Open Access Article
Open Access ArticleEnhanced sunlight photocatalytic activity of Ag3PO4 decorated novel combustion synthesis derived TiO2 nanobelts for dye and bacterial degradation
Neerugatti KrishnaRao
Eswar
a,
Praveen Chandrashekarapura
Ramamurthy
ab and
Giridhar
Madras
 *c
*c
aCentre for Nanoscience and Engineering, Indian Institute of Science, Bangalore-560012, India
bDepartment of Materials Engineering, Indian Institute of Science, Bangalore-560012, India
cDepartment of Chemical Engineering, Indian Institute of Science, Bangalore-560012, India. E-mail: giridhar@chemeng.iisc.ernet.in; Fax: +91 80 23600683; Tel: +91 80 22932321
First published on 20th May 2015
Abstract
This study demonstrates the synthesis of TiO2 nanobelts using solution combustion derived TiO2 with enhanced photocatalytic activity for dye degradation and bacterial inactivation. Hydrothermal treatment of combustion synthesized TiO2 resulted in unique partially etched TiO2 nanobelts and Ag3PO4 was decorated using the co-precipitation method. The catalyst particles were characterized using X-ray diffraction analysis, BET surface area analysis, diffuse reflectance and electron microscopy. The photocatalytic properties of the composites of Ag3PO4 with pristine combustion synthesized TiO2 and commercial TiO2 under sunlight were compared. Therefore the studies conducted proved that the novel Ag3PO4/unique combustion synthesis derived TiO2 nanobelt composites exhibited extended light absorption, better charge transfer mechanism and higher generation of hydroxyl and hole radicals. These properties resulted in enhanced photodegradation of dyes and bacteria when compared to the commercial TiO2 nanocomposite. These findings have important implications in designing new photocatalysts for water purification.
1. Introduction
As a result of radical urbanization, the quality of water has been compromised. Further, improper waste water treatment in industries dumping of wastes causes water pollution. Besides chemical contamination, bacterial infections pertaining to water contamination must also be considered seriously. Fecal bacteria such as Escherichia, fecal coliforms, Staphylococcus, Pseudomonas, and Streptococcus species in water cause various infections1 and are a menace to public health.2 The primary objective of any disinfection process in water treatment is the control of water-borne diseases through inactivation of pathogenic microorganisms in the water.3Generation of reactive oxygen species to degrade the chemical contaminants and pathogens by an advanced oxidation process using nanoparticles is one of the solutions for water purification. Over the past few years, research on nanomaterials is increasing rapidly because of their valuable properties. There are several methods for the synthesis of nanomaterials and varying morphologies such as nanorods, spheres, discs, nanoflowers, platelets etc.4,5 have been obtained and used for different applications like photocatalysis, electrocatalysis, supercapacitors etc.6,7
Metal oxides such as ZnO, TiO2etc. and metals like Ag and Au have been used for various catalytic applications.8,9 Among various routes such as sol–gel, hydrothermal, solvothermal, aerosol, inert gas condensation, combustion synthesis of nanomaterials is considered to be quick and efficient. Combustion synthesized TiO2 has proved to be an efficient catalyst compared to its commercial counterpart.10,11 However, many studies have attempted to increase the photoactivity of TiO2, since its efficiency depends on parameters such as particle size, crystallinity, pore size12etc. Efforts have been made to increase the photoactivity by doping it with various transition elements.13
Silver and silver based compounds such as AgBr, AgI, Ag3PO4, have been used for photocatalytic and antibacterial applications.14–16 Silver is being used as an effective antimicrobial agent because it exhibits strong cytotoxic activity against a broad range of microbial organisms.17 Recently, in addition to pristine silver, silver based photocatalysts are used widely for photocatalytic applications. AgBr on AgVO3 nanobelts has shown efficient plasmonic photocatalytic activity against Rhodamine-B with good stability.16 Surfactant assisted highly-crystalline AgI nanoplates showed better photocatalytic activity under visible light.14 Ag3PO4 and Ag/Ag3PO4 showed better absorption of visible light and exhibit excellent antibacterial activity.18 Recent studies on the activity of the AgBr composite with iron oxide have reported enhanced photocatalytic activity under visible light.19,20 Similarly the Ag3PO4 composite with TiO2·Fe2O3 and GO has shown better and synergistic photocatalytic activity compared to its pristine composition, also by facilitating magnetic separation of the photocatalyst.21–24
In this study, TiO2 (CST) was prepared by the solution combustion method using ascorbic acid as a reducer. Recent reports have shown that as the nature of the TiO2 precursors varies, the attributes of the nanobelts derived from them change significantly.25 The TiO2 nanobelts (CSTNB) were synthesized using combustion synthesized TiO2 as a novel precursor. Ag3PO4 has been impregnated on these TiO2 nanobelts by the simple co-precipitation technique. This is the first study to report impregnation of Ag3PO4 on TiO2 nanobelts prepared using combustion synthesized TiO2 as a precursor for photocatalysis under natural sunlight. The photocatalytic degradation of organic pollutants and antibacterial activity of Ag3PO4 impregnated combustion synthesized TiO2 and TiO2 nanobelt compounds have been compared with commercial TiO2 (Degussa P-25).
