Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Avoiding compositional drift during the RAFT copolymerization of N-(2-hydroxypropyl)methacrylamide and N-acryloxysuccinimide: towards uniform platforms for post-polymerization modification
John
Moraes
,
Ioana-Maria
Simionca†
,
Hedi
Ketari
and
Harm-Anton
Klok
*
École Polytechnique Fédérale de Lausanne (EPFL), Institut des Matériaux and Institut des Sciences et Ingénierie Chimiques, Laboratoire des Polymères, Bâtiment MXD, Lausanne, Switzerland. E-mail: harm-anton.klok@epfl.ch
First published on 1st October 2015
Abstract
Correction for ‘Avoiding compositional drift during the RAFT copolymerization of N-(2-hydroxypropyl)methacrylamide and N-acryloxysuccinimide: towards uniform platforms for post-polymerization modification’ by John Moraes et al., Polym. Chem., 2015, 6, 3245–3251.
The original manuscript (John Moraes et al., Polym. Chem., 2015, 6, 3245–3251) discussed the reactivity ratios and copolymerization kinetics of the N-(2-hydroxypropyl)methacrylamide (HPMA) and N-acryloxysuccinimide (NAS) comonomer pair and the post-polymerization modification of a poly(HPMA-stat-NAS) copolymer. The aim of this correction is to point out and correct a mistake that was made in the peak assignments in Fig. S1A of the original manuscript. While this mis-assignment does not affect the analysis of the copolymerization behavior of the HPMA–NAS comonomer pair and the determination of the reactivity ratios for these two monomers, which are the key points of the paper, it does not accurately describe the post-polymerization modification of the poly(HPMA-stat-NAS) copolymer, which is described at the end of the manuscript.
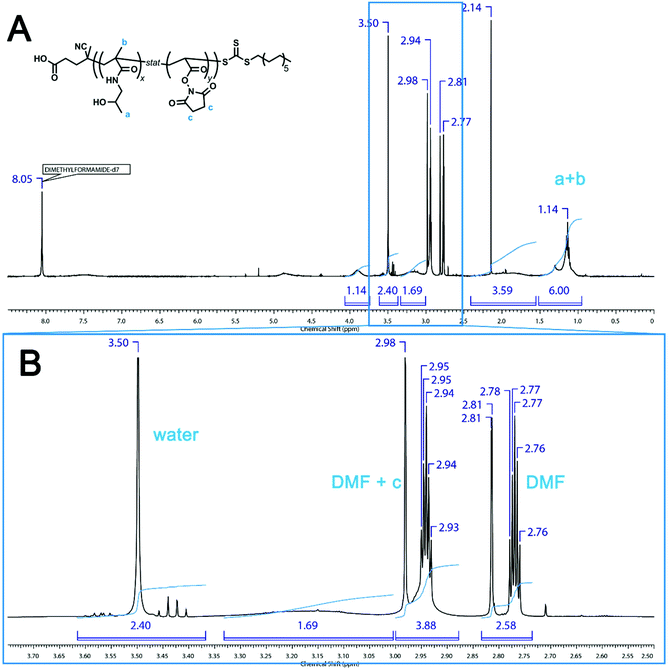
In Fig. S1A in the ESI for our original manuscript, the peak at 3.5 ppm in the 1H NMR spectrum of poly(HPMA-stat-NAS) was assigned to the CH2 groups of NAS. This signal, however, should be assigned to H2O. The CH2 groups of NAS appear at 2.94 ppm. This region (2.87–3.03 ppm) is also where the solvent DMF protons appear (Fig. 1, this document). However, the solvent contribution to the peak can be subtracted using the protons at 2.72–2.86 ppm (also CH3 from DMF). Doing this calculation yields an integral value of 1.30 for the CH2 protons of the NAS group (cf. 2.16 as in the original manuscript Fig. S1A). This leads to a NAS composition of the final polymer of 25% (as opposed to 35%, which was stated in the original manuscript). The lower value suggests that some hydrolysis of the active esters may be taking place as previously noted by Relogio et al.,1 however it is worth noting that the authors in this case obtained the 1H NMR spectrum in D2O, which is expected to have a much larger potential for hydrolysis.
