The effect of Z-group modification on the RAFT polymerization of N-vinylpyrrolidone controlled by “switchable” N-pyridyl-functional dithiocarbamates†
Abstract
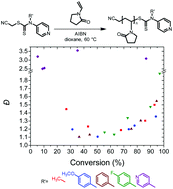
The ability of a RAFT agent to control the polymerization of a monomer is dictated by the structures of both the monomer and the RAFT agent. In this paper, the polymerization of N-vinylpyrrolidone was examined with a series of cyanomethyl N-aryl-N-pyridyldithiocarbamates [(4-R′Ph)N(py)C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)SCH2CN] varying in the substituent (R′) at the 4-position on the phenyl ring. The polymerization of N-vinylpyrrolidone was best controlled when R′ was methoxy; one of the least active RAFT agents in the series. The preservation of RAFT agent functionality was demonstrated by chain extension experiments with further N-vinylpyrrolidone. Again best control again was found for the RAFT agent with R′ = MeOPh. The utility of this RAFT agent was also proved with the preparation of poly(N-isopropylacrylamide)-block-poly(N-vinylpyrrolidone).
S)SCH2CN] varying in the substituent (R′) at the 4-position on the phenyl ring. The polymerization of N-vinylpyrrolidone was best controlled when R′ was methoxy; one of the least active RAFT agents in the series. The preservation of RAFT agent functionality was demonstrated by chain extension experiments with further N-vinylpyrrolidone. Again best control again was found for the RAFT agent with R′ = MeOPh. The utility of this RAFT agent was also proved with the preparation of poly(N-isopropylacrylamide)-block-poly(N-vinylpyrrolidone).


 Please wait while we load your content...
Please wait while we load your content...