Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A recognition-mediated reaction drives amplification within a dynamic library†
Jan W.
Sadownik‡
and
Douglas
Philp
*
School of Chemistry and EaStCHEM, University of St Andrews, North Haugh, St Andrews, Fife KY16 9ST, UK. E-mail: d.philp@st-andrews.ac.uk; Fax: +44 (0)1334 463808; Tel: +44 (0)1334 467264
First published on 25th August 2015
Abstract
A single, appropriately designed, recognition event targets and transforms one of two reactive members of an exchanging pool of compounds through a recognition-mediated irreversible cycloaddition reaction, altering dramatically the final composition and kinetic behaviour of the dynamic library.
Introduction
Classically, dynamic covalent chemistry1 has created and exploited networks of interconverting compounds, so-called dynamic combinatorial libraries (DCLs), which operate under thermodynamic control. Within a DCL, the relative abundances of library members are therefore governed by the total free energy of the system. Recognition events that are capable of altering the free energy relationships2 within a DCL can alter the final distribution of compounds in the library and this approach has been used for development3 of synthetic receptors and sensors, the creation4 of supramolecular assemblies and in the search5 for ligands for biomacromolecules. The behaviour of complex network of interactions that exist between all of the compounds present in a DCL is amenable to simulation2d,e,6 computationally and this approach has identified emergent phenomena, such as pattern generation, which may appear spontaneously within such libraries. Such complexity, beyond the simple sum of the individual components, means that DCLs will become a critical component in the development7 of Systems Chemistry.One method of achieving selectivity8 within a DCL is the receptor-assisted combinatorial approach. In this approach, a receptor is present during the generation of the dynamic combinatorial library, and, therefore, the creation of the compounds that associate strongly with the receptor is favored. Synthesis and screening therefore occur in the same step. Alternatively, amplification can be accomplished9 using pseudo-dynamic combinatorial chemistry. After the synthesis of the library, certain members bind to the receptor present in the library. Subsequently, a degradative reaction removes the compounds that did not bind, or are bound only weakly, to the receptor rapidly. Library members that are bound strongly to the receptor are destroyed slowly. In this case, thermodynamics govern initial binding to the receptor, while the kinetics of the degradative reaction governs the overall selectivity achieved in the library.
Selection strategies for DCLs based on thermodynamic control often have limited effectiveness, since selectivity is based only on the differences in the strengths of interactions between library members. Employing kinetic effects10,11 in DCLs can improve selectivity, but this approach is currently underexploited. We have become interested in exploiting recognition-mediated reactions12 as a tool for selection and amplification of target molecules within DCLs. Previously, we described13 the application of autocatalysis, mediated by a self-replicating template, to successfully amplify a single product from an exchanging pool of reagents. In this approach, we exploited an irreversible chemical reaction in a constructive manner, using recognition to direct reaction selectively to one library member, and, hence, influence the composition of the library. The design14 of autocatalytic templates can be problematic and we wished to study a system in which a simpler recognition-mediated reaction, operating through a binary reactive complex,12 could operate in a constructive manner in order to influence the distribution of products within in a DCL. Additionally, we wished to compare the efficiency of this simpler kinetic selection strategy with more complex autocatalytic scenarios. Here, we report the design of an extremely simple exemplar system, based on a recognition-mediated reaction that operates through a reactive binary complex, that demonstrates the power of this approach whilst simultaneously permitting the detailed real-time tracking of the system-wide effects of the single recognition event used to direct the system.
Results and discussion
In order to allow direct comparison with our previous work, we employed the same dynamic library (Fig. 1), constructed from two aldehydes, one of which bears an amidopyridine recognition site, as used previously. The library contains two nucleophiles – 4-fluoroaniline and 4-fluorophenylhydroxyl amine. The aniline reacts to form imines 1 and 3 and the hydroxylamine reacts to form nitrones 2 and 4. The DCL will therefore contain all of the compounds within the area marked Exchange Pool in Fig. 1, namely the two imines, 1 and 3, and the two nitrones, 2 and 4, together with the components necessary to synthesise all of these compounds. Transfer of material from the Exchange Pool to the Product Pool occurs irreversibly through the 1,3-dipolar cycloaddition reactions of either nitrone 2 or nitrone 4 with a maleimide, either 5a or 5b. The cycloaddition reactions between 2 and 5 affords the diastereoisomeric trans- and cis-6 and the cycloaddition reaction between 2 and 5 affords the diastereoisomeric trans- and cis-7. Critically, within the Exchange Pool, only one compound, nitrone 4, bears a carboxylic acid recognition site and is reactive towards maleimides. This compound is, therefore, the target for the recognition-mediated irreversible chemical reaction that will ultimately resolve the DCL.Previously, we have demonstrated12 that it is possible to accelerate cycloaddition reactions significantly through the formation of a reactive binary complex between the 4π (diene or 1,3-dipole) and the 2π components. Hence, we needed to identify the most appropriate structure for a dipolarophile that could recognise 4 and, subsequently, participate in a 1,3-dipolar cycloaddition reaction through a recognition-mediated pathway. We used electronic structure calculations to design maleimide 5b. Our calculations indicated (Fig. 2a) that 5b was capable of binding to nitrone 4 and this complex was capable of accessing the transition state leading to cis-7b. This expectation was verified experimentally (Fig. 2b) – the reaction between 4 and 5b proceeds 125× faster that the corresponding reaction between 4 and maleimide 5a, in which recognition has been disabled. Further, the reaction between 4 and 5b is highly diastereoselective – trans-7b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis-7b = 1
cis-7b = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 – compared to the control reaction between 4 and 5a – trans-7a
35 – compared to the control reaction between 4 and 5a – trans-7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis-7a = 3
cis-7a = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (see ESI†).
