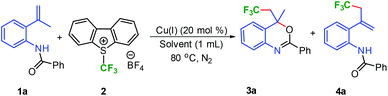Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalyzed trifluoromethylation of alkenes: synthesis of trifluoromethylated benzoxazines†
Sadhan
Jana
,
Athira
Ashokan
,
Shailesh
Kumar
,
Ajay
Verma
 and
Sangit
Kumar
*
and
Sangit
Kumar
*
Department of Chemistry, Indian Institute of Science Education and Research (IISER) Bhopal, Bhopal By-Pass Road, Bhauri, Bhopal, Madhya Pradesh 462066, India. E-mail: sangitkumar@iiserb.ac.in
First published on 3rd July 2015
Abstract
A simple base and ligand free copper catalyzed method for the construction of trifluoromethylated benzoxazines has been developed by using Umemoto's reagent. It involves the oxidative difunctionalization of alkenes through tandem C–O and C–CF3 bond formations. Furthermore, synthesized benzoxazines were selectively converted into trifluoromethylated allylic and (E)-vinylic benzamides by the treatment of KOtBu and CH3Li, respectively.
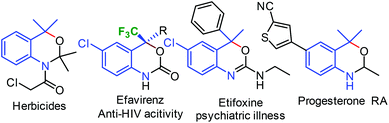
Benzoxazines, N,O-containing six membered heterocycles, are present in many drug molecules and herbicides and are also widely used as building blocks for bioactive molecules. They show interesting biological and pharmaceutical properties such as progesterone receptor (PR) modulation, and anti-anxiety, anti-HIV, agonist, and antagonist activities (Fig. 1).1,2
Incorporation of the CF3 group in the organic molecule enhances several biological properties such as solubility, lipophilicity, metabolic stability, binding selectivity etc.3 As a result, several trifluoromethylated heterocycles such as efavirenz and celecoxib were used as potential drugs for the treatment of HIV infection, arthritis, and spondylitis.4
As a consequence, versatile methodologies are being established for the introduction of the CF3 moiety into heterocycles and related organic molecules.5–12 However, the synthesis of CF3-containing heterocycles through intramolecular cyclization has been less explored. In 2013, Buchwald et al. reported copper-catalyzed intramolecular oxytrifluoromethylation of unactivated alkenes using Togni's reagent for the synthesis of trifluoromethylated lactones.6 Subsequently, intramolecular aminotrifluoromethylation7 and carbotrifluoromethylation8 of simple alkenes have been successfully established. The Fu group had developed transition metal-free synthesis of trifluoromethylated oxazolines exploiting CF3SO2Na as a CF3 source.9
Construction of the benzoxazine ring has been achieved by the intramolecular cyclization of alkenes using electrophiles such as Br+, I+, and KSCN and K2S2O8 combination.13,14 Benzoxazines comprising the CF3 group has remained an unexplored area. Recently, Xiao et al. has demonstrated photocatalytic oxytrifluoromethylation of N-allylamides for the synthesis of trifluoromethylated benzoxazines using the expensive Ru(bpy)3(PF6)2 catalyst and a base by a radical pathway.15d
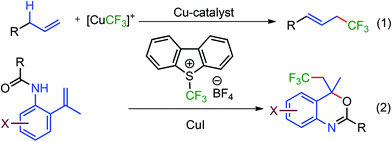
Copper-catalyzed trifluoromethylation of terminal alkenes through allylic C–H bond activation has been accomplished by Fu and Liu et al. exploiting the copper catalyst, Umemoto's reagent, and the 2,4,6-trimethyl pyridine reaction system (Scheme 1, eqn (1)).10a In view of the recent advances in Cu-catalyzed functionalization of alkenes,16,17 we envisioned, trifluoromethylation of the terminal alkene followed by intramolecular addition of oxygen for the synthesis of trifluoromethylated benzoxazines keeping the allylic C–H bond intact (eqn (2)). Herein, we present a simple base and ligand-free Cu-catalyzed synthesis of trifluoromethylated benzoxazine heterocycles from N-(2-(prop-1-en-2yl)benzamide substrates 1 using Umemoto's reagent 2 (Scheme 1, eqn (2)). Synthesized trifluoromethylated benzoxazines were also selectively converted into trifluoromethylated allylic and (E)-vinylic benzamides.
