Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fast and effective inactivation of Bacillus atrophaeus endospores using light-activated derivatives of vitamin B2
Anja
Eichner
*a,
Anita
Gollmer
a,
Andreas
Späth
b,
Wolfgang
Bäumler
a,
Johannes
Regensburger
a,
Burkhard
König
b and
Tim
Maisch
a
aDepartment of Dermatology, Regensburg University Hospital, 93053 Regensburg, Germany. E-mail: anja.eichner@klinik.uni-regensburg.de
bDepartment of Organic Chemistry, University of Regensburg, 93053 Regensburg, Germany
First published on 11th November 2014
Abstract
Highly resistant endospores may cause severe problems in medicine as well as in the food and packaging industries. We found that bacterial endospores can be inactivated quickly with reactive oxygen species (ROS) that were generated by a new generation of flavin photosensitizers. Flavins like the natural compound vitamin B2 are already known to produce ROS but they show a poor antimicrobial photodynamic killing efficacy due to the lack of positive charges. Therefore we synthesized new flavin photosensitizers that have one (FLASH-01a) or eight (FLASH-07a) positive charges and can hence attach to the negatively charged surface of endospores. In this study we used standardized Bacillus atrophaeus endospores (ATCC 9372) as a biological surrogate model for a proof-of-concept study of photodynamic inactivation experiments using FLASH-01a and FLASH-07a. After incubation of spores with different flavin concentrations, the flavin derivatives were excited with blue light at a light dose of 70 J cm−2. The inactivation of spores was investigated either in suspension or after attachment to polyethylene terephthalate (PET) surfaces. Incubation of spores suspended in Millipore water with 4 mM FLASH-01a for 10 seconds and irradiation with blue light for 10 seconds caused a biologically relevant decrease of spore survival of 3.5 log10 orders. Using FLASH-07a under the same conditions we achieved a decrease of 4.4 log10 orders. Immobilized spores on PET surfaces were efficiently killed with 7.0 log10 orders using 8 mM FLASH-07a. The total treatment time (incubation + irradiation) was as short as 20 seconds. The results of this study show evidence that endospores can be fastly and effectively inactivated with new generations of flavin photosensitizers that may be useful for industrial or medical applications in the future.
Introduction
The photodynamic approach is widely used in clinical applications such as the treatment of cancer or infectious diseases.1,2 Spore forming bacteria like Bacillus or Clostridium cause severe infections (diarrhea, colitis) in humans which could arise from contaminated food and beverage products. Thus, these bacteria and their corresponding endospores provoke massive problems in the food and packaging industries as well as in medical and biotechnological processes. It is known that dried foods like cereals or spices are often contaminated with bacterial endospores.3 When spore-contaminated food products were exposed to a humidified area (e.g. during food preparation or in a closed package), spores will germinate and the corresponding bacteria produce toxins leading to food poisoning and severe diseases in humans.Exposed to unfavorable conditions, some bacterial species produce spores which are highly resistant against a variety of stress factors including biocides, UV and gamma radiation, wet and dry heat, oxidizing agents, desiccation and even toxic chemicals.4,5 To date, only strong chemical or physical agents (e.g. peracetic acid, hypochlorite solution, chlorine dioxide, formaldehyde gas) show a satisfactory result in spore decontamination.6–9 However, these measures show harmful potential to humans in particular when the used chemicals remain in the food or beverage product or on the surface of the food package. Moreover, chemical approaches for spore decontamination (e.g. hydrogen peroxide vapor) are highly intensive in consumption of water or other resources and are harmful for the environment.
Alternatively, the photodynamic inactivation (PDI) of microorganisms presents several positive aspects regarding the killing efficacy of microorganisms. PDI has been independent of the resistance pattern of microorganisms so far, PDI can be applied for various microorganisms, and PDI show no selection of photo-resistant cells.10 The photodynamic principle is based on the concept that visible light, oxygen and a non-toxic dye (known as a photosensitizer) generate reactive oxygen species, which cause massive oxidative stress and lethal damage to the microorganisms.11–14 For sufficient inactivation it is necessary that photosensitizers have at least one positive charge to attach to the negatively charged cell wall of bacteria or the surface of spores.15,16 The photodynamic approach shows very good results in killing of different types of bacteria.17–20 Recently we showed that PDI is able to kill bacterial suspensions of Bacillus atrophaeus, Staphylococcus aureus and Escherichia coli completely within milliseconds (flashes of intense pulse light) after a 10 second incubation time period.13 In contrast, the inactivation of bacterial endospores should be more difficult because of the multi-layered and robust composition of the spore's coat. Long incubation or very long irradiation times are generally needed for sufficient inactivation of bacterial endospores with PDI. Demidova et al. achieved a biologically relevant decrease (>5 log10 orders) of Bacillus spores with 3 hour incubation and a 100–200 second irradiation period at least.14
Typical photosensitizer classes (e.g. porphyrins, phenothiazines, phthalocyanins) with different properties are known, but these photosensitizers have disadvantages when used in the photodynamic approach. Porphyrins show toxic effects without light or have only a poor spore killing efficacy (e.g. amine-modified protoporphyrin IX21 or a tricationic porphyrin Tri-Py+-Me-PF22). Thus, we searched for non-toxic and food-safe photosensitizers, which can be safely applied especially in food and food processing without environmental hazards. In this study we considered vitamins like riboflavin which are known as potential photosensitizers.23 Riboflavin is a natural compound and shows a ability to generate singlet oxygen,24–26 but unfortunately it has a very low killing efficacy against bacteria due to the lack of one positive charge.27,28 By chemical modification we created new flavin photosensitizers with different numbers of positive charges.
Spores of the non-pathogenic Gram-positive bacterium Bacillus atrophaeus were chosen as surrogate spores in our study as Sagripanti et al. clearly established that B. subtilis, B. cereus, and B. anthracis show similar or comparable sensitivity to chemical disinfectants.27 Furthermore Sagripanti and colleagues concluded that decontamination and sterilization data obtained with non-pathogenic spore simulants can be safely extrapolated to virulent spores of other Bacillus species.27 Thus, the main goal of our investigations was a proof-of-concept study that provides evidence for fast and effective inactivation of B. atrophaeus spores in vitro while attached to food-related packaging surfaces using newly developed flavin photosensitizers.
