Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of polymeric janus nanoparticles and their application in surfactant-free emulsion polymerizations
Binh T. T.
Pham
 a,
Chris H.
Such
b and
Brian S.
Hawkett
*a
a,
Chris H.
Such
b and
Brian S.
Hawkett
*a
aKey Centre for Polymers and Colloids, School of Chemistry – F11, The University of Sydney, NSW 2006, Australia. E-mail: brian.hawkett@sydney.edu.au; Fax: +61 2 9351 8651; Tel: +61 2 9351 6973
bDuluxGroup, 1970 Princess Hwy, Clayton VIC 3168, Australia
First published on 1st October 2014
Abstract
A robust and simple synthesis of nano-size oblate to dumbbell shaped polymeric anisotropic particles using RAFT mediated emulsion polymerization is presented. The particle synthesis relies on the property that monomer swollen cross-linked polymer seed particles shrink and expel some of the monomer when heated. Thus, upon heating for polymerization, some of the swelling monomer is expelled and subsequently polymerizes to form a bulge on the side of the original crosslinked seed particle. The shape of the bulge, and the degree of contact that the expelled monomer maintains with the original seed particle, is controlled by controlling the wettability of the seed surface by the expelled monomer. Very small monodisperse cross-linked polymer particles are initially prepared by RAFT mediated emulsion polymerization, then swollen with monomer and further polymerized to form anisotropic particles with a long dimension as little as 25 nm. Both the shape of the anisotropic bulge and the polymer composition, of each end of the final Janus nanoparticle can be finely controlled. The surface active properties of the Janus nanoparticles are demonstrated by their ability to contribute as stabilizers and influence particle formation in a surfactant free ab-initio emulsion polymerization. The method provides a simple and reproducible process for the production of Janus colloidal nanoparticles readily achievable in a normal latex plant where batch size would be limited only by the size of the reactor.
Introduction
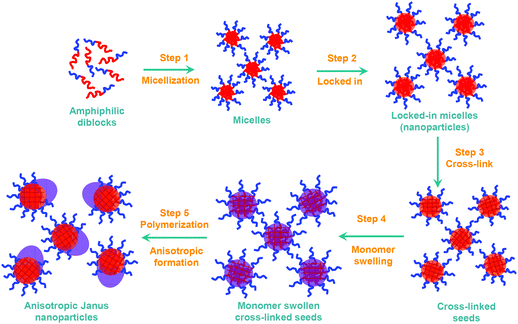
It is well recognized that anisotropic and/or Janus particles have the potential to self-assemble to form nano-scale devices. As a consequence there has been considerable interest in anisotropic particles in recent times and articles on their formation have been published in prestigious journals.1–4 Current methods for forming anisotropic particles are generally multi step and/or only capable of forming very small quantities at a time. Moreover, the current general and common approach to forming anisotropic particles usually involves using templates1–4 to generate organic–inorganic hybrid particles, in most cases, that are greater than 100 nm, more usually greater than 1 micron in diameter.5–21 Sheu et al.22 synthesized anisotropic micron size particles by swelling crosslinked particles with monomer, raising the reaction temperature, and initiating free-radical polymerization. This approach relies on the characteristic of solvent swollen crosslinked polymer particles that they contract as the temperature is raised and expel some of the solvent. When the solvent is monomer, that monomer can be subsequently polymerized to form an anisotropic bulge on the surface of the original crosslinked particle. Our group has previously developed a method to form anisotropic polystyrene particles on a submicron scale (of order 300 to 500 nm in diameter).23 Although the particles formed by this process were anisotropic in shapes, they were not anisotropic particles in other respects as the particle surface and chemical composition was relatively uniform throughout each particle. In addition to the complicated and multiple step synthesis of Janus particles, typically at a very low solid content, the micron and sub-micron size of the Janus particles imposes significant limitations in the application of the Janus particles in bio-medical fields.24Reversible Addition Fragmentation chain Transfer (RAFT) is a versatile controlled radical process that is applicable to a wide range of monomers at very accessible polymerization temperatures.25 Our group has pioneered the use of RAFT to mediate the emulsion polymerization processes.26–31 Formation of aqueous based particles by living radical polymerization, especially by RAFT polymerization, has proved to be a very versatile and powerful technique to produce a range of particle morphologies and coating nanotechnology.32–34 The particle formation mechanism for the RAFT mediated emulsion polymerization process has recently been investigated and the knowledge gained enables us to exert very fine control over the desired properties of the particles formed.35 This approach is based on forming micelles from amphiphilic macro-RAFT agents, which are all turned into monodisperse latex particles that can be less than 20 nm in diameter.
In this study, we report a facile and robust water-based synthesis of polymeric anisotropic and Janus nanoparticles (NPs) that are appreciably smaller than previously reported. The particles can have longitudes of as short as 25 nm, which can consist of two halves of different surface properties and/or different polymer compositions, using RAFT mediated emulsion polymerization. We also demonstrate the surface active properties of these Janus nanoparticles by controlling the size of latex particles in a surfactant free ab-initio emulsion polymerization.
