Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
IBX-mediated oxidation of unactivated cyclic amines: application in highly diastereoselective oxidative Ugi-type and aza-Friedel–Crafts reactions†
C.
de Graaff
,
L.
Bensch
,
Matthijs J.
van Lint
,
E.
Ruijter
and
R. V. A.
Orru
*
Department of Chemistry & Pharmaceutical Sciences and Amsterdam Institute for Molecules Medicines and Systems (AIMMS), VU University Amsterdam, De Boelelaan 1083, 1081 HV Amsterdam, The Netherlands. E-mail: r.v.a.orru@vu.nl
First published on 8th September 2015
Abstract
The first o-iodoxybenzoic acid (IBX) mediated oxidation of unactivated amines to imines is described. A range of meso-pyrrolidines were shown to be suitable substrates. The chemical space was further explored with one-pot oxidative Ugi-type and aza-Friedel–Crafts reactions, which proved to be highly diastereoselective.
The chemistry of hypervalent iodine reagents (Fig. 1) has received major interest in recent years as a result of the increasing number of new reagents and their application in diverse chemical transformations.1 In particular, o-iodoxy-benzoic acid (IBX)2 has experienced increasing attention owing to its broad applicability and high chemoselectivity. Santagostino et al. developed a convenient and cost-efficient synthesis of high purity IBX by the treatment of 2-iodobenzoic acid with oxone in aqueous medium,3 which renders IBX an easily accessible reagent. Chemical transformations mediated by IBX include the selective oxidation of primary and secondary alcohols to the corresponding aldehydes and ketones as well as dehydrogenation of aldehydes and ketones to α,β-unsaturated carbonyl compounds. Nicolaou et al. described the oxidation of benzylic amines as well as amines that aromatize upon oxidation,4,5 however, the oxidation of unactivated aliphatic amines with IBX was not investigated.6
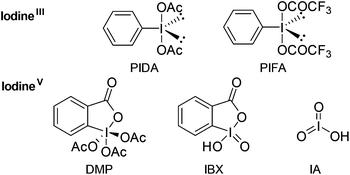 | ||
| Fig. 1 A selection of commonly used and commercially available hypervalent iodine reagents and their acronyms. | ||
The direct α-functionalization of amines is a highly interesting transformation that attracted great attention in recent years.7 Consequently, transition metal-catalyzed oxidative versions of important C–C bond-forming reactions such as Mannich, Strecker, aza-Henry and aza-Friedel–Crafts reactions have been developed.8 A limited number of examples of the application of IBX in oxidative multicomponent reactions has been described, i.e. oxidative Strecker,9 Passerini,10 Ugi and Ugi-type11 reactions. Although unactivated alcohols were suitable substrates, the scope of the amine component is basically unexplored with the exception of a small selection of α-activated amines, such as benzylic amines, α-amino nitriles and α-amino esters.
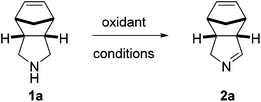
Herein, we report a mild and selective oxidation of unactivated cyclic amines with IBX to access the corresponding imines. The oxidation can be combined in one pot with the addition of C-nucleophiles, providing oxidative Ugi-type and aza-Friedel–Crafts reactions.
