Towards a general understanding of hydrothermal polymerization of polyimides†
Abstract
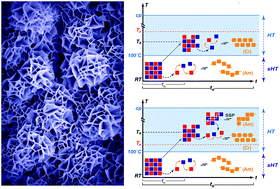
Hydrothermal polymerization (HTP) has been recently established as a novel route to synthesize polyimides of outstanding crystallinity. In this contribution, we lay out the basic theoretical and experimental framework for understanding the mechanistic underpinnings of this process. For this purpose, we hydrothermally synthesize two representative polyimides that are known to form amorphous polymers when synthesized classically. Hydrothermal polymerization, in contrast, yields unprecedented crystallinity after only two hours. The co-monomers diamine and dianhydride form monomer salts via acid–base reaction when brought in contact in water. We show that the physicochemical properties of the crystalline monomer salts (i.e. solubility, solid-state polymerization temperature) are important factors for the crystallinity and the morphology of the corresponding hydrothermally synthesized polyimide. We develop a mechanistic model of hydrothermal polymerization processes allowing us to relate the polymerization parameters (concentration, reaction temperature, reaction time) to the obtained polyimide crystallinity and morphology. By adjusting the parameters, the achieved crystallinity can be further increased and high morphological homogeneity can be obtained. We believe that the developed mechanistic picture is applicable for the hydrothermal polymerization of any polyimide.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...