 | 0.0156 |
| GOF | 1.067 | 1.041 | 1.027 | 1.067 | 1.018 |
| R 1 [I > 2σ(I)] | 0.0416 | 0.0451 | 0.0435 | 0.0329 | 0.0297 |
| wR2b (all data) | 0.1220 | 0.1128 | 0.1234 | 0.0959 | 0.0747 |
| Complex | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|. b wR2 = ∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]1/2. | |||||
| Empirical formula | C31H28CoN4O12 | C28H18NiN4O6 | C28H18CuN4O6 | C31H20ZnN4O8 | C25H17CdN4O4Cl |
| Formula weight | 707.50 | 565.17 | 570.00 | 641.88 | 585.28 |
| Crystal system | Triclinic | Trigonal | Trigonal | Monoclinic | Trigonal |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c c |
P21/c |
P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) |
| a (Å) | 10.0522(16) | 16.7246(7) | 18.942(2) | 11.8470(4) | 14.1344(19) |
| b (Å) | 11.4431(18) | 16.7246(7) | 18.942(2) | 16.2260(5) | 14.1344(19) |
| c (Å) | 15.205(2) | 59.874(5) | 38.976(4) | 14.7859(5) | 20.396(3) |
| α (°) | 96.493(3) | 90 | 90 | 90 | 90 |
| β (°) | 102.803(3) | 90 | 90 | 109.5450(10) | 90 |
| γ (°) | 105.101(2) | 120 | 120 | 90 | 120 |
| V (Å 3) | 1619.3(4) | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 503.8(15) 503.8(15) |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 111(2) 111(2) |
2678.51(15) | 3528.9(9) |
| Z | 2 | 18 | 18 | 4 | 6 |
| D c (g cm−3) | 1.451 | 1.165 | 1.407 | 1.592 | 1.652 |
| μ (mm−1) | 0.599 | 0.642 | 0.860 | 0.981 | 1.082 |
| F (000) | 730 | 5220 | 5238 | 1312 | 1752 |
| Reflection collected | 8304 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 885 885 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 278 278 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 361 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 101 101 |
| Unique reflections | 5671 | 5712 | 2488 | 4707 | 4887 |
| Parameters | 433 | 352 | 181 | 397 | 316 |
| R int | 0.0156 | 0.0569 | 0.0739 | 0.0148 | 0.0809 |
| GOF | 1.084 | 1.095 | 1.077 | 1.026 | 1.026 |
| R 1 [I > 2σ(I)] | 0.0443 | 0.0637 | 0.1104 | 0.0274 | 0.0406 |
| wR2b (all data) | 0.1501 | 0.2021 | 0.3212 | 0.0752 | 0.0987 |
Preparation of complexes 1–10
Preparation of 1–3 and 6–8-CPEs
The complexes 1–3 and 6–8 bulk-modified carbon paste electrodes (1-CPE, 2-CPE, 3-CPE, 6-CPE, 7-CPE, 8-CPE) were fabricated by the following steps. Graphite powder (0.11 g) and complexes 1, 2, 3, 6, 7, or 8 (0.022 g) were mixed together with an agate mortar for about 30 min to obtain a mixture; then 0.05 mL paraffin oil was added and stirred with a glass rod.11 The homogenized mixture was packed into a 3 mm inner diameter glass tube, and a copper stick was used as the electrical contact. The surfaces of the modified electrodes were polished on a piece of weighing paper to achieve a mirror finish before use.Preparation of 4a, 5a, 9a and 10a
After immersing in CH3OH for 3 days with the soaking solution being replaced every 6 h, the solvent-exchanged samples of 4a, 5a, 9a and 10a for 4, 5, 9 and 10 were evacuated at 60 °C for 1 h and then at 120 °C for over 3 h.Results and discussion
Structural description of the title complexes
Each 3-DPNA showing a μ2-bridging mode (via ligation of two pyridyl nitrogen atoms, Table 3) connects the Co(II) ions to form 1D left-/right-hand helical [Co-(3-DPNA)]n chains along the b-axis (Fig. 1b). The 1,3,5-HBTC anion adopts a chelating-monodentate bridging mode connecting the Co(II) ions from both sides of 1D [Co(3-DPNA)]n helical chains to form two [Co-(1,3,5-HBTC)]n chains along the b-axis (Fig. S2† and 1c). Thus, complex 1 shows a 1D belt-like chain containing two 1D [Co-(1,3,5-HBTC)]n linear chains and a 1D [Co(3-DPNA)]n helical chain. Furthermore, the hydrogen-bonding interactions exist between the N4 of the amide group and the O6 of the carboxyl group from 1,3,5-HBTC (N4–H4A⋯O6 = 2.8717 Å), which extend the neighboring belt-like chains to a 2D supramolecular layer (Fig. S3†).
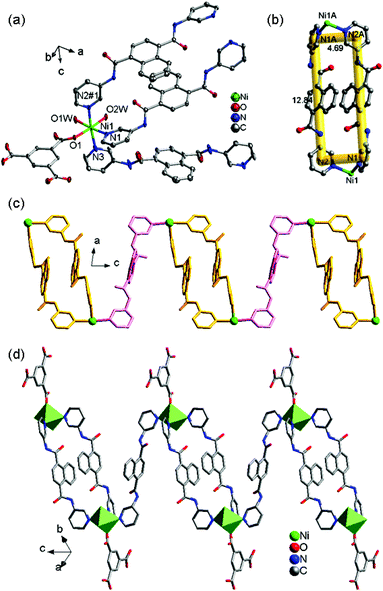
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group and displays a 1D chain structure. As shown in Fig. 2a, the fundamental structure of 2 contains one Ni(II) ion, one and a half 3-DPNA ligands, one 1,3,5-HBTC anion, and two coordination water molecules. In 2, each distorted octahedral Ni(II) center is coordinated by one O atom from 1,3,5-HBTC, two O atoms of coordinated water molecules [Ni(1)–O(1) = 2.0375(18), Ni(1)–O(1W) = 2.107(2), and Ni(1)–O(2W) = 2.069(2) Å], and three N atoms originating from three 3-DPNA ligands [Ni(1)–N(1) = 2.113(2), Ni(1)–N(2)#1 = 2.158(2) and Ni(1)–N(3) = 2.135(3) Å].
space group and displays a 1D chain structure. As shown in Fig. 2a, the fundamental structure of 2 contains one Ni(II) ion, one and a half 3-DPNA ligands, one 1,3,5-HBTC anion, and two coordination water molecules. In 2, each distorted octahedral Ni(II) center is coordinated by one O atom from 1,3,5-HBTC, two O atoms of coordinated water molecules [Ni(1)–O(1) = 2.0375(18), Ni(1)–O(1W) = 2.107(2), and Ni(1)–O(2W) = 2.069(2) Å], and three N atoms originating from three 3-DPNA ligands [Ni(1)–N(1) = 2.113(2), Ni(1)–N(2)#1 = 2.158(2) and Ni(1)–N(3) = 2.135(3) Å].