Experimental section
Materials
Milli-Q water was used in the synthesis of all latexes. Acrylic acid (AA, Sumika) and 1,4-dioxane (Fluka) were distilled under reduced pressure. Styrene (Sty, Synthetic Resins), divinyl benzene (DVB, Fluka), butyl acrylate (BA, Aldrich) and methyl methacrylate (MMA, Aldrich) were passed through an inhibitor removal column (Aldrich). 2,2′-Azobis-isobutyronitrile (AIBN, Fluka) was recrystallized from ethanol. Acrylamide (AAm, Aldrich), 4,4′-azobis(4-cyanovaleric acid) (V-501, Wako), potassium persulfate (KPS, Aldrich), sodium dodecyl sulphate (SDS, Aldrich), sodium hydroxide (NaOH, Aldrich), methanol (MeOH, Ajax, HPLC grade) and tetrahydrofuran (THF, Ajax, HPLC grade) were used as received. The RAFT agent 2-{[(butylsulfanyl)carbonothioyl]-sulfanyl}propanoic acid (PABTC, Scheme 1A), was synthesized as previously described.26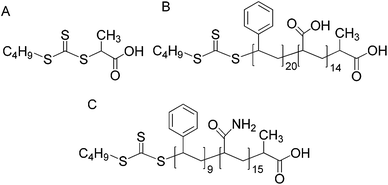 | ||
| Scheme 1 Structure of RAFT agent (A) and RAFT diblock of styrene and acrylic acid (B) or styrene and acrylamide (C). | ||
Analysis
Electrospray mass spectrometry was carried out on a Finnigan LCQ MS detector with Finnigan LCQ Data Processing using Instrument Control Software. 10 uL of solution (10 μg mL−1 in methanol or methanol–water) was injected.Dynamic light scattering (DLS) : a Zetasizer NS, Malvern Instruments with a detection angle of 173° was used to measure particle size (hydrodynamic diameter Z-Ave) and polydispersity index (PDI).
Hydrodynamic chromatography (HDC) : a Particle Size Distribution Analyzer (Polymer Laboratories) with a PSDA-Type 2 column, PL-PSDA eluent at a flow rate of 1.7 mL min−1 was used to measure particle size distribution. Polystyrene latexes standards (Duke Scientific Corporation, from 20 to 350 nm diameter) were used for the calibration.
Transmission electron microscopy (TEM) images were recorded on the Philips CM120 Biofilter, Electron Microscope Unit, University of Sydney, using a CCD camera and an accelerating voltage of 120 kV. The samples (ca. 0.01 wt% in water) were deposited on carbon coated copper grids and dried under ambient conditions. RuO4 was used as a positive stain for polystyrene.
Gel permeation chromatography (GPC, Shimadzu) was used to measure molecular weights and molecular weight distributions of RAFT-Sty20-b-AA14 diblocks. The method has been described previously.26,28,29
Synthesis of amphiphilic macro-RAFT agents
Stage one, synthesis of the hydrophilic acrylic acid block: RAFT agent (0.80 g, 3.36 × 10−3 mol), V501 (0.05 g, 0.17 × 10−3 mol), acrylic acid (4.84 g, 67.15 × 10−3 mol) and dioxane (22.03 g) were mixed in a round-bottom flask. The mixture was stirred at room temperature until both RAFT agent and initiator were completely dissolved, sparged with nitrogen for 20 minutes and then immersed in an 80 °C oil bath under constant stirring for 2 hours. The flask was then cooled down to room temperature and a sample was withdrawn for electrospray mass spectrometry.
Stage two, synthesis of the hydrophobic block: styrene (10.25 g, 98.45 × 10−3 mol), V-501 (0.24 g, 0.84 × 10−3 mol) and 24.52 g dioxane were added. The mixture was again sparged with nitrogen and magnetically stirred in an 80 °C oil bath under constant stirring for at least 2 hours. The length of styrene hydrophobic block was checked by gravimetry and GPC. Most of the dioxane was removed from the final copolymer solutions by sparging nitrogen through the solution while it was being stirred overnight on a magnetic stirrer in a fume hood. The copolymer was then dried in a vacuum oven at 60 °C until solvent-free copolymer was obtained. Yellow powder of RAFT-Sty20AA14 amphiphilic diblock was obtained.
Stage one, synthesis of the hydrophilic block: RAFT agent (0.80 g, 3.36 × 10−3 mol), V-501 (0.10 g, 0.36 × 10−3 mol), acrylamide (3.71 g, 52.21 × 10−3 mol), water (4.41 g) and dioxane (6.61 g) were mixed in a round-bottom flask. The mixture was stirred at room temperature (until both RAFT agent and initiator were completely dissolved), sparged with nitrogen for 20 minutes, immersed in a 70 °C oil bath and magnetically stirred for 5 hours. The flask was then cooled down to room temperature. The length of the acrylamide block was determined by electrospray mass spectrometry.
Stage two, synthesis of the hydrophobic block: styrene (3.16 g, 30.32 × 10−3 mol), V501 (0.19 g, 0.69 × 10−3 mol), water (6.14 g) and dioxane (21.15 g) were added to the macro-RAFT solution (0.80 g) from stage 1 to obtain a clear solution. The mixture was sparged with nitrogen for 10 minutes, immersed in a 70 °C oil bath and magnetically stirred overnight. Conversion was checked by gravimetry.