Materials and methods
Spores
Bacillus atrophaeus endospores (ATCC 9372) were purchased from Simicon, Inc. (Munich, Germany) at concentrations of ∼108 and ∼109 spores per mL distilled H2O. The purity of spore suspensions was checked using a Schaeffer and Fulton spore stain kit (malachite green 50 g L−1 in H2O, safranin O 5 g L−1 in H2O; Sigma Aldrich, Taufkirchen, Germany). Endospores were stored at −20 °C for a maximum of 6 months according to the guideline of the manufacturer. To avoid germination all aliquots were kept on ice during each experiment.Light source and irradiation parameters
A non-coherent light source (OmniCure Series 2000, igb-tech GmbH, Friedelsheim, Germany) was used in this study with a nominative excitation filter set of 320–500 nm. The effective radiant exposure of the light source was calculated as follows (eqn (1)):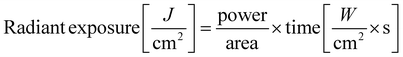 | (1) |
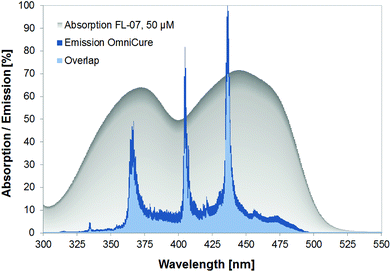
The tip of the lamp was fixed on a tripod, the lamp power was measured using a thermal sensor (model 30A-P-SH, Ophir Spiricon Europe Inc., Darmstadt, Germany) and a Nova power meter (Ophir Spiricon Europe Inc., Darmstadt, Germany). The emitted spectrum of the light source was recorded with a spectrometer (270M, Jobin Yvon Inc., Longjumeau, France) with 300 grid-lines per mm and a spectral resolution of approximately 0.4 nm. The detection range was 300 to 1000 nm. The recorded spectral data were corrected regarding the spectral sensitivity of the spectrometer. The emission spectrum of the OmniCure light source was normalized to its corresponding maximum at 436 nm. The absorption spectrum of 50 μM FLASH-07a was measured in distilled water with a Beckman DU460 spectrophotometer (Beckman Coulter Biomedical Inc., Munich, Germany). The absorption spectra of both flavin derivatives are the same within experimental accuracy. The difference of the molecular weights is caused by different chemical side chains that do not affect the absorption characteristics of the flavin core structure. The absorption peaks of both flavin derivatives are at 371 nm and 443 nm, respectively. The spectral overlap of 50 μM of FLASH-07a absorption and emission of the OmniCure light source is 61.5%; the absorption of our new flavin photosensitizer matches closely with the emission spectrum of the light source (Fig. 1).
Detection of singlet oxygen generated by flavin photosensitizers
The ability of flavin photosensitizers to generate singlet oxygen was qualitatively evaluated using a tunable laser system (NT242, Ekspla Inc., Vilnius, Lithuania) with an excitation beam at 443 nm in the range of 10 ± 1 mW to 200 ± 10 mW for 20 seconds. The reference photosensitizer was 5,10,15,20-tetrakis(1-methyl-pyridinium-4-yl)-porphyrin tetra p-toluenesulfonate (TMPyP). Singlet oxygen luminescence generated by the flavin photosensitizers and TMPyP was detected with an IR-sensitive photomultiplier (R5509-42, Hamamatsu Photonics Inc., Herrsching, Germany) at different wavelengths from 1150 to 1400 nm using interference filters.28 Depending on the absorption coefficient of the photosensitizers, different concentrations of flavin derivatives (7 to 10 μM) and TMPyP (2.5 to 7 μM) were used for singlet oxygen luminescence measurements aligning absorbed photons from each photosensitizer.Photosensitizer
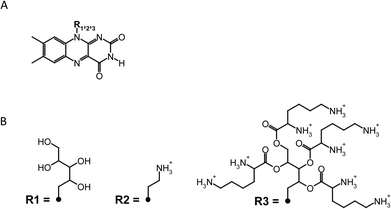
FLASH-01a and -07a were generated as previously described.29 Briefly, FLASH-01a was prepared by attachment of one positive charge to the riboflavin chromophore by standard protocols.30,31 FLASH-07a was prepared by esterification of the alcohol groups of the ribose chain with lysine using the Steglich protocol.32 Both flavin derivatives were synthesized at the Department of Chemistry, University of Regensburg, Germany. Riboflavin was purchased from Sigma Aldrich Inc., Steinheim, Germany. Riboflavin: MW 376.6 g mol−1, purity >98% for biochemical application. FLASH-01a-hydrochloride: MW 321.77 g mol−1, purity >98% as determined by NMR spectroscopy. FLASH-07a-hydrochloride: MW 1180.74 g mol−1, purity >95% as determined by NMR spectroscopy.pH values of both photosensitizers dissolved in distilled water were measured at different concentrations (8 mM, 1 mM, 50 μM). The determined pH values of FLASH-01a were 5.4 ± 0.1 (8 mM), 6.0 ± 0.1 (1 mM) and 6.8 ± 0.1 (50 μM), pH values of FLASH-07a were 4.4 ± 0.1 (8 mM), 5.3 ± 0.1 (1 mM) and 6.5 ± 0.1 (50 μM), respectively. The chemical structures of riboflavin and both flavin photosensitizers are depicted in Fig. 2.