In complex 2, the ligand 3-DPNA displays two different conformations, 3-DPNAa and 3-DPNAb (Table 3). Two 3-DPNAa ligands link two Ni(II) ions forming a [Ni2(3-DPNAa)2] dinuclear loop with the size of 12.84 × 4.69 Å2 (Fig. 2b). The adjacent [Ni2(3-DPNAa)2] dinuclear loops are connected by the 3-DPNAb ligands to build a 1D [Ni(3-DPNA)1.5]n “loop + line” chain, in which the 3-DPNAb ligands and [Ni2(3-DPNAa)2] dinuclear loops are alternating and the non-bonding Ni⋯Ni distances are 16.124 Å (3-DPNAa) and 16.108 Å (3-DPNAb), respectively (Fig. 2c, Table 3). The 1,3,5-HBTC anion adopts a monodentate mode (Table 3) coordinating to Ni(II) ions and hanging on both sides of the 1D [Ni(3-DPNA)1.5]n chain (Fig. 2d). The N–H⋯O hydrogen bonds are observed between the N6 of the amide group from 3-DPNA and the O6 of the carboxyl group from 1,3,5-HBTC (N6–H6A⋯O6 = 3.0209 Å), expanding the 1D chain to a 2D architecture (Fig. S4†). Finally, the neighboring 2D layers are connected by O1W–H1WB⋯O5 (2.7466 Å) hydrogen-bonding interactions between coordination water molecule and carboxyl group from 1,3,5-HBTC to produce a 3D supramolecular network (Fig. S5†).
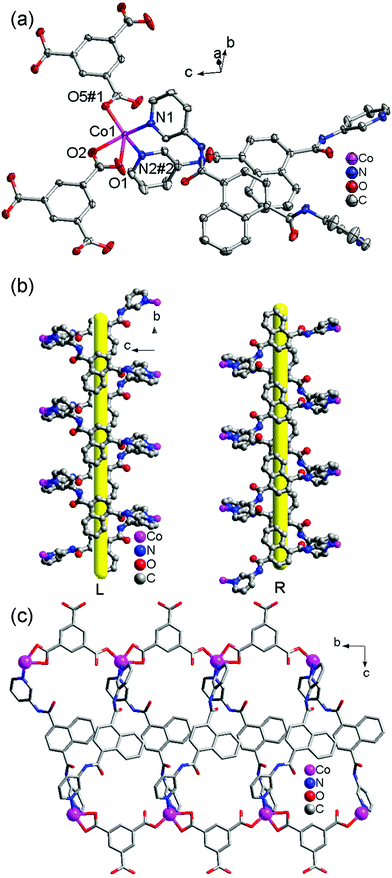
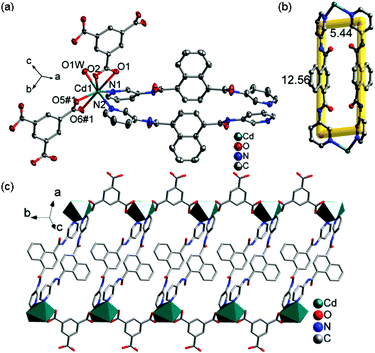
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) and its asymmetric unit consists of one independent Cd(II) ion, one 3-DPNA ligand, one 1,3,5-HBTC anion, one coordinated water molecule, and two lattice water molecules (Fig. 4a). The Cd(II) ion is seven-coordinated by four carboxyl O atoms from two 1,3,5-HBTC anions, one O atom from a coordinated water molecule [Cd(1)–O(1) = 2.5512(16), Cd(1)–O(2) = 2.4050(16), Cd(1)–O(5)#1 = 2.3091(16), Cd(1)–O(6)#1 = 2.5756(16), and Cd(1)–O(1W) = 2.3655(16) Å], and two pyridyl N atoms from two 3-DPNA ligands [Cd(1)–N(1) = 2.3303(18) and Cd(1)–N(2) = 2.3470(19) Å] in a distorted pentagonal bipyramidal geometry. Two carboxyl groups of each 1,3,5-HBTC anion chelate to two Cd(II) centers to form a 1D [Cd(1,3,5-HBTC)]n linear chain (Fig. S9†). A pair of 3-DPNA ligands, adopting a μ2-bridging coordination mode, connects two Cd(II) ions to generate a [Cd2(3-DPNA)2] loop with the dimension of 5.44 × 12.56 Å2 (Fig. 4b). Two [Cd(1,3,5-HBTC)]n chains are connected by [Cd2(3-DPNA)2] loops to construct a 1D ladder-like chain (Fig. 4c). There are intermolecular hydrogen bonds between O1W and carboxyl O atoms (O1W–H1WA⋯O6 = 2.737 Å), which further link neighboring ladder-like chains to form a 2D supramolecular layer (Fig. S10†).
and its asymmetric unit consists of one independent Cd(II) ion, one 3-DPNA ligand, one 1,3,5-HBTC anion, one coordinated water molecule, and two lattice water molecules (Fig. 4a). The Cd(II) ion is seven-coordinated by four carboxyl O atoms from two 1,3,5-HBTC anions, one O atom from a coordinated water molecule [Cd(1)–O(1) = 2.5512(16), Cd(1)–O(2) = 2.4050(16), Cd(1)–O(5)#1 = 2.3091(16), Cd(1)–O(6)#1 = 2.5756(16), and Cd(1)–O(1W) = 2.3655(16) Å], and two pyridyl N atoms from two 3-DPNA ligands [Cd(1)–N(1) = 2.3303(18) and Cd(1)–N(2) = 2.3470(19) Å] in a distorted pentagonal bipyramidal geometry. Two carboxyl groups of each 1,3,5-HBTC anion chelate to two Cd(II) centers to form a 1D [Cd(1,3,5-HBTC)]n linear chain (Fig. S9†). A pair of 3-DPNA ligands, adopting a μ2-bridging coordination mode, connects two Cd(II) ions to generate a [Cd2(3-DPNA)2] loop with the dimension of 5.44 × 12.56 Å2 (Fig. 4b). Two [Cd(1,3,5-HBTC)]n chains are connected by [Cd2(3-DPNA)2] loops to construct a 1D ladder-like chain (Fig. 4c). There are intermolecular hydrogen bonds between O1W and carboxyl O atoms (O1W–H1WA⋯O6 = 2.737 Å), which further link neighboring ladder-like chains to form a 2D supramolecular layer (Fig. S10†).
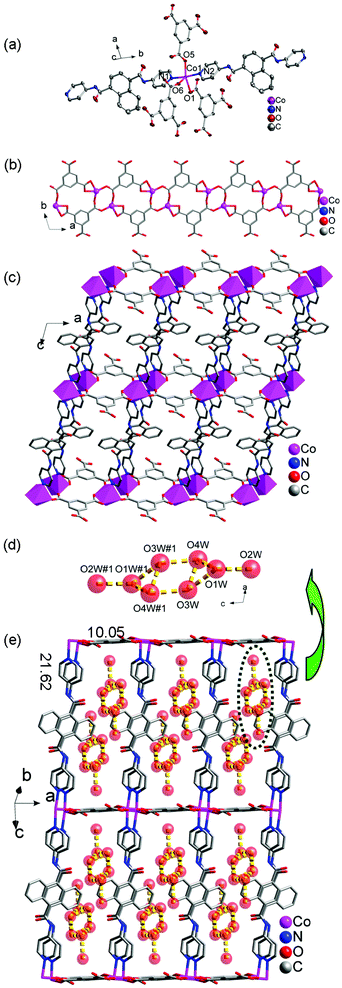
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The asymmetric unit of 6 contains one Co(II) ion, one 4-DPNA ligand, one 1,3,5-HBTC anion and four lattice water molecules (Fig. 5a). In 6, each Co(II) ion is coordinated by three carboxyl O atoms from three 1,3,5-HBTC anions and two pyridine N atoms from two 4-DPNA ligands. The Co–O and Co–N bond lengths are in the range of 2.0129(18)–2.409(2) and 2.142(2)–2.153(2) Å, respectively.
space group. The asymmetric unit of 6 contains one Co(II) ion, one 4-DPNA ligand, one 1,3,5-HBTC anion and four lattice water molecules (Fig. 5a). In 6, each Co(II) ion is coordinated by three carboxyl O atoms from three 1,3,5-HBTC anions and two pyridine N atoms from two 4-DPNA ligands. The Co–O and Co–N bond lengths are in the range of 2.0129(18)–2.409(2) and 2.142(2)–2.153(2) Å, respectively.