1 (see ESI†).
 | ||
| Fig. 2 (a) Stick representation of the calculated transition state (B3LYP/6-31G+(d,p)) for the recognition mediated reaction between nitrone 4 and maleimide 5b to afford cis-7b. Carbon atoms are coloured green, oxygen atoms red, nitrogen atoms blue, the fluorine atom yellow-green and hydrogen atoms grey. Most hydrogen atoms are omitted for clarity. Dashed lines represent hydrogen bonds and solid lines represent the location of covalent bonds formed during the reaction. (b) Kinetic data for the reaction between nitrone 4 and maleimide 5b. Starting conditions: [4] = [5b] = 20 mM; CD2Cl2 saturated with p-toluenesulfonic acid monohydrate; 273 K. Concentrations determined by 470.4 MHz 19F NMR spectroscopy. Dark blue circles represent cis-7b and light blue circles represent trans-7b. The dashed line represents the best fit of the data to the appropriate kinetic model (see ESI†). | ||
We were now in a position to establish the effect of this rapid and selective reaction on the set of equilibria present within the Exchange Pool. However, in the first instance, it is important to establish the dynamic behaviour15 of the Exchange Pool itself and determine its equilibrium composition. A CD2Cl2 solution of imine 1 and nitrone 2 ([1] = [2] = 20 mM), saturated with p-toluene sulfonic acid monohydrate (PTSA), was incubated at 273 K. As expected, analysis of this mixture after 16 hours showed the presence of all four condensation products, 1 to 4, with some selectivity for the nitrones 2 and 4 as a result of their higher hydrolytic stability. At equilibrium, some 4-fluoroaniline and benzaldehyde can still be detected in the mixture by 1H and 19F NMR spectroscopy and the distribution of compounds 1 to 4 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 = 1.0
4 = 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4
1.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7. Reassuringly, if the equilibration process is started from imine 3 and nitrone 4 ([3] = [4] = 20 mM, CD2Cl2/sat. PTSA, 273 K, 16 h), the same mixture of compounds 1 to 4 is formed establishing this composition as the equilibrium position of this small library.
1.7. Reassuringly, if the equilibration process is started from imine 3 and nitrone 4 ([3] = [4] = 20 mM, CD2Cl2/sat. PTSA, 273 K, 16 h), the same mixture of compounds 1 to 4 is formed establishing this composition as the equilibrium position of this small library.
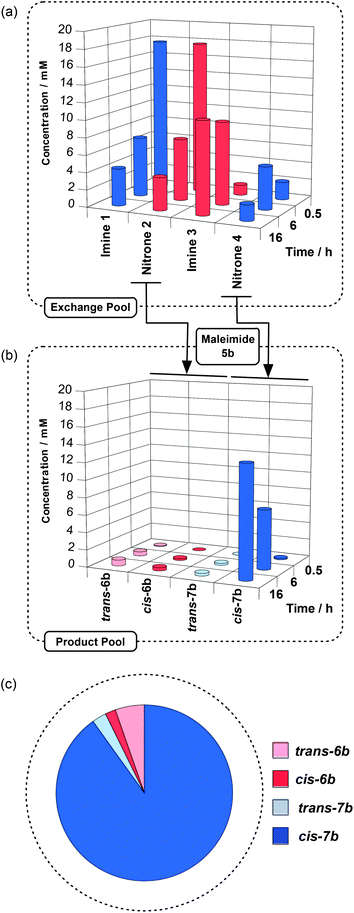
These experiments demonstrate that the mixture of imines and nitrones is, indeed, dynamic and that all four condensation products are present in similar amounts at equilibrium. We were now in a position to couple this exchange process to our recognition-mediated cycloaddition reaction. Initially, we wished to perform a control experiment to determine the effect of providing an exit route through irreversible reaction to the nitrones within the Exchange Pool. Methyl ester 5a is incapable of recognising the amidopyridines present in 1 and 4 and, hence, reactions involving 5a cannot proceed through any recognition-mediated pathway. A CD2Cl2 solution of imine 1 and nitrone 2 ([1] = [2] = 20 mM) was saturated with PTSA and maleimide 5a was added immediately as the dipolarophile ([5a] = 20 mM). The composition of this mixture was monitored by 1H and 19F NMR spectroscopy at 273 K every 30 minutes for 16 hours. These spectra were used to determine the concentrations of all of the compounds present in the mixture as a function of time (see ESI†). The results of this experiment are summarised in Fig. 3.