Phototoxicity assay of Bacillus spore suspensions
The original spore solution (∼108 spores per mL) was mixed with an equal volume of a double concentrated solution of FLASH-01a or FLASH-07a. 25 μL of the original spore solution was placed into a sterile 96-well microtiter plate and incubated with different concentrations of flavin photosensitizers (final concentrations: 0/0.5/1/2/4 mM) for 10 seconds in the dark. Immediately at the end of the incubation period, the suspensions were illuminated with 7 W cm−2 for 10 seconds corresponding to a radiant exposure of 70 J cm−2. After illumination, the suspensions were serially diluted and plated on Mueller–Hinton agar using the Miles, Misra and Irwin technique.33 After incubation at 37 °C for 24 h the survival of the germinated spores was determined by counting the number of colony-forming units (CFU). Controls were neither sensitized with flavin derivatives nor exposed to the light source (reference control) or were incubated with the photosensitizer only (dark control) or illuminated only (light control).Phototoxicity assay of Bacillus spores on inert surfaces
A total of ∼107 spores were used for photokilling studies of Bacillus spores dried on polyethylene terephthalate (PET) coupons (thickness 0.1 mm; 12 × 12 mm). At first, the test coupons were wiped with 70% isopropanol. Autoclaving of the test coupons was not applicable due to the surface damage of the test coupons. A 12.5 μL aliquot of the original spore suspension was placed as a little drop onto the test coupon and dried for 2 hours in a lamina flow hood to avoid any contamination. Irradiated samples and control samples (only light; only photosensitizer; no light and no photosensitizer) consisted of 8 PET coupons, respectively. Each coupon was incubated for 10 seconds with a 50 μL photosensitizer solution (final concentrations: 0/2/4/8 mM) and was illuminated with 7 W cm−2 light for 10 seconds. After illumination, the coupons of one condition (8 pieces) were collected in 3 mL Mueller–Hinton broth and sonicated for 2 × 5 minutes with intermediately rigorous vortexing for 1 minute. The recovery efficiency of the spores attached to the PET coupon was controlled by applying 100 μL of the original spore suspension into 3 mL Mueller–Hinton broth in parallel (recovery control). The resolved spores from the PET coupons and the initial inoculum were serially diluted and plated on Mueller–Hinton agar using the Miles, Misra and Irwin technique.33 After incubation at 37 °C for 24 h the survival of the germinated spores was determined by counting the numbers of CFU. The bacterial count of the recovery control of three independent experiments was 1.49 × 107. Controls were neither sensitized with flavin photosensitizers nor exposed to the light source (reference control) or were incubated with the photosensitizer only (dark control) or illuminated only (light control).Transmission electron microscopy
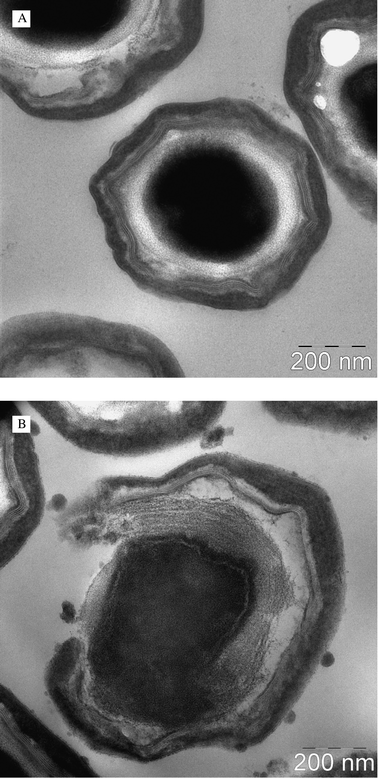
An original spore suspension of approximately 109 per mL was mixed with an 8 mM stock solution of FLASH-07a (final concentration 4 mM). The suspension was subsequently illuminated for 8 seconds at an intensity of 7 W cm−2, yielding a total light dose of 56 J cm−2. The irradiated samples were centrifuged at 3000 rpm (Heraeus Sepatech Megafuge 1.0, swing-out rotor #2705) for 10 min. The remaining pellet was resuspended in 1.5 mL distilled water and transferred into 1.5 mL reaction tubes. The suspension was centrifuged again for 10 min at 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (Eppendorf Centrifuge 5415R, rotor F45-24-11). Spore samples were routinely fixed in 0.1 M cacodylate-buffered Karnovsky solution (2.5% glutaraldehyde and 2% paraformaldehyde; overnight; room temperature) and postfixed for 2 h in 1% osmium tetroxide at pH 7.3. The samples were dehydrated in graded ethanol solutions and embedded in EmBed-812 epoxy resin (all reagents from Science Services, Munich, Germany). After 48 h heat polymerisation at 60 °C, semi-thin sections (0.8 μm) were cut from epon blocks and stained with toluidine blue/basic fuchsin. Ultrathin sections (80 nm) were cut with a diamond knife on a Reichert Ultracut S microtome and double contrasted with aqueous 2% uranyl acetate and lead citrate solutions for 10 min each. The sections were examined on a LEO912AB transmission electron microscope (Zeiss Inc., Oberkochen, Germany) operating at 100 kV. Images were recorded using OSIS-Software iTEM (Olympus Soft Imaging Solutions Inc., Münster, Germany).
000 rpm (Eppendorf Centrifuge 5415R, rotor F45-24-11). Spore samples were routinely fixed in 0.1 M cacodylate-buffered Karnovsky solution (2.5% glutaraldehyde and 2% paraformaldehyde; overnight; room temperature) and postfixed for 2 h in 1% osmium tetroxide at pH 7.3. The samples were dehydrated in graded ethanol solutions and embedded in EmBed-812 epoxy resin (all reagents from Science Services, Munich, Germany). After 48 h heat polymerisation at 60 °C, semi-thin sections (0.8 μm) were cut from epon blocks and stained with toluidine blue/basic fuchsin. Ultrathin sections (80 nm) were cut with a diamond knife on a Reichert Ultracut S microtome and double contrasted with aqueous 2% uranyl acetate and lead citrate solutions for 10 min each. The sections were examined on a LEO912AB transmission electron microscope (Zeiss Inc., Oberkochen, Germany) operating at 100 kV. Images were recorded using OSIS-Software iTEM (Olympus Soft Imaging Solutions Inc., Münster, Germany).