In light of our continued interest in the functionalization of imines, in particular 1-pyrrolines,12 we envisioned a clean and fast oxidation of unactivated meso-pyrrolidines. Examination of the currently available methods revealed that the oxidation of unactivated secondary amines is rather challenging.13 Given the recent regained interest in hypervalent iodine reagents, we decided to explore their ability to oxidize aliphatic amines. We started our investigation with the oxidation of meso-pyrrolidine 1a with different commercially available hypervalent iodine reagents at room temperature in the typically used solvent DMSO (Table 1, entries 1–4).4 The desired 1-pyrroline (2a) was obtained in high yield (Table 1, entry 4) with only one equivalent of IBX, while oxidants such as (diacetoxy)iodobenzene (PIDA), [bis(trifluoroacetoxy)]iodobenzene (PIFA) and Dess-Martin periodinane (DMP) gave poor to moderate yields (Table 1, entries 1–3). A solvent screen revealed that the oxidation proceeds well in a range of protic as well as aprotic solvents in which IBX is virtually insoluble (see ESI†), although an increased temperature and reaction time were required (60 °C, 1 h). Superior results were obtained using CH2Cl2 (Table 1, entry 5),14 which prompted us to select this solvent for a screening of the substrate scope.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Oxidant | Conditions | Yieldb (%) |
|---|---|---|---|
| a Conditions: amine (0.25 mmol), oxidant (1 eq.), solvent (0.2 M). b Yield determined by adding 2,5-dimethylfuran (0.5 eq.) after the reaction workup as standard for NMR spectroscopy. c Closed vessel. | |||
| 1 | PIDA | DMSO, RT, 30 min | 38 |
| 2 | PIFA | DMSO, RT, 30 min | 33 |
| 3 | DMP | DMSO, RT, 30 min | 55 |
| 4 | IBX | DMSO, RT, 30 min | 90 |
| 5c | IBX | CH2Cl2, 60 °C, 1 h | 95 |
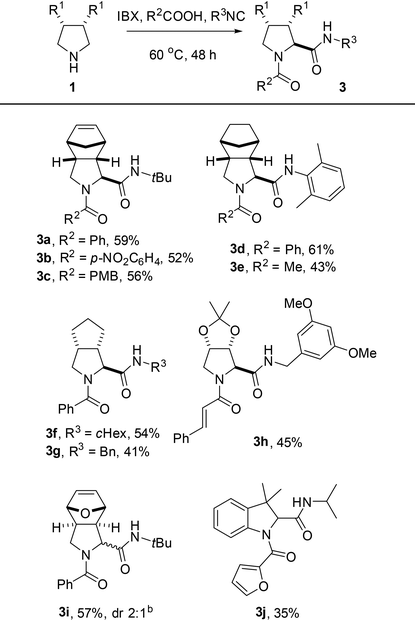
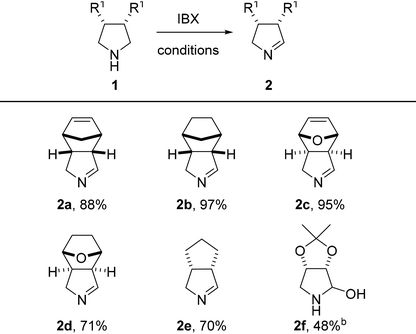
To our delight, a range of aliphatic meso-pyrrolidines was selectively oxidized by IBX towards the corresponding 1-pyrrolines in good to excellent yield (Table 2, 70–97%). Hemiaminal 2f is a highly interesting building block for the synthesis of aza-sugars, but was obtained in moderate yield presumably as a result of instability due to its increased electrophilicity.15 Under the same conditions, reactions of monocyclic pyrrolidines gave mixtures of the corresponding pyrrolines and pyrroles.16 We hypothesize that this overoxidation is caused by tautomerization of the initially formed imines to the corresponding enamines, which subsequently undergo a second oxidation leading to pyrroles. In case of bi- and tricyclic 1-pyrrolines 2a–f, tautomerization is prevented and overoxidation does not occur.