Formation of Janus nanoparticles (Janus NPs) from cross-linked polystyrene seed particles stabilized by RAFT-Sty20-b-AA14
Two different types of cross-linked polystyrene (PSty) seed nanoparticles stabilized by the poly(acrylic acid)-b-polystyrene macro-RAFT agents were synthesized, one using V-501 as initiator and the other KPS. The particles were expected to have a more hydrophilic surface when KPS was used as initiator relative to when V501 was used.Synthesis of DVB cross-linked PSty seeds using V-501 as initiator (V-501 derived PSty seeds)
Stage one, formation of micelles (Scheme 2, step 1): a mixture of RAFT-Sty20AA14 amphiphilic diblock (0.50 g), sodium hydroxide (0.15 g) and water (15 g) was stirred on a magnetic stirrer for 60 minutes, followed by sonication in a sonic bath until a clear yellow micellar solution was obtained, with the diameter Z-Ave of 19.9 nm by DLS or the average volume mean diameter of 21 nm with the coefficient of variance of 7.8% by HDC (see Table 1).| Micelles | V-501 derived PSty seeds | KPS derived PSty seeds | ||||
|---|---|---|---|---|---|---|
| Before cross-link | After cross-link | Before cross-link | After cross-link | |||
| Zetasizer (DLS) data | Z-Ave (nm) | 19.9 | 23.8 | 27.6 | 25.0 | 27.3 |
| PdI | 0.386 | 0.152 | 0.221 | 0.096 | 0.098 | |
| HDC data | Volume mean diameter (nm) | 21.0 | — | 25.8 | — | 29.1 |
| Coefficient of variance (%) | 7.8 | — | 7.9 | — | 7.5 | |
Stage two, extension of hydrophobic block length to lock in micelles to form particles (Scheme 2, step 2): To the micellar solution (2.46 g) in a 25 ml round bottom flask, styrene (0.25 g) and water (8.03 g) were then added and stirred overnight. Initiator V-501 (0.01 g) was added to the monomer swollen micelles. The flask was sealed, sparged with nitrogen for 15 minutes and immersed in an oil bath with a temperature setting of 80 °C for 4 hours under constant magnetic stirring. The reaction reached approximately 80% conversion.
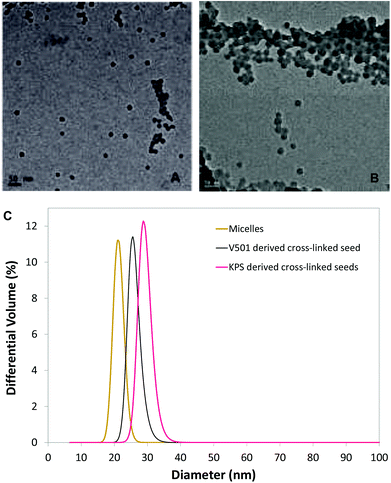
Stage three, formation of crosslinked particles (Scheme 2, step 3), (Since the particles are largely made up of living polymers we are able to conduct the crosslinking step after the particles have been formed): DVB (0.10 g) and initiator AIBN (0.014 g) were then added. The flask was stirred to mix for 2 hours at room temperature and immersed overnight in an oil bath with a temperature setting of 75 °C under constant magnetic stirring. The final latex particles had an average diameter of 27.6 nm by DLS and the volume mean diameter of 25.8 nm (coefficient of variance of 7.9%) by HDC. Transmission electron microscopy showed that the latex contained nanoparticles with narrow size distribution with a diameter of approximately 17 nm (Fig. 1A).
Synthesis of DVB cross-linked PSty seeds stabilized by polyAA chains and initiator derived sulfate groups (KPS derived PSty seeds)
Stage one, formation of micelles (Scheme 2, step 1): a mixture of RAFT-Sty20AA14 amphiphilic diblock (0.379 g), sodium hydroxide (0.075 g) and water (7.57 g) in a 25 mL round bottom flask was stirred on a magnetic stirrer for 60 minutes, followed by sonication in a sonic bath for 30 minutes.Stage two, extension of hydrophobic block length to lock in micelles to form particles (Scheme 2, step 2): To the micellar solution, styrene (0.855 g) and sodium hydrogen carbonate (8.7 mg) were added and stirred overnight. Initiator KPS (0.02 g) was added to the monomer swollen micelles. The flask was sealed, sparged with nitrogen for 15 minutes and immersed in an oil bath with a temperature setting of 80 °C for 4 hours under constant magnetic stirring. The reaction reached approximately 80% conversion.
Stage three, formation of crosslinked particles (Scheme 2, step 3): DVB (0.346 g) and initiator AIBN (0.032 g) were then added, stirring to mix for 1 hour at room temperature. The flask was then immersed overnight in an oil bath with a temperature setting of 75 °C under constant magnetic stirring. The final latex particles had an average diameter of 27.3 nm by DLS and the volume mean diameter of 29.1 nm (coefficient of variance of 7.5%) by HDC. Transmission electron microscopy showed that the latex contained nanoparticles with narrow size distribution (Fig. 1B).