2. Experimental
2.1 Materials
Titanium iso-propoxide (>97% purity) was purchased from Sigma-Aldrich. L-Ascorbic acid-LR, silver nitrate, di-sodium hydrogen phosphate and methylene blue, methyl orange, neutral red dyes were purchased from SD Fine Chemicals Ltd (India). Sodium hydroxide, sulphuric acid (98%), hydrochloric acid (35%) and nitric acid (69%) were purchased from Merck (India). The Neubauer-chamber was purchased from Pristine Scientific Ltd (India). Luria nutrient broth was purchased from Hi Media (India). Double distilled Millipore water was used for all the experiments.2.2 Catalyst preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 nitric acid (by volume) was added to obtain a transparent solution of titanyl nitrate. An equimolar oxidizer to fuel ratio was maintained by adding titanyl nitrate with a stoichiometric amount of L-ascorbic acid. The combustion reaction is;
2 nitric acid (by volume) was added to obtain a transparent solution of titanyl nitrate. An equimolar oxidizer to fuel ratio was maintained by adding titanyl nitrate with a stoichiometric amount of L-ascorbic acid. The combustion reaction is;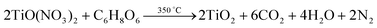 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 of Ag3PO4
10 of Ag3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2) was added dropwise to the TiO2 nanobelt dispersed mixture while continuing with vigorous stirring at 60 °C. The pH of the mixture was kept at 5 using 0.1 M nitric acid.
TiO2) was added dropwise to the TiO2 nanobelt dispersed mixture while continuing with vigorous stirring at 60 °C. The pH of the mixture was kept at 5 using 0.1 M nitric acid.
For comparison, the same process was carried out for the preparation of Ag3PO4/CST and Ag3PO4/P-25 using combustion synthesized TiO2 and commercial Degussa P-25 instead of TiO2 nanobelts. Di-sodium hydrogen phosphate solution was added slowly into the above mixture until the solution turned pale yellow indicating the formation of Ag3PO4 on CST and CSTNB. Pristine Ag3PO4 was prepared by the same procedure but without the addition of TiO2 nanoparticles.
3. Characterization
X-ray diffraction spectra were obtained from a Rigaku diffractometer using Cu-Kα radiation with a scan rate of 1° min−1 in the scan range of 10°–80°. Scanning electron micrographs were captured using ULTRA55 FESEM, Carl Zeiss. The samples were dispersed in absolute ethanol and sonicated for 10 min. These dispersed samples were drop-casted on silica wafers which were stuck to a carbon tape on a SEM aluminium stub. The prepared samples were kept under vacuum for 12 h; after that samples were gold sputtered using a Quaram sputtering machine to prevent the sample charging effect and SEM images were taken. Transmission electron micrographs were acquired with a Tecnai T20 operated at 180 kV. Samples for TEM analysis were prepared by dispersing it in isopropanol and subjected to ultrasonication. Later, the samples were drop casted on copper grid and kept under vacuum for 12 h. Diffuse reflectance spectra were obtained using a solid state UV-visible spectrophotometer (Perkin Elmer). Photoluminescence measurements were performed using a PL-spectrophotometer (Perkin Elmer). The absorbance measurements for photocatalysis experiments were measured using a UV-Visible spectrophotometer (Shimadzu-UV 1700). Samples were regenerated at 120 °C for 2 h prior to BET surface analysis using Nova-1000 Quantachrome. The data were obtained by using a Belsorb surface area analyzer (Smart instruments) with the help of liquid nitrogen (77 K) and water (300 K) atmospheres for both adsorption and desorption of N2.4. Photochemical reactor and photocatalysis
100 ml quartz reactors were used to perform photocatalytic experiments. Photocatalytic dye degradation and antibacterial degradation experiments were conducted under direct sunlight between 11:00 am and 2:00 pm, when the solar intensity fluctuations are less and the intensity was ∼975 W m−2. The reaction mixture was stirred vigorously using a magnetic stirrer and the samples were collected from the reactor at a specific time to measure absorbance. Experiments were performed in the dark to measure the adsorption of the dye over catalyst particles.Photocatalysis was performed using a 20 ppm aqueous solution of methylene blue and a 30 ppm aqueous solution of methyl orange. The catalyst concentration was maintained at 1 g l−1 for all the experiments. The catalyst particles were initially suspended in the dye solution and kept in the dark for 1 h to achieve stable absorption–desorption equilibrium. Samples were collected from the reactor at regular intervals and centrifuged at 5000 rpm for 5 min to separate catalyst particles from the solution and absorbance was measured using a UV-visible spectrophotometer.