Each 1,3,5-HBTC anion adopts a chelating-bis(monodentate) bridging mode connecting the neighboring Co(II) ions to form a 1D [Co-(1,3,5-HBTC)]n ladder-like chain (Fig. 5b). The μ2-bridging 4-DPNA ligands link the Co(II) ions from the adjacent [Co-(1,3,5-HBTC)]n ladder-like chains to construct a 2D double layer (Fig. 5c), which contains a big window with the dimensions of 10.05 × 21.62 Å2. It is interesting that there exist (H2O)8 clusters in the windows, which are constructed from the guest water molecules through hydrogen bonding interactions (Fig. 5d and 5e). In addition, the (H2O)8 clusters further interact with the host framework through hydrogen bonds to construct a 3D supramolecular structure (Fig. S12†). Hydrogen bonding geometries of 6 are summarized in Table S11.†
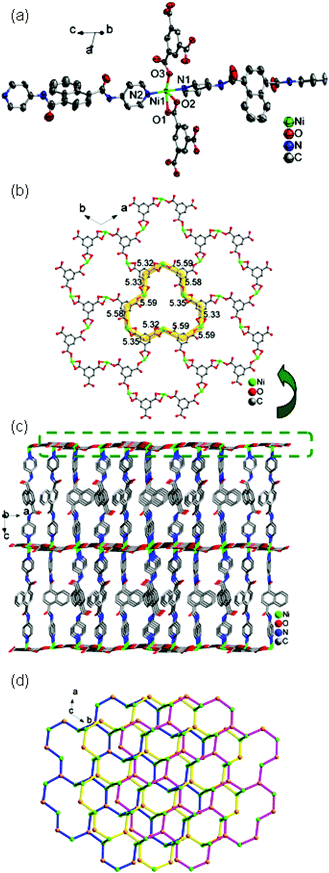
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) . The asymmetric unit contains one Ni(II) ion, one 4-DPNA ligand, and two-thirds of one 1,3,5-BTC anion (Fig. 6a). The Ni(II) ion is five-coordinated by three carboxyl oxygen atoms from two different 1,3,5-BTC anions [Ni(1)–O(1) = 2.148(3), Ni(1)–O(2) = 2.125(3), and Ni(1)–O(3) = 1.982(3) Å] and two nitrogen atoms from two 4-DPNA ligands [Ni(1)–N(1) = 2.086(4) and Ni(1)–N(2) = 2.109(4) Å], showing a pyramid geometry. In 7, the 1,3,5-BTC anions exhibit two types of coordination modes: tri(chelating) bridging mode and tri(monodentate) bridging mode (Table 3). Six Zn(II) ions are alternately linked by six 1,3,5-BTC anions in two types of modes to form a large dodecagon-shape 47-membered ring [Ni6(1,3,5-BTC)6], finally leading to the formation of an intriguing 2D [Ni-(1,3,5-BTC)2/3]n network (Fig. 6b). The adjacent 2D networks are further supported by 4-DPNA ligands to form a 3D framework (Fig. 6c). Each Ni(II) ion is surrounded by two bridging 4-DPNA ligands and two bridging 1,3,5-BTC anions, which can simply be regarded as a 4-connected node. Each 1,3,5-BTC is linked to three Ni(II) ions, and therefore, is defined as a 3-connected node. The 4-DPNA ligand serves as a linear linker. Thus, the overall topology of the 3D framework is best described as a unique binodal (3,4)-connected network with dodecagon-shape cavities, and its Schläfli symbol is (63)2(64·8·10)3 (Fig. 6d).
. The asymmetric unit contains one Ni(II) ion, one 4-DPNA ligand, and two-thirds of one 1,3,5-BTC anion (Fig. 6a). The Ni(II) ion is five-coordinated by three carboxyl oxygen atoms from two different 1,3,5-BTC anions [Ni(1)–O(1) = 2.148(3), Ni(1)–O(2) = 2.125(3), and Ni(1)–O(3) = 1.982(3) Å] and two nitrogen atoms from two 4-DPNA ligands [Ni(1)–N(1) = 2.086(4) and Ni(1)–N(2) = 2.109(4) Å], showing a pyramid geometry. In 7, the 1,3,5-BTC anions exhibit two types of coordination modes: tri(chelating) bridging mode and tri(monodentate) bridging mode (Table 3). Six Zn(II) ions are alternately linked by six 1,3,5-BTC anions in two types of modes to form a large dodecagon-shape 47-membered ring [Ni6(1,3,5-BTC)6], finally leading to the formation of an intriguing 2D [Ni-(1,3,5-BTC)2/3]n network (Fig. 6b). The adjacent 2D networks are further supported by 4-DPNA ligands to form a 3D framework (Fig. 6c). Each Ni(II) ion is surrounded by two bridging 4-DPNA ligands and two bridging 1,3,5-BTC anions, which can simply be regarded as a 4-connected node. Each 1,3,5-BTC is linked to three Ni(II) ions, and therefore, is defined as a 3-connected node. The 4-DPNA ligand serves as a linear linker. Thus, the overall topology of the 3D framework is best described as a unique binodal (3,4)-connected network with dodecagon-shape cavities, and its Schläfli symbol is (63)2(64·8·10)3 (Fig. 6d).
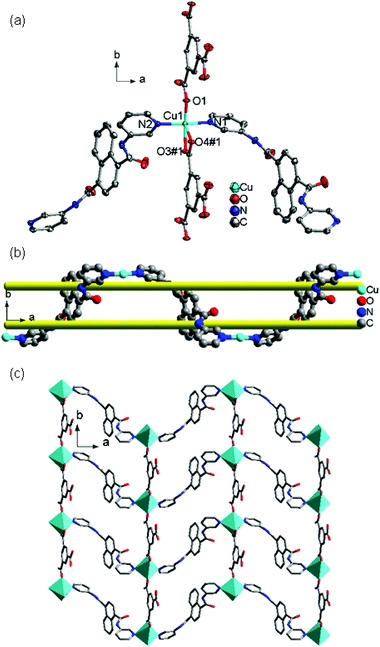
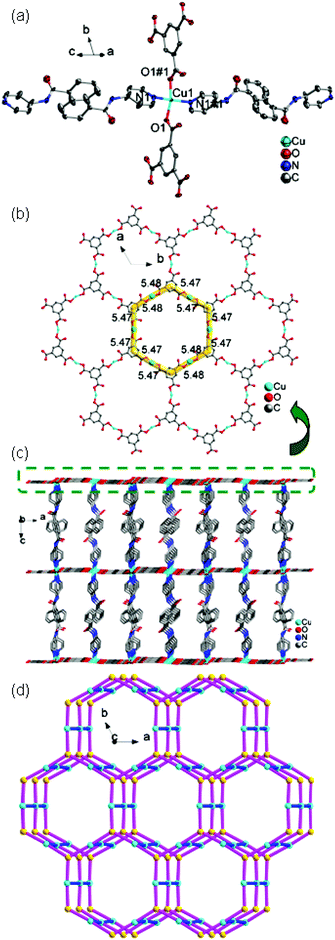
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c. The asymmetric unit contains one Cu(II) ion, one 4-DPNA ligand, and two-thirds of one 1,3,5-BTC anion. As depicted in Fig. 7a, the Cu(II) ion is four-coordinated in a distorted quadrangle geometry by two carboxyl oxygen atoms from two 1,3,5-BTC anions with Cu(1)–O(1) and Cu(1)–O(1)#1 distances of 1.928(5) Å, and two nitrogen atoms from two different 4-DPNA ligands with Cu(1)–N(1) and Cu(1)–N(1)#1 distances of 1.999(7) Å. In 8, all of the carboxyl groups of 1,3,5-BTC anions exhibit a μ1–η1:η0 coordination mode. In this mode, the 1,3,5-BTC anions link the Cu(II) ions to give a 2D network containing the regular hexagon windows [Cu6(1,3,5-BTC)6] with 51-membered rings (Fig. 7b), which is different from those in 7. Such 2D layers are connected by 4-DPNA pillars in a μ2-bridging mode to generate a 3D network (Fig. 7c). Topologically, if the Cu(II) ions and 1,3,5-BTC anions are viewed as four- and three-connected nodes, respectively, the 4-DPNA ligands are regarded as the linkers, and then the whole 3D framework can be simplified as a 3,4-connected net with the Schläfli symbol of (63)2(64·8·10)3 (Fig. 7d).