Synthesis of ellipsoidal nanoparticles using the V-501 derived seed latex (Scheme 2, step 4)
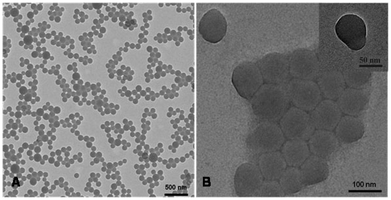
A mixture of the V-501 derived polystyrene seed latex (6.02 g), milliQ water (9.75 g), styrene monomer (1.62 g) and SDS (0.11 g) was prepared in a 25 mL round bottom flask and stirred overnight. Initiator V-501 (0.068 g) was added to the monomer swollen latex particles. The flask was sealed, stirred at room temperature for 30 minutes and sparged with nitrogen for 15 minutes and immersed in an oil bath with a temperature setting of 80 °C under constant magnetic stirring for 4 hours. TEM micrographs as shown in Fig. 2A showed that the final latex contained monodisperse ellipsoidal nanoparticles with average dimensions of 23 nm width and 35 nm length.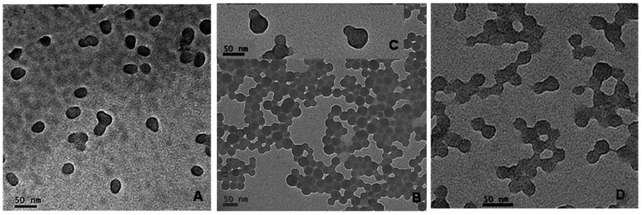 | ||
| Fig. 2 TEM micrographs of (A) ellipsoidal Janus nanoparticles with both halves of PSty, which were synthesized from the cross-linked V-501 derived seeds (as shown in Fig. 1A); of (B, C) snowman shaped Janus nanoparticles with both ends of PSty or (D) dumbbell shaped particles with one half of polystyrene and the other of poly(butyl acrylate-co-methyl methacrylate), which were synthesized from the cross-linked KPS derived PSty seeds (as shown in Fig. 1B). | ||
Synthesis of Janus nanoparticles using the KPS derived seed latex (Scheme 2, step 4)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (0.50 g) and water (4.68 g) were prepared in a 25 mL flask and stirred overnight. Initiator AIBN (0.015 g) was added to the monomer swollen latex particles and the flask was sealed, stirred at room temperature for 2 hours, sparged with nitrogen for 10 minutes and immersed in an oil bath with a temperature setting of 75 °C under constant magnetic stirring for 5 hours. TEM micrographs showed that the final latex contained monodisperse dumbbell shaped nanoparticles with each half having an average diameter of approximately 23 nm (see Fig. 2D).
3 (0.50 g) and water (4.68 g) were prepared in a 25 mL flask and stirred overnight. Initiator AIBN (0.015 g) was added to the monomer swollen latex particles and the flask was sealed, stirred at room temperature for 2 hours, sparged with nitrogen for 10 minutes and immersed in an oil bath with a temperature setting of 75 °C under constant magnetic stirring for 5 hours. TEM micrographs showed that the final latex contained monodisperse dumbbell shaped nanoparticles with each half having an average diameter of approximately 23 nm (see Fig. 2D).
Formation of Janus nanoparticles (Janus NPs) from a cross-linked AIBN derived PSty seed sterically stabilized by polyacrylamide (sterically stabilized seed latex)
Stage three, formation of crosslinked particles (Scheme 2, step 3): to a 50 mL round bottom flask containing the sterically stabilized polystyrene latex particles (10.03 g) DVB (0.22 g), initiator AIBN (0.032 g) and water (2.42 g) were then added and stirred to mix for 5 hours at room temperature. The flask was then sealed, sparged with nitrogen for 15 minutes and immersed in an oil bath with a temperature setting of 75 °C under constant magnetic stirring overnight. The final latex particles had an average diameter of 109 nm and PDI of 0.078 by light scattering. Transmission electron microscopy showed that the latex contained polyacrylamide stabilized nanoparticles with low polydispersity (Fig. 3A).
Synthesis of surfactant free polystyrene or poly(butyl acrylate-co-methyl methacrylate) particles using the Janus nanoparticles as stabilizers affecting particle sizes of the final latex particles
Recipes used to synthesize polystyrene or poly(butyl acrylate-co-methyl methacrylate) latex particles in a surfactant-free ab-initio emulsion polymerization are given in Table 2. As a proof-of-concept experiment, only one type of Janus polystyrene nanoparticles, which were formed from the cross-liked V-501 derived polystyrene seeds using RAFT-Sty20AA14 diblocks, was employed.| Particles | Janus particles (wt% to monomer) | Monomer mass (g) | V-501 (mM) | NaOH (mM) | H2O (g) | Conversion by gravimetry (%) |
|---|---|---|---|---|---|---|
| PSty-1 | 0 | 5.6035 | 15.0 | 106 | 22.29 | 75 |
| PSty-2 | 1 | 5.6363 | 12.5 | 115 | 25.91 | 84 |
| P(BA-co-MMA)-1 | 0 | 1.4276 | 16.1 | 125 | 10.41 | 70 |
| P(BA-co-MMA)-2 | 1 | 2.8408 | 13.0 | 127 | 23.41 | 87 |
| P(BA-co-MMA)-3 | 5 | 1.1252 | 12.6 | 120 | 11.51 | 85 |
A desired amount of Janus particle dispersion, styrene or a mixture of (butyl acrylate-co-methyl methacrylate) (1/1 mass ratio), NaOH, water and V-501 as initiator were weighed into a 50 mL round bottom flask. The flask was sealed, sparged with nitrogen for 15 minutes and immersed in an oil bath with a temperature setting of 70 °C under constant magnetic stirring overnight. The final conversion was determined by gravimetry. The particle size and morphology of the final latex particles was determined by DLS (or HDC) and TEM, respectively.