We initially examined the reaction of N-(2-(prop-1-en-2-yl)benzamide 1a with CuI (20 mol%) and various trifluoromethylating reagents in DCE (Table 1, entries 1–4). TMSCF3 was observed to be sluggish and the formation of the desired product was not realized. Togni's reagent gave a mixture of the desired trifluoromethylated product 3a in low yield (30%) along with 45% allylic trifluoromethylated product 4a (entry 1, Table 1). Shreeve's reagent, a triflate analogue of 2 provided 37% yield of 3a. The use of Umemoto's reagent 2, led to further increase in the yield by 15% of the desired product 3a. The presence of copper is crucial for trifluoromethylation as the reaction failed to provide even traces of 3a in the absence of copper (entry 5, Table 1). Although, various Cu sources (Table 1, entries 6–9, 14), ligands, and bases were screened (see ESI, Table S1, pp. S5–S6† for detailed optimization study), CuI alone was observed to be effective. The change in solvent from DCE to DMF, DMAc, NMP, and DMSO (Table 1, entries 10–13) led to further improvement in the yield and the best yield (68%) was obtained in DMSO (entry 13, Table 1).
| Entry | Catalyst + CF3 source | Solvent | Yield of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
|---|---|---|---|
| a All reactions were carried out using 0.2 mmol of 1a, 0.35 mmol of 2 in 1 mL solvent at 80 °C in a Schlenk tube under nitrogen and the progress of the reaction was monitored by TLC up to 35 h. b Percentage yield was determined by 19F-NMR using fluorobenzene as an internal standard. c Yield of 4a. ND = not detected, CuTc = thiophene-2-carbonyloxylate copper(I). | |||
| 1 | CuI + Togni's reagent | DCE | 30b, 45c |
| 2 | CuI + TMSCF3 | DCE | ND |
| 3 | CuI + Shreeve's reagent | DCE | 37 |
| 4 | CuI + 2 | DCE | 52 |
| 5 | – + 2 | DCE | ND |
| 6 | CuTc + 2 | DCE | 17 |
| 7 | CuCN + 2 | DCE | 26 |
| 8 | [Cu(CH3CN)4]BF4 + 2 | DCE | 5 |
| 9 | [Cu(CH3CN)4]PF6 + 2 | DCE | 6 |
| 10 | CuI + 2 | DMF | 52 |
| 11 | CuI + 2 | DMAc | 58 |
| 12 | CuI + 2 | NMP | 39 |
| 13 | CuI + 2 | DMSO | 68 |
| 14 | Cu | DMSO | 18 |
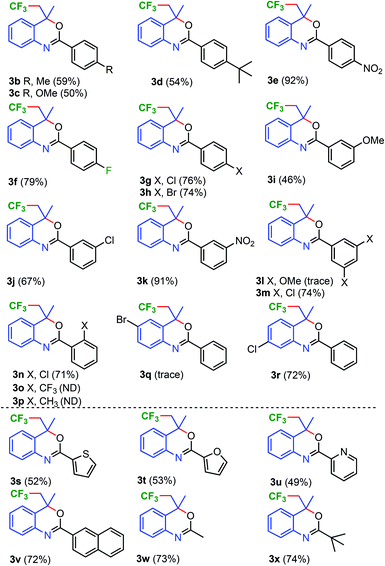
Next, the substrate scope of trifluoromethylation of the alkenes using 20 mol% of CuI at 80 °C in DMSO was studied. The substitution in the amide ring of 1a was explored (Scheme 2). Alkenes with electron donating substituents such as CH3, OCH3, and tBu at the para-position of the benzamide ring, gave trifluoromethylated benzoxazines 3b–3d in moderate yields (50–59%) whereas the electron withdrawing groups such as NO2, F, Cl, and Br favoured the trifluoromethylation which indeed led to good yields (74–92%) of 3e–3h.