Eukaryotic cells and cell culture
Normal human epidermal keratinocytes (NHEKs) were purchased from ATCC (ATCC-PCS-200-010, American Type Culture Collection, Manassas, USA) and seeded into a T75 cell culture flask with 10 ml of dermal cell basal medium supplemented with a keratinocyte growth kit (ATCC PCS-200-030/PCS-200-040). Cells were incubated at 37 °C under a humidified atmosphere with 5% CO2 (v/v). The medium was replaced every two days. The NHEK cells were washed once with 10 ml PBS (Biochrom, Berlin, Germany) and removed from the flask bottom with a 2 ml 0.1% trypsin-EDTA solution (Gibco Life Technologies, Eggenstein, Germany).For incubation with 100 μL FLASH-07a for 60 seconds, the cells were seeded into 96-well microtiter plates (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well) and were incubated at 37 °C and 5% CO2 overnight. On the next day, cells were incubated with different concentrations of FLASH-07a (final concentrations 0/2/4/8 mM). FLASH-07a was dissolved in DMEM medium (Dulbecco's Modified Eagle Medium, PAN Biotech Inc., Aidenbach, Germany) without serum and phenol red. The photosensitizer was used as an irradiated (7 W cm−2, 10 seconds) or non-irradiated solution to see whether the decomposition compounds of FLASH-07a show a toxic effect against the keratinocytes. After incubation, the flavin solution was removed from each well, cells were washed two times to remove all PS solution and were incubated with 100 μL fresh dermal cell basal medium overnight at 37 °C and 5% CO2. Control cells were not incubated with FLASH-07a. To evaluate the effects of incubation with irradiated and non-irradiated FLASH-07a on NHEK cells, the cell viability was directly estimated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) test as described by Mosmann.34
000 cells per well) and were incubated at 37 °C and 5% CO2 overnight. On the next day, cells were incubated with different concentrations of FLASH-07a (final concentrations 0/2/4/8 mM). FLASH-07a was dissolved in DMEM medium (Dulbecco's Modified Eagle Medium, PAN Biotech Inc., Aidenbach, Germany) without serum and phenol red. The photosensitizer was used as an irradiated (7 W cm−2, 10 seconds) or non-irradiated solution to see whether the decomposition compounds of FLASH-07a show a toxic effect against the keratinocytes. After incubation, the flavin solution was removed from each well, cells were washed two times to remove all PS solution and were incubated with 100 μL fresh dermal cell basal medium overnight at 37 °C and 5% CO2. Control cells were not incubated with FLASH-07a. To evaluate the effects of incubation with irradiated and non-irradiated FLASH-07a on NHEK cells, the cell viability was directly estimated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) test as described by Mosmann.34
Statistical methods
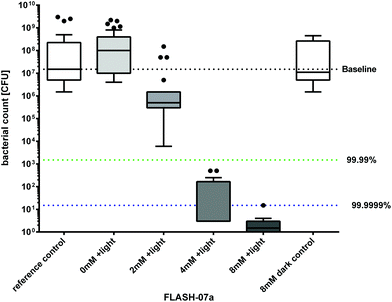
All results are shown as medians including the 25% and 75% quartiles which were calculated from the values of at least 3 independent experiments (Prism 6 for Windows, GraphPad Software Inc., San Diego, CA, USA). Each experiment was conducted in triplicate. The calculation of inactivation was done for untreated controls, which were neither incubated with flavin photosensitizers nor illuminated (reference control). In Fig. 3 medians below the red dotted horizontal line represent >99.9% efficacy of killing. In Fig. 5 medians below the green dotted line represent >99.99% killing efficacy, medians below the blue dotted line represent >99.9999% killing efficacy corresponding to at least more than 4 or 6 orders of log10 reduction compared to untreated controls, respectively. A reduction of at least 3 magnitudes of viable spores was stated as biologically relevant according to the guidelines of hand hygiene.35 The percentage of phototoxicity was calculated as follows (eqn (2)):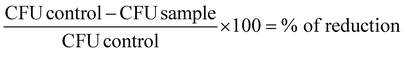 | (2) |
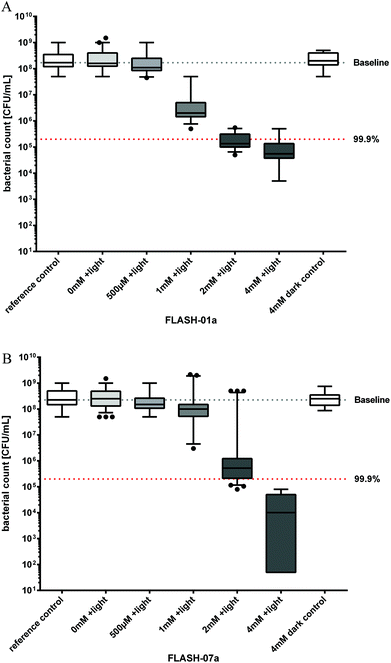 | ||
| Fig. 3 Photosensitized inactivation of B. atrophaeus spores in vitro. Survival of B. atrophaeus spores incubated with flavin derivatives FLASH-01a (Fig. 3A) or FLASH-07a (Fig. 3B) for 10 seconds in the dark followed by irradiation with 7 W cm−2 light for 10 seconds (grey boxes). Box–whisker plots represent the median including the interquartile range and the whiskers. Controls: spores alone (white box, 0 mM FLASH, reference control) or incubated with the flavin photosensitizer only (white box, 4 mM FLASH, dark control), but not irradiated. Black dots represent outliers calculated with Prism 6 for Windows (GraphPad Software Inc., San Diego, CA, USA) that were not included in the calculations. The black line within the boxes represents the median of at least three independent experiments. Values below the red dotted horizontal line represent >99.9% efficacy of spore killing which was referred to untreated controls (= Baseline). | ||
Results
Singlet oxygen generation and the photostability of flavin photosensitizers
The singlet oxygen quantum yields of the flavin photosensitizers were 0.75 ± 0.05 (FLASH-01a) and 0.78 ± 0.05 (FLASH-07a), respectively. The quantum yield was calculated by the luminescence integral of singlet oxygen generated by the excited photosensitizers with different energies (200–4000 mJ).To estimate the photostability of the flavin derivatives, 50 μL of each sample (4 mM) was irradiated for 10 seconds with an intensity of 7 W cm−2. Both photosensitizers showed a decrease of the absorption maxima (Table 1).
| FLASH-01a | Maximum 371 nm | Maximum 443 nm |
|---|---|---|
| Before irradiation | 100 | 100 |
| After irradiation | 89 | 94 |
| FLASH-07a | Maximum 371 nm | Maximum 443 nm |
|---|---|---|
| Before irradiation | 100 | 100 |
| After irradiation | 93 | 92 |
Killing of Bacillus atrophaeus endospores in vitro
A total of approximately 108Bacillus spores were incubated for 10 seconds with different concentrations of FLASH-01a and FLASH-07a, respectively. Suspensions were subsequently irradiated with 7 W cm−2 light for 10 seconds yielding a total light dose of 70 J cm−2. A FLASH-01a concentration of 2 mM resulted in a spore killing efficacy of 3.1 log10 orders (Fig. 3A). The killing effect of FLASH-01a was enhanced up to 3.5 log10 orders by increasing the concentration from 2 to 4 mM.Using FLASH-07a, a concentration of 2 mM achieved no biologically relevant spore killing (2.6 log10 orders; Fig. 3B). Again, an increase in the photosensitizer concentration from 2 to 4 mM FLASH-07a demonstrated an enhanced spore killing efficacy of 4.4 log10 orders upon light activation. Irradiation of the spores in the presence of 4 mM FLASH-01a or FLASH-07a resulted in an increasing spore reduction rate with regard to their charge numbers: 3.5 (FLASH-01a, one positive charge) < 4.4 (FLASH-07a, eight positive charges) log10 orders. Thus, an increasing number of positive charges of the flavin derivatives obviously enhanced the photodynamic spore killing efficacy when the highest flavin concentration of 4 mM was used.
All spore samples that were incubated with the highest concentrations of the flavin derivatives but without any irradiation (dark control) or irradiation only (light control) exhibited normal spore germination and the following bacterial growth, demonstrating that the maximal applied light dose of 70 J cm−2 alone as well as the photosensitizers alone (8 mM) had no toxic effects against spores and bacteria.