4.1. Antibacterial evaluation
The bacterial culture of wild type Escherichia coli strain was prepared using liquid nutrient broth. Glass ware, media, test tubes, Eppendorf tubes, micro tips etc. and all other accessories required for culture preparation and antibacterial evaluation were autoclaved and kept under UV light inside a laminar chamber. The bacterial culture was centrifuged at 5000 rpm for 10 minutes to separate bacterial cells as pellets. Later, the pellets were re-suspended in phosphate buffer saline solution in order to maintain the ionic concentration for the bacteria. Control experiments were conducted in the dark using 0.25 g l−1 catalyst and bacterial suspension alone without keeping the reactor under solar irradiation. Furthermore, the catalysts were added into a fresh bacterial suspension and kept under solar radiation. Sample mixtures of bacterial and catalyst suspensions were collected at regular intervals in order to measure antibacterial activity. Various methods such as broth dilution, turbidity assay, colony-counting, disk-diffusion, growth inhibition and minimum inhibitory concentration were used. In addition to these, Neubauer-chamber counting can be used for the quantitative or qualitative determination of anti-microbial activity.29,30 100 μl of serially diluted aliquots were mixed with 0.4% of a neutral red solution according to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio. 20 μl of the above stained bacterial suspension was pipetted out on a Neubauer counting chamber. The viable bacterial cells were stained by neutral red because live cells incorporate the stain into lysosomes. Live bacterial cells were counted on respective squares under a microscope and averaged for calculating bacterial cell density.
10 ratio. 20 μl of the above stained bacterial suspension was pipetted out on a Neubauer counting chamber. The viable bacterial cells were stained by neutral red because live cells incorporate the stain into lysosomes. Live bacterial cells were counted on respective squares under a microscope and averaged for calculating bacterial cell density.
5. Results and discussion
5.1. X-ray diffraction analysis
X-ray diffraction patterns of combustion synthesized TiO2 and acid etched TiO2 nanobelts are shown in Fig. 1(a) and (b). The synthesized product with ascorbic acid as the fuel for combustion has shown the possibility of formation of a rutile phase at 2θ values of 36.0° and 54.2° (JCPDS no: 00-001-1292) besides a predominant anatase phase. The anatase can be observed at a 2θ value of 25.4° (JCPDS no: 00-004-0477) with (101) as <hkl> parameters. It has been reported that combustion synthesis of TiO2 using titanyl nitrate and ascorbic acid of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio between the oxidizer and the fuel resulted in a pure anatase phase.31 However, if the stoichiometric ratio is 2
3 molar ratio between the oxidizer and the fuel resulted in a pure anatase phase.31 However, if the stoichiometric ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the rutile phase is formed which co-exists with anatase.
1, the rutile phase is formed which co-exists with anatase.
 | ||
| Fig. 1 (a) X-ray diffraction pattern of combustion synthesized TiO2 and acid etched TiO2 nanobelts and (b) X-ray diffraction pattern of Ag3PO4 and Ag3PO4 coupled with acid etched TiO2 nanobelts. | ||
The X-ray diffraction pattern of nanobelts prepared out of combustion synthesized TiO2 shows a huge peak that corresponds to the anatase phase (JCPDS no: 00-004-0477) and adjacent to it a small peak at 27.6° corresponding to the rutile phase (JCPDS no: 00-001-1292). The diffraction peaks for Ag3PO4 nanoparticles are denoted with filled dots (Fig. 1(c)) and at 33.4° a huge peak was observed which corresponds to (210) plane diffraction. Further peaks at 36.7°, 55.0°, 57.5°, and 61.9°, can be assigned to the diffractions from the (2 1 0), (3 1 0), (3 2 0), (3 2 1), and (4 0 0) planes of Ag3PO4 (JCPDS (00-006-0505)). This pattern was also manifested in the Ag3PO4/TiO2 nano heterostructures (Fig. 1(d)). Therefore, successful impregnation of Ag3PO4 over acid etched TiO2 nanobelts can be corroborated based on these observations.