c. The asymmetric unit contains one Cu(II) ion, one 4-DPNA ligand, and two-thirds of one 1,3,5-BTC anion. As depicted in Fig. 7a, the Cu(II) ion is four-coordinated in a distorted quadrangle geometry by two carboxyl oxygen atoms from two 1,3,5-BTC anions with Cu(1)–O(1) and Cu(1)–O(1)#1 distances of 1.928(5) Å, and two nitrogen atoms from two different 4-DPNA ligands with Cu(1)–N(1) and Cu(1)–N(1)#1 distances of 1.999(7) Å. In 8, all of the carboxyl groups of 1,3,5-BTC anions exhibit a μ1–η1:η0 coordination mode. In this mode, the 1,3,5-BTC anions link the Cu(II) ions to give a 2D network containing the regular hexagon windows [Cu6(1,3,5-BTC)6] with 51-membered rings (Fig. 7b), which is different from those in 7. Such 2D layers are connected by 4-DPNA pillars in a μ2-bridging mode to generate a 3D network (Fig. 7c). Topologically, if the Cu(II) ions and 1,3,5-BTC anions are viewed as four- and three-connected nodes, respectively, the 4-DPNA ligands are regarded as the linkers, and then the whole 3D framework can be simplified as a 3,4-connected net with the Schläfli symbol of (63)2(64·8·10)3 (Fig. 7d).
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) space group. The asymmetric unit of 10 consists of one Cd(II) ion, one 4-DPNA ligand, one-third of 1,3,5-BTC anions and one μ2-Cl− anion. The Cd(II) ion is located in a six-coordinated octahedral center. As depicted in Fig. 8a, the Cd(II) ion is coordinated by two pyridyl nitrogen atoms from two individual 4-DPNA ligands, two oxygen atoms of one carboxyl from 1,3,5-BTC anions, and two μ2-Cl− anions [Cd1–N1 = 2.344(3) Å, Cd1–N2 = 2.335(3) Å, Cd1–O1 = 2.352(2) Å, Cd1–O2 = 2.397(2) Å, Cd1–Cl1 = 2.577 (9) Å, Cd1–Cl1#1 = 2.561 (9) Å]. Each 1,3,5-BTC anion adopts a tri(chelating) bridging mode, and links three Cd(II) ions to form a [Cd3(1,3,5-BTC)]3+ subunit, which is further connected by μ2-Cl− anions to form a 2D network (Fig. 8c and 8b). The 4-DPNA ligands adopting a μ2-bridging mode link the neighboring Cd(II) ions from adjacent 2D layers to generate a 3D framework (Fig. 8e and 8d). Topologically, if the Cd(II) ions and 1,3,5-BTC anions are considered as five- and three-connected nodes, respectively, the 4-DPNA ligands and μ2-Cl− anions are regarded as the linkers, and the whole structure can be simplified as a 3,5-connected net with the Schläfli symbol of (3·44·63·82)3(63) (Fig. 8e).
space group. The asymmetric unit of 10 consists of one Cd(II) ion, one 4-DPNA ligand, one-third of 1,3,5-BTC anions and one μ2-Cl− anion. The Cd(II) ion is located in a six-coordinated octahedral center. As depicted in Fig. 8a, the Cd(II) ion is coordinated by two pyridyl nitrogen atoms from two individual 4-DPNA ligands, two oxygen atoms of one carboxyl from 1,3,5-BTC anions, and two μ2-Cl− anions [Cd1–N1 = 2.344(3) Å, Cd1–N2 = 2.335(3) Å, Cd1–O1 = 2.352(2) Å, Cd1–O2 = 2.397(2) Å, Cd1–Cl1 = 2.577 (9) Å, Cd1–Cl1#1 = 2.561 (9) Å]. Each 1,3,5-BTC anion adopts a tri(chelating) bridging mode, and links three Cd(II) ions to form a [Cd3(1,3,5-BTC)]3+ subunit, which is further connected by μ2-Cl− anions to form a 2D network (Fig. 8c and 8b). The 4-DPNA ligands adopting a μ2-bridging mode link the neighboring Cd(II) ions from adjacent 2D layers to generate a 3D framework (Fig. 8e and 8d). Topologically, if the Cd(II) ions and 1,3,5-BTC anions are considered as five- and three-connected nodes, respectively, the 4-DPNA ligands and μ2-Cl− anions are regarded as the linkers, and the whole structure can be simplified as a 3,5-connected net with the Schläfli symbol of (3·44·63·82)3(63) (Fig. 8e).
Effect of the N-donor ligands on the title frameworks
In this work, two bis-pyridyl-bis-amide ligands, namely, 3-DPNA and 4-DPNA, were used to investigate the effect of N-complexes 1 and 6 based on the same Co(II) ion and 1,3,5-donor positions of the pyridyl groups on the structures. For H3BTC, the difference in N-donor positions of the pyridine groups is the main factor that influences the final structures of 1 and 6. In 1, each 3-DPNA showing a μ2-bridging mode connects the Co(II) ions to form a 1D helical chain, which is further connected by 1,3,5-HBTC anions along the same direction to construct a belt-like chain. In complex 6, the 4-DPNA ligand acts as a linear linker connecting the adjacent [Cd-(1,3,5-HBTC)]n ribbon-like chains to form a 2D double layer, in which the big void space is occupied by guest (H2O)8 clusters. For 2 and 7, 3-DPNA with a μ2-bridging mode connects the Ni(II) ions to form a 1D [Ni-(3-DPNA)1.5]n “loop + line” chain with 1,3,5-HBTC anions hanging on both sides in 2, while in 7, the 4-DPNA ligands act as a linear pillar linking the adjacent [Ni-(1,3,5-HBTC)2/3]n 2D networks to construct a 3D (3,4)-connected framework with the (63)2(64·8·10)3 topology. For 3 and 8, 3-DPNA connects the Cu(II) ions to form 1D [Cu-(3-DPNA)]nmeso-helical chains, which are connected by 1,3,5-HBTC anions to form a 2D sql network in 3, while in 8, the 4-DPNA ligands also act as a linear pillar linking the adjacent [Cu-(1,3,5-HBTC)2/3]n 2D networks to construct a 3D (3,4)-connected framework with the (63)2(64·8·10)3 topology. Complex 4 exhibits a similar 1D belt-like chain to that of 1, which is constructed from 1D left- and right-hand [Zn-3-DPNA]n helical chains and [Zn-(1,3,5-HBTC)]n linear chains. In complex 9, 4-DPNA adopts a monodentate mode with one pyridine nitrogen atom of 4-DPNA protonated, finally resulting in a 2D network. For 5 and 10, a pair of 3-DPNA connects the Cd(II) ions to form [Cd2(3-DPNA)2] loops in 5, which further link the adjacent [Cd-1,3,5-HBTC]n linear chains to construct a 1D ladder-like chain. In 10, the 4-DPNA ligands act as a linear pillars linking the adjacent [Cd-(1,3,5-HBTC)1/3Cl]n 2D networks to construct a 3D (3,5)-connected framework with (3·44·63·82)3(63) topology.From the structural comparison above, we can see that the 3-DPNA ligands tend to construct helical/meso-helical chains or dinuclear rings, finally resulting in low-dimensional (1D or 2D) networks, while 4-DPNA ligands usually act as linear pillars to extend 2D networks to 3D frameworks with cavities. Thus, it can be concluded that the bis-pyridyl-bis-amide ligands with different N-donor positions play important roles in the construction of the various structures. The N-donor positions of the semi-rigid bis-pyridyl-bis-amide ligands show a great effect on the final structures of the title complexes.