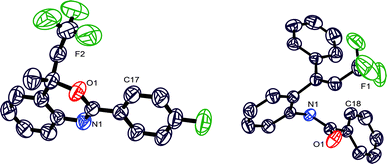
Structures of CF3–benzoxazines 3f and 3z are also established by the single crystal structure study (Fig. 2, for 3z and crystallographic details, please see the ESI, pp. S202–S232†). meta-Methoxy substituted benzoxazine 3i was obtained in moderate yield (46%) whereas Cl and NO2 substituted benzoxazines 3j and 3k were obtained in 67 and 91% yields, respectively. The reaction of the meta-di-OCH3-subtituted substrate was observed to be sluggish and only trace amounts of the respective trifluoromethylated benzoxazine 3l were isolated. On the other hand, meta-di-chloro-substituted benzoxazine 3m was obtained in 74% yield.
Similarly, the substrate with ortho-chloro substitution in the amide ring, gave the desired trifluoromethylated benzoxazine 3n in good yield (71%). ortho-Methyl and trifluoromethyl substituted substrates failed to yield trifluoromethylated benzoxazines 3o and 3p and recovered quantitatively and this could be due to the steric effect of CH3 and CF3 which may prevent the coordination of the –CONHPh group with CuCF3 (vide infra).
On the other hand, bromo-substitution in the aniline ring para to NH has a negative effect on the trifluoromethylation reaction as only trace amounts of 3q were obtained. The substrate with a chloro substituent in the aniline ring, para to the alkene, underwent trifluoromethylation smoothly and yielded chloro benzoxazines 3r in 72% yield.
Next, the substrates with various N-aromatics such as naphthyl heteroaromatics; thiophenyl, furanyl, and pyridinyl were subjected to trifluoromethylation reaction. Indeed, the reaction also showed compatibility with naphthyl and heteroaryl containing substrates and respective trifluoromethyl benzoxazines 3s–3v were obtained in 49–72% yields.
Substrates not only with various aryl benzamides but also with alkyl amides such as methyl and tert-butyl substituents were tolerated and yielded trifluormethylated benzoxazines 3w and 3x in 73 and 74% yields, respectively.
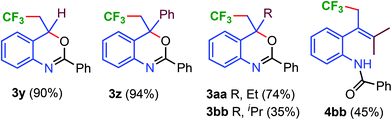
After studying various N-aryl, alkyl and benzamide substrates, C-2 substituted alkenes were explored in the copper-catalyzed C–CF3 and C–O bond formation reaction (Scheme 3). Alkenes with H and Ph substituents at the C-2 position provided excellent yields (90 and 94%) of trifluoromethylated benzoxazines 3y and 3z. C-2 substituted methyl, ethyl, and iso-propyl benzoxazines (3a, vide supra), 3aa, and 3bb were obtained in 68, 74, and 35% yields, respectively. In the case of 3bb, formation of trifluoromethylated, the uncyclized product 4bb in 45% yield was also observed.
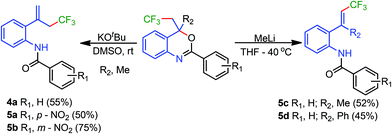
Next, the synthetic utility of trifluoromethylated benzoxazines was explored (Scheme 4). An addition of KOtBu to benzoxazines 3a, 3e, and 3k provided trifluoromethylated allylic benzamides 4a, 5a, and 5b, respectively. Interestingly, treatment of 3a and 3y with CH3Li provided trifluoromethylated (E)-vinyl benzamides 5c and 5d