After 16 h, the Product Pool contains all four possible cycloadducts at relatively low concentrations. The cycloadducts formed between nitrone 2 and maleimide 5a – trans-6a and cis-6a – are present in the Product Pool at a total concentration of around 3 mM and the diastereoselectivity (trans-6a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis-6a) is 2
cis-6a) is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Similarly, the cycloadducts formed between nitrone 4 and maleimide 5a – trans-7a and cis-7a – are present at a lower concentration – 1.4 mM. Once again the diastereoselectivity is very modest (trans-7a
1. Similarly, the cycloadducts formed between nitrone 4 and maleimide 5a – trans-7a and cis-7a – are present at a lower concentration – 1.4 mM. Once again the diastereoselectivity is very modest (trans-7a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis-7a = 2.5
cis-7a = 2.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). After 16 h, all cycloaddition reactions within the library have converted only 22% of maleimide 5a. Compounds 1 to 4 are present (Fig. 3a) in similar amounts ([1] = 8.1 mM, [2] = 7.1 mM, [3] = 7.6 mM, [4] = 8.5 mM). The composition of the Exchange Pool after 16 h is similar (1
1). After 16 h, all cycloaddition reactions within the library have converted only 22% of maleimide 5a. Compounds 1 to 4 are present (Fig. 3a) in similar amounts ([1] = 8.1 mM, [2] = 7.1 mM, [3] = 7.6 mM, [4] = 8.5 mM). The composition of the Exchange Pool after 16 h is similar (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 = 1.1
4 = 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0
1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) to that observed at equilibrium when maleimide 5a is absent from the reaction mixture. The main compositional difference is the depletion of nitrones 2 and 4 as a consequence of their reaction with maleimide 5a. Since the rates of the cycloaddition reactions between 2 or 4 and 5a are significantly slower than the rates of the exchange processes, these irreversible reactions have little influence on the Exchange Pool and, since the diastereoselectivity of both cycloaddition reactions is poor, all four cycloadducts are present in the Product Pool (Fig. 3b and c). It is worth noting that, although nitrone 4 is not present in the Exchange Pool at the start of the experiment, after 16 hours, the ratio of cycloadducts arising from nitrone 2 (trans-6a and cis-6a) to those arising from nitrone 4 (trans-7a and cis-7a) is 2
1.2) to that observed at equilibrium when maleimide 5a is absent from the reaction mixture. The main compositional difference is the depletion of nitrones 2 and 4 as a consequence of their reaction with maleimide 5a. Since the rates of the cycloaddition reactions between 2 or 4 and 5a are significantly slower than the rates of the exchange processes, these irreversible reactions have little influence on the Exchange Pool and, since the diastereoselectivity of both cycloaddition reactions is poor, all four cycloadducts are present in the Product Pool (Fig. 3b and c). It is worth noting that, although nitrone 4 is not present in the Exchange Pool at the start of the experiment, after 16 hours, the ratio of cycloadducts arising from nitrone 2 (trans-6a and cis-6a) to those arising from nitrone 4 (trans-7a and cis-7a) is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. It is clear from these results that coupling exchange to the irreversible cycloaddition reaction does not generate significant chemo- or diastereoselectivity, beyond that inherent in the cycloaddition reaction, in either the Exchange Pool or in the Product Pool.
1. It is clear from these results that coupling exchange to the irreversible cycloaddition reaction does not generate significant chemo- or diastereoselectivity, beyond that inherent in the cycloaddition reaction, in either the Exchange Pool or in the Product Pool.
Next, we examined the effect that the recognition process has on the cycloaddition reactions within the context of the exchanging DCL. We anticipated that maleimide 5b, which possesses a carboxylic acid recognition site complementary with the amidopyridine recognition site present in nitrone 4, could exploit the >100-fold acceleration engendered by the recognition-mediated reaction process to drive the exchange process between the nitrones and imines. Therefore, we prepared a solution of compounds 1, 2 and 5b, saturated with PTSA ([1] = [2] = [5b] = 20 mM), and the composition of this mixture was monitored by 1H and 19F NMR spectroscopy at 273 K every 30 minutes for 16 hours. These spectra were used to determine the concentrations of all of the compounds present in the solution as a function of time (see ESI†). The results of this experiment are summarised in Fig. 4.