Effect of the central metal ions on the final frameworks
It is well known that the central metal ions play an important role in the formation of the final frameworks and various structures. In this work, ten coordination polymers based on 3-DPNA/4-DPNA with 1,3,5-H3BTC mixed ligands and different metal centers (Co(II), Ni(II), Cu(II), Zn(II), Cd(II)) have been synthesized under similar hydrothermal conditions, which exhibit distinct coordination networks. In complexes 1–5, 3-DPNA ligands act as μ2-bridging ligands connecting the metal ions to construct helical chains or dinuclear loops. The central metal ions with different atomic radii belong to the VIIIB/IB/IIB groups and show different coordination numbers and coordination modes, which have an obvious effect on the coordination modes of 1,3,5-HBTC and finally influence the structures of complexes 1–5. For example, the Co(II) ion in 1 shows coordination number of 5, and the 1,3,5-HBTC exhibits a monodentate-chelating bridging mode, finally resulting in a 1D belt-like chain. The coordination number of Ni(II) ions in 2 is 6, and the 1,3,5-HBTC exhibits a monodentate mode. As a result, a 1D “loop + line” chain was obtained. When the Cu(II) ion was selected as the central metal in 3, a 2D network was formed, in which the Cu(II) ions exhibit a coordination number of 5, and the 1,3,5-HBTC anion adopts a chelating-monodentate bridging mode. In 4, the Zn(II) ions adopt the coordination number of 4, and 1,3,5-HBTC adopts bis(monodentate) bridging mode, and finally a 1D belt-like chain containing a [Zn(3-DPNA)]n helical chain and two [Zn(1,3,5-HBTC)]n linear chains was obtained. When the Cd(II) ion, which belong to the same IIB group as the Zn(II) ions but have a bigger atomic radius, was employed as the central metal in complex 5, the coordination number of Cd(II) ions was 7, the 1,3,5-HBTC anion with a bis(chelating) mode connected the [Cd2(3-DPNA)2] loops to construct a 1D ladder-like chain. It is the central metal that determines the final structures of complexes 1–5, as well as influences the coordination modes of the 1,3,5-HBTC ligand. Similarly, for complexes 6–10, 4-DPNA ligands act as μ2-bridging pillars connecting the metal ions to construct linear chains and extending the metal-carboxylate subunits to 2D or 3D networks, except for 9, in which the 4-DPNA was protonated and adopted a monodentate mode. The central metals also show different coordination numbers and 1,3,5-HBTC/1,3,5-BTC anions exhibit different coordination modes. As a result, various architectures of 6–10 were obtained. Thus, it can be concluded that the differences in the coordination numbers and atomic radii of the central metal ions resulted in the various coordination modes of 1,3,5-HBTC/1,3,5-BTC anions and finally the construction of diverse structures for 1–10.IR spectra of complexes 1–10
As shown in Fig. S15,† the IR spectra of complexes 1–10 are determined in the frequency range of 500–4000 cm−1. The strong peaks at 1376 and 1109 cm−1 for 1, 1366 and 1105 cm−1 for 2, 1367 and 1107 cm−1 for 3, 1382 and 1107 cm−1 for 4, 1383 and 1107 cm−1 for 5, 1372 and 1108 cm−1 for 6, 1382 and 1107 cm−1 for 7, 1382 and 1102 cm−1 for 8, 1367 and 1104 cm−1 for 9, 1375 and 1107 cm−1 for 10 may be attributed to the νC–N stretching vibrations of the pyridyl ring of the 3-DPNA or 4-DPNA ligands.12a The bands around 1615 and 1424 cm−1 for 1, 1611 and 1423 cm−1 for 2, 1617 and 1422 cm−1 for 3, 1615 and 1427 cm−1 for 4, 1608 and 1449 cm−1 for 5, 1613 and 1434 cm−1 for 6, 1615 and 1446 cm−1 for 7, 1629 and 1439 cm−1 for 8, 1619 and 1454 cm−1 for 9, 1619 and 1427 cm−1 for 10 suggest to the asymmetric and symmetric vibrations of carboxyl groups.12b The presence of the characteristic bands at 1660 cm−1 for 1, 1650 cm−1 for 2, 1687 cm−1 for 3, 1678 cm−1 for 4, 1648 cm−1 for 5, 1647 cm−1 for 6, 1679 cm−1 for 7, 1675 cm−1 for 8, 1657 cm−1 for 9, and 1680 cm−1 for 10 are characteristic of the carbonyl groups12. For complexes 1–6, the strong absorption peaks observed at around 3237, 3299, 3394, 3266, 3267 and 3271 cm−1 indicate the presence of –OH groups of water molecules, while the bands at 1705, 1703, 1706, 1711, 1704 and 1700 cm−1 are assigned to stretching and bending vibrations of –COOH groups, respectively.12Powder X-ray diffraction (PXRD) and thermogravimetric analysis (TGA)
The powder X-ray diffraction (PXRD) patterns for complexes 1–10 are presented in Fig. S16.† The as-synthesized patterns are in good agreement with the corresponding simulated ones, indicating the phase purities of the samples.The thermogravimetric analysis (TGA) was performed under a N2 atmosphere with a heating rate of 10 °C·min−1, and the TG curves of complexes 1–10 are shown in Fig. S17.† The TGA curves for 1–10 suggested that their host networks were stable up to 306, 279, 271, 240, 272, 314, 258, 316, 352 and 299 °C, respectively, and then they started to gradually lose their ligands as a result of thermal decomposition, leading to the formation of CoO/NiO/CuO/ZnO/CdO for 1–10. The removal of water molecules was observed for 1–6 with weight losses of 5.27% (calcd 5.36%) in the temperature range of 68–113 for 1, 4.26% (calcd 4.20%) between 169 and 241 for 2, 2.63% (calcd 2.73%) between 52 and 151 for 3, 5.22% (calcd 5.31%) between 57 and 116 for 4, 7.22% (calcd 7.28%) between 59 and 158 for 5 and 10.27% (calcd 10.18%) between 68 and 200 for 6, respectively. Further verified by the TGA of the title complexes, there are two regions of slope for weight losses between 300 and 500 °C. The first step mass loss may be attributed to the decomposition of 1,3,5-BTC anions, and the second may be assigned to the degradation of 3-/4-DPNA ligands. As described above, the different slopes of TGA curves in complexes 1–10 are different from each other, which may be ascribed to the different coordination fashions of organic ligands and their different components and architectures.
