Transesterification of dimethyl carbonate with phenol to diphenyl carbonate over hexagonal Mg(OH)2 nanoflakes
Qiang
Wang
*a,
Chunhong
Li
b,
Ming
Guo
a,
Shengjun
Luo
c and
Changwen
Hu
*d
aLaboratory for Micro-sized Functional Materials & College of Elementary Education, Capital Normal University, Beijing, P R China. E-mail: qwchem@gmail.com; Fax: +86 10 68901751; Tel: +86 10 68902523
bNational Laboratory for Superconductivity, Institute of Physics and Beijing National Laboratory for Condensed Matter Physics, Chinese Academy of Sciences, Beijing, P R China
cQingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao, P R China
dKey Laboratory of Cluster Science of Ministry of Education of China, The Institute for Chemical Physics and Department of Chemistry, Beijing Institute of Technology, Beijing, P R China. E-mail: cwhu@bit.edu.cn; Fax: +86 10 68912631
First published on 12th November 2014
Abstract
Hexagonal Mg(OH)2 nanoflakes were synthesized via a hydrothermal method in the presence of polyethylene glycol 20000 (PEG-20000). X-ray diffraction (XRD) and field-emission scanning electron microscopy (FE-SEM) were applied to characterize the composition, morphologies and structure of the Mg(OH)2 nanoflakes. Brunauer–Emmett–Teller (BET) analysis was performed to investigate the porous structure and surface area of the as-obtained nanoflakes. The transesterification of dimethyl carbonate (DMC) with phenol to produce diphenyl carbonate (DPC) was carried out over the hexagonal Mg(OH)2 nanoflakes. The evaluation results showed that the hexagonal Mg(OH)2 nanoflakes had better activity and excellent selectivity to target products compared with many conventional ester exchange catalysts. Compared with other catalysts, such as AlCl3, ZnCl2, and irregular Mg(OH)2 synthesized via a hydrothermal method without PEG-20000, which all have been widely used as catalysts for this transesterification reaction, Mg(OH)2 nanoflakes were more stable and showed a relatively high activity with a low catalyst amount. The transesterification reaction process was also analyzed with the classic thermodynamic theory, and when the reaction was carried out at 453 K, with a molar ratio of phenol to DMC of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a reaction time of 13 h, and a catalyst amount of 0.2% (molar ratio to phenol), the selectivity of the transesterification reaction reached 92.3%. Moreover, the deactivated hexagonal Mg(OH)2 nanoflakes could be easily reactivated by calcination under vacuum, and the regenerated Mg(OH)2 nanoflakes showed the catalytic activity almost as high as that of the fresh sample.
1, a reaction time of 13 h, and a catalyst amount of 0.2% (molar ratio to phenol), the selectivity of the transesterification reaction reached 92.3%. Moreover, the deactivated hexagonal Mg(OH)2 nanoflakes could be easily reactivated by calcination under vacuum, and the regenerated Mg(OH)2 nanoflakes showed the catalytic activity almost as high as that of the fresh sample.
Introduction
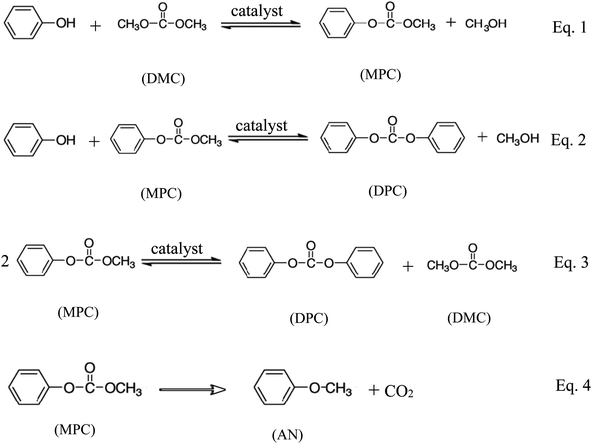
Transesterification is an important organic transformation and provides necessary synthons for a number of applications in organic processes.1–5 The synthesis of aromatic carbonates from dimethyl carbonate (DMC) and phenols is one of the most important transesterification reactions. DMC is an important intermediate for organic synthesis because its molecular structure contains several functional groups, such as carbonyl, methyl and methoxy. Moreover, it is nontoxic for human health and environment. Meanwhile, aromatic carbonates, especially diphenyl carbonate (DPC), are precursors for the production of aromatic polycarbonates by the melt polymerization process which is now the highlight in the polycarbonate industry.6–31 DPC is widely used as a starting material for phosgene-free polycarbonate production by interfacial polycondensation. Because of the increasing demand for a safe and environmentally friendly process of DPC synthesis, several non-phosgene methods have been developed.1–31 Among them, the most suitable method route is the oxidative carbonylation of phenol, which is a single-step process with water as the sole byproduct.1 This route is a two-step process, which involves the transesterification of DMC and phenol to methyl phenyl carbonate (MPC) (eqn (1)) and the further transesterification of MPC and phenol (eqn (2)) or disproportion of MPC to DPC (eqn (3)) and the O-methylation reaction (eqn (4)) as shown in Scheme 1.The synthesis of DPC by transesterification of DMC with phenol is usually catalyzed by organometallic compounds containing titanium, aluminium, tin, and conventional Lewis acid catalysts such as aluminium chloride and zinc chloride.1–29 The homogeneous catalysts such as conventional Lewis acids,16–19 titanium esters,18 tin compounds,19 titanocene dichloride (Cp2TiCl2),20 samarium halogen,7,21 and mesoporous MCM-4122 have been used as effective catalysts for the transesterification of DMC with phenol. But the reuse of these catalysts is very difficult because it is not easy to separate and recover these catalysts. Furthermore, the titanium and tin compounds are very poisonous and harmful to the environment. Consequently, many heterogeneous catalysts such as MoO3/SiO2,23 TiO2/SiO2,24 Mg–Al-hydrotalcite,25 PbO/MgO,26,27 NNC28 and MCN–TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 were investigated for this reaction. Disappointedly, these catalysts not only produce the byproduct anisole more or less due to their alkalescence, but they also terminate the reaction in the first step making MPC the main product, as shown in eqn (1). Also, Tundo et al.30 reported that the equilibrium constant for eqn (1) was 3 × 10−4 at 453 K. This indicates that the transesterification reactions are thermodynamically unfavorable, while the O-methylation eqn (4) is thermodynamically favorable due to the production of gaseous CO2. To overcome the thermodynamic limitation of the transesterification reactions, many processes have been proposed.1–31 Therefore, it is desirable to find more efficient, innocuous and cheap catalysts for transesterification of DMC with phenol in the application of commercial production.
29 were investigated for this reaction. Disappointedly, these catalysts not only produce the byproduct anisole more or less due to their alkalescence, but they also terminate the reaction in the first step making MPC the main product, as shown in eqn (1). Also, Tundo et al.30 reported that the equilibrium constant for eqn (1) was 3 × 10−4 at 453 K. This indicates that the transesterification reactions are thermodynamically unfavorable, while the O-methylation eqn (4) is thermodynamically favorable due to the production of gaseous CO2. To overcome the thermodynamic limitation of the transesterification reactions, many processes have been proposed.1–31 Therefore, it is desirable to find more efficient, innocuous and cheap catalysts for transesterification of DMC with phenol in the application of commercial production.
Here, hexagonal Mg(OH)2 nanoflakes with a thickness of about ∼10 nm and a lateral size of about ∼100 nm in the presence of PEG-20000 were synthesized via a hydrothermal process. More importantly, the hexagonal Mg(OH)2 nanoflakes showed excellent catalytic activities for the transesterification of DMC and phenol. Although the synthesis of Mg(OH)2 has been studied by many researchers,32 to the best of our knowledge, there has been no report on the catalytic behavior of Mg(OH)2 nanostructures yet.
Experimental
Materials
Dimethyl carbonate and phenol were obtained from Alfa Aesar. Magnesium nitrate hexahydrate (AR), sodium hydroxide (AR), polyethylene glycol (MW ≈ 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), ethanol (AR), and acetone (AR) were purchased from Beijing Chemical Reagent Company, China. All of the materials and reagents were used directly without further purification. Distilled water was purified using a Milli-Q system (Millipore, Billerica, MA).
000), ethanol (AR), and acetone (AR) were purchased from Beijing Chemical Reagent Company, China. All of the materials and reagents were used directly without further purification. Distilled water was purified using a Milli-Q system (Millipore, Billerica, MA).
Preparation of hexagonal Mg(OH)2 nanoflakes
The hexagonal Mg(OH)2 nanoflakes were prepared by the classical methods. In a typical synthesis, 0.2 g of hydrated magnesium nitrate (Mg(NO3)2·6H2O) and 0.1 g of PEG-20000 were each dissolved in 30 mL of deionized water. Then the aqueous solution of PEG-20000 was added dropwise to the aqueous Mg2+ solution under constant stirring. The mixture was stirred until a clear and homogeneous solution formed, and 1 mL of 1.0 mol L−1 NaOH solution was added to the homogeneous solution. Then the newly formed suspension was transferred to an 80 mL Teflon-lined autoclave, which was sealed and maintained at 433 K for 12 h. After the autoclave was cooled to room temperature, the resulting white precipitate was collected, washed several times with distilled water and absolute ethanol and dried at 373 K for 4 h.Characterization of Mg(OH)2 samples