After 16 h, the Product Pool contains both trans-6b and cis-6b – the products of reaction between nitrone 2 and maleimide 5b – at a total concentration of 1.1 mM and exhibiting the same modest diastereoselectivity as the experiment with maleimide 5a (trans-6b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cis-6b = 2
cis-6b = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Although, as expected, the diastereoselectivity is the same as in the control experiment involving malieimide 5a, the overall concentration of products has decreased significantly (3 mM→1.1 mM). By contrast, trans-7b and cis-7b – the products of reaction between nitrone 4 and maleimide 5b – are present (Fig. 4b) at a total concentration of 13.3 mM ([trans-7b]
1). Although, as expected, the diastereoselectivity is the same as in the control experiment involving malieimide 5a, the overall concentration of products has decreased significantly (3 mM→1.1 mM). By contrast, trans-7b and cis-7b – the products of reaction between nitrone 4 and maleimide 5b – are present (Fig. 4b) at a total concentration of 13.3 mM ([trans-7b]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [cis-7b] = 1
[cis-7b] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43). There has been a dramatic increase in the overall conversion. Cycloadduct cis-7b now constitutes more than 90% of the total cycloadduct present in the Product Pool (Fig. 4c) and the conversion of maleimide 5b is 72%. The effects of the introduction of the recognition process into the system are equally significant (Fig. 4a) in the Exchange Pool. After 16 h, the relative concentrations of compounds 1 to 4 present in the Exchange Pool are significantly different compared to the same exchange process in the presence of the control maleimide 5a (Fig. 3). Given the large increase in overall conversion to cycloadducts, it is unsurprising that the concentrations of both of the nitrones, 2 and 4, are depressed significantly ([2] = 3.8 mM, [4] = 1.9 mM). Whilst the rate of the cycloaddition reaction between 2 and 5b is still slower than the rate of exchange, the cycloaddition reaction between nitrone 4 and 5b is comparable in rate to the exchange processes. Thus, once the exchange processes generates a concentration of nitrone 4 that is close to the Kd (∼3 mM) of the [4·5b] complex, rapid reaction to form cis-7b occurs through this binary complex, thereby removing nitrone 4 from the Exchange Pool. This rapid depletion of one component of the Exchange Pool drives the equilibration of compounds 1–4 to regenerate the depleted species. Since 4-fluorophenyl hydroxylamine is required to form both 2 and 4, the concentration of 2 is suppressed indirectly by the consumption of nitrone 4 in the recognition-mediated reaction. The results presented in Fig. 4 establish unambiguously the irreversible recognition-mediated cycloaddition reaction, when coupled to exchange, generates significant selectivity in both the Exchange Pool and in the Product Pool.
43). There has been a dramatic increase in the overall conversion. Cycloadduct cis-7b now constitutes more than 90% of the total cycloadduct present in the Product Pool (Fig. 4c) and the conversion of maleimide 5b is 72%. The effects of the introduction of the recognition process into the system are equally significant (Fig. 4a) in the Exchange Pool. After 16 h, the relative concentrations of compounds 1 to 4 present in the Exchange Pool are significantly different compared to the same exchange process in the presence of the control maleimide 5a (Fig. 3). Given the large increase in overall conversion to cycloadducts, it is unsurprising that the concentrations of both of the nitrones, 2 and 4, are depressed significantly ([2] = 3.8 mM, [4] = 1.9 mM). Whilst the rate of the cycloaddition reaction between 2 and 5b is still slower than the rate of exchange, the cycloaddition reaction between nitrone 4 and 5b is comparable in rate to the exchange processes. Thus, once the exchange processes generates a concentration of nitrone 4 that is close to the Kd (∼3 mM) of the [4·5b] complex, rapid reaction to form cis-7b occurs through this binary complex, thereby removing nitrone 4 from the Exchange Pool. This rapid depletion of one component of the Exchange Pool drives the equilibration of compounds 1–4 to regenerate the depleted species. Since 4-fluorophenyl hydroxylamine is required to form both 2 and 4, the concentration of 2 is suppressed indirectly by the consumption of nitrone 4 in the recognition-mediated reaction. The results presented in Fig. 4 establish unambiguously the irreversible recognition-mediated cycloaddition reaction, when coupled to exchange, generates significant selectivity in both the Exchange Pool and in the Product Pool.
These comparisons give us insight into the endpoints of these processes. However, during these experiments, concentration-time data was acquired at 30 min resolution. Therefore, we are in a position to examine in detail the rates of the processes that take place in both the Exchange Pool and the Product Pool during these experiments. Therefore, we analysed the time course NMR data collected during these experiments and extracted the rates of reaction (d[X]/dt) for each species in the system by fitting polynomial expressions to the concentration-time data and generating the rate-time data for each species by differentiation of these best-fit polynomials.
For the recognition-inactive experiment, i.e. that performed in the presence of maleimide 5a, the results are shown in Fig. 5. In this case, the cycloaddition reactions are effectively decoupled from the exchange processes – no cycloaddition has a maximum rate greater than 0.08 μM s−1 (Fig. 5, right side). It is clear that any selectivity for cis- and trans-6a in the Product Pool is only generated as a result of the initial presence of nitrone 2 in the reaction mixture. The formation of cis- and trans-7a in the Product Pool relies on the formation of nitrone 4 through exchange. Consequently, the rate maximum for the formation of cis- and trans-7a occurs after around 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s and the peak rate for the formation of cis- and trans-7a in the Product Pool (0.03 μM s−1) is lower than that for the formation of cis- and trans-6a because the concentration of nitrone 4 never reaches 20 mM – the starting concentration of nitrone 2.
000 s and the peak rate for the formation of cis- and trans-7a in the Product Pool (0.03 μM s−1) is lower than that for the formation of cis- and trans-6a because the concentration of nitrone 4 never reaches 20 mM – the starting concentration of nitrone 2.