| a Conditions: amine (0.25 mmol), IBX (1 eq.), DCM (0.2 M), 60 °C, 1 h, closed vessel. Isolated yield, unless stated otherwise. b Yield determined by adding 2,5-dimethylfuran (0.5 eq.) after the reaction workup as standard for NMR spectroscopy and proposed structure (2f) was not fully characterized (see ESI). MeCN as solvent. |
|---|
|
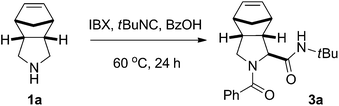
Imines are interesting inputs for a wide variety of complexity-generating reactions as a result of their electrophilic as well as nucleophilic properties. For example, imines serve as templates for many multicomponent reactions (MCRs), a category of reactions that create a high degree of diversity and complexity in a single step.17 One of the most studied and widely applied MCRs is the Ugi reaction, which has proven to be a powerful tool for rapid synthesis of lead compounds in drug discovery.18 Consequently, we envisioned a diastereoselective oxidative Ugi-type three-component reaction for the synthesis of N-acylprolyl amide derivatives.19
For a screening of the reaction conditions, the reaction between meso-pyrrolidine 1a, benzoic acid and tert-butyl isocyanide was investigated (Table 3). In agreement with earlier reports,10,11 a high concentration of reagents proved to be beneficial for the reaction outcome (see ESI†). The solvent of choice for most Ugi reactions, MeOH, was used as reaction medium with modest efficiency (Table 3, entry 1). Competing oxidation of the solvent could pose a problem, although previous reports suggest that the oxidation of amines is generally much faster than alcohols.4a,b,11b Considering the complexity of the reaction, satisfactory results were obtained using either MeCN or CH2Cl2 as the solvent (Table 3, entries 2 and 3). As a consequence of the superior results obtained for the oxidation of a variety of meso-pyrrolidines in CH2Cl2, this solvent was selected for investigation of the reaction scope. The one-pot reaction could be performed with a range of meso-pyrrolidines, affording the corresponding dipeptides in modest to good yields (Table 4, 41–61%). A range of electronically diverse carboxylic acids were suitable reaction partners, although it should be noted that lower yields were observed for aliphatic acids such as acetic acid (3e, 43%). Various aliphatic and aromatic isocyanides were compatible with the oxidative Ugi-type reaction as well. Although hemiaminal 2f was found to be unstable upon isolation, Ugi adduct 3h could be obtained in 45% yield as a single diastereoisomer. Notably, 3,3-dimethylindoline – with blocked benzylic positions to prevent benzylic oxidation20 – could also be applied with modest efficiency (3j, 35%).21
| Entry | Solvent | Yieldb (%) |
|---|---|---|
| a Conditions: amine (0.25 mmol), benzoic acid (1.5 eq.), tert-butyl isocyanide (1.5 eq.), IBX (1 eq.), solvent (0.5 M), 60 °C, 24 h, closed vessel. b Yield determined by adding 2,5-dimethylfuran (0.5 eq.) after the reaction workup as standard for NMR spectroscopy. | ||
| 1 | MeOH | 25 |
| 2 | MeCN | 50 |
| 3 | CH2Cl2 | 54 |
For the majority of examples, a single diastereoisomer of the dipeptide was isolated (3a–h). However, exo-configured dipeptide 3i was obtained as a mixture of diastereoisomers (Fig. 2).22 The increased diastereoselectivity with endo-configured imines11a such as 2a is explained by steric congestion on the concave face of the molecule, facilitating selective attack of the nucleophile from the other side (Fig. 2a). For exo-configured 2c, the steric effect is expected to be less pronounced.
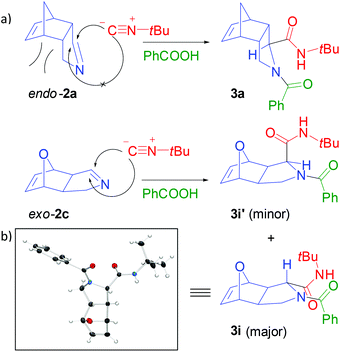 | ||
| Fig. 2 (a) Steric rationalization for superior diastereoselectivity for the Ugi-type reaction of endo-configured 2a compared to exo-2c. (b) Plot of the molecular structure of major diastereoisomer 3i with displacement ellipsoids drawn at 50% probability.† | ||
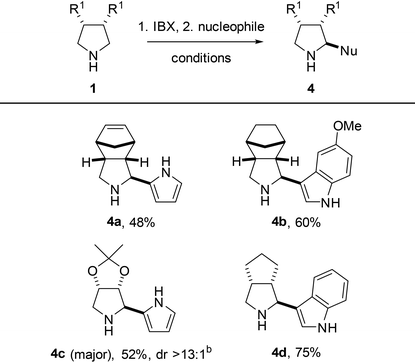
In order to expand the molecular diversity available by reactions of in situ-generated bicyclic pyrrolines, we decided to explore the aza-Friedel–Crafts reaction.23 Our one-pot methodology showed to be effective in an oxidative aza-Friedel–Crafts reaction with pyrrole and indoles as a two-step procedure, giving 2-substituted pyrrolidines in modest to good yield (Table 5, 48–75%) and high to excellent diastereoselectivity. The yield of pyrrolidine 4c even exceeded the yield of its hemiaminal intermediate 2f, supporting our hypothesized instability of the latter species. Unfortunately, 6-nitro- and 3-methyl-substituted indoles were not suitable reaction partners, presumably as a result of their reduced nucleophilicity.