The composition, morphologies and structures of Mg(OH)2 samples were characterized by X-ray powder diffraction (XRD, Shimadzu XRD-6000, Cu Kα radiation (λ = 1.54 Å)) and field-emission scanning electron microscopy (FE-SEM, Hitachi S-4800) with an accelerating voltage of 10 kV. The specific surface structures of the Mg(OH)2 samples were determined on constant volume adsorption apparatus (CHEMBET-3000) by the N2-BET method at liquid nitrogen temperature. The catalytic properties of the Mg(OH)2 samples were examined on-line using a gas-chromatogram (GC) (Shimadzu GC-2014). The surface composition and structure of the catalyst were studied by X-ray photoelectron spectroscopy (XPS, ThermoFisher Scientific, USA, ESCALAB 250) with Al Kα radiation (1487 eV) at a power of 300 W under a vacuum of 1.0 × 10−5 Pa. Data were analyzed by the use of XPSpeak 41 software.Typical procedure for transesterification
The typical procedure for the transesterification of DMC and phenol is as follows: all transesterification reactions were carried out in a 100 mL four-necked round-bottomed flask equipped with a magnetic stirring bar, a nitrogen inlet, a precise plunger pump for DMC instillation, and a Vigreux fractionating column connected to a liquid dividing head. Typical conditions and procedures were as follows: phenol (0.2 mol) and the catalyst (0.5 mmol) were charged into the reactor under a nitrogen atmosphere, and the temperature was gradually raised to and kept at 453 K. Then DMC was added drop-wise into the reactor at a flow rate of 0.05 ml min−1 using the precise plunger pump. The reaction mixture was under refluxing conditions during the period of reaction. The occurrence of reaction was indicated by attainment of a temperature of about 333 K at the top of the column. During the reaction, this distillation was analyzed by GC one time per hour.1–7 The reaction mixture was identified by the absolute retention time and quantified with ethyl benzoate as the internal standard. After the reaction, the mixture was cooled to room temperature, the catalyst was filtered and washed. The deactivated hexagonal Mg(OH)2 nanoflakes could be easily reactivated by calcination at 573 K under vacuum, and the regenerated Mg(OH)2 nanoflakes showed the catalytic activity almost as high as that of the fresh sample.Results and discussion
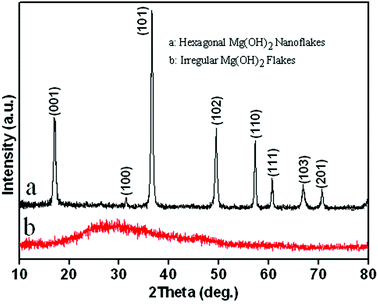
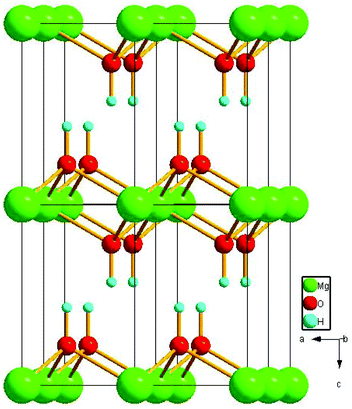
The composition and purity of the as-synthesized Mg(OH)2 flakes were examined by X-ray diffraction (Fig. 1). All of the diffraction peaks in Fig. 1a can be indexed as the pure hexagonal phase of Mg(OH)2 [space group: P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1[164]] with lattice constants of a = b = 3.144 Å and c = 4.777 Å (JCPDS card no. 44-1482) and angle constants of α = β = 90° and γ = 120°. As shown in Fig. 2, the hexagonal Mg(OH)2 nanoflakes have a layered structure. Fig. 1b shows the amorphous structure of magnesium hydroxide synthesized in the absence of PEG-20000.
m1[164]] with lattice constants of a = b = 3.144 Å and c = 4.777 Å (JCPDS card no. 44-1482) and angle constants of α = β = 90° and γ = 120°. As shown in Fig. 2, the hexagonal Mg(OH)2 nanoflakes have a layered structure. Fig. 1b shows the amorphous structure of magnesium hydroxide synthesized in the absence of PEG-20000.
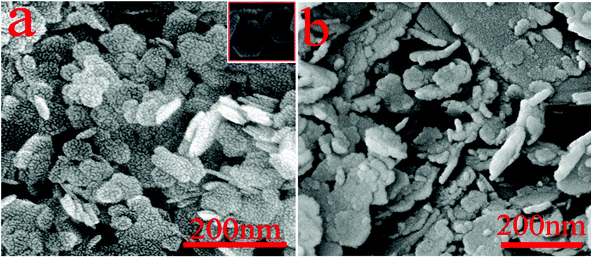
Representative field-emission scanning electron micrographs (FE-SEM) of the as-synthesized Mg(OH)2 samples are shown in Fig. 3. An overview image (Fig. 3a) discloses that the sample entirely consists of flake-shaped particles in the absence of other morphological structures. The higher magnification FE-SEM image inserted in Fig. 3a clearly reveals that each particle consists almost entirely of well-defined hexagonal nanoflakes with a thickness of ∼10 nm and a lateral dimension of ∼100 nm. The overall hexagonal morphology of these Mg(OH)2 particles is consistent with the hexagonal crystallographic characteristics of brucite. The amorphous structures of magnesium hydroxide synthesized in the absence of PEG-20000 are shown in Fig. 3b, which are disorganized morphologies.