Phototoxicity of flavin irradiated Bacillus spores immobilized on inert surfaces
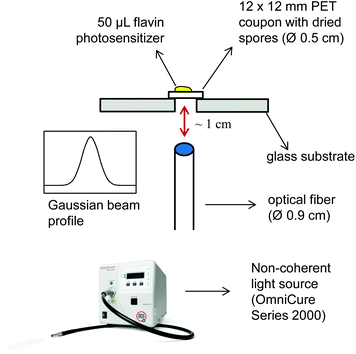
Next we investigated the ability of the new flavin photosensitizers to effectively kill spores that were dried on a polyethylene terephthalate (PET) surface. In order to incubate flavin photosensitizers with immobilized spores and to irradiate the samples, a new setup was developed to enable the reproducibility and the standardization of the experiments (Fig. 4). Dried Bacillus spores were incubated with FLASH-01a or FLASH-07a for 10 seconds, respectively. Samples were then subsequently irradiated for 10 seconds with 7 W cm−2 yielding a total light dose of 70 J cm−2.First of all, the sporicidal effect of FLASH-01a was tested with concentrations of up to 8 mM. The data indicate that Bacillus spores were effectively killed depending on the flavin concentration. There was a considerable reduction of CFU of >3 log10 orders when a FLASH-01a concentration of 4 mM was used. On changing the flavin concentration of FLASH-01a to 8 mM we could detect only a moderate increase of the spore killing efficacy (≤4 log10 orders) (data not shown).
In Germany, the VDMA (German Engineering Federation) states that a minimum of 4 log10 orders is required for disinfection of packaging substrates and/or product pipelines.36 Incubation of Bacillus spores for 10 seconds with 4 mM of FLASH-07a caused a biologically relevant decrease of 6.7 log10 orders upon irradiation with 70 J cm−2 which is in line with the VDMA statement. Incubation with 8 mM of FLASH-07a showed the highest decrease in spore survival of 7.0 log10 orders equivalent to high level disinfection.37 All spore samples that were irradiated only (= light control) or were incubated with 8 mM FLASH-07a only (= dark control) exhibited unaffected germination and bacterial growth after incubation at 37 °C compared to the untreated controls (no light, no photosensitizer = reference control) demonstrating that the maximal applied radiant exposure of 70 J cm−2 and a maximal concentration of 8 mM showed no toxic effects (Fig. 5). Additionally we could not detect any macroscopic alteration of the PET surface properties after the photodynamic treatment (data not shown).
Toxicity of FLASH-07a against normal human epidermal keratinocytes (nHEK)
Solutions of FLASH-07a with different concentrations (0/2/4/8 mM) were irradiated (7 W cm−2, 10 seconds) or non-irradiated to see whether decomposition compounds of FLASH-07a show a toxic effect against nHEKs. Thereafter 100 μL of each flavin solution (irradiated/non-irradiated) was applied to nHEK cells for 60 seconds. The results of the MTT assay showed that the nHEK cell viability decreased from 100% (no photosensitizer) to 90.5 ± 7.3% (2 mM), 90.9 ± 8.5% (4 mM) and 92.8 ± 3.8% when FLASH-07a was irradiated before incubation. When FLASH-07a was not irradiated before incubation, the cell viability decreased in a similar range as before (within the experimental accuracy): 89.7 ± 9.3% (2 mM), 92.4 ± 10.1% (4 mM) and 87.6 ± 5.2% (8 mM). The decrease of cell viability was not dependent on the flavin concentration (Table 2).Transmission electron microscopy images of flavin sensitized Bacillus spores
Images of flavin treated spores were exemplarily recorded with FLASH-07a using transmission electron microscopy (Fig. 6). Control experiments showed an unaffected structure of the coat, the outer membrane, the cortex and the core (Fig. 6A). After photodynamic treatment with an applied radiant exposure of 56 J cm−2 and a FLASH-07a concentration of 4 mM a clear disruption of the coat and the outer membrane of the spore could be observed (Fig. 6B), which might be a possibility of the flavin photosensitizer to penetrate more easily into the spore interior during irradiation. Additionally, spores showed damage of the cortex layer and the inner core. In contrast, both the light control (spores were irradiated only) and the dark control (spores were incubated with FLASH-07a only at the highest concentration of 4 mM) showed no structural changes (data not shown).Discussion
Vitamins are known as potential photosensitizers.23 Some vitamins of the B group like riboflavin (vitamin B2) effectively generate singlet oxygen (ΦΔ riboflavin = 0.54) after exposure to blue light.26 Riboflavin is important for mitochondrial energy metabolism in humans and is a precursor for two key coenzymes of the respiratory chain, flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD). Riboflavin application to humans seems to be safe because of its restricted solubility in water (up to 8 mg riboflavin per liter deionized water38) and in line with this, the decreased gastrointestinal reabsorption. Moreover, a randomized placebo-controlled study of Schoenen et al. showed that high-dose riboflavin administration (400 mg per day) to migraine patients over 3 months had almost no side effects, only 2 out of 55 patients suffered from non-serious diarrhea and polyuritis.39 For comparison, the estimated daily nutrient uptake of adult humans is about 1.1–1.3 mg riboflavin per day.40 Thus flavin-based photosensitizers should be good candidates for efficient, safe, and sustainable photosensitizers that could be used for PDI in medical applications, food industry and environment.When irradiated with appropriate light, photosensitizers transfer (i) charge to biomolecules or (ii) energy to molecular oxygen generating reactive oxygen species like hydroxyl radicals, super oxide anions or highly reactive singlet oxygen. The new flavin photosensitizers effectively generate singlet oxygen (ΦΔ FLASH-01a = 0.75; ΦΔ FLASH-07a = 0.78). In view of the high quantum yields, singlet oxygen is considered the most important species in PDI. To effectively destroy microorganisms, viruses and spores via PDI, singlet oxygen has to be generated near the cell wall, virus capsule or spore coat because of the short lifetime of a few microseconds (e.g. 3.5 μs in water41) and the small diffusion length (<1 μm).42 Despite sufficient singlet oxygen generation, riboflavin shows a poor antibacterial killing efficacy.43,44 This is because riboflavin is an uncharged molecule and cannot attach well to the negatively charged surface of microorganisms. Thus, an efficient photosensitizer should be positively charged to allow the attachment to negatively charged cell walls. For this reason we added one (FLASH-01a) or eight (FLASH-07a) positive charges to riboflavin to create our new positively charged flavin photosensitizers.