Photophysical properties
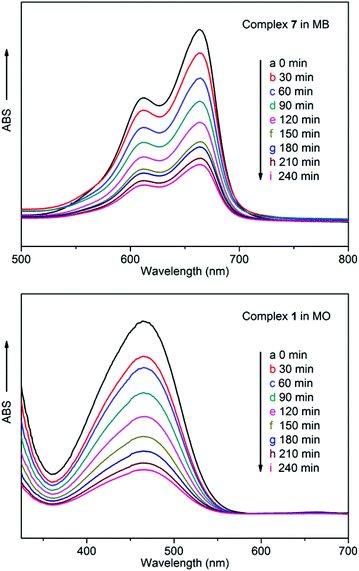
To examine the fluorescence properties of the title complexes, the fluorescence spectra of complexes 1–10, together with the free ligands in the solid state, were recorded. It was found that the 3-DPNA/4-DPNA ligand displays an emission band at 400 nm (λex = 320 nm) (Fig. 9), which may be ascribed to the π*→π or π*→n transitions.13 In addition, it was considered that the 1,3,5-H3BTC ligand has no significant contribution to the fluorescence emission of the MOCPs in the presence of N-donor ligands.13c | ||
| Fig. 9 The emission spectra of complexes 1–10 and the free ligands 3-DPNA and 4-DPNA in the solid state. | ||
Complexes 1–10 emit at 417, 465, 417, 435, 415, 415, 417, 411, 411 and 415 nm upon excitation at 320 nm (Fig. 9), respectively, which show different degrees of red-shift compared with the free 3-DPNA/4-DPNA ligand (17 nm for 1, 65 nm for 2, 17 nm for 3, 35 nm for 4, 15 nm for 5, 415 nm for 6, 17 nm for 7, 11 nm for 8, 11 nm for 9, 15 nm for 10). Although the Cu(II)/Ni(II)/Co(II) complexes do not contain d10 metal centers, a series of fluorescence Cu(II)/Ni(II)/Co(II) complexes have been reported, and the fluorescence of Cu(II)/Ni(II)/Co(II) complexes may be mainly attributed to the ligand-to-ligand charge transfer (LLCT) or intraligand charge transfer.14 Meanwhile, the fluorescence behaviors of 4, 5, 9 and 10 can be best ascribed to the ligand-to-metal charge-transfer (LMCT)13, 14d
In order to investigate the guest solvents on the fluorescence properties of the Zn(II) and Cd(II) complexes, dehydrated samples for 4, 5, 9 or 10 (defined as 4a, 5a, 9a or 10a) and their corresponding solvent-containing samples (4a-, 5a-, 9a- or 10a-solvent) were prepared and their fluorescence spectra were recorded. The 4a-, 5a-, 9a- or 10a-solvent samples were prepared as follows: complexes 4, 5, 9 and 10 were immersed in CH3OH for 3 days with the soaking solution being replaced every 6 h, the solvent-exchanged samples were evacuated at 60 °C for 1 h and then 120 °C for over 3 h, then desolvated samples 4a, 5a, 9a or 10a were obtained. The finely ground sample of 4a, 5a, 9a or 10a (5 mg) was immersed in 5 mL of different organic solvents (MeOH, EtOH, CH3CN, CH2Cl2, DMF, DMSO, THF, acetone, diethyl ether, and cyclohexane); after sonication treatment, aging for over 24 h, shaking, filtering, and drying in air, fluorescent samples of complex 4a-/5a-/9a-/10a-solvent were obtained. The fluorescence spectra are shown in Fig. 10 and S18.† It can be clearly seen that the fluorescence intensities of 5a-acetone, 5a-MeOH, 9a-acetone, 9a-THF, and 9a-DMF were enhanced. For the other 4a-/5a-/9a-/10a-solvent, different degrees of fluorescence decrease can be observed. Only the fluorescence intensity of 4a-DMF shows no obvious change. The results indicate that various guest solvent molecules in the voids of 4a, 5a, 9a or 10a networks exhibit a great effect on their fluorescence intensity. It is assumed that the different binding interactions between the host networks and guest solvent molecules play an important role in enhancing or quenching the fluorescence intensity.14e The fluorescence response of 5a-acetone was the largest, while the fluorescence intensity of 5a-DMSO was the lowest. The results show that 5a possesses good fluorescence selectivity for different solvent molecules.
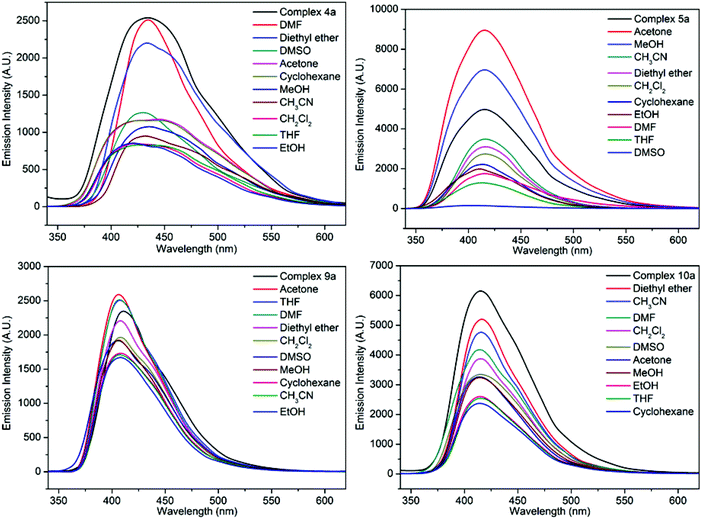 | ||
| Fig. 10 The solid-state fluorescence spectra of complexes 4a-solvent, 5a-solvent, 9a-solvent and 10a-solvent. | ||
Electrochemical properties
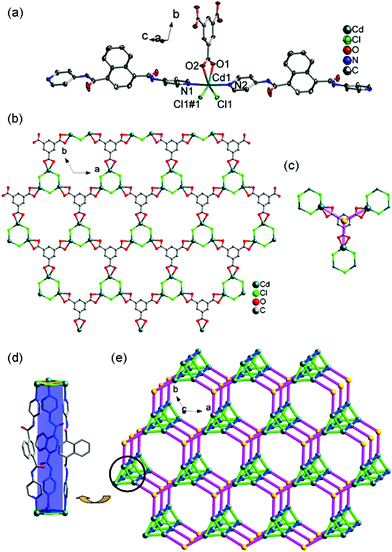
In recent years, the electrochemical and electrocatalytic activities of Co(II)/Ni(II)/Cu(II)-based MOCPs have attracted significant interest in both the scientific and industrial fields because of their easy fabrication and good performance, in which the redox peaks are ascribed to the one-electron Co(III)/Co(II), Ni(III)/Ni(II) or Cu(II)/Cu(I) redox processes15. For example, Mukherjee et al. reported the electrochemical properties of several Co(II), Ni(II) and Cu(II) MOCPs containing the hexadentate pyridine amide ligand, in which these MOCPs exhibited a one-electron quasi-reversible Co(III)/Co(II), Ni(III)/Ni(II) or Cu(II)/Cu(I) response with E1/2 = 0.48/1.17/−0.88 V in CH2Cl2 (0.1 M in TBAP) at a platinum electrode, respectively.15a More recently, our group has also investigated the electrochemical behaviors of a series of Co(II), Ni(II) and Cu(II) MOCPs, all of which display excellent electrocatalytic activities towards the reduction of nitrite.15cThe electrochemical studies of 1–3-CPEs and 6–8-CPEs were carried out in 0.01 M H2SO4 + 0.5 M Na2SO4 aqueous solution, in which the MOCPs were stable. Fig. 11 and S19† show the cyclic voltammograms of 1–3-CPE and 6–8-CPE at different scan rates, respectively. It can be clearly seen that in the potential ranges of 500 to 0 mV, 600 to 0 mV, and 500 to −500 mV, 600 to 0 mV, 800 to −200 mV, and 300 to −300 mV, a quasi-reversible redox peak is observed at 1–3-CPE and 6–8-CPE, respectively, which could be attributed to the redox couple of Co(III)/Co(II), Ni(III)/Ni(II) or Cu(II)/Cu(I), respectively.16–19 The mean peak potentials E1/2 = (Epa + Epc)/2 are +286 mV for 1-CPE, +258 mV for 2-CPE, and +10 mV for 3-CPE, 259 mV for 6-CPE, +240 mV for 7-CPE, and +15 mV for 8-CPE (50 mV s−1), respectively.
