A rational approach to activated polyacrylates and polymethacrylates by using a combination of model reactions and SET-LRP of hexafluoroisopropyl acrylate and methacrylate
Abstract
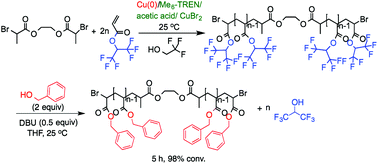
The transesterification of hexafluoroisopropyl esters mediated by two mild bases, 1,8-diazabicycloundec-7-ene (DBU) and 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) was investigated as model reaction for the transesterification of poly(1,1,1,3,3,3-hexafluoroisopropyl acrylate) [poly(HFIPA)] and poly(1,1,1,3,3,3-hexafluoropropyl methacrylate) [poly(HFIPM)]. Unexpectedly, the rate of transesterification of the hexafluoroisopropyl esters was higher than that of the more reactive pentafluorophenyl esters although the rate of uncatalyzed aminolysis followed the reverse trend. Subsequently SET-LRP of HFIPA up to [M]0/[I]0 = 92 at 25 °C and of HFIPM up to [M]0/[I]0 = 86 at 50 °C with activated Cu(0) wire as catalyst, Me6-TREN as ligand, and trifluoroethanol as solvent in the presence of acetic acid to generate the corresponding polymers with well-defined molecular weight, narrow molecular weight distribution and near-quantitative chain-end functionality was elaborated. Using reaction conditions established with model compounds a highly efficient transesterification of poly(HFIPA) and of poly(HFIPM) using DBU as catalyst at 25 °C has been developed. Therefore, this combination of experiments demonstrated that hexafluoroisopropyl esters provide a new class of activated carboxylic acids with applications in functional group transformation of interest both to organic and macromolecular synthesis.

 Please wait while we load your content...
Please wait while we load your content...