The new flavin derivatives especially the chemical core groups used here in this study for the first time are all from natural origin. It is commonly accepted that the first step of flavin degradation occurs via lumiflavin and in succession lumichrom.45 Both are omnipresent in nature and do not have toxic potential. From this point of view, many different fragments can arise upon flavin irradiation that is not specifically defined. There are also several possible points of bond breaks that are competing with each other and that can also happen simultaneously. During disintegration of the chromophore, oxidation and hydrolysis processes also take place and contribute to the formation of a variety of compounds. Due to the daily consumption of such compounds derived from food (e.g. in milk46), the toxicity of these compounds originating from flavin degradation under natural, heat or lightning conditions should be negligible.
In the present study we focused on the inactivation of bacterial endospores because they show a high intrinsic resistance to many chemical and physical stress factors. In 2001, contaminated letters with B. anthracis spores caused massive security and bioterrorism threats in the United States. The possible use of such biological weapons by terrorists is a leading cause of concern all over the world. Thus, studies of fast and effective inactivation of spores reinforce the application of PDI in military and national security.47 It is known that the surface of Bacillus spores is negatively charged when the pH value is above 4.5.48 In our experiments we used flavin–water solutions with a pH range of 5.3–6.8 ± 0.1 (FLASH-01a) and 4.4–6.5 ± 0.1 (FLASH-07a) expecting that positively charged flavin photosensitizers attach well to the negatively charged spore surface. In line with this fact, we could effectively kill B. atrophaeus endospores in vitro as well as on PET surfaces probably due to the perfect attachment of the photosensitizer to the spore surface.
Different approaches have been used to inactivate bacterial endospores. Physical49,50 or chemical51,52 approaches show several disadvantages regarding safety, energy consumption and environmental hazard. For example, Dauphin et al. used gamma irradiation for B. anthracis spore inactivation to achieve 6 log10 orders of spore killing.53 However, this approach represents a good killing efficacy against spores on inert surfaces, but needs higher safety precautions since gamma irradiation is very harmful to humans and leads to DNA damage with possible consequences like cancer. PDI-mediated killing of microorganisms and spores could be a worthwhile alternative. So far, PDI showed moderate results in spore decontamination when porphyrins or phenothiazines were used as the corresponding photosensitizers. Banerjee et al. demonstrated that 20 μg mL−1meso-tetra-(N-methyl-4-pyridyl)-porphine (TMPyP) incubated with Bacillus spores and irradiated for 30 min caused only a 2.8 log10 reduction in spore survival.21 Using toluidine blue (TBO), tri- or tetra-cationic porphyrins and an incubation time of 3 h, the irradiation time could be diminished to 1 minute reaching a 3–3.6 log10 reduction of Bacillus spores.54 An interesting result in spore killing (>5 log10 steps) was achieved by Demidova et al. when using TBO or new methylene blue N for inactivation of B. cereus endospores.14 To achieve an efficient sporicidal effect, incubation times of up to 3 hours of the photosensitizer were necessary followed by irradiation times of a minimum of 100 seconds.14 In contrast to that, the use of FLASH-07a yielded a 4.4 log10 reduction of B. atrophaeus spores in vitro within an irradiation time of 10 seconds. Only 10 seconds for incubation and only 10 seconds for irradiation with 7 W cm−2 light were necessary to achieve sporicidal effects without washing the flavin–spore solution. In view of the high light intensity, a heating effect that might contribute to the spore killing efficacy and thus micro modifications of the PET surface cannot be fully excluded. Furthermore, we did not recognize any shielding effects of the tested photosensitizers although the flavin concentration was 4 mM for in vitro experiments. This is in contrast to Demidova and colleagues. They reported that washing of a B. atrophaeus TBO mix achieved a better result in spore killing.14 These differences may be due to the different experimental setups and the different absorption of the used photosensitizers.
Alves et al. showed that an increase of the positive charge number of cationic porphyrins directly correlates with a improved killing efficacy of Gram-negative bacteria.30 They used 7 types of porphyrins with different charge numbers (1–4 positive charges) and charge distributions as well as different structures regarding the meso-substituent groups. They demonstrated that porphyrins with three or four positive charges showed the highest log10 reduction (>7 log10 orders) against Enterococcus faecalis and Escherichia coli. In our study, we used two flavin photosensitizers with different charge numbers (one, FLASH-01a; eight FLASH-07a). In line with results of Alves et al. we also observed that the increase of positive charges of the flavin molecules leads to a more effective spore killing in vitro. The reason for this phenomenon may be the number of multiple positive charges of the photosensitizer which might improve the attachment to the negatively charged spore surface.
In general, spores appear in the whole environment on inert and living surfaces with limited water resources. Spores are able to adhere to surfaces much better than their natural counterpart55 and this is a major problem not only in the medical field but also in the food and beverage industries.56 Contamination of industrial products or packaging pipelines with bacterial endospores is greatly feared because spores will germinate when the conditions will be favorable and the bacteria will then cause serious foodborne diseases. Actually, several groups used foodborne bacteria like Staphylococcus aureus, Listeria monocytogenes and Bacillus cereus as a model organism to test the photodynamic approach in vitro, in special formulations/coatings, on packaging materials or on spices and meat.57–60 The photodynamic killing efficacy differed from 2 to 7 log10 orders using chlorophyllin (porphyrin) or curcumin as the corresponding photosensitizers. Our new riboflavin derivatives are also from natural origin and we investigated the sporicidal potential of the flavin photosensitizer FLASH-07a against B. atrophaeus endospores which were dried on the PET material which is often used for food and beverage packaging. There exist several approaches to decontaminate inert surfaces from bacterial endospores. Li et al. showed a fumigation approach with chlorine dioxide gas to decontaminate different surfaces which were contaminated with B. subtilis var. niger spores (actual name: B. atrophaeus).6 The log10 reduction of viable spores greatly differed from 1.8 to 6.6 depending on the used surface (cotton cloth: 1.8; glass: 6.6). To obtain these results they treated the material coupons for 3 h in a chamber which contained approximately 0.08% chlorine dioxide gas.6 Rogers et al. demonstrated a 6–8 log10 reduction of B. subtilis spores immobilized on 7 different indoor surface materials.7 They used formaldehyde gas for spore killing with a spore-gas contact time of 10 hours. Udompijitkul et al. investigated the efficacy of common disinfectants (e.g. 70% ethanol) against Clostridium perfringens spores attached to stainless steel.61 Although they used germinated spores for their experiments, they only achieved a spore inactivation of 1.5–2.7 log10 orders. Approaches to inactivate bacterial endospores attached to surfaces showed either a good efficacy when long treatment times were used or the spore killing efficacy was not biologically relevant. In contrast, our experiments demonstrated that PDI mediated killing of dried endospores is fast and effective at the same time. We only needed a total treatment time of 20 seconds (10 second incubation, 10 second irradiation) to obtain 7 log10 orders killing efficacy against spores attached to food related surfaces.