By contrast, the exchange processes that interconvert compounds 1 through 4 have maximum rates of around ±0.40 μM s−1 (Fig. 5, left side). The rate-time profiles for compounds 1 through 4 are essentially mirror images of each other indicating that the progress of the exchange process towards equilibrium is unperturbed by the very slow bimolecular transformations of the two nitrones to the corresponding cycloadducts.
The results of the same analysis for the recognition-active experiment, i.e. that performed in the presence of maleimide 5b, are shown in Fig. 6. In this case, the exchange reactions are now being driven at significantly faster rates by the rapid recognition-mediated cycloaddition between 4 and 5b. This cycloaddition requires nitrone 4, however, at the start of the process the concentration of this compound is zero. Nitrone 4 is formed by exchange (Fig. 6, bottom left, solid line), but as soon as it forms, it interacts with maleimide 5b, forming the reactive binary complex [4·5b].
Cycloadduct cis-7b is formed rapidly (Fig. 6, bottom right, dashed line) within this complex, removing nitrone 4 from the Exchange Pool. The effect of this rapid removal of 4 from the Exchange Pool can be seen clearly in the rate-time profiles for the exchange processes that interconvert compounds 1 to 4. These processes now have maximum rates of around ±1 μM s−1 (Fig. 6, left side) – more than twice as fast as in the case of the recognition-inactive experiment (Fig. 5, left side). The accelerated formation of cis-7b also distorts the shapes of the rate-time profiles in the Exchange Pool – in particular, that of the nitrone 4 (Fig. 6, bottom left). Until 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, the concentration of nitrone 4 is increasing (rate > 0), however, its maximum rate of formation (+0.9 μM s−1) is passed after only 5000 s. After 10
000 s, the concentration of nitrone 4 is increasing (rate > 0), however, its maximum rate of formation (+0.9 μM s−1) is passed after only 5000 s. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 s, the concentration of nitrone 4 is always falling (rate < 0) – this point corresponds to the rate maximum (+0.45 μM s−1) for the formation of cis-7b. After this point, the rate of reaction of 4 with 5b, mediated by the reactive binary complex [4·5b] is limited by the availability of 4, hence, exchange has become rate limiting.
000 s, the concentration of nitrone 4 is always falling (rate < 0) – this point corresponds to the rate maximum (+0.45 μM s−1) for the formation of cis-7b. After this point, the rate of reaction of 4 with 5b, mediated by the reactive binary complex [4·5b] is limited by the availability of 4, hence, exchange has become rate limiting.
In order to understand the broader effect of this single, recognition-mediated reaction process within the pool of exchanging species, we can make an overall comparison (Fig. 7) between the compositions of the exchange and Product Pools after 16 h in the absence and in the presence of recognition. This data can in turn be compared with our data13 obtained previously when exploiting an autocatalytic reaction in order to resolve the library.
 | ||
| Fig. 7 Calculated enhancement factors (EFs) for each member of the exchange and Product Pools after 16 h in the experiment where recognition is active (data shown in Fig. 4). | ||
For the purposes of comparison, we define the enhancement factor, EF, for a given species as being log10 of the ratio of the concentration of that species after 16 h in the experiment where recognition is active (data in Fig. 4) to that of the experiment where recognition is inactive (data in Fig. 3). Within the Exchange Pool (Fig. 7), only imine 3 is enhanced (EF = +0.15). This small enhancement arises because neither of the compounds required to form imine 3 – 4-fluoroaniline and benzaldehyde – are necessary for the recognition-mediated reaction. By contrast, imine 1 and nitrones 2 and 4 are all suppressed (EF(1) = −0.27; EF(2) = −0.27; EF(4) = −0.66), since all three compounds contain at least one component required for the recognition-mediated reaction. Nitrone 4 is suppressed particularly strongly as it is, of course, consumed in the reaction that forms cis- and trans-7b. In the reaction pool, the formation of one cycloadduct, cis-7b, is enhanced very strongly (Fig. 7) and the other three cycloadducts are all suppressed. The enhancement factor for cycloadduct cis-7b is +1.51 (an increase in concentration of more than 30×) and those of the other three cycloadducts, trans-6b, cis-6b and trans-7b, are −0.34, −0.37 and −0.30 respectively. These changes generate selectivity for cis-7b of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 over the next most abundant cycloadduct in the Product Pool, namely trans-6b.
1 over the next most abundant cycloadduct in the Product Pool, namely trans-6b.
At a molecular level, the difference in the system whose behaviour is represented in Fig. 5 and that presented in Fig. 6 is the presence of a single recognition event driven by the formation of two hydrogen bonds. The influence of this recognition event extends well beyond its immediate molecular environment. The formation of the two hydrogen bonds results directly in the acceleration in the rate of formation of cis-7b through the complex [4·5b]. Since the starting concentration of nitrone 4 is zero, this fact is, in itself, irrelevant. Nitrone 4 can only be formed through the exchange processes that involve compounds 1 through 4 and it is the indirect effect that the formation of complex [4·5b] and, in turn, cis-7b that is perhaps most striking. By accelerating strongly the irreversible reaction between 4 and 5b, the exchange reactions themselves are also increased in rate by a factor of two and the composition of the Exchange Pool is directed strongly towards the formation of nitrone 4.