| a Conditions: 1. amine (0.25 mmol), IBX (1 eq.), DCM (0.2 M), 60 °C, 1 h; 2. nucleophile (2.0 eq.), TFA (2.0 eq.), 60 °C, 1 h, closed vessel. Isolated yield. Only a single diastereoisomer was observed, unless stated otherwise. b Diastereoisomers were inseparable; ratio determined by NMR analysis of the crude mixture. |
|---|
|
In conclusion, we have developed the first IBX-mediated oxidation of unactivated aliphatic amines to give 1-pyrrolines. Moreover, an efficient one-pot protocol for diastereoselective α-functionalization of meso-pyrrolidines in oxidative Ugi-type and aza-Friedel–Crafts reactions is presented.
Acknowledgements
The Netherlands Organization of Scientific Research (NWO) is gratefully acknowledged for financial support by means of a TOP grant. The authors thank Christophe Vande Velde (University of Antwerp, Belgium) for the X-ray structure determination, and Matthias Zeller (Youngstown State University, USA) for the collection of the X-ray diffraction data. The diffractometer was funded by NSF Grant 1337296. We thank Elwin Janssen for technical assistance, Sanne Bouwman for HRMS measurements, and Dr Andreas W. Ehlers for NMR maintenance.Notes and references
- For selected reviews, see: (a) T. Wirth, Angew. Chem., Int. Ed., 2005, 44, 3656 CrossRef CAS PubMed; (b) V. V. Zhdankin and P. J. Stang, Chem. Rev., 2008, 108, 5299 CrossRef CAS PubMed; (c) T. Dohi and Y. Kita, Chem. Commun., 2009, 2073 RSC; (d) V. V. Zhdankin, J. Org. Chem., 2011, 76, 1185 CrossRef CAS PubMed; (e) L. F. Silva and B. Olofsson, Nat. Prod. Rep., 2011, 28, 1722 RSC; (f) A. Parra and S. Reboredo, Chem. – Eur. J., 2013, 19, 17244 CrossRef CAS PubMed.
- For selected reviews, see: (a) U. Ladziata and V. V. Zhdankin, ARKIVOC, 2006, 26 CAS; (b) V. Satam, A. Harad, R. Rajule and H. Pati, Tetrahedron, 2010, 66, 7659 CrossRef CAS PubMed; (c) A. Duschek and S. F. Kirsch, Angew. Chem., Int. Ed., 2011, 50, 1524 CrossRef CAS PubMed.
- M. Frigerio, M. Santagostino and S. Sputore, J. Org. Chem., 1999, 64, 4537 CrossRef CAS.
- (a) K. C. Nicolaou, C. J. N. Mathison and T. Montagnon, Angew. Chem., Int. Ed., 2003, 42, 4077 CrossRef CAS PubMed; (b) K. C. Nicolaou, C. J. N. Mathison and T. Montagnon, J. Am. Chem. Soc., 2004, 126, 5192 CrossRef CAS PubMed; (c) S. N. Murthy and Y. V. D. Nageswar, Tetrahedron Lett., 2011, 52, 4481 CrossRef PubMed.
- A single case of oxidation at a nonactivated position was unexpectedly observed for N-methylbenzylamine; see ref. 4a.
