Green polymer chemistry: synthesis of symmetric and asymmetric telechelic ethylene glycol oligomers†
Abstract
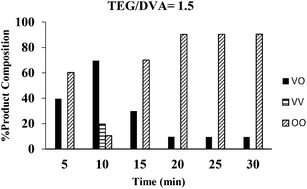
In this work we investigated the kinetics of the transesterification of divinyl adipate (DVA) with tetraethylene glycol (TEG) using Candida antarctica lipase B (CALB) as a biocatalyst at 50 °C under solventless conditions. We examined the time-dependence of the reactions with various DVA/TEG ratios. Samples were taken at the specified times, and then the composition and end group structures were analyzed by MALDI-ToF mass spectrometry. We found conditions under which polycondensation was minimized and symmetric and asymmetric telechelic TEGs were obtained. Specifically, at DVA/TEG = 1.5 molar ratio 100% of the oligomers had divinyl end groups after 20 minutes reaction time. At DVA/TEG = 3 only vinyl end groups were detected after 5 minutes. HO-(TEG)n-Vinyl was maximized at 70% at DVA/TEG 1/1.5 molar ratio at 10 minutes reaction time, while ∼90% HO-(TEG)n-OH was obtained in 20 minutes with TEG excess.

 Please wait while we load your content...
Please wait while we load your content...