It is also instructive to compare the performance of library resolution using the method reported here with the autocatalytic system we reported13 previously. The two systems are very closely related structurally – the only difference being the location of the acid recognition site on the maleimide. In this work, the acid recognition site in 5b is meta with respect to the maleimide ring, whereas in our autocatalytic system,13 the acid recognition site is para to the maleimide ring. Comparing the EF values for the cycloadduct product targeted specifically in each case by the recognition-mediated reaction, we find that, for the autocatalytic system, the EF is +0.89, compared to +1.51 for cis-7b in this work. That is, the autocatalytic system is more than four times less efficient at selecting from the library, despite the fact that the recognition-mediated reaction itself is more efficient (effective molarity = 11 M for the autocatalytic system as compared to 2.4 M for the binary complex [4·5b] reported here). This poorer efficiency can be readily understood when one considers that the maximal rate of an autocatalytic reaction is not at t = 0, whereas binary complex reactions, such as the one involving [4·5b], reach maximal rate as soon as the binary complex is formed. Therefore, the temporal profiles of these two methods with respect to resolution of the DCL are complementary.
Conclusions
The work presented here demonstrates that the fate of an exchanging pool of compounds can be influenced profoundly by a single, appropriately designed, recognition event that operates on a single chemical reaction. In the system presented here, one of two reactive members of an exchanging pool of compounds can be targeted and transformed rapidly by a recognition-mediated irreversible cycloaddition reaction, altering dramatically the final composition and kinetic behaviour of the entire system. We have been able to demonstrate, through close to real time monitoring that the fate of the library is determined not only by the direct acceleration of the cycloaddition reaction, but also by the effect that this acceleration has on the rest of the system. Comparison with data from resolution of the same library using an autocatalytic process reveals that the different kinetic profile of an autocatalytic reaction leads to significantly poorer resolution of the library. These results indicate that the construction of more complex systems that integrate both minimal7b,16 and reciprocal17 replication within the type of framework presented here will require careful design to achieve optimum library resolution allowing the generation of systems that can express more complex18 behaviour, e.g. act as primitive models of metabolism through template-directed synthesis of specific products in response to chemical inputs. These strategies are currently under development in our laboratory.Acknowledgements
We thank EaStCHEM (Graduate Studentship to JWS) and the University of St Andrews for financial support.Notes and references
- (a) J.-M. Lehn, Chem. – Eur. J., 1999, 5, 2455–2463 CrossRef CAS; (b) S. J. Cantrill, G. R. L. Cousins, J. K. M. Sanders and J. F. Stoddart, Angew. Chem., Int. Ed., 2002, 41, 898–952 CrossRef; (c) K. Severin, Chem. – Eur. J., 2004, 10, 2565–2580 CrossRef CAS PubMed; (d) R. T. S. Lam, A. Belenguer, S. L. Roberts, C. Naumann, T. Jarrosson, S. Otto and J. K. M. Sanders, Science, 2005, 308, 667–669 CrossRef CAS PubMed; (e) P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J. L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652–3711 CrossRef CAS PubMed; (f) J. R. Nitschke, Acc. Chem. Res., 2007, 40, 103–112 CrossRef CAS PubMed; S. Ladame, Org. Biomol. Chem., 2008, 6, 219–226 Search PubMed.
- (a) R. F. Ludlow and S. Otto, J. Am. Chem. Soc., 2010, 132, 5984–5986 CrossRef CAS PubMed; (b) P. T. Corbett, J. K. M. Sanders and S. Otto, J. Am. Chem. Soc., 2005, 127, 9390–9392 CrossRef CAS PubMed; (c) K. Severin, Chem. – Eur. J., 2004, 10, 2565–2580 CrossRef CAS PubMed; (d) P. T. Corbett, S. Otto and J. K. M. Sanders, Org. Lett., 2004, 11, 1825–1827 CrossRef PubMed; (e) P. T. Corbett, S. Otto and J. K. M. Sanders, Chem. – Eur. J., 2004, 10, 3139–3143 CrossRef CAS PubMed.
- (a) S. Otto and K. Severin, Top. Curr. Chem., 2007, 277, 267–288 CrossRef CAS; (b) F. Bulos, S. L. Roberts, R. L. E. Furlan and J. K. M. Sanders, Chem. Commun., 2007, 3092–3093 RSC; (c) T. Haino, H. Mitsuhashi, Y. Ishizu and Y. Fukazawa, Tetrahedron Lett., 2006, 47, 7915–7918 CrossRef CAS PubMed; (d) A. Buryak and K. Severin, J. Comb. Chem., 2006, 8, 540–543 CrossRef CAS PubMed; (e) P. T. Corbett, J. K. M. Sanders and S. Otto, J. Am. Chem. Soc., 2005, 127, 9390–9392 CrossRef CAS PubMed; (f) S. M. Voshell, S. J. Lee and M. R. Gagne, J. Am. Chem. Soc., 2006, 128, 12422–12423 CrossRef CAS PubMed; (g) G. Gasparini, F. Rastrelli and L. J. Prins, Org. Biomol. Chem., 2013, 11, 6580–6587 RSC; (h) G. Gasparini, M. Dal Molin and L. J. Prins, Eur. J. Org. Chem., 2010, 2429–2440 CrossRef CAS PubMed; (i) M. Eisenberg, I. Shumacher, R. Cohen-Luria and G. Ashkenasy, Bioorg. Med. Chem., 2013, 21, 3450–3457 CrossRef CAS PubMed.