We also tested the cell toxicity of FLASH-07a against normal human epidermal keratinocytes (nHEKs). In cell culture experiments we focused on FLASH-07a because this derivative was the most efficient photosensitizer against spores in vitro and dried on PET surfaces. In view of the high polarity (eightfold charged) and the high concentrations used in the experiments (up to 8 mM), the decrease of nHEK viability of maximal 12.4% in comparison with untreated cells was remarkably small. After incubation the FLASH-07a solution was removed and cells were washed two times with medium. This procedure immediately dilutes the high concentrations in the experiment to an extent which do not affect the keratinocytes. In addition, we recently showed that photodynamic experiments on human keratinocytes with flavin concentrations of up to 500 μM have no effect on the cell viability of nHEKS.29
Conclusions
PDI can be considered a worthwhile procedure to kill bacterial endospores either in aqueous suspension or anhydrously attached to surfaces. The use of our novel flavin photosensitizers showed a high efficacy in Bacillus atrophaeus spore inactivation within 20 seconds of total treatment time in vitro as well as on food related inert surfaces. In addition, our flavin photosensitizers are based on natural vitamin B2 and hence might offer great potential for a safe and sustainable use not only in the food and packaging industries but also in medical applications.Acknowledgements
The excellent technical assistance of Ewa Kowalewski is gratefully acknowledged. We thank Dr. Josef Schroeder and Heiko Siegmund for excellent technical assistance regarding electron microscopic studies (Institute for Pathology, University of Regensburg). Dr. Johannes Regensburger and Dr. Anita Gollmer were supported by a grant from the German Research Foundation (DFG-RE-3323/2-1; DFG-GO-2340/1-1). Andreas Späth and part of this work were supported by a grant from the Bavarian Research Foundation (AZ 952-10). No conflict of interest is declared.Notes and references
- I. Baldea and A. G. Filip, J. Physiol. Pharmacol., 2012, 63, 109–118 CAS.
- T. G. St Denis, T. Dai, L. Izikson, C. Astrakas, R. R. Anderson, M. R. Hamblin and G. P. Tegos, Virulence, 2011, 2, 509–520 CrossRef PubMed.
- K. L. Brown, Br. Med. Bull., 2000, 56, 158–171 CrossRef CAS.
- P. Setlow, J. Appl. Microbiol., 2006, 101, 514–525 CrossRef CAS PubMed.
- W. L. Nicholson, P. Fajardo-Cavazos, R. Rebeil, T. A. Slieman, P. J. Riesenman, J. F. Law and Y. Xue, Antonie Van Leeuwenhoek, 2002, 81, 27–32 CrossRef CAS.
- Y. J. Li, N. Zhu, H. Q. Jia, J. H. Wu, Y. Yi and J. C. Qi, J. Zhejiang Univ., Sci. B, 2012, 13, 254–260 CrossRef CAS PubMed.
- J. V. Rogers, Y. W. Choi, W. R. Richter, D. C. Rudnicki, D. W. Joseph, C. L. Sabourin, M. L. Taylor and J. C. Chang, J. Appl. Microbiol., 2007, 103, 1104–1112 CrossRef CAS PubMed.
- N. Sudhaus, M. C. Pina-Perez, A. Martinez and G. Klein, Foodborne Pathog. Dis., 2012, 9, 442–452 CrossRef CAS PubMed.
- F. Barbut, D. Menuet, M. Verachten and E. Girou, Infect. Control Hosp. Epidemiol., 2009, 30, 507–514 CrossRef CAS PubMed.
- G. Jori, C. Fabris, M. Soncin, S. Ferro, O. Coppellotti, D. Dei, L. Fantetti, G. Chiti and G. Roncucci, Lasers Surg. Med., 2006, 38, 468–481 CrossRef PubMed.
- M. Wainwright, J. Antimicrob. Chemother., 1998, 42, 13–28 CrossRef CAS.
- T. Maisch, Lasers Med. Sci., 2007, 22, 83–91 CrossRef PubMed.
- T. Maisch, F. Spannberger, J. Regensburger, A. Felgenträger and W. Bäumler, J. Ind. Microbiol. Biotechnol., 2012, 39, 1013–1021 CrossRef CAS PubMed.
- T. N. Demidova and M. R. Hamblin, Appl. Environ. Microbiol., 2005, 71, 6918–6925 CrossRef CAS PubMed.
- J. S. Dickson and M. Koohmaraie, Appl. Environ. Microbiol., 1989, 55, 832–836 CAS.
- L. M. He and B. M. Tebo, Appl. Environ. Microbiol., 1998, 64, 1123–1129 CAS.
- F. Cieplik, A. Späth, J. Regensburger, A. Gollmer, L. Tabenski, K. A. Hiller, W. Bäumler, T. Maisch and G. Schmalz, Free Radical Biol. Med., 2013, 65C, 477–487 CrossRef PubMed.
- A. Felgenträger, T. Maisch, D. Dobler and A. Späth, BioMed Res. Int., 2013, 2013, 482167 Search PubMed.
- A. Eichner, F. P. Gonzales, A. Felgenträger, J. Regensburger, T. Holzmann, W. Schneider-Brachert, W. Bäumler and T. Maisch, Photochem. Photobiol. Sci., 2013, 12, 135–147 CAS.
- A. Späth, C. Leibl, F. Cieplik, K. Lehner, J. Regensburger, K. A. Hiller, W. Bäumler, G. Schmalz and T. Maisch, J. Med. Chem., 2014, 57, 5157–5168 CrossRef PubMed.
- I. Banerjee, K. K. Mehta, J. S. Dordick and R. S. Kane, J. Appl. Microbiol., 2012, 113, 1461–1467 CrossRef CAS PubMed.
- R. N. da Silva, A. C. Tome, J. P. Tome, M. G. Neves, M. A. Faustino, J. A. Cavaleiro, A. Oliveira, A. Almeida and A. Cunha, Microbiol. Immunol., 2012, 56, 692–699 CrossRef CAS PubMed.
- A. Knak, J. Regensburger, T. Maisch and W. Bäumler, Photochem. Photobiol. Sci., 2014, 13(5), 820–829 CAS.
- W. Bäumler, J. Regensburger, A. Knak, A. Felgenträger and T. Maisch, Photochem. Photobiol. Sci., 2012, 11, 107–117 Search PubMed.
- J. Regensburger, A. Knak, T. Maisch, M. Landthaler and W. Bäumler, Exp. Dermatol., 2012, 21, 135–139 CrossRef CAS PubMed.