5.2. Diffuse reflectance spectrophotometry
The UV-vis diffuse reflectance spectra of the Ag3PO4, acid etched TiO2 nanobelts and Ag3PO4 coupled acid etched TiO2 nanobelts were measured using a UV-Vis spectrophotometer (Perkin Elmer). The formation of Ag3PO4/TiO2 has accounted for a remarkable increase in light absorption in the visible region compared to pristine TiO2 nanobelts. As shown in Fig. 2, UV-vis diffuse reflectance spectroscopy measurements rendered that the acid-etched TiO2 nanobelts exhibited a strong absorption at about 390 nm, which can correspond to the band gap of multi-phase titania having both anatase and rutile phases (3.0–3.2 eV).32 Similarly, a strong absorption peak was recorded at around 550 nm which can be matched to Ag3PO4 nanoparticles whose in-direct band gap was calculated to be around 2.4 eV.33 The absorption edge of the Ag3PO4 impregnated TiO2 heterostructures was unaltered compared to the pristine acid etched TiO2 nanobelts. However, there was a strong absorbance in the visible region between 400 and 700 nm. This can confirm that Ag3PO4 was successfully impregnated over the acid etched TiO2 nanobelts.33,345.3. Microscopy analysis
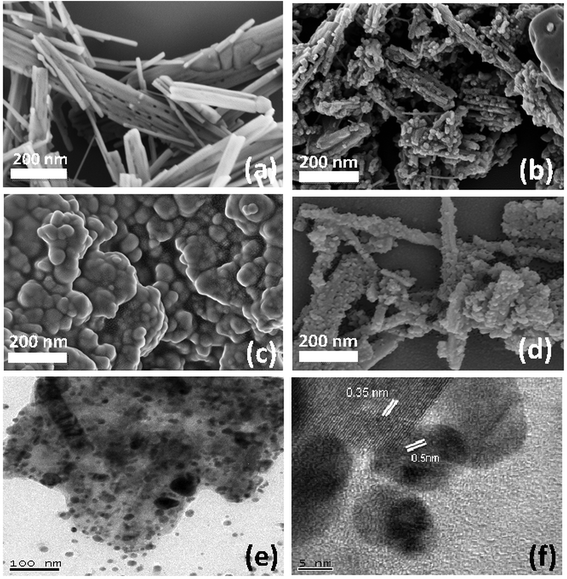
The morphological features of acid etched TiO2 nanobelts and Ag3PO4 impregnated TiO2 nanobelts were studied using SEM and HRTEM micrographs. The SEM image of acid etched TiO2 nanobelts before acid treatment is shown in Fig. 3(a). An acid corrosion treatment led to partial etching of nanobelts and subsequent formation of several TiO2 islands on existing nanobelts and also on partially etched nanobelts, as shown in Fig. 3(b). The surface area of TiO2 nanobelts was 35 m2 g−1 and it increases to 75 m2 g−1 after the acid etching process.35 It can be suggested that the etched surfaces could serve as nucleating sites for Ag3PO4 nanoparticles, resulting in the formation of Ag3PO4/TiO2 composites, as shown in (Fig. 3(c)). One can observe that the Ag3PO4 nanoparticles, with an approximate diameter between 10 and 20 nm, were deposited onto the acid-etched TiO2 nanobelt surface, consistent with the TEM image shown in Fig. 3(e). Ag3PO4 nanoparticles were also prepared without the acid etched TiO2 nanobelt support, following similar experimental conditions excluding the addition of TiO2 nanobelts. Ag3PO4 nanoparticles were spherical in shape and mostly agglomerated with other particles, as shown in Fig. 3(d). The structural analysis of the Ag3PO4/TiO2 heterostructures was done by HRTEM.As shown in Fig. 3(f), the lattice of both Ag3PO4 and TiO2 can be clearly distinguished. Ag3PO4 particles have a d-spacing of 0.56 nm, matching with the structure of Ag3PO4, whereas a d-spacing of 0.35 nm was observed for the TiO2 nanobelts, which corresponds to anatase TiO2. It can be clearly observed that the Ag3PO4 was impregnated firmly into the TiO2 nanobelt substrate, which promotes a synergistic effect towards photocatalysis by promoting a wide optical absorption and also ensures structural stability of the composite.
5.4. X-ray photoelectron spectroscopy
XPS results of silver in Ag3PO4/CSTNB show the existence of Ag in two bands corresponding to 368.2 and 374.1 eV, respectively, ascribed to Ag 3d5/2 and Ag 3d3/2 (Fig. 4(a)). These bands correspond to the Ag+ of Ag3PO4. The bands observed in Fig. 4(b) at 369.2 and 374.9 eV exhibit the binding energy of silver in the Ag0 state. This proves that after exposing Ag3PO4 (either in pristine or composite form) to light, part of Ag+ becomes Ag0. In the case of just Ag3PO4, the Ag0 formation is very high and hence stability is low. However, when Ag3PO4 is in the composite form, there will be a formation of the Ag/Ag3PO4/substrate36 and hence a prolonged activity of Ag3PO4 is maintained.5.5. Photoluminescence spectroscopy
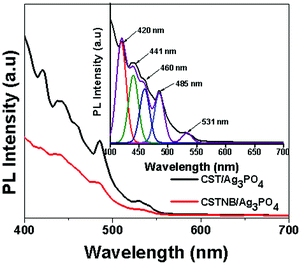
The photoluminescence spectra of Ag3PO4 impregnated combustion synthesized TiO2 and acid etched TiO2 nanobelts are shown in Fig. 5. The presence of intrinsic states instead of surface states can be corroborated to the peak originating at 419 nm because of charge transfer transitions between the 2p orbitals of oxygen and the empty d orbital of the silver ion which could possibly lead to recombination during photoluminescence.37 Recombination of self-trapped excitons in the PO4 anionic complex could also be the reason for the emission peak at 419 nm. The peak at 533 nm which is approximately equal to the band gap of Ag3PO4 can be matched to the recombination of electrons at the conduction band and holes at valence band edges.38,39 Surface deposited silver or Ag doped into the TiO2 lattice can act as efficient traps for the photo-induced electrons that prevent recombination. However, the presence of excess Ag0 could lead to reduction in the traps and increase in photoluminescence intensity as observed at 441 nm and 485 nm. The peak at 530 nm can also correspond to charge transfer between oxygen vacancies and Ti4+ including the peak at 460 nm which acts as a trap for charge.40 In the case of Ag3PO4 impregnated with acid etched TiO2 nanobelts the photoluminescence intensity decreased significantly which can be attributed due to an efficient charge transfer and charge separation mechanism.5.6. Photocatalysis