- (a) J.-M. Lehn, Chem. Soc. Rev., 2007, 36, 151–160 RSC; (b) R. F. Ludlow, J. Liu, H. Li, S. L. Roberts, J. K. M. Sanders and S. Otto, Angew. Chem., Int. Ed., 2007, 46, 5762–5764 CrossRef CAS PubMed; (c) P. C. Haussmann, S. I. Khan and J. F. Stoddart, J. Org. Chem., 2007, 72, 6708–6713 CrossRef CAS PubMed; (d) E. Stulz, S. M. Scott, A. D. Bond, S. J. Teat and J. K. M. Sanders, Chem. – Eur. J., 2003, 9, 6039–6048 CrossRef CAS PubMed; (e) J. M. C. A. Kerckhoffs, M. A. Mateos-Timoneda, D. N. Reinhoudt and M. Crego-Calama, Chem. – Eur. J., 2007, 13, 2377–2385 CrossRef CAS PubMed; (f) R. J. Sarma, S. Otto and J. R. Nitschke, Chem. – Eur. J., 2007, 13, 9542–9546 CrossRef CAS PubMed; (g) Y. R. Hristova, M. M. J. Smulders, J. K. Clegg, B. Breiner and J. R. Nitschke, Chem. Sci., 2011, 2, 638–641 RSC.
- (a) B. Shi, R. Stevenson, D. J. Campopiano and M. F. Greaney, J. Am. Chem. Soc., 2006, 128, 8459–8467 CrossRef CAS PubMed; (b) L. Milanesi, C. A. Hunter, S. E. Sedelinkova and J. P. Waltho, Chem. – Eur. J., 2006, 12, 1081–1087 CrossRef CAS PubMed; (c) O. Ramstrom, S. Lohmann, T. Bunyapaiboonsri and J.-M. Lehn, Chem. – Eur. J., 2004, 10, 1711–1715 CrossRef PubMed; (d) M. Hochgürtel, R. Biesinger, H. Kroth, D. Piecha, M. W. Hofmann, S. Krause, O. Schaaf, C. Nicolau and A. V. Eliseev, J. Med. Chem., 2003, 46, 356–358 CrossRef PubMed.
- (a) P. T. Corbett, J. K. M. Sanders and S. Otto, Angew. Chem., Int. Ed., 2007, 46, 8858–8861 CrossRef CAS PubMed; (b) R. D. Clark, J. Kar, L. Akella and F. Soltanshahi, J. Chem. Inf. Comput. Sci., 2003, 43, 829–836 CrossRef CAS PubMed.
- (a) E. Mattia and S. Otto, Nat. Nanotechnol., 2015, 10, 111–119 CrossRef CAS PubMed; (b) R. F. Ludlow and S. Otto, Chem. Soc. Rev., 2008, 37, 101–108 RSC; (c) M. Kindermann, I. Stahl, M. Reimold, W. M. Pankau and G. von Kiedrowski, Angew. Chem., Int. Ed., 2005, 44, 6750–6755 CrossRef CAS PubMed; (d) J. Stankiewicz and L. H. Eckardt, Angew. Chem., Int. Ed., 2006, 45, 342–344 CrossRef CAS PubMed.
- (a) J. D. Cheeseman, A. D. Corbett, J. L. Gleason and R. J. Kazlauskas, Chem. – Eur. J., 2005, 11, 1708–1716 CrossRef CAS PubMed; (b) A. V. Eliseev and M. I. Nelen, J. Am. Chem. Soc., 1997, 119, 1147–1148 CrossRef CAS; (c) Y. Kubota, S. Sakamoto, K. Yamaguchi and M. Fujita, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4854–4856 CrossRef CAS PubMed.
- (a) J. D. Cheeseman, A. D. Corbett, R. Shu, J. Croteau, J. L. Gleason and R. J. Kazlauskas, J. Am. Chem. Soc., 2002, 124, 5692–5701 CrossRef CAS PubMed; (b) A. D. Corbett and J. L. Gleason, Tetrahedron Lett., 2002, 43, 1369–1372 CrossRef CAS; (c) A. D. Corbett, J. D. Cheeseman, R. J. Kazlauskas and J. L. Gleason, Angew. Chem., Int. Ed., 2004, 43, 2432–2436 CrossRef CAS PubMed.