- J. Baier, T. Maisch, M. Maier, E. Engel, M. Landthaler and W. Bäumler, Biophys. J., 2006, 91, 1452–1459 CrossRef CAS PubMed.
- J. L. Sagripanti, M. Carrera, J. Insalaco, M. Ziemski, J. Rogers and R. Zandomeni, J. Appl. Microbiol., 2007, 102, 11–21 CrossRef PubMed.
- J. Regensburger, T. Maisch, A. Felgenträger, F. Santarelli and W. Bäumler, J. Biophotonics, 2010, 3, 319–327 CrossRef CAS PubMed.
- T. Maisch, A. Eichner, A. Späth, A. Gollmer, J. Regensburger, B. König and W. Bäumler, PLoS One, 2014 DOI:10.1371/journal.pone.0111792.
- E. Alves, L. Costa, C. M. B. Carvalho, J. P. C. Tome, M. A. Faustino, M. G. P. M. S. Neves, A. C. Tome, J. A. S. Cavaleiro, A. Cunha and A. Almeida, BMC Microbiol., 2009, 9, 70 CrossRef PubMed.
- R. Kuhn and F. Weygand, Ber. Dtsch. Chem. Ges. A/B, 1935, 68, 1282–1288 CrossRef.
- B. Neises and W. Steglich, Angew. Chem., Int. Ed. Engl., 1978, 17, 522–524 CrossRef.
- A. A. Miles, S. S. Misra and J. O. Irwin, J. Hygiene, 1938, 38, 732–749 CrossRef CAS.
- T. Mosmann, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
- J. M. Boyce and D. Pittet, Am. J. Infect. Control, 2002, 30, S1–46 CrossRef.
- VDMA , Hygienische Abfüllmaschinen der Klasse IV nach VDMA für flüssige und pastöse Nahrungsmittel.
- FDA , Class II Special Controls Guidance Document: Medical Washers and Medical Washer-Disinfectors. Guidance for the Medical Device Industry and FDA Review Staff.
- P. W. Morrison, C. J. Connon and V. V. Khutoryanskiy, Mol. Pharm., 2013, 10, 756–762 CrossRef CAS PubMed.
- J. Schoenen, J. Jacquy and M. Lenaerts, Neurology, 1998, 50, 466–470 CrossRef CAS.
- W. D. Lienhart, V. Gudipati and P. Macheroux, Arch. Biochem. Biophys., 2013, 535, 150–162 CrossRef CAS PubMed.
- T. Maisch, J. Baier, B. Franz, M. Maier, M. Landthaler, R. M. Szeimies and W. Bäumler, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7223–7228 CrossRef CAS PubMed.
- T. Maisch, J. Wagner, V. Papastamou, H. J. Nerl, K. A. Hiller, R. M. Szeimies and G. Schmalz, J. Appl. Microbiol., 2009, 107, 1569–1578 CrossRef CAS PubMed.
- R. T. Kashiwabuchi, Y. Khan, F. R. Carvalho, F. Hirai, M. S. Campos and P. J. McDonnell, Arq. Bras. Oftalmol., 2012, 75, 423–426 CrossRef PubMed.
- P. S. Thakuri, R. Joshi, S. Basnet, S. Pandey, S. D. Taujale and N. Mishra, Nepal Med. Coll. J., 2011, 13, 281–284 CAS.
- I. Ahmad and F. Vaid, in Comprehensive Series in Photochemistry and Photobiology, ed. D. P. Häder and G. Jori, RSC Publishing, http://pubs.rsc.org/en/content/chapter/bk9780854043316-00013/978-0-85404-331-6#!divabstract, 2006 Search PubMed.
- E. Tagliaferri, R. Sieber, U. Bütikofer, P. Eberhard and J. O. Bosset, Mitteilungen aus dem Gebiet der Lebensmittel-Untersuchung und -Hygiene, 1992, 83, 467–491 CAS.
- F. Vatansever, C. Ferraresi, M. V. de Sousa, R. Yin, A. Rineh, S. K. Sharma and M. R. Hamblin, Virulence, 2013, 4, 796–825 CrossRef PubMed.
- G. Pesce, G. Rusciano, A. Sasso, R. Isticato, T. Sirec and E. Ricca, Colloids Surf., B, 2014, 116C, 568–575 CrossRef PubMed.
- W. H. Coleman, D. Chen, Y. Q. Li, A. E. Cowan and P. Setlow, J. Bacteriol., 2007, 189, 8458–8466 CrossRef CAS PubMed.
- S. E. Fiester, S. L. Helfinstine, J. C. Redfearn, R. M. Uribe and C. J. Woolverton, Int. J. Microbiol., 2012, 2012, 579593 CAS.
- J. D. Hemmer, M. J. Drews, M. LaBerge and M. A. Matthews, J. Biomed. Mater. Res., Part B, 2007, 80, 511–518 CrossRef PubMed.
- A. Mohan, J. Dunn, M. C. Hunt and C. E. Sizer, J. Food Sci., 2009, 74, M411–M417 CrossRef CAS PubMed.
- L. A. Dauphin, B. R. Newton, M. V. Rasmussen, R. F. Meyer and M. D. Bowen, Appl. Environ. Microbiol., 2008, 74, 4427–4433 CrossRef CAS PubMed.
- A. Oliveira, A. Almeida, C. M. Carvalho, J. P. Tome, M. A. Faustino, M. G. Neves, A. C. Tome, J. A. Cavaleiro and A. Cunha, J. Appl. Microbiol., 2009, 106, 1986–1995 CrossRef CAS PubMed.
- A. Harimawan, S. Zhong, C. T. Lim and Y. P. Ting, J. Colloid Interface Sci., 2013, 405, 233–241 CrossRef CAS PubMed.
- A. Andersson, U. Ronner and P. E. Granum, Int. J. Food Microbiol., 1995, 28, 145–155 CrossRef CAS.
- G. Lopez-Carballo, P. Hernandez-Munoz, R. Gavara and M. J. Ocio, Int. J. Food Microbiol., 2008, 126, 65–70 CrossRef CAS PubMed.
- Z. Luksiene, I. Buchovec and E. Paskeviciute, J. Appl. Microbiol., 2010, 109, 1540–1548 CAS.
- Z. Luksiene and E. Paskeviciute, J. Photochem. Photobiol., B, 2011, 105, 69–74 CrossRef CAS PubMed.
- N. Tortik, A. Spaeth and K. Plaetzer, Photochem. Photobiol. Sci., 2014, 13, 1402–1409 CAS.
- P. Udompijitkul, M. Alnoman, D. Paredes-Sabjaa and M. R. Sarker, Food Microbiol., 2013, 34, 328–336 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and Owner Societies 2015 |