| Catalyst | Rate constant, k (×10−4 min−1) | |
|---|---|---|
| Methylene blue | Methyl orange | |
| Ag3PO4/Degussa P-25 | 802 ± 2.3 | 759 ± 87.9 |
| Ag3PO4/Combustion synthesized TiO2 | 1092 ± 2.1 | 762 ± 7.2 |
| Ag3PO4/Acid etched TiO2 nanobelts | 1840 ± 3.3 | 1354 ± 6.7 |
| Ag3PO4 | 3096 ± 15.3 | 445 ± 3.2 |
 | ||
| Fig. 7 Antimicrobial activity of Ag3PO4, Ag3PO4/P-25, Ag3PO4/CST and Ag3PO4/CSTNB under (a) dark conditions (b) under solar irradiation. | ||
Under dark conditions with Ag3PO4 in a bacterial suspension, a bactericidal effect was observed because of the release of Ag+ ions into the suspension.47 However, this process occurs at time scales greater than 2 h. Thus the release of Ag does not influence photocatalytic activity for the degradation of dyes but shows some activity towards the inactivation of bacteria. Therefore, it is considered best to employ Ag3PO4 as a photocatalyst and investigate the bactericidal effect under UV/solar light. Similarly, using pristine Ag3PO4 for methylene blue degradation showed best activity initially but the photoactivity of Ag3PO4 decreased over subsequent trials, as discussed in the later section on reusability. This was observed by the decrease in photocatalytic activity and also by observing a change in the color of Ag3PO4 from yellow to black because of the formation of Ag nanoparticles upon shining light. But this was not observed when Ag3PO4 was impregnated with nanobelt structures, where the latter act as the support preventing decrease in the photoactivity of Ag3PO4. Thus Ag3PO4 decorated TiO2 nanobelts are the best for the photocatalytic degradation of dyes and bacteria.
5.7. Scavenger reactions
It is known that the principal factor of photocatalysis is the generation of reactive radicals. Therefore, it is necessary to evaluate the nature of the reactive radicals responsible for the catalytic degradation. The photocatalytic reactant mixture was mixed with various scavengers and the degradation was carried out. EDTA-2Na+ (hole scavenger),49 tertiary-butyl alcohol (˙OH scavenger),50 DMSO (e− scavenger),51 and benzoquinone (O˙−2 scavenger)52 were the scavengers added to the reaction mixture containing methylene blue and catalyst particles and it was subjected to photocatalytic degradation under sunlight. From Fig. 8, it is observed that photocatalytically generated holes and hydroxyl radicals formed during the reaction of Ag3PO4 and acid etched TiO2 nanobelt composites are responsible for the efficient degradation. There is a huge probability for the holes generated from TiO2 excitation to get transported towards Ag3PO4, and thus the generation of hydroxyl radicals is highly favorable. The excited electrons of Ag3PO4, in addition to transported electrons from anatase, also take part in the generation of O˙−2 radicals.5.8. Stability
The reusability of the catalyst has been tested by photo-degrading methylene blue under solar radiation. The catalyst particles were centrifuged and dried at 100 °C after each cycle. The catalytic stability is shown in Fig. 9 as a 3D profile representing methylene blue degradation with respect to each cycle. As compared to the pristine Ag3PO4, Ag3PO4 impregnated on acid-etched TiO2 nanobelts shows consistent photocatalytic activity even after 3 cycles. This might be due to the tight anchoring of Ag3PO4 on the surface of acid etched nanobelts that enhances the stability of the composites.6. Conclusions
This study indicates the outstanding properties exhibited by the novel combustion synthesis derived TiO2 nanobelts compared to commercial TiO2. TiO2 nanobelts were synthesized using combustion synthesized TiO2 for the first time. The synthesis and photodegradation experiments of composite Ag3PO4 coupled with acid etched combustion synthesized TiO2 nanobelts were performed. The photocatalytic activity of Ag3PO4/CSTNB composites was significantly higher compared to Ag3PO4/CST and Ag3PO4/P-25. The efficient and excess generation of hydroxyl radicals by the Ag3PO4/CSTNB due to lower recombination and better charge transfer led to enhanced photoactivity. TiO2 islands and Ag3PO4 impregnation on top of acid etched TiO2 nanobelts have not only contributed towards enhanced charge separation but also efficient charge transfer and utilization of a broad spectrum of light for a better photocatalytic activity for the degradation of dyes and inactivation of bacteria.Acknowledgements
The authors are thankful to the Department of Science and Technology, India for financial support. The authors thank Prof. Jayant Modak, Department of Chemical Engineering, IISc, for extending facilities to perform antibacterial experiments.References
- T. Schwartz, W. Kohnen, B. Jansen and U. Obst, Detection of antibiotic-resistant bacteria and their resistance genes in wastewater, surface water, and drinking water biofilms, FEMS Microbiol. Ecol., 2003, 43, 325–335 CrossRef CAS PubMed.