The stoichiometric equation for the transesterification of DMC with phenol is as follows:
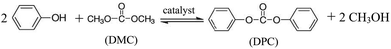 | (5) |
The chemical equilibrium and the reaction kinetics were probed in the expected operating range of the transesterification process, during which the temperature was varied between 298 K and 523 K. On the basis of ref. 25, 33–36, the thermodynamic data for eqn (5) and (1) were calculated and are shown in Tables 1 and 2. According to Table 1, transesterification equation (5) is endothermic at temperatures below 453 K, and the equilibrium constant (K⊖eq) increases with the increase of temperature. When the temperature is above 453 K, phenol is vaporized, and eqn (5) becomes exothermic. Between 423 K and 523 K, K⊖eq remains almost the same. Thus, the suitable temperature for eqn (5) should be 423 K–453 K. Because eqn (1) is endothermic, a higher temperature is preferable for this reaction (see Table 2). The thermodynamic data in Tables 1 and 2 show that the transesterification of DMC with phenol is thermodynamically unfavorable even at 523 K, while the reaction of O-methylation of DMC with phenol is thermodynamically favorable at room temperature. Thus, to choose a suitable temperature and to design an efficient reaction process are the keys to increase not only the yields of DPC and MPC but also the selectivities to them. According to Tables 1 and 2, the temperature of the transesterification of DMC with phenol was selected as 453 K, and the standard enthalpy for the exothermic reaction has been determined which was subsequently integrated into the energy balance of the transesterification process model accounting for the heat supplied by the chemical reaction.36
| T/K | ΔrH⊖m (kJ mol−1) | ΔrS⊖m (J mol−1 K−1) | ΔrG⊖m (kJ mol−1) | K ⊖eq |
|---|---|---|---|---|
| 298 | 64.4 | −3.6 | 65.4 | 3.5 × 10−12 |
| 373 | 49.6 | 65.3 | 25.2 | 2.9 × 10−4 |
| 423 | 45.9 | 56.0 | 22.2 | 1.8 × 10−3 |
| 453 | 43.0 | 49.1 | 20.8 | 4.0 × 10−3 |
| 493 | −45.2 | −146.1 | 26.8 | 1.4 × 10−3 |
| 523 | −41.2 | −133.9 | 28.8 | 1.3 × 10−3 |
| T/K | ΔrH⊖m (kJ mol−1) | ΔrS⊖m (J mol−1 K−1) | ΔrG⊖m (kJ mol−1) | K ⊖eq |
|---|---|---|---|---|
| 298 | 25.9 | 192.9 | −32.6 | 5.1 × 105 |
| 373 | 10.7 | 151.1 | −45.7 | 2.5 × 106 |
| 423 | 13.4 | 158.2 | −53.5 | 4.1 × 106 |
| 453 | 52.5 | 247.6 | −59.7 | 7.6 × 106 |
| 493 | 10.7 | 154.9 | −65.7 | 9.1 × 106 |
| 523 | 14.8 | 165.6 | −71.8 | 1.5 × 107 |
The catalytic properties of hexagonal Mg(OH)2 nanoflakes for the transesterification of DMC with phenol were also investigated (Fig. 4a). For comparison, the catalytic behavior of irregular Mg(OH)2 flakes (Fig. 4b), AlCl3 (Fig. 4c) and ZnCl2 (Fig. 4d) for the transesterification was also provided. The yield of ester, and selectivities to MPC and DPC are shown in Fig. 4, and the detailed results are summarized in Table 3. The synthesis of DPC is usually performed in two steps, as shown in eqn (1)–(4).
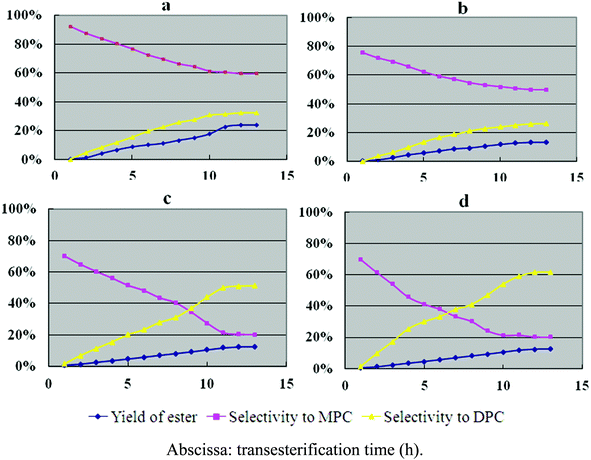 | ||
| Fig. 4 Catalytic activity of hexagonal Mg(OH)2 nanoflakes (a), irregular Mg(OH)2 flakes (b), AlCl3 (c) and ZnCl2 (d). | ||
| Catalyst | Yield of MPCd (%) | Yield of DPCe (%) | Yield of anisole (%) | Selectivity to MPC (%) | Selectivity to DPC (%) | Transesterification selectivity (%) |
|---|---|---|---|---|---|---|
a Reaction conditions: molar ratio of phenol-to-DMC 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaction time: 13 h, temperature: 453 K. DPC yield was based on the amount of DMC.
b Hexagonal Mg(OH)2 nanoflakes.
c Irregular Mg(OH)2 flakes.
d MPC: methyl phenyl carbonate.
e DPC: diphenyl carbonate. 1, reaction time: 13 h, temperature: 453 K. DPC yield was based on the amount of DMC.
b Hexagonal Mg(OH)2 nanoflakes.
c Irregular Mg(OH)2 flakes.
d MPC: methyl phenyl carbonate.
e DPC: diphenyl carbonate.
|
||||||
| Mg(OH)2b | 6.1 | 17.8 | 1.3 | 59.7 | 32.6 | 92.3 |
| Mg(OH)2c | 4.3 | 8.9 | 3.7 | 49.6 | 26.1 | 75.7 |
| AlCl3 | 3.2 | 15.1 | 1.1 | 20.3 | 51.1 | 71.4 |
| ZnCl2 | 1.5 | 11.0 | 1.2 | 8.8 | 61.5 | 71.3 |
In the first step, phenol is converted into methyl phenyl carbonate (MPC), and in the second step, MPC is converted into DPC by the reaction with phenol. MPC is the intermediate product during the transesterification, which is also a valuable industrial product, and in some cases, anisole (AN) may be produced as a byproduct in the second step (eqn (4)).