Discussions
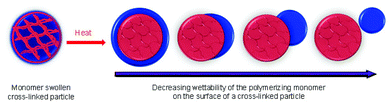
The formation of polymeric nanoparticles from micelles in ab initio RAFT mediated emulsion polymerization has been previously studied in our group.35 In this study, micelles were first formed from the amphiphilic macro-RAFT-Sty20AA14 diblocks. These micelles were then further polymerized using either V-501 or KPS as an initiator to form poly(acrylic acid) stabilized PSty seeds, using recipes stated in the Experimental section. Our previous studies showed that polymerization using these diblocks under similar conditions were under RAFT control.28,29 The particle size and size distribution of the micelles and PSty seeds by DLS and HDC is presented in Table 1. The HDC particle sizing method is based on the principle of chromatography that the smaller particles are eluted after the bigger ones. The elution is independent of the particle morphology. The results given by the HDC measurements for the micelles and cross-linked seeds (see Fig. 1C) showed that they all had narrow size distribution with the coefficient of variance of less than 8%. The particle size distribution was further investigated using the TEM. TEM micrographs (see Fig. 1A and B) showed that both types of cross-linked PSty seeds had narrow particle size distribution. The cross-linked V-501 derived seeds (see Fig. 1A) appeared slightly smaller in size than the cross-linked KPS derived seeds (see Fig. 1B), in a good agreement with the TEM result.The cross-linked PSty seeds were then used to synthesize Janus nanoparticles. The particle morphologies obtained when polymerizing monomer swollen cross-linked seed particles, depends on the surface wetting of the seed particle by the polymerizing monomer, which has been discussed in detail by Mock et al.23Scheme 3 illustrates a number of possible morphologies that can be formed during the subsequent polymerization of the swelling monomer.
The wettability of the seed surface by the polymerizing monomer(s) can be controlled by the type of stabilizer used to prepare the cross-linked seed particles and the type of initiator used in their preparation. When AIBN was used as the initiator no charged initiator residues were expected on the surface and the particle surface between the stabilizers was expected to be most hydrophobic. Using V501 initiator to yield carboxylic acid residues on the surface was expected to produce a more hydrophilic surface, while the sulphate residues derived from the persulphate initiator KPS was expected to yield a more hydrophilic surface again. The stabilisers derived from the original micelles are expected to play a very limited role as they are very sparsely distributed over the surface by the time the micelles are grown up tp crosslinked seeds. The compatibility of the monomer used to swell the crosslinked seed particles with their surface was further changed by changing the monomer from styrene to a blend of MMA and BA. TEM was used to examine morphologies of the claimed Janus nanoparticles.
When Janus NPs were synthesised from the two cross-linked seed particles in accordance with Scheme 2, step 4, the polymerizing monomer from the monomer swollen particles was expelled and protruded out of the original seed particles, forming different bulge configurations based on the interactions between the expelled monomer and the surface of the seed particles. When V-501 was used as an initiator to grow the cross-linked seed particles (Scheme 2, step 3), the polymerization of the swollen styrene formed PSty Janus NPs with ellipsoidal shapes (see Fig. 2A). This indicated that, despite the carboxylic charge derived from the V501 on the surface, the expelled styrene monomer wetted the surface reasonably well.
When the cross-linked KPS derived seed particles were used to grow Janus NPs with both ends of PSty, snowman shaped anisotropic particles were obtained (see Fig. 2B, and the insert 2-C). This is consistent with our original hypothesis that these particles would be less easily wetted by the styrene monomer than those obtained from the V-501 derived seeds. When styrene was replaced by a blend of MMA/BA monomers to swell the cross-linked KPS derived seeds, we were initially surprised to observe the formation of dumbbell shaped Janus NPs (see Fig. 2D) on the polymerization. This blend was expected to be more hydrophilic than pure styrene but the particle morphology indicated poorer surface wetting. It seems that we were under estimating the influence of the compatibility of the underlying polymer surface with the polymer of the newly formed bulge. Indeed, the incompatibility and phase separation of the two polymers may well have forced a complete detachment of the newly formed bulge had it not have been for many of the acrylic/methacrylic polymer chains still being linked to the original seed particle because the whole particle was grown under some measure of RAFT control.
Moving from the electrostatic stabilized to sterically stabilized seeds, RAFT-Sty9AAm15 diblocks were used instead of the -RAFT-Sty20AA14 diblocks. However, the RAFT-Sty9AAm15 amphiphilic diblock is not dispersible in the aqueous phase due to the hydrophobicity imparted by the styrene block. We therefore employed a small amount of styrene monomer to solubilize the styrene block and enabled the formation of the monomer swollen micelles from the RAFT-Sty9AAm15 amphiphilic diblock. These monomer swollen micelles were polymerized and formed sterically stabilized PSty seeds with Z-Ave diameter of 84 nm, PDI 0.16. The TEM micrograph shows that we achieved a reasonably narrow particle size distribution for the polyacrylamide (sterically) stabilized PSty seeds (see Fig. 3A).
As the polyacrylamide stabilized crosslinked seeds were prepared using AIBN as an initiator there would have been no initiator derived charge on the surface. Only the polyacrylamide chains would have reduced the compatibility of the surface with the expelled monomer. The ellipsoidal shape of the derived Janus NPs suggests that the incompatibility of the polyacrylamide with both the styrene monomer and the polymer formed from it was such as to prevent the complete wetting of the entire seed surface and enable the formation of ellipsoidal Janus NPs.
Another important achievement from all processes was that no secondary population of new particles was observed in any experiments. Particle size and particle size distribution measurements by HDC (see Fig. 5) showed that the monodisperse cross-linked PSty seeds led to monodisperse ellipsoidal Janus NPs.