- D. D. Mara, S. Cairncross and W. H. Organization, Guidelines for the safe use of wastewater and excreta in agriculture and aquaculture: measures for public health protection/prepared by Duncan Mara & Sandy Cairncross, 1989 Search PubMed.
- M. D. Sobsey, Inactivation of health-related microorganisms in water by disinfection processes, Water Sci. Technol., 1989, 21, 179–195 CAS.
- G. Korotcenkov, The role of morphology and crystallographic structure of metal oxides in response of conductometric-type gas sensors, Mater. Sci. Eng., R, 2008, 61, 1–39 CrossRef PubMed.
- T. M. Shang, J. H. Sun, Q. F. Zhou and M. Y. Guan, Controlled synthesis of various morphologies of nanostructured zinc oxide: flower, nanoplate, and urchin, Cryst. Res. Technol., 2007, 42, 1002–1006 CrossRef CAS PubMed.
- K. M. Kim, K.-Y. Kang, S. Kim and Y.-G. Lee, Electrochemical properties of TiO2 nanotube-Li4Ti5O12 composite anodes for lithium-ion batteries, Curr. Appl. Phys., 2012, 12, 1199–1206 CrossRef PubMed.
- X. Wu, W. Xing, L. Zhang, S. Zhuo, J. Zhou, G. Wang and S. Qiao, Nickel nanoparticles prepared by hydrazine hydrate reduction and their application in supercapacitor, Powder Technol., 2012, 224, 162–167 CrossRef CAS PubMed.
- A. Leelavathi, G. Madras and N. Ravishankar, Origin of enhanced photocatalytic activity and photoconduction in high aspect ratio ZnO nanorods, Phys. Chem. Chem. Phys., 2013, 15, 10795–10802 RSC.
- V. Subramanian, E. Wolf and P. V. Kamat, Semiconductor-metal composite nanostructures. To what extent do metal nanoparticles improve the photocatalytic activity of TiO2 films?, J. Phys. Chem. B, 2001, 105, 11439–11446 CrossRef CAS.
- K. Nagaveni, M. Hegde, N. Ravishankar, G. Subbanna and G. Madras, Synthesis and structure of nanocrystalline TiO2 with lower band gap showing high photocatalytic activity, Langmuir, 2004, 20, 2900–2907 CrossRef CAS.
- K. Patil, M. Hegde, T. Rattan and S. Aruna, Chemistry of nanocrystalline oxide materials-Combustion synthesis, properties and applications, 2008 Search PubMed.
- Z. Zhang, C.-C. Wang, R. Zakaria and J. Y. Ying, Role of particle size in nanocrystalline TiO2-based photocatalysts, J. Phys. Chem. B, 1998, 102, 10871–10878 CrossRef CAS.
- K. Nagaveni, M. Hegde and G. Madras, Structure and Photocatalytic Activity of Ti1−xM xO2±δ (M = W, V, Ce, Zr, Fe, and Cu) Synthesized by Solution Combustion Method, J. Phys. Chem. B, 2004, 108, 20204–20212 CrossRef CAS.
- W. Jiang, C. An, J. Liu, S. Wang, L. Zhao, W. Guo and J. Liu, Facile aqueous synthesis of β-AgI nanoplates as efficient visible-light-responsive photocatalyst, Dalton Trans., 2014, 43, 300–305 RSC.
- M. Ge, N. Zhu, Y. Zhao, J. Li and L. Liu, Sunlight-assisted degradation of dye pollutants in Ag3PO4 suspension, Ind. Eng. Chem. Res., 2012, 51, 5167–5173 CrossRef CAS.
- Y. Sang, L. Kuai, C. Chen, Z. Fang and B. Geng, Fabrication of a Visible-Light-Driven Plasmonic Photocatalyst of AgVO3@AgBr@Ag Nanobelt Heterostructures, ACS Appl. Mater. Interfaces, 2014, 6, 5061–5068 CAS.
- J. S. Kim, E. Kuk, K. N. Yu, J.-H. Kim, S. J. Park, H. J. Lee, S. H. Kim, Y. K. Park, Y. H. Park and C.-Y. Hwang, Antimicrobial effects of silver nanoparticles, Nanomedicine, 2007, 3, 95–101 CrossRef CAS PubMed.
- Y. Liu, L. Fang, H. Lu, L. Liu, H. Wang and C. Hu, Highly efficient and stable Ag/Ag3PO4 plasmonic photocatalyst in visible light, Catal. Commun., 2012, 17, 200–204 CrossRef CAS PubMed.