- For selected examples of the oxidation of unactivated secondary amines to the corresponding imine with other oxidants including hypervalent iodine reagents, see: (a) S. Furukawa, A. Suga and T. Komatsu, Chem. Commun., 2014, 50, 3277 RSC; (b) T. Sonobe, K. Oisaki and M. Kanai, Chem. Sci., 2012, 3, 3249 RSC; (c) V. Köhler, K. R. Bailey, A. Znabet, J. Raftery, M. Helliwell and N. J. Turner, Angew. Chem., Int. Ed., 2010, 49, 2182 CrossRef PubMed; (d) B. Zhu and R. J. Angelici, Chem. Commun., 2007, 2157 RSC; (e) X.-Q. Gu, W. Chen, D. Morales-Morales and C. M. Jensen, J. Mol. Catal. A: Chem., 2002, 189, 119 CrossRef CAS; (f) S. Minakata, Y. Ohshima, A. Takemiya, I. Ryu, M. Komatsu and Y. Ohshiro, Chem. Lett., 1997, 311 CrossRef CAS; (g) M. Ochiai, D. Kajishima and T. Sueda, Heterocycles, 1997, 46, 71 CrossRef CAS; (h) J. Larsen and K. A. Jørgensen, J. Chem. Soc., Perkin Trans. 2, 1992, 1213 RSC; (i) M. Ochiai, M. Inenaga, Y. Nagao, R. M. Moriarty, R. K. Vaid and M. P. Duncan, Tetrahedron Lett., 1988, 29, 6917 CrossRef CAS; (j) H. B. Henbest and P. Slade, J. Chem. Soc., 1960, 1558 RSC.
- For selected reviews, see: (a) C. J. Li, Acc. Chem. Res., 2009, 42, 335 CrossRef CAS PubMed; (b) E. A. Mitchell, A. Peschiulli, N. Lefevre, L. Meerpoel and B. U. W. Maes, Chem. – Eur. J., 2012, 18, 10092 CrossRef CAS PubMed.
- For a selected review, see: S. A. Girard, T. Knauber and C. J. Li, Angew. Chem., Int. Ed., 2014, 53, 74 CrossRef CAS PubMed.
- P. Fontaine, A. Chiaroni, G. Masson and J. Zhu, Org. Lett., 2008, 10, 1509 CrossRef CAS PubMed.
- (a) T. Ngouansavanh and J. P. Zhu, Angew. Chem., Int. Ed., 2006, 45, 3495 CrossRef CAS PubMed; (b) F. De Moliner, S. Crosignani, A. Galatini, R. Riva and A. Basso, ACS Comb. Sci., 2011, 13, 453 CrossRef CAS PubMed.
- For an IBX-mediated oxidative Ugi four-component reaction, see: (a) F. Drouet, G. Masson and J. P. Zhu, Org. Lett., 2013, 15, 2854 CrossRef CAS PubMed; For IBX-mediated oxidative Ugi-type three-component reactions, see: (b) T. Ngouansavanh and J. Zhu, Angew. Chem., Int. Ed., 2007, 46, 5775 CrossRef CAS PubMed; (c) D. Zhu, L. Xia, L. Pan, S. Li, R. Chen, Y. Mou and X. Chen, J. Org. Chem., 2012, 77, 1386 CrossRef CAS PubMed.
- (a) A. Znabet, E. Ruijter, F. J. J. de Kanter, V. Kohler, M. Helliwell, N. J. Turner and R. V. A. Orru, Angew. Chem., Int. Ed., 2010, 49, 5289 CrossRef CAS PubMed; (b) A. Znabet, M. M. Polak, E. Janssen, F. J. J. de Kanter, N. J. Turner, R. V. A. Orru and E. Ruijter, Chem. Commun., 2010, 46, 7918 RSC; (c) A. Znabet, J. Zonneveld, E. Janssen, F. J. J. De Kanter, M. Helliwell, N. J. Turner, E. Ruijter and R. V. A. Orru, Chem. Commun., 2010, 46, 7706 RSC; (d) A. Znabet, S. Blanken, E. Janssen, F. J. J. de Kanter, M. Helliwell, N. J. Turner, E. Ruijter and R. V. A. Orru, Org. Biomol. Chem., 2012, 10, 941 RSC.