Scan rate's effect on the electrochemical behaviors of 1–3-CPE and 6–8-CPE can be seen in Fig. 11 and S19.† With the scan rates increasing from 10 to 100 mV s−1, the peak potentials of the 1–3-CPE and 6–8-CPE change gradually: the cathodic peak potentials shift to the negative direction and the corresponding anodic peak potentials shift to the positive direction. The plots of peak currents versus scan rates/square root of scan rates are shown in Fig. S20† and the inset of Fig. 11 and S19.† For the linearity of peak currents vs. scan rates, R values are 0.99598 for 1-CPE, 0.99996 for 2-CPE, 0.75330 for 3-CPE, 0.99380 for 6-CPE, 0.99894 for 7-CPE and 0.98909 for 8-CPE, respectively. For the plots of peak currents vs. square root of scan rates, R values are 0.97941 for 1-CPE, 0.98832 for 2-CPE, 0.83659 for 3-CPE, 0.97139 for 6-CPE, 0.98707 for 7-CPE and 0.99293 for 8-CPE, respectively (Table S12†). Comparing the R2 for both plots, we can conclude that the redox process is more close to the surface controlled process for 1-CPE, 2-CPE, 6-CPE and 7-CPE, as well as more close to the diffusion controlled process for 3-CPE and 8-CPE.
Photocatalytic properties
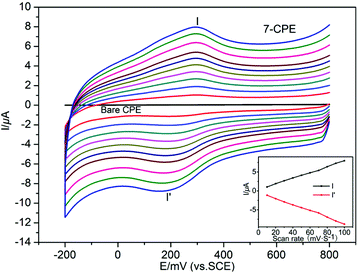
In order to investigate the influence of introducing a naphthalene group into the bis-pyridyl-bis-amide ligand of MOCPs on the photocatalytic properties of title compounds, in this work, we also selected methylene blue (MB), methyl orange (MO) and rhodamine B (RhB) as model pollutants in aqueous media to evaluate the photocatalytic effectiveness of complexes 1–10. The photocatalytic performances of complexes 1–10 for the degradation of MB, MO and RhB were investigated through a typical process: 50 mg powder of the title complexes was dispersed in the MB/MO/RhB solution (10.0 mg L−1). Then, the mixture was stirred and continuously exposed to UV irradiation from a 15 W high pressure mercury vapor lamp. A sample was taken every 30 min from the vessel, and subsequently analyzed by UV–visible spectroscopy. The photocatalytic properties of title complexes are shown in Fig. 12, 13, S21 and S22.† In addition, control experiments on the photodegradation of MB/MO/RhB were carried out. No significant change in the degradation of MB/MO was observed under the following reaction conditions: (1) in the dark; (2) without catalyst; (3) only CoCl2·6H2O/NiCl2·6H2O/CuCl2·2H2O/ZnCl2/CdCl2·2.5H2O; (4) only the 3-DPNA/4-DPNA ligand; (5) only the 1,3,5-H3BTC. The results indicate that all the complexes 1–10 are active for the decomposition of MB/MO under UV irradiation. However, there is no obvious photocatalytic degradation of RhB. Notably, the photocatalytic efficiency of 7 in MB as well as 1 in MO under UV irradiation is higher than others, respectively.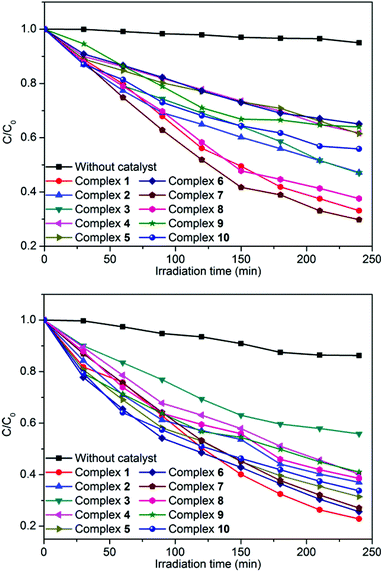 | ||
| Fig. 13 Photocatalytic decomposition rates of MB (top) and MO (bottom) solutions under UV irradiation with the use of the title complexes and no crystal under the same conditions. | ||
Comparatively, the photocatalytic performance of 7 was better than that of the low dimensional framework [Cu3(4-bpah)4(1,3,5-BTC)2]·8H2O20 (4-bpah = N,N′-bis(4-pyridinecarboxamide)-1,2-cyclohexane) for the decomposition of MB under UV irradiation. For 7, approximately 71% of MB was decomposed after 240 min, whereas only 51% of MB was degraded after 240 min for [Cu3(4-bpah)4(1,3,5-BTC)2]·8H2O. However, the photocatalytic efficiency of 7 was lower than that of [Cu2(4-DPNA)2(2,5-tdca)2]9b (2,5-H2tdca = thiophene-2,5-dicarboxylic acid) for the decomposition of RhB. For 7, there is no obvious photocatalytic degradation of RhB after 4 h, whereas 93% of RhB was degraded after 4 h for [Cu2(4-DPNA)2(2,5-tdca)2]. The results indicate that 7 has an advantage during the photocatalytic decomposition of MB under UV irradiation. As is known, the differences of the photocatalytic activities for MOCPs are mainly related to the nature of their central metals, organic ligands, as well as the final framework structures of the MOCPs.9b,20
As is known, in the presence of UV light, there is electron transfer from the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). The HOMO is mainly contributed by oxygen and/or nitrogen 2p bonding orbitals (valence band) and the LUMO by empty Co/Ni/Cu/Zn/Cd orbitals (conduction band), and charge transfer actually takes place from oxygen and (or) nitrogen to Co/Ni/Cu/Zn/Cd on photoexcitation7. The HOMO strongly demands one electron to return to its stable state. Therefore, one electron was captured from water molecules, which was oxygenated into the ˙OH active species. Then the ˙OH radicals could cleave MB effectively to complete the photocatalytic process.7
In addition, the stability of complexes 1–10 was monitored using IR spectra and PXRD during the course of photocatalytic reactions (Fig. S15†). After photocatalysis, the IR spectra of complexes 1–10 are nearly identical to those of the original complexes. The PXRD patterns of complexes 1 and 7 are also consistent with the simulated ones. In other words, they reveal good stability toward photocatalysis. The differences in catalytic activity under UV irradiation between 1–10 may be traced back to the discrepancy in various structures or central metal atoms of the ten complexes.
Conclusions
In summary, ten metal–organic coordination polymers based on the tricarboxylic acid 1,3,5-H3BTC and two isomeric semi-rigid bis-pyridyl-bis-amide ligands 3-DPNA/4-DPNA have been hydrothermally synthesized. The title complexes display versatile coordination features with 1D, 2D and 3D frameworks. These various structures from 1D to 3D reveal that the N-donor ligands and the metal cations play important roles in the construction of the final complexes. The structural features of the title complexes suggest that the semi-rigid 4-DPNA ligand is a good candidate for the construction of microporous metal–organic coordination polymers. The title complexes show good fluorescence, electrochemical and photocatalytic properties, and can be good candidates as potential functional materials.Acknowledgements
The support from the National Natural Science Foundation of China (no. 21171025 and 21471021), New Century Excellent Talents in University (NCET-09-0853), and Program of Innovative Research Team in University of Liaoning Province (LT2012020) is gratefully acknowledged.Notes and references
- (a) A. Morsali and M. Y. Masoomi, Coord. Chem. Rev., 2009, 253, 1882 CrossRef CAS PubMed; (b) S. T. Zheng, T. Wu, B. Irfanoglu, F. Zuo, P. Feng and X. H. Bu, Angew. Chem., Int. Ed., 2011, 50, 8034 CrossRef CAS PubMed; (c) M. H. Zeng, Q. X. Wang, Y. X. Tan, S. Hu, H. X. Zhao, L. S. Long and M. Kurmoo, J. Am. Chem. Soc., 2010, 132, 2561 CrossRef CAS PubMed; (d) Y. Y. Qin, J. Zhang, Z. J. Li, L. Zhang, X. Y. Cao and Y. G. Yao, Chem. Commun., 2008, 2532 RSC.