- H. Zhao, L. Zhang, X. Gu, S. Li, B. Li, H. Wang, J. Yang and J. Liu, Fe2O3–AgBr nonwoven cloth with hierarchical nanostructures as efficient and easily recyclable macroscale photocatalysts, RSC Adv., 2015, 5, 10951–10959 RSC.
- H. Yu, L. Xu, P. Wang, X. Wang and J. Yu, Enhanced photoinduced stability and photocatalytic activity of AgBr photocatalyst by surface modification of Fe(III) cocatalyst, Appl. Catal., B, 2014, 144, 75–82 CrossRef CAS PubMed.
- X. Yang, J. Qin, Y. Jiang, K. Chen, X. Yan, D. Zhang, R. Li and H. Tang, Fabrication of P25/Ag3PO4/graphene oxide heterostructures for enhanced solar photocatalytic degradation of organic pollutants and bacteria, Appl. Catal., B, 2015, 166, 231–240 CrossRef PubMed.
- J.-W. Xu, Z.-D. Gao, K. Han, Y. Liu and Y.-Y. Song, Synthesis of Magnetically Separable Ag3PO4/TiO2/Fe3O4 Heterostructure with Enhanced Photocatalytic Performance under Visible Light for Photoinactivation of Bacteria, ACS Appl. Mater. Interfaces, 2014, 6, 15122–15131 CAS.
- X. Yang, H. Cui, Y. Li, J. Qin, R. Zhang and H. Tang, Fabrication of Ag3PO4-Graphene composites with highly efficient and stable visible light photocatalytic performance, ACS Catal., 2013, 3, 363–369 CrossRef CAS.
- H. Cui, X. Yang, Q. Gao, H. Liu, Y. Li, H. Tang, R. Zhang, J. Qin and X. Yan, Facile synthesis of graphene oxide-enwrapped Ag3PO4 composites with highly efficient visible light photocatalytic performance, Mater. Lett., 2013, 93, 28–31 CrossRef CAS PubMed.
- V. Bellat, R. Chassagnon, O. Heintz, L. Saviot, D. Vandroux and N. Millot, A multi-step mechanism and integrity of titanate nanoribbons, Dalton Trans., 2015, 44, 1150–1160 RSC.
- N. Wu, J. Wang, D. N. Tafen, H. Wang, J.-G. Zheng, J. P. Lewis, X. Liu, S. S. Leonard and A. Manivannan, Shape-enhanced photocatalytic activity of single-crystalline anatase TiO2 (101) nanobelts, J. Am. Chem. Soc., 2010, 132, 6679–6685 CrossRef CAS PubMed.
- Z.-Y. Yuan, J.-F. Colomer and B.-L. Su, Titanium oxide nanoribbons, Chem. Phys. Lett., 2002, 363, 362–366 CrossRef CAS.
- Y. Wang, G. Du, H. Liu, D. Liu, S. Qin, N. Wang, C. Hu, X. Tao, J. Jiao and J. Wang, Nanostructured Sheets of Ti-O Nanobelts for Gas Sensing and Antibacterial Applications, Adv. Funct. Mater., 2008, 18, 1131–1137 CrossRef CAS PubMed.
- R. Hernandez-Delgadillo, D. Velasco-Arias, D. Diaz, K. Arevalo-Niño, M. Garza-Enriquez, M. A. De la Garza-Ramos and C. Cabral-Romero, Zerovalent bismuth nanoparticles inhibit Streptococcus mutans growth and formation of biofilm, Int. J. Nanomed., 2012, 7, 2109 CAS.
- R. Hernandez-Delgadillo, D. Velasco-Arias, J. J. Martinez-Sanmiguel, D. Diaz, I. Zumeta-Dube, K. Arevalo-Niño and C. Cabral-Romero, Bismuth oxide aqueous colloidal nanoparticles inhibit Candida albicans growth and biofilm formation, Int. J. Nanomed., 2013, 8, 1645 Search PubMed.
- A. D. Mani, P. M. K. Reddy, M. Srinivaas, P. Ghosal, N. Xanthopoulos and C. Subrahmanyam, Facile synthesis of efficient visible active C-doped TiO2 nanomaterials with high surface area for the simultaneous removal of phenol and Cr (VI), Mater. Res. Bull., 2015, 61, 391–399 CrossRef CAS PubMed.
- F. M. Hossain, L. Sheppard, J. Nowotny and G. E. Murch, Optical properties of anatase and rutile titanium dioxide: Ab initio calculations for pure and anion-doped material, J. Phys. Chem. Solids, 2008, 69, 1820–1828 CrossRef CAS PubMed.
- S. B. Rawal, S. D. Sung and W. I. Lee, Novel Ag3PO4/TiO2 composites for efficient decomposition of gaseous 2-propanol under visible-light irradiation, Catal. Commun., 2012, 17, 131–135 CrossRef CAS PubMed.