As shown in Fig. 4 and Table 3, the transesterification selectivity for hexagonal Mg(OH)2 nanoflakes is 92.3%, which is the highest of the four catalysts, while the selectivities for AlCl3, ZnCl2, and irregular Mg(OH)2 flakes range from 70% to 80%. The selectivities to DPC for Mg(OH)2 hexagonal nanoflakes’ and irregular Mg(OH)2 flakes’ selectivities toward DPC are 32.6% and 26.1%, but the selectivities toward MPC are 59.7% and 49.6%, respectively. Table 3 shows an overview of the selectivities to DPC and MPC for the various catalysts tested. When compared, the hexagonal Mg(OH)2 nanoflakes have the highest selectivity towards MPC while ZnCl2 has the highest selectivity toward DPC. It is also worth noting that AlCl3 had the highest yield of the byproduct anisole.
It is known that the activities and selectivities of the various catalysts may be influenced by the acidity or alkalinity of the catalysts.1–27 AlCl3 and ZnCl2 are typical Lewis acid catalysts, which are known to accelerate eqn (2) and (3) in Scheme 1, while the hexagonal Mg(OH)2 nanoflakes and irregular Mg(OH)2 flakes are alkaline compounds, which are known to speed up eqn (1) in Scheme 1. It is generally accepted that the more alkaline a catalyst is, the higher the yield and selectivity of the relevant products will be. The appropriate alkalescence is also an important factor for transesterification activity. As shown above, hexagonal Mg(OH)2 nanoflakes have the highest overall transesterification selectivity, which may be due to its high alkalinity on the surface of the nanoflakes. In addition, in the above catalytic process, the hexagonal Mg(OH)2 nanoflakes are not dissolved or decomposed during the transesterification and once collected, 97.8% of the nanoflakes can be recycled. The transesterification catalyzed by hexagonal Mg(OH)2 nanoflakes is also an unpolluted process. Hence, hexagonal Mg(OH)2 nanoflakes are more suitable for the transesterification of DMC with phenol when compared with AlCl3, ZnCl2 and irregular Mg(OH)2 flakes.
One of the important criteria for heterogeneous catalysis is the reusability. The possibility and extent of catalyst deactivation was investigated by employing the recycled catalyst sample washed with DMC and dried at 373 K in a number of reaction cycles, which is given in Table 4. Table 4 shows that the hexagonal Mg(OH)2 nanoflakes exhibited a decline in activity with reuse. According to the total yield of the esters (MPC and DPC), the activity of the catalyst used once reduced by only 5%, from 23.9 to 22.7%. When it was used for the fifth time, the activity of the catalyst reduced by about a half, from 23.9 to 13.1%. It was interesting that the total selectivity of MPC and DPC increased slightly with the decrease of the total yield of the esters, which might suggest some structure sensitivity in terms of transesterification selectivity. Moreover, the hexagonal Mg(OH)2 nanoflakes used for the fifth time were regenerated by calcination at 573 K under vacuum, the result is also shown in Table 4. The total yield of the esters reached 21.7%, which was a little lower than that of the fresh hexagonal Mg(OH)2 nanoflakes. It presented that the catalytic activity could almost be recovered by calcination of the used catalyst.
| Numbers of catalyst reuse | Yield of MPCb (%) | Yield of DPCc (%) | Total yield of esters (%) | Transesterification selectivity (%) |
|---|---|---|---|---|
a Reaction conditions: molar ratio of phenol-to-DMC 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaction time: 13 h, temperature: 453 K.
b MPC: methyl phenol carbonate.
c DPC: diphenyl carbonate.
d Catalyst used for the fourth time and then calcined at 573 K under vacuum. 1, reaction time: 13 h, temperature: 453 K.
b MPC: methyl phenol carbonate.
c DPC: diphenyl carbonate.
d Catalyst used for the fourth time and then calcined at 573 K under vacuum.