The synthesized Janus NPs are expected to impose surface active properties due to the anisotropic surface properties of the two halves. Based on the principles of emulsification and emulsion polymerization, surface active molecules influence particle/droplet sizes of emulsion droplets and/or of polymerizing particles.
For a proof of concept characterisation, we used the synthesised Janus NPs as stabilising agents in a surfactant free emulsion polymerization of styrene or acrylate/methacrylate monomers to study the influence of Janus NPs on particle formation and final particle size. The ellipsoidal Janus NPs (as shown in Fig. 2A) with one half stabilised by poly(acrylic acid) were employed in all surfactant-free syntheses (see Table 2). If the Janus NPs were surface active, the Janus NPs were expected to localize on the surface of a latex particle during surfactant free emulsion polymerization, with poly(acrylic acid) stabilised half protruding further out into to the aqueous phase.
Using the recipe given in Table 2, the surfactant free PSty particles (PSty-1) had an average diameter of 610 nm by light scattering. Our previous study found out that the PSty particles in a surfactant free ab-initio emulsion polymerization were formed by the aggregation of smaller precursor particles.36 In this case a population of the small precursor particles that were not collected up into the main population of larger particles were also observed in the TEM micrograph (see Fig. 4A and B, indicated by dotted circle). In the presence of Janus NPs at a concentrations as low as 1 wt% (based on monomer mass), the final PSty particles (PSty-2) were significantly reduced in size and had a narrower size distribution (see Table 3). The TEM micrograph (Fig. 4C and D) shows the presence of Janus NPs on the surface of PSty-2 particles (black arrows) with a maximum of 2 Janus NPs on each PSty-2 particle. In some PSty-2 particles, Janus NPs were not apparent, probably due to their hidden position on the TEM grid when dried.
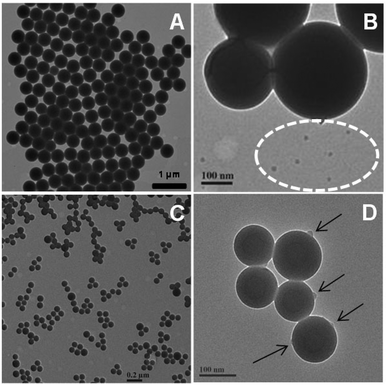 | ||
| Fig. 4 TEM micrographs of the surfactant free PSty-1 particles in the absence (A and B) and PSty-2 particles in the presence (C and D) of the Janus NPs. Janus NPs were obtained from the cross-linked V-501 derived PSty seeds shown in Fig. 2A. | ||
| Particles | wt% of Janus NPs (to monomer) | Z-Ave diameter by Zetasizer (nm) | Volume mean diameter by HDC (nm) |
|---|---|---|---|
| PSty-1 | 0 | 610 (PDI = 0.207) | — |
| PSty-2 | 1 | 215 (PDI = 0.045) | 196.2 (CV = 17.5%) |
| P(BA-co-MMA)-1 | 0 | 812 (PDI = 0.374) | — |
| P(BA-co-MMA)-2 | 1 | 229 (PDI = 0.027) | 186.7 (CV = 12.4%) |
| P(BA-co-MMA)-3 | 5 | 147 (PDI = 0.008) | 117.4 (CV = 8.4%) |
In TEM studies, poly(acrylates/methacrylates) have much lower electron density than polystyrene especially when the PSty is positively stained (with RuO4 for example), which makes poly(BA/MMA) particles appear brighter than PSty particles. Styrene monomer was therefore replaced by a mixture of (BA/MMA) monomers to achieve a better contrast between the Janus NPs and the final latex particles. A mixture of 1/1 mass ratio BA/MMA was used to obtain glassy copolymer particles which avoided coalescence on the TEM grid. The P(BA-co-MMA)-1 latex made by surfactant free emulsion polymerization without Janus NPs had a Z-average diameter of 812 nm and a broad particle distribution. Similar to PSty-2 particles, with 1 wt% of Janus NPs, P(BA-co-MMA)-2 particles were obtained with significantly smaller size and narrower size distribution than P(BA-co-MMA)-1 (see Table 3). P(BA-co-MMA)-2 and PSty-2 latex particles have similar size and size distribution. At a higher concentration of the Janus NPs, 5 wt% on monomer, P(BA-co-MMA)-3 latex particles were further reduced in size and size distribution as shown in Table 3 and Fig. 5.