- R. Liu, P. Hu and S. Chen, Photocatalytic activity of Ag3PO4 nanoparticle/TiO2 nanobelt heterostructures, Appl. Surf. Sci., 2012, 258, 9805–9809 CrossRef CAS PubMed.
- W. Zhou, G. Du, P. Hu, G. Li, D. Wang, H. Liu, J. Wang, R. I. Boughton, D. Liu and H. Jiang, Nanoheterostructures on TiO2 nanobelts achieved by acid hydrothermal method with enhanced photocatalytic and gas sensitive performance, J. Mater. Chem., 2011, 21, 7937–7945 RSC.
- W. Teng, X. Li, Q. Zhao, J. Zhao and D. Zhang, In situ capture of active species and oxidation mechanism of RhB and MB dyes over sunlight-driven Ag/Ag3PO4 plasmonic nanocatalyst, Appl. Catal., B, 2012, 125, 538–545 CrossRef CAS PubMed.
- Y. Lei, L.-D. Zhang, G.-W. Meng, G.-H. Li, X. Zhang, C. Liang, W. Chen and S. Wang, Preparation and photoluminescence of highly ordered TiO2 nanowire arrays, Appl. Phys. Lett., 2001, 78, 1125–1127 CrossRef CAS PubMed.
- J. Liu, X. Fu, S. Chen and Y. Zhu, Electronic structure and optical properties of Ag3PO4 photocatalyst calculated by hybrid density functional method, Appl. Phys. Lett., 2011, 99, 191903 CrossRef PubMed.
- X. Ma, B. Lu, D. Li, R. Shi, C. Pan and Y. Zhu, Origin of photocatalytic activation of silver orthophosphate from first-principles, J. Phys. Chem. C, 2011, 115, 4680–4687 CAS.
- B. Xin, L. Jing, Z. Ren, B. Wang and H. Fu, Effects of simultaneously doped and deposited Ag on the photocatalytic activity and surface states of TiO2, J. Phys. Chem. C, 2005, 109, 2805–2809 CrossRef CAS PubMed.
- J. Kiwi and V. Nadtochenko, Evidence for the mechanism of photocatalytic degradation of the bacterial wall membrane at the TiO2 interface by ATR-FTIR and laser kinetic spectroscopy, Langmuir, 2005, 21, 4631–4641 CrossRef CAS.
- S. Sontakke, C. Mohan, J. Modak and G. Madras, Visible light photocatalytic inactivation of Escherichia coli with combustion synthesized TiO2, Chem. Eng. J., 2012, 189, 101–107 CrossRef PubMed.
- G. Gogniat and S. Dukan, TiO2 photocatalysis causes DNA damage via Fenton reaction-generated hydroxyl radicals during the recovery period, Appl. Environ. Microbiol., 2007, 73, 7740–7743 CrossRef CAS PubMed.
- Q. Li, S. Mahendra, D. Y. Lyon, L. Brunet, M. V. Liga, D. Li and P. J. Alvarez, Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications, Water Res., 2008, 42, 4591–4602 CrossRef CAS PubMed.
- G. A. Sotiriou and S. E. Pratsinis, Antibacterial activity of nanosilver ions and particles, Environ. Sci. Technol., 2010, 44, 5649–5654 CrossRef CAS PubMed.
- M. Rai, A. Yadav and A. Gade, Silver nanoparticles as a new generation of antimicrobials, Biotechnol. Adv., 2009, 27, 76–83 CrossRef CAS PubMed.
- A. Wu, C. Tian, W. Chang, Y. Hong, Q. Zhang, Y. Qu and H. Fu, Morphology-controlled synthesis of Ag3PO4 nano/microcrystals and their antibacterial properties, Mater. Res. Bull., 2013, 48, 3043–3048 CrossRef CAS PubMed.
- X. Yang, J. Qin, Y. Jiang, R. Li, Y. Li and H. Tang, Bifunctional TiO2/Ag3PO4/graphene composites with superior visible light photocatalytic performance and synergistic inactivation of bacteria, RSC Adv., 2014, 4, 18627–18636 RSC.
- R. Sapkal, S. Shinde, T. Waghmode, S. Govindwar, K. Rajpure and C. Bhosale, Photo-corrosion inhibition and photoactivity enhancement with tailored zinc oxide thin films, J. Photochem. Photobiol., B, 2012, 110, 15–21 CrossRef CAS PubMed.
- S. Yan, Z. Li and Z. Zou, Photodegradation of rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed.
- D. Wang, Y. Duan, Q. Luo, X. Li, J. An, L. Bao and L. Shi, Novel preparation method for a new visible light photocatalyst: mesoporous TiO2 supported Ag/AgBr, J. Mater. Chem., 2012, 22, 4847–4854 RSC.
- W. Li, D. Li, S. Meng, W. Chen, X. Fu and Y. Shao, Novel Approach To Enhance Photosensitized Degradation of Rhodamine B under Visible Light Irradiation by the ZnxCd1−x S/TiO2 Nanocomposites, Environ. Sci. Technol., 2011, 45, 2987–2993 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2015 |