- Standard procedures for the oxidation of aliphatic amines to imines are performed using a two-step protocol of N-halogenation followed by base-mediated elimination. Unfortunately, we experienced these procedures to be highly dependent on concentration and batch of halogenation reagent and therefore difficult to reproduce.
- The oxidation was amenable to scale up (4 mmol) with minor changes to the general procedure (0.4 M, 2 h), affording imine 2a in 76% yield.
- T. M. Chapman, S. Courtney, P. Hay and B. G. Davis, Chem. – Eur. J., 2003, 9, 3397 CrossRef CAS PubMed.
- Piperidine derivatives and acyclic secondary amines were not converted under these conditions.
- For selected reviews, see: (a) C. de Graaff, E. Ruijter and R. V. A. Orru, Chem. Soc. Rev., 2012, 41, 3969 RSC; (b) A. Dömling, W. Wang and K. Wang, Chem. Rev., 2012, 112, 3083 CrossRef PubMed; (c) G. van der Heijden, E. Ruijter and R. V. A. Orru, Synlett, 2013, 666 CAS; (d) R. C. Cioc, E. Ruijter and R. V. A. Orru, Green Chem., 2014, 16, 2958 RSC.
- For selected reviews, see: (a) A. Dömling and I. Ugi, Angew. Chem., Int. Ed., 2000, 39, 3169 CrossRef; (b) A. Dömling, Chem. Rev., 2006, 106, 17 CrossRef PubMed.
- For the corresponding diastereoselective Ugi-type reaction from 1-pyrrolines, see ref. 11a. For examples of photoredox, aerobic and transition metal-catalyzed oxidative Ugi-type three component reactions with either carboxylic acid or water, see (a) Y. Chen and G. Feng, Org. Biomol. Chem., 2015, 13, 4260 RSC; (b) S. U. Dighe, S. Kolle and S. Batra, Eur. J. Org. Chem., 2015, 4238 CrossRef CAS PubMed; (c) C. Xie and L. Han, Tetrahedron Lett., 2014, 55, 240 CrossRef CAS PubMed; (d) C. Vila and M. Rueping, Green Chem., 2013, 15, 2056 RSC; (e) X. Ye, C. Xie, R. Huang and J. Liu, Synlett, 2012, 409 CAS; (f) G. Jiang, J. Chen, J.-S. Huang and C.-M. Che, Org. Lett., 2009, 11, 4568 CrossRef CAS PubMed.
- K. C. Nicolaou, T. Montagnon, P. S. Baran and Y. L. Zhong, J. Am. Chem. Soc., 2002, 124, 2245 CrossRef CAS PubMed.
- The oxidation of dihydroindoline and tetrahydroquinoline was only selective when using an excess of IBX (2 eq.) and a stoichiometric amount of radical inhibitor TEMPO (1–1.5 eq.), providing the aromatized products. This observation supports previous hypotheses by Nicolaou et al. that the oxidation of amines with IBX proceeds via an ionic mechanism, while benzylic oxidation towards acetophenone derivatives follows a single electron transfer (SET) mechanism. TEMPO itself does not facilitate the oxidation.
- The relative configurations of the major diastereoisomer 3i, determined by X-ray crystallography, and minor diastereoisomer 3i′ are depicted in Figure 2.
- For examples of transition metal-catalyzed oxidative aza-Friedel–Crafts reactions with a stoichiometric oxidant, see ref. 6b and references therein, as well as: (a) A. J. Catino, J. M. Nichols, B. J. Nettles and M. P. Doyle, J. Am. Chem. Soc., 2006, 128, 5648 CrossRef CAS PubMed; (b) Z. Li, D. S. Bohle and C.-J. Li, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8928 CrossRef CAS PubMed; (c) Z. Li and C.-J. Li, J. Am. Chem. Soc., 2005, 127, 6968 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Optimization data, experimental procedures, compound characterization data, copies of 1H and 13C spectra, and X-ray crystallographic data of compound 3i. CCDC 1400648. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ob01519g |
| This journal is © The Royal Society of Chemistry 2015 |