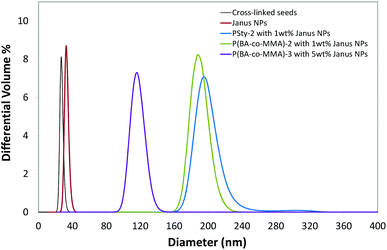 | ||
| Fig. 5 HDC measurements for particle size distributions of the cross-linked seeds (shown in Fig. 1A), the corresponding Janus PSty NPs (shown in Fig. 2A) and the composite latex particles of PSty or P(BA-co-MMA) from the surfactant free emulsion polymerizations in the presence of Janus NPs. | ||
TEM micrographs (Fig. 6) of the P(BA-co-MMA) latex particles positively stained by RuO4 showed that when Janus NPs were present in the surfactant free emulsion polymerization, darker regions/dots appeared clearly on the surface of both P(BA-co-MMA)-2 and P(BA-co-MMA)-3 latex particles (Fig. 6B and C). These black dots were only given by the Janus polystyrene NPs. Similar to PSty-2, a maximum of 2 Janus NPs were observed on each P(BA-co-MMA)-2 latex particle when using 1 wt% Janus NPs. When the concentration of the Janus NPs was increased to 5 wt%, a maximum of 4 Janus NPs on each P(BA-co-MMA)-3 latex particle was found. The results presented here strongly support the surface active properties of our claimed Janus particles with two halves of different hydrophilic/hydrophobic surfaces. It was an unexpected and interesting discovery that it takes very few Janus nanoparticles to significantly influence the size of the particles that result from this surfactant free emulsion polymerization. The presence of this few Janus particles on the surface the final particle not only made the final composite particles much smaller but also more monodisperse. It is also interesting to note that there does not appear to be any Janus particles engulfed within the much larger surfactant free particles. The Janus NPs appear to play an important role in the nucleation and stabilization of the particles formed in an ab-initio surfactant free emulsion polymerization and have considerable control over particle size and particle size distribution. Further investigations are required to fully understand this exciting discovery.
 | ||
| Fig. 6 TEM micrographs of the RuO4 stained surfactant free poly(BA-co-MMA) particles: P(BA-co-MMA)-1 without Janus NPs (A); P(BA-co-MMA)-2 (B) and P(BA-co-MMA)-3 (C) in the presence of 1 wt% and 5 wt% Janus NPs, respectively. Janus NPs were obtained from the cross-linked V-501 derived PSty seeds (shown in Fig. 2A). | ||
Conclusions
We have demonstrated that monodisperse surfactant-free anisotropic Janus nanoparticles with a long dimension of as small as 25 nm with desired surface hydrophobicity/hydrophilicity and polymer compositions can be successfully designed, tuned and synthesized employing facile, reproducible and robust procedures, using RAFT mediated emulsion polymerizations. By controlling the wettability of the surface of the crosslinked seed particles by the swelling monomer(s) and/or the compatibility of the bulge polymer with the surface of the seed particles, we can control the morphology of the particle obtained on polymerization. The overall size and dimensions of the final Janus particles were controlled by adjusting the size of the original seed particles, the initiator and the type and amount of swelling monomer. The Janus NPs were successfully used as stabilisers in the ab-initio emulsion polymerization to control the particle size and particle size distribution. We have offered a very simple, versatile and environmentally friendly method to produce surfactant free latex polymer(s) particles with decorated/functional surface.Acknowledgements
We acknowledge the support of Dr Algi Serelis of DuluxGroup Australia for preparing the RAFT agent; Dr Meiliana Siauw for the synthesis of the RAFT-Sty9-b-AAm15 diblock; Dr Chiara Neto and Dr Duc Nguyen for helpful discussion. The authors acknowledge the facilities, and the technical assistance, of the Australian Microscopy & Microanalysis Research Facility at the Australian Centre for Microscopy and Microanalysis, The University of Sydney. The Key Centre for Polymers and Colloids was established under the Australian Research Council's Research Centres Program.References
- K. K. Zhang, M. Jiang and D. Y. Chen, Self-assembly of particles The regulatory role of particle flexibility, Prog. Polym. Sci., 2012, 37(3), 445–486 CrossRef CAS PubMed.
- J. Z. Du and R. K. O'Reilly, Anisotropic particles with patchy: multicompartment and Janus architectures: preparation and application, Chem. Soc. Rev., 2011, 40(5), 2402–2416 RSC.
- K. J. Lee, J. Yoon and J. Lahann, Recent advances with anisotropic particles, Curr. Opin. Colloid Interface Sci., 2011, 16(3), 195–202 CrossRef CAS PubMed.
- M. Lattuada and T. A. Hatton, Synthesis, properties and applications of Janus nanoparticles, Nano Today, 2011, 6(3), 286–308 CrossRef CAS PubMed.
- V. N. Paunov and O. J. Cayre, Supraparticles and \”Janus\” particles fabricated by replication of particle monolayers at liquid surfaces using a gel trapping technique, Adv. Mater., 2004, 16(9–10), 788–791 CrossRef CAS.
- A. Perro, et al., Design and synthesis of Janus micro- and nanoparticles, J. Mater. Chem., 2005, 15(35–36), 3745–3760 RSC.
- K.-H. Roh, D. C. Martin and J. Lahann, Biphasic Janus particles with nanoscale anisotropy, Nat. Mater., 2005, 4(10), 759–763 CrossRef CAS PubMed.
- L. Hong, S. Jiang and S. Granick, Simple Method to Produce Janus Colloidal Particles in Large Quantity, Langmuir, 2006, 22(23), 9495–9499 CrossRef CAS PubMed.
- T. Nisisako, et al., Synthesis of monodisperse bicolored Janus particles with electrical anisotropy using a microfluidic co-flow system, Adv. Mater., 2006, 18(9), 1152–1156 CrossRef CAS.
- L. Nie, et al., One-pot synthesis of amphiphilic polymeric Janus particles and their self-assembly into supermicelles with a narrow size distribution, Angew. Chem., Int. Ed., 2007, 46(33), 6321–6324 CrossRef CAS PubMed.
- A. Walther, et al., Janus Discs, J. Am. Chem. Soc., 2007, 129(19), 6187–6198 CrossRef CAS PubMed.