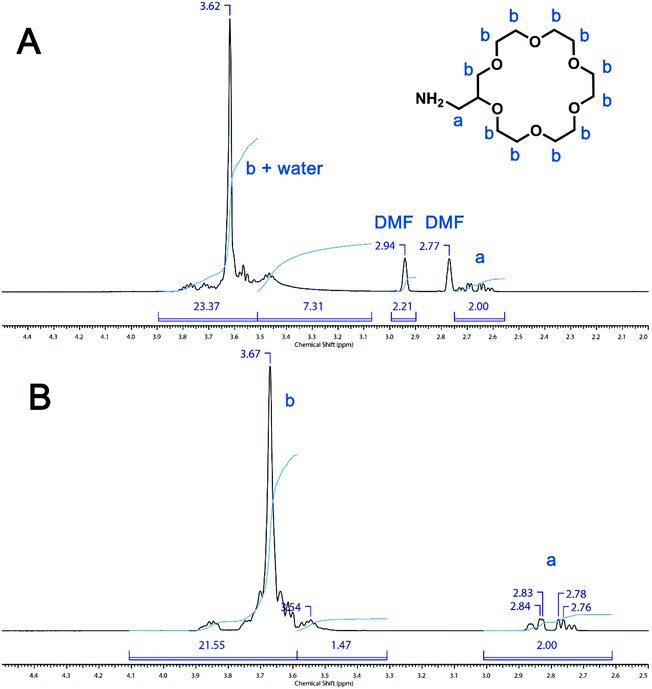
In the original manuscript, we reported the post-polymerization modification of a poly(HPMA-stat-NAS) copolymer with 2-aminomethyl-18-crown-6. This point remains as evidenced by the presence of the crown ether peaks in the 1H NMR spectrum (3.36–3.74 ppm, original manuscript Fig. S1B). Once again however, it is worth considering the H2O contribution to these peaks. As seen in Fig. 2A (this document), the residual water peak coincides with the crown ether peaks of 2-aminomethyl-18-crown-6 resulting in a higher than expected integral (compared to the 1H NMR spectrum of the same compound in chloroform (Fig. 2B, this document)). In chloroform, where there is no overlap of a water peak with the crown ether resonances, the 1H NMR signals between 3.6 and 3.9 ppm represent approximately 94% of the total crown ether integrals.
Knowing that the 1H NMR resonances between 3.6 and 3.9 ppm account for 94% of the crown ether signal, the spectrum in Fig. S1B of the original manuscript can be re-examined. If the region from 3.60 to 3.74 ppm is integrated and considered as 94% of the crown ether integral (Fig. 3), it is found that the crown ether group is incorporated into 21% of the total number of repeat units. As the starting polymer contains 25% NAS units, this means that there is an apparent 4% loss of functionality on conversion of the active ester moieties into crown ether moieties. While these re-calculations do not affect the salient points in the paper, especially with regard to compositional drift and reactivity ratios, it is important that they are corrected so that a more accurate depiction of the polymerization and post-polymerization modification is portrayed.
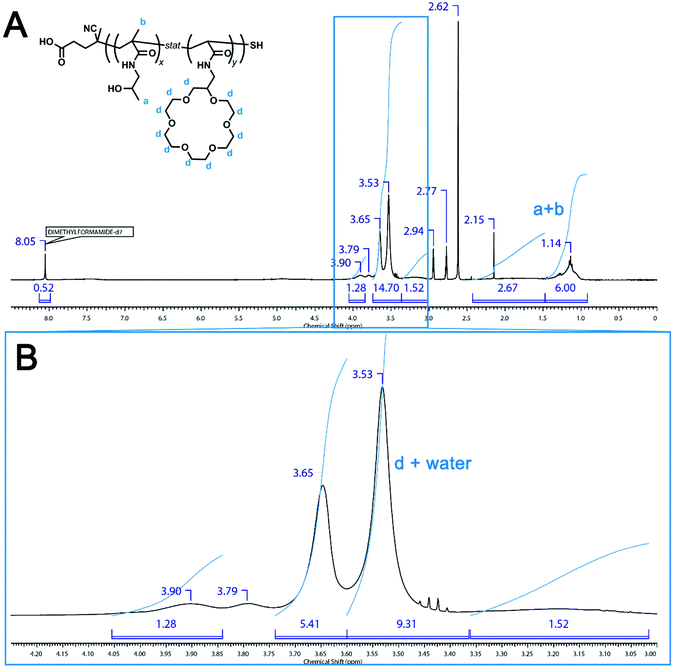 | ||
| Fig. 3 1H NMR spectrum of poly(HPMA-stat-NAS) after post-polymerization modification with 2-aminomethyl-18-crown-6: (A) full spectrum and (B) zoomed in region. | ||
In summary, a re-examination of the 1H NMR spectra in Fig. S1 in the ESI of our original manuscript points to the distinct possibility of some hydrolysis of the active ester groups of the NAS in the poly(HPMA-stat-NAS) copolymer. The extent of hydrolysis is difficult to quantify, especially given the complexity of the region in the 1H NMR spectra. However, the point in the original manuscript that near quantitative conversion of the NAS groups by the amino crown ether remains, even under this re-examination.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
Acknowledgements
The authors would like to thank Dr. Nicholas A. Burke of McMaster University for bringing the issue of peak assignments to our attention.References
- P. Relogio, M. T. Charreyre, J. P. S. Farinha, J. M. G. Martinho and C. Pichot, Polymer, 2004, 45, 8639–8649 Search PubMed.
Footnote |
| † Current address: Center of Advanced Research in Bionanoconjugates and Biopolymers, Petru Poni Institute of Macromolecular Chemistry, 41A Grigore Ghica Voda Alley, 700487, Iasi, Romania. |
| This journal is © The Royal Society of Chemistry 2015 |