- (a) Y. B. Xie, H. Yang, Z. Y. U. Wang, Y. Y. Liu, H. C. Zhou and J. R. Li, Chem. Commun., 2014, 50, 563 RSC; (b) C. Perego and R. Millini, Chem. Soc. Rev., 2013, 42, 3956 RSC; (c) G. Y. Wang, L. L. Yang, Y. Li, H. Song, W. J. Ruan, Z. Chang and X. H. Bu, Dalton Trans., 2013, 42, 12865 RSC; (d) J. Boeckmann and C. Näther, Chem. Commun., 2011, 47, 7104 RSC; (e) S. Pandey, P. P. Das, A. K. Singh and R. Mukherjee, Dalton Trans., 2011, 40, 10758 RSC.
- N. N. Adarsh and P. Dastidar, Chem. Soc. Rev., 2012, 41, 3039 RSC.
- (a) B. C. Tzeng, T. Y. Chang, S. L. Wei and H. S. Sheu, Chem. – Eur. J., 2012, 18, 5105 CrossRef CAS PubMed; (b) F. Luo, Z. Z. Yuan, X. F. Feng, S. R. Batten, J. Q. Li, M. B. Luo, S. J. Liu, W. Y. Xu, G. M. Sun, Y. M. Song, H. X. Huang and X. Z. Tian, Cryst. Growth Des., 2012, 12, 3392 CrossRef.
- J. Park, J. R. Li, Y. P. Chen, J. M. Yu, A. A. Yakovenko, Z. Y. U. Wang, L. B. Sun, P. B. Balbuena and H. C. Zhou, Chem. Commun., 2012, 48, 9995 RSC.
- C. H. Lee, H. Y. Huang, Y. H. Liu, T. T. Luo, G. H. Lee, S. M. Peng, J. C. Jiang, I. Chao and K. L. Lu, Inorg. Chem., 2013, 52, 3962 CrossRef CAS PubMed.
- Y. Q. Chen, S. J. Liu, Y. W. Li, G. R. Li, K. H. He, Y. K. Qu, T. L. Hu and X. H. Bu, Cryst. Growth Des., 2012, 12, 5426 CAS.
- (a) S. Y. Huang, J. Q. Li, S. J. Liu, Y. Ning, L. N. Meng, J. Y. Li, M. B. Luo and F. Luo, CrystEngComm, 2014, 16, 5608 RSC.
- (a) X. M. Zhang, Y. Ning, L. Meng, J. Q. Li, M. B. Luo and F. Luo, CrystEngComm, 2014, 16, 7440 RSC; (b) X. L. Wang, M. Le, H. Y. Lin, J. Luan, G. C. Liu, J. W. Zhang and A. X. Tian, Inorg. Chem. Commun., 2014, 49, 19 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- X. L. Wang, J. Luan, H. Y. Lin, Q. L. Lu, C. Xu and G. C. Liu, Dalton Trans., 2013, 42, 8375 RSC.
- (a) G. Mukherjee and K. Biradha, Cryst. Growth Des., 2013, 13, 4100 CrossRef CAS; (b) H. E. Bakkali, A. Castiñeiras, I. G. Santos, J. M. G. Pérez and J. N. Gutiérrez, Cryst. Growth Des., 2014, 14, 249 CrossRef; (c) B. Dolenský, R. Konvalinka, M. Jakubek and V. Král, J. Mol. Struct., 2013, 1035, 124 CrossRef PubMed.
- (a) Y. Gong, T. Wu and J. H. Lin, CrystEngComm, 2012, 14, 3727 RSC; (b) S. S. Chen, Y. Zhao, J. Fan, T. Okamura, Z. S. Bai, Z. H. Chen and W. Y. Sun, CrystEngComm, 2012, 14, 3564 RSC; (c) S. l. Huang, X. X. Li, X. J. Shi, H. W. Hou and Y. T. Fan, J. Mater. Chem., 2010, 20, 5695 RSC; (d) C. Y. Xu, L. K. Li, Y. P. Wang, Q. Q. Guo, X. J. Wang, H. W. Hou and Y. T. Fan, Cryst. Growth Des., 2011, 11, 4667 CrossRef CAS.
- (a) Y. Bai, G. J. He, Y. G. Zhao, C. Y. Duan, D. B. Dang and Q. J. Meng, Chem. Commun., 2006, 1530 RSC; (b) J. P. Zou, Q. Peng, Z. H. Wen, G. S. Zeng, Q. J. Xing and G. C. Guo, Cryst. Growth Des., 2010, 10, 2613 CrossRef CAS; (c) S. S. Xiong, S. J. Wang, X. J. Tang and Z. Y. Wang, CrystEngComm, 2011, 13, 1646 RSC; (d) Y. J. Cui, Y. F. Yue, G. D. Qian and B. L. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed; (e) G. L. Liu, Y. J. Qin, L. Jing, G. Y. Wei and H. Li, Chem. Commun., 2013, 49, 1699 RSC.
- (a) A. K. Singh and R. Mukherjee, Dalton Trans., 2005, 2886 RSC; (b) S. Hazra, S. Majumder, M. Fleck, N. Aliaga-Alcalde and S. Mohanta, Polyhedron, 2009, 28, 3707 CrossRef CAS PubMed; (c) X. L. Wang, H. Y. Zhao, H. Y. Lin, G. C. Liu, J. N. Fang and B. K. Chen, Electroanalysis, 2008, 20, 1055 CrossRef CAS.
- (a) S. Pandey, P. P. Das, A. K. Singh and R. Mukherjee, Dalton Trans., 2011, 40, 10758 RSC; (b) X. L. Wang, L. L. Hou, J. W. Zhang, G. C. Liu and S. Yang, CrystEngComm, 2012, 14, 3936 RSC.
- (a) M. Shamsipur, M. Najafi, M. M. Hosseini and H. Sharghi, Electroanalysis, 2007, 19, 1661 CrossRef CAS; (b) P. Bhunia, D. Sardar, K. K. Sarker, U. S. Ray, J. S. Wu, T. H. Lu and C. Sinha, J. Coord. Chem., 2009, 62, 552 CrossRef CAS.
- (a) X. L. Wang, H. Y. Lin, B. Mu, A. X. Tian and G. C. Liu, Dalton Trans., 2010, 39, 6187 RSC; (b) X. L. Wang, J. Luan, H. Y. Lin, M. Le and G. C. Liu, Dalton Trans., 2014, 43, 8072 RSC.
- X. L. Wang, J. X. Zhang, G. C. Liu, H. Y. Lin, Y. Q. Chen and Z. H. Kang, Inorg. Chim. Acta, 2011, 368, 207 CrossRef CAS PubMed.
- (a) J. Guo, J. Yang, Y. Y. Liu and J. F. Ma, CrystEngComm, 2012, 14, 6609 RSC; (b) T. Wen, D. X. Zhang, J. Liu, R. Lin and J. Zhang, Chem. Commun., 2013, 49, 5660 RSC; (c) T. Wen, D. X. Zhang and J. Zhang, Inorg. Chem., 2013, 52, 12 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: IR spectra, TG, PXRD, and additional figures. CCDC 1005163–1005172. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00218k |
| This journal is © the Partner Organisations 2015 |