|
||||
| 1 | 6.1 | 17.8 | 23.9 | 92.3 |
| 2 | 5.2 | 17.5 | 22.7 | 95.4 |
| 3 | 4.4 | 14.1 | 18.5 | 95.2 |
| 4 | 3.9 | 13.0 | 16.9 | 95.0 |
| 5 | 3.6 | 12.5 | 13.1 | 94.9 |
| 6d | 5.5 | 16.2 | 21.7 | 91.8 |
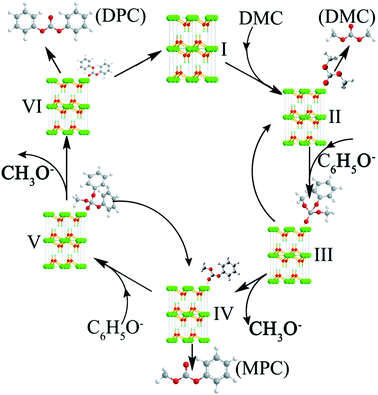
Based on the above experimental results, the transesterification mechanism catalyzed by the hexagonal Mg(OH)2 nanoflakes was suggested as the following procedure (Fig. 5).1,20 At first, the Mg2+ was exposed(I) in the crystal structure of Mg(OH)2. According to the charge distribution and the theory of coordination chemistry, the weak interaction between the O atom of carbonyl in DMC and the Mg2+ would allow DMC and Mg(OH)2 to form Mg(OH)2(DMC)(II). The transesterification of DMC and phenol belongs to a reaction of a soft base binding to a hard acid, the active center sp2-hybridized carbonyl carbon in DMC was regarded as a hard acid center. The carbonyl carbon in DMC was affected by the carbonyl oxygen with larger electronegativity, so that electrons would tend to carbonyl oxygen resulting in the electron cloud density on carbon atom decreasing and the positively charged carbonyl carbon. So the carbonyl carbon had a strong electrophilic activity and was the electrophilic center. Meanwhile, the negatively charged oxygen atoms of C6H5O− contain lone electron pairs and the ability to attract electrons is stronger than the carbon atoms on the benzene ring, the oxygen atoms prone to nucleophilic reactions. As a result, the carbonyl carbon in DMC should be attacked more easily by C6H5O− to produce intermediate (III). After the rearrangement, it might either demethoxylate to give Mg(OH)2(MPC)(IV) or dephenoxylate to yield Mg(OH)2(DMC)(II). The DMC or MPC can leave from the intermediate (II) or (IV), respectively, in agreement with eqn (1) in Scheme 1. The transformation of Mg(OH)2(MPC)(IV) to intermediate (V) is similar to the procedure of (II)–(III). The intermediate (V) may also demethoxylate to produce Mg(OH)2(DPC)(VI) or dephenoxylate to return Mg(OH)2(DMC)(II). The MPC or DPC also leaves from the (IV) or (VI), respectively, as shown in eqn (2). The intermediates (II, IV and VI) can return to (I) with the leaving of DMC, MPC or DPC, respectively, resulting in a complete catalytic cycle. This mechanism explains the high selectivity of esters in this transesterification.
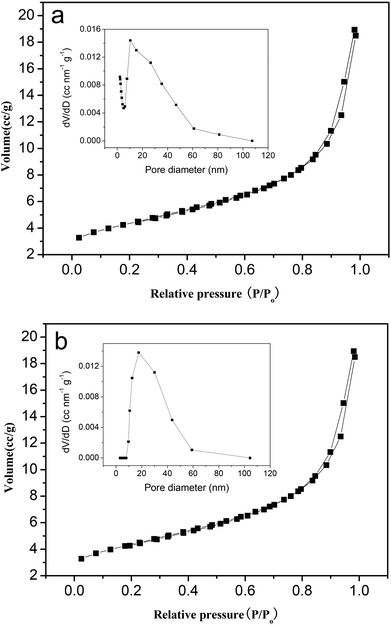
In addition, the higher activity of the hexagonal Mg(OH)2 nanoflakes when compared to the irregular Mg(OH)2 flakes can be ascribed to their relatively higher surface area. The isotherms of nitrogen adsorption and desorption at 77 K for hexagonal Mg(OH)2 nanoflakes and irregular Mg(OH)2 flakes are plotted in Fig. 6a and 6b, respectively. The isotherms for the hexagonal Mg(OH)2 nanoflakes and irregular Mg(OH)2 flakes are characteristic of a type IV isotherm with a type H3 hysteresis loop. Also, the pore volume and pore diameter distribution plots of hexagonal Mg(OH)2 nanoflakes and irregular Mg(OH)2 flakes are given in Fig. 6, which indicate the presence of mesopores in the size range 2–110 nm. The pore size distribution testing presented that the average pore sizes of hexagonal Mg(OH)2 nanoflakes and irregular Mg(OH)2 flakes were 10.39 nm and 17.67 nm, respectively, Therefore, the number of holes per unit area of the hexagonal Mg(OH)2 nanoflakes was more than the irregular Mg(OH)2 flakes. On the basis of the isotherms, it is possible to calculate the values of the BET surface area (SBET); SBET values of the hexagonal Mg(OH)2 nanoflakes and irregular Mg(OH)2 flakes are 68.87 and 42.48 m2 g−1, respectively. The SBET values of the hexagonal Mg(OH)2 nanoflakes are larger than that of irregular Mg(OH)2 flakes, because the hexagonal Mg(OH)2 nanoflakes are smaller than the irregular Mg(OH)2 flakes and the latter easily tend to agglomerate (Fig. 3). On the basis of the catalytic tests and the surface area measurements shown in Fig. 4, 6 and Table 3, it can be concluded that between these two samples, the sample with the largest surface area may possess the highest amount of active centers which then offers the highest selectivity towards DPC. So the hexagonal Mg(OH)2 nanoflakes with a high surface area and suitable pores are more effective as catalysts than the irregular counterpart in the transesterification.
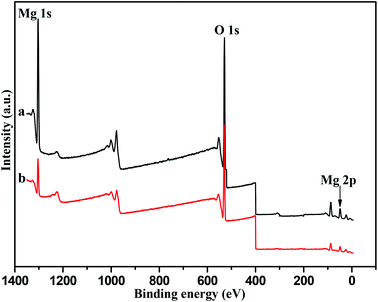
XPS results for the hexagonal Mg(OH)2 nanoflakes and irregular Mg(OH)2 flakes are shown in Fig. 7. XPS analysis was used to determine the elements of the surface of the samples.37–39Fig. 7a shows sharp peaks with a binding energy of 56.8 eV, 530.4 eV and 1309.6 eV. Taking the known binding energy of Mg(OH)2 into consideration, the relative content of Mg(+2, 2p) and Mg(+2, 1s) was 27.13 and 25.13%, respectively. Fig. 7b also shows similar peaks to Fig. 7a, the relative content of Mg(+2, 2p) and Mg(+2, 1s) was about 18.44 and 10.40%, respectively. The high-resolution O(1s) XPS spectra of the film surfaces revealed one component at a binding energy of 530.4 eV associated with the OH−. According to Fig. 7, the content of the Mg(+2) ions on the surface of the hexagonal nanoflakes was higher than that of the irregular Mg(OH)2 flakes, which was consistent with the BET analysis and their appropriate catalytic behavior. Also, this further provided a basis for the transesterification mechanism (shown in Fig. 5) that Mg2+ played a key role in the process of the formation of intermediate products, such as Mg(OH)2(DMC)(II), Mg(OH)2(MPC)(IV) and Mg(OH)2(DPC)(VI).
Conclusions
The hexagonal Mg(OH)2 nanoflakes were successfully synthesized in large quantities via a hydrothermal method in the presence of PEG-20000. The composition, morphology and structure of the hexagonal Mg(OH)2 nanoflakes were characterized by XRD, FE-SEM, and BET. The hexagonal Mg(OH)2 nanoflakes were active catalysts for the synthesis of DPC from the transesterification of DMC with phenol. The transesterification selectivity for hexagonal Mg(OH)2 nanoflakes is 92.3%. The selectivities to DPC for Mg(OH)2 hexagonal nanoflakes’ and irregular Mg(OH)2 flakes’ selectivities toward DPC are 32.6% and 26.1%, but the selectivities toward MPC are 59.7% and 49.6%, respectively. In general, the hexagonal Mg(OH)2 nanoflakes were found to be one novel and efficient heterogeneous catalyst for the synthesis of DPC by transesterification of DMC with phenol. Moreover, the deactivated hexagonal Mg(OH)2 nanoflakes could be easily reactivated by calcination under vacuum and can be reused.Acknowledgements
This work was supported by the Natural Science Foundation of China (NSFC no. 21471103, 21471100, 51002180, 21001074, 20731002, 20871016, 10876002, 91022006 and 20973023), the 111 Project (B07012), the Fundamental Research Funds for the Central Universities (2013NT13), the Project of Excellent Talents of Beijing (203135407707), and the Scientific Research Base Development Program of the Beijing Municipal Commission of Education.References
- S. J. Luo, Y. N. Chi, L. N. Sun, Q. Wang and C. W. Hu, Catal. Commun., 2008, 9, 2560 CrossRef CAS PubMed.
- P. Braustein, M. Lakkis and D. Matt, J. Mol. Catal., 1987, 42, 353 CrossRef.
- A. G. Shaikh and S. Sivaram, Ind. Eng. Chem. Res., 1992, 31, 1167 CrossRef CAS.
- H. Y. Niu, J. Yao, Y. Wang and G. Y. Wang, J. Mol. Catal. A: Chem., 2005, 235, 240 CrossRef CAS PubMed.
- Q. Wang, S. J. Luo, M. H. Cao and C. W. Hu, J. Beijing Inst. Technol., 2008, 17, 87 CAS.
- Q. Wang, K. L. Wang, X. L. Wu, S. J. Luo and C. W. Hu, Chem. Res. Chin. Univ., 2007, 23, 641 CrossRef CAS.
- F. M. Mei, G. X. Li, J. Nie and H. B. Xu, J. Mol. Catal. A: Chem., 2002, 184, 465 CrossRef CAS.
- Y. Watanabe and T. Tatsumi, Microporous Mesoporous Mater., 1998, 22, 399 CrossRef CAS.
- C. H. Yang, W. B. Li, Y. Q. Liu, Y. S. Tan and Y. Z. Han, J. Fuel Chem. Technol., 2002, 30, 551 CAS.
- Z. H. Li, Z. F. Qin and J. G. Wang, Petrochem. Technol., 2006, 35, 528 CAS.
- F. Y. Pan, S. P. Wang, X. B. Ma, Z. H. Li and G. H. Xu, Chem. Ind. Eng., 2004, 21, 174 CAS.