- C.-C. Ho, et al., Novel Fabrication of Janus Particles from the Surfaces of Electrospun Polymer Fibers, Langmuir, 2008, 24(11), 5663–5666 CrossRef CAS PubMed.
- S. Jiang, et al., Solvent-Free Synthesis of Janus Colloidal Particles, Langmuir, 2008, 24(18), 10073–10077 CrossRef CAS PubMed.
- A. Walther and A. H. E. Mueller, Janus particles, Soft Matter, 2008, 4(4), 663–668 RSC.
- F. S. Romanski, et al., Production and Characterization of Anisotropic Particles from Biodegradable Materials, Langmuir, 2012, 28(8), 3756–3765 CrossRef CAS PubMed.
- N. Chaturvedi, et al., Simple fabrication of snowman-like colloids, J. Colloid Interface Sci., 2012, 371, 28–33 CrossRef CAS PubMed.
- Y. H. Wang, et al., Emulsion Interfacial Synthesis of Asymmetric Janus Particles, Macromolecules, 2011, 44(10), 3787–3794 CrossRef CAS.
- T. Nisisako and T. Hatsuzawa, A microfluidic cross-flowing emulsion generator for producing biphasic droplets and anisotropically shaped polymer particles, Microfluid. Nanofluid., 2010, 9(2–3), 427–437 CrossRef.
- T. Tanaka, et al., Preparation of “Mushroom-like” Janus Particles by Site-Selective Surface-Initiated Atom Transfer Radical Polymerization in Aqueous Dispersed Systems, Langmuir, 2010, 26(11), 7843–7847 CrossRef CAS PubMed.
- N. Saito, et al., Formation of “Snowmanlike” polystyrene/poly(methyl methacrylate)/toluene droplets dispersed in an aqueous solution of a nonionic surfactant at thermodynamic equilibrium, Langmuir, 2007, 23(23), 11506–11512 CrossRef CAS PubMed.
- D. J. Kraft, et al., Patchy Polymer Colloids with Tunable Anisotropy Dimensions, J. Phys. Chem. B, 2011, 115(22), 7175–7181 CrossRef CAS PubMed.
- H. R. Sheu, M. S. El-Aasser and J. W. Vanderhoff, Phase separation in polystyrene latex interpenetrating polymer networks, J. Appl. Polym. Sci., Part A: Polym. Chem., 1990, 28(3), 22 Search PubMed.
- E. B. Mock, et al., Synthesis of Anisotropic Nanoparticles by Seeded Emulsion Polymerization, Langmuir, 2006, 22(9), 4037–4043 CrossRef CAS PubMed.
- C. Kaewsaneha, et al., Janus Colloidal Particles: Preparation, Properties, and Biomedical Applications, ACS Appl. Mater. Interfaces, 2013, 5(6), 1857–1869 CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Radical addition-fragmentation chemistry in polymer synthesis, Polymer, 2008, 49(5), 1079–1131 CrossRef CAS PubMed.
- C. J. Ferguson, et al., Effective Ab Initio Emulsion Polymerization Under RAFT Control, Macromolecules, 2002, 35, 9243–9245 CrossRef CAS.
- C. J. Ferguson, et al., Ab Initio Emulsion Polymerization by RAFT-Controlled Self-Assembly, Macromolecules, 2005, 38(6), 2191–2204 CrossRef CAS.
- B. T. T. Pham, et al., Miniemulsion Polymerization Stabilized by Amphipathic Macro RAFT Agents, Macromolecules, 2003, 36(24), 8907–8909 CrossRef CAS.
- B. T. T. Pham, et al., Miniemulsion Polymerization with Arrested Ostwald Ripening Stabilized by Amphiphilic RAFT Copolymers, Macromolecules, 2010, 43(19), 7950–7957 CrossRef CAS.
- D. Nguyen, C. Such and B. Hawkett, Polymer-TiO2 Composite Nanorattles via RAFT-Mediated Emulsion Polymerization, J. Polym. Sci., Part A: Polym. Chem., 2012, 50(2), 346–352 CrossRef CAS.
- D. Nguyen, et al., Pigment Encapsulation by Emulsion Polymerization Using Macro-RAFT Copolymers, Langmuir, 2008, 24(5), 2140–2150 CrossRef CAS PubMed.
- B. Charleux, et al., Polymerization-Induced Self-Assembly: From Soluble Macromolecules to Block Copolymer Nano-Objects in One Step, Macromolecules, 2012, 45(17), 6753–6765 CrossRef CAS.
- M. J. Monteiro and M. F. Cunningham, Polymer Nanoparticles via Living Radical Polymerization in Aqueous Dispersions: Design and Applications, Macromolecules, 2012, 45(12), 4939–4957 CrossRef CAS.
- D. Nguyen, C. H. Such and B. S. Hawkett, Polymer coating of carboxylic acid functionalized multiwalled carbon nanotubes via reversible addition-fragmentation chain transfer mediated emulsion polymerization, J. Polym. Sci., Part A: Polym. Chem., 2013, 51(2), 250–257 CrossRef CAS.
- D. E. Ganeva, et al., Particle Formation in ab Initio RAFT Mediated Emulsion Polymerization Systems, Macromolecules, 2007, 40(17), 6181–6189 CrossRef CAS.
- A. M. Telford, et al., Micron-sized polystyrene particles by surfactant-free emulsion polymerization in air: Synthesis and mechanism, J. Polym. Sci., Part A: Polym. Chem., 2013, 51(19), 3997–4002 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |