End-quenching of tert-chloride-terminated polyisobutylene with alkoxybenzenes: comparison of AlCl3 and TiCl4 catalysts†
Abstract
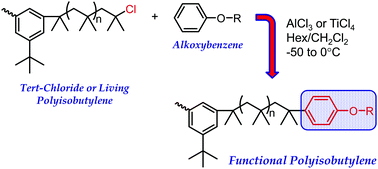
Alkoxybenzenes, including (3-bromopropoxy)benzene, anisole, and isopropoxybenzene, were used to end-quench polyisobutylene, activated with either AlCl3 or TiCl4 at different temperatures (−50, −25 and 0 °C) in 70/30 and 55/45 (v/v) hexane–methylene chloride (Hex–CH2Cl2). Quenching reactions were performed on pre-formed difunctional tert-chloride PIB, which was produced from 5-tert-butyl-1,3-di(1-chloro-1-methylethyl)benzene–TiCl4 at −70 °C in 40/60 (v/v) hexane–methyl chloride. For (3-bromopropoxy)benzene and anisole, quantitatively end-capped products were achieved if the alkoxybenzene/AlCl3 molar ratio was greater than unity. Under these conditions, alkylations were generally quantitative and occurred exclusively in the para position; neither multiple alkylations on the same alkoxybenzene nor polymer chain degradation were observed. Carbocation rearrangement was observed if the alkoxybenzene/AlCl3 molar ratio was less than unity. No such specific alkoxybenzene/Lewis acid molar ratio was required to obtain quantitatively alkylated products using TiCl4 catalyst. The alkylation rate of (3-bromopropoxy)benzene with AlCl3 was 2.6 times faster than with TiCl4 under the same reaction conditions. The alkylation rate of (3-bromopropoxy)benzene was faster than that of anisole using AlCl3. For isopropoxybenzene quencher only, using AlCl3 catalyst, a small fraction of exo-olefin was formed during the initial stage of reaction, and the quenching reaction failed to reach completion. Chain end functionality, a mixture of para and meta isomers (3 : 1), reached only 1.72 (86.4% conversion) after 45 h. Increasing temperature to −25 and 0 °C did not greatly affect the rate of alkylation, but it did increase carbocation rearrangement and decreased regioselectivity; at 0 °C, 25% alkylation occurred at the meta or ortho positions of (3-bromopropoxy)benzene. Increasing solvent polarity to 55/45 (v/v) Hex–CH2Cl2 also did not greatly affect alkylation rate but increased carbocation rearrangement.

 Please wait while we load your content...
Please wait while we load your content...