- Y. Liu, S. P. Wang and X. B. Ma, Ind. Eng. Chem. Res., 2007, 46, 1045 CrossRef CAS.
- D. X. Yin, Z. H. Li, T. L. Xiang, B. W. Wang, X. B. Ma and G. H. Xu, Nat. Gas Chem. Ind., 2004, 29, 20 CAS.
- Y. Z. Li and S. J. Kim, J. Phys. Chem. B, 2005, 109, 12309 CrossRef CAS PubMed.
- A. Prakash, A. V. McCormick and M. R. Zachariah, Chem. Mater., 2004, 16, 1466 CrossRef CAS.
- Z. Li and Z. Qin, J. Mol. Catal. A: Chem., 2007, 264, 255 CrossRef CAS PubMed.
- K. M. Deshmukh, Z. S. Qureshi, K. P. Dhake and B. M. Bhanage, Catal. Commun., 2010, 12, 207 CrossRef CAS PubMed.
- H. Lee, S. J. Kima, B. S. Ahna and W. K. Lee, Catal. Today, 2003, 87, 139 CrossRef CAS PubMed.
- A. Vimont, A. Travert, C. Binet, C. Pichon, P. Mialane, F. Sécheresse and J. C. Lavalley, J. Catal., 2006, 241, 221 CrossRef CAS PubMed.
- H. Y. Niu, J. Yao, Y. Wang and G. Y. Wang, Catal. Commun., 2007, 8, 355 CrossRef CAS PubMed.
- H. Y. Niu, H. M. Guo, J. Yao, Y. Wang and G. Y. Wang, J. Mol. Catal. A: Chem., 2006, 259, 292 CrossRef CAS PubMed.
- Y. Liu, G. M. Zhao, G. Liu, S. J. Wu, G. H. Chen, W. X. Zhang, H. Y. Sun and M. J. Jia, Catal. Commun., 2008, 9, 2022 CrossRef CAS PubMed.
- Z. H. Fu and Y. Ono, J. Mol. Catal. A: Chem., 1997, 118, 293 CrossRef CAS.
- R. Z. Tang, T. Chen, Y. Chen, Y. Z. Zhang and G. Y. Wang, Chin. J. Catal., 2014, 35, 457 CrossRef CAS.
- F. M. Mei, Z. Pei and G. X. Li, Org. Process Res. Dev., 2004, 8, 372 CrossRef CAS.
- W. Q. Zhou, X. Q. Zhao, Y. J. Wang and J. Y. Zhang, Appl. Catal., A, 2004, 260, 19 CrossRef CAS PubMed.
- Z. H. Li, Y. J. Wang, X. S. Ding and X. Q. Zhao, J. Nat. Gas Chem., 2009, 18, 104 CrossRef CAS.
- X. L. Yuan, M. Zhang, X. D. Chen, N. H. An, G. Liu, Y. Liu, W. X. Zhang, W. F. Yan and M. J. Jia, Appl. Catal., A, 2012, 439–440, 149 CrossRef CAS PubMed.
- X. Zhou, X. Ge, R. Z. Tang, T. Chen and G. Y. Wang, Chin. J. Catal., 2014, 35, 481 CrossRef CAS.
- P. Tundo, F. Trotta, G. Moraglio and F. Ligorati, Ind. Eng. Chem. Res., 1988, 27, 1565–1571 CrossRef CAS.
- Y. Watanabe and T. Tatsumi, Microporous Mesoporous Mater., 1998, 22, 399 CrossRef CAS.
- G. W. Beall, E. M. Duraia, F. El-Tantawy, F. Al-Hazmi and A. A. Al-Ghamdi, Powder Technol., 2013, 234, 26 CrossRef PubMed.
- G. C. Sinke, D. L. Hildenbrand, R. A. McDonald, W. R. Kramer and D. R. Stull, J. Phys. Chem., 1958, 62, 1461 CrossRef CAS.
- W. V. Steele, R. D. Chirico, S. E. Knipmeyer and A. Nguyen, J. Chem. Eng. Data, 1997, 42, 1008 CrossRef CAS.
- W. V. Steele, R. D. Chirico, S. E. Knipmeyer, A. Nguyen and N. K. Smith, J. Chem. Eng. Data, 1997, 42, 1037 CrossRef CAS.
- J. Holtbruegge, S. Heile, P. Lutze and A. Górak, Chem. Eng. J., 2013, 234, 448 CrossRef CAS PubMed.
- D. S. Tong, J. Yao, Y. Wang, H. Y. Niu and G. Y. Wang, J. Mol. Catal. A: Chem., 2007, 268, 120 CrossRef CAS PubMed.
- F. Z. Zhang, H. Zhang and Z. X. Su, Appl. Surf. Sci., 2007, 253, 7393 CrossRef CAS PubMed.
- T. Ishizaki, R. Kudo, T. Omi, K. Teshima, T. Sonoda, I. Shigematsu and M. Sakamoto, Electrochim. Acta, 2012, 62, 19 CrossRef CAS PubMed.
| This journal is © the Partner Organisations 2015 |