Amphiphilic polyether-based block copolymers as crosslinkable ligands for Au-nanoparticles†
Abstract
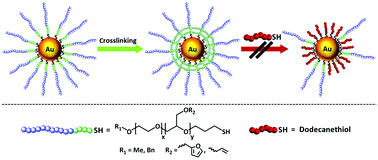
We report on the synthesis of thiol-terminated, polyether-based amphiphilic block copolymers with a hydrophilic poly(ethylene oxide) (PEO) segment and a second crosslinkable block of either poly(furfuryl glycidyl ether) (PFGE) or poly(allyl glycidyl ether) (PAGE). Both block copolymers could be synthesized with narrow dispersities (Đ ≤ 1.07) via living anionic ring-opening polymerization (AROP). Introduction of the thiol-moiety enables the application of these block copolymers as ligands for the preparation of Au-nanoparticles (Au-NPs) by direct reduction of suitable precursors in N,N-dimethylacetamide (DMAc). The ligands of the obtained hybrid nanoparticles featuring an Au core and a block copolymer shell were crosslinked either via Diels–Alder reactions for the PFGE segment or via hydrosilylation chemistry targeting the PAGE segment. In this way, shell-crosslinked Au-NPs with enhanced stability against ligand exchange reactions in the presence of competitive ligands like alkyl thiols could be prepared.
- This article is part of the themed collection: Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...