- (a) Y. Zhang and O. Ramström, Chem. – Eur. J., 2014, 20, 3288–3291 CrossRef CAS PubMed; (b) L. Hu and O. Ramström, Chem. Commun., 2014, 50, 3792–3794 RSC; (c) Y. Zhang, L. Hu and O. Ramström, Chem. Commun., 2013, 49, 1805–1807 RSC; (d) P. Vongvilai and O. Ramström, J. Am. Chem. Soc., 2009, 131, 14419–14425 CrossRef CAS PubMed; (e) M. Angelin, P. Vongvilai, A. Fischer and O. Ramström, Chem. Commun., 2008, 768–770 RSC; (f) P. Vongvilai, R. Larsson and O. Ramström, Adv. Synth. Catal., 2008, 350, 448–452 CrossRef CAS PubMed; (g) P. Vongvialai, M. Angelin, R. Larsson and O. Ramström, Angew. Chem., Int. Ed., 2007, 46, 948–950 CrossRef PubMed; (h) W. L. Noorduin, T. Izumi, A. Millemaggi, M. Leeman, H. Meekes, W. J. P. Van Enckevort, R. M. Kellogg, B. Kaptein, E. Vlieg and D. G. Blackmond, J. Am. Chem. Soc., 2008, 130, 1158–1159 CrossRef CAS PubMed.
- (a) M. Sakulsombat, Y. Zhang and O. Ramström, Top. Curr. Chem., 2012, 322, 55–86 CrossRef CAS; (b) V. del Amo and D. Philp, Chem. – Eur. J., 2010, 13304–13318 CrossRef CAS PubMed.
- (a) R. Bennes and D. Philp, Org. Lett., 2006, 8, 3651–3654 CrossRef CAS PubMed; (b) E. Kassianidis, R. J. Pearson and D. Philp, Chem. – Eur. J., 2006, 12, 8798–8812 CrossRef PubMed; (c) R. J. Pearson, E. Kassianidis, A. M. Z. Slawin and D. Philp, Chem. – Eur. J., 2006, 12, 6829–6840 CrossRef CAS PubMed; (d) S. M. Turega and D. Philp, Chem. Commun., 2006, 3684–3686 RSC; (e) E. Kassianidis, R. J. Pearson and D. Philp, Org. Lett., 2005, 7, 3833–3836 CrossRef CAS PubMed; (f) R. J. Pearson, E. Kassianidis and D. Philp, Tetrahedron Lett., 2004, 45, 4777–4780 CrossRef CAS PubMed.
- J. W. Sadownik and D. Philp, Angew. Chem., Int. Ed., 2008, 47, 9965–9970 CrossRef CAS PubMed.
- (a) A. Vidonne and D. Philp, Eur. J. Org. Chem., 2009, 593–610 CrossRef CAS PubMed; (b) V. Patzke and G. von Kiedrowski, ARKIVOC, 2007, 322, 293–310 Search PubMed.
- S. M. Turega, C. Lorenz, J. W. Sadownik and D. Philp, Chem. Commun., 2008, 4076–4078 RSC ; (a) B. Wang and I. O. Sutherland, Chem. Commun., 1997, 1495–1496 RSC; (b) V. C. Allen, D. Philp and N. Spencer, Org. Lett., 2001, 3, 777 CrossRef CAS PubMed; (c) E. Kassianidis and D. Philp, Angew. Chem., Int. Ed., 2006, 45, 6334–6348 CrossRef PubMed.
- E. Kassianidis and D. Philp, Chem. Commun., 2006, 4072–4074 RSC.
- (a) S. Xu and N. Giuseppone, J. Am. Chem. Soc., 2008, 130, 1826–1827 CrossRef CAS PubMed; (b) V. del Amo and D. Philp, Org. Lett., 2008, 10, 4589–4592 CrossRef CAS PubMed.
- (a) M. Colomb-Delsuc, E. Mattia, J. W. Sadownik and S. Otto, Nat. Commun., 2015, 6, 7427 CrossRef CAS PubMed; (b) P. Nowak, V. Saggiomo, F. Salehian, M. Colomb-Delsuc, Y. Han and S. Otto, Angew. Chem., Int. Ed., 2015, 54, 4192–4197 CrossRef CAS PubMed; (c) J. Li, P. Nowak and S. Otto, Angew. Chem., Int. Ed., 2015, 54, 833–837 CrossRef CAS PubMed; (d) J. Li, I. Cvrtila, M. Colomb-Delsuc, E. Otten and S. Otto, Chem. – Eur. J., 2014, 20, 15709–15714 CrossRef CAS PubMed; (e) M. A. Carnall, C. A. Waudby, A. M. Belenguer, M. C. A. Stuart, J. J.-P. Peyralans and S. Otto, Science, 2010, 327, 1502–1506 CrossRef PubMed; (f) E. Kassianidis, R. J. Pearson, E. A. Wood and D. Philp, Faraday Discuss., 2010, 145, 235–254 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of kinetic experiments, computational methods and characterisation data for all compounds. See DOI: 10.1039/c5ob01621e |
| ‡ Current address: University of Groningen, Centre for Systems Chemistry, Stratingh Institute for Chemistry, Nijenborgh 4, 9747 AG Groningen, The Netherlands. |
| This journal is © The Royal Society of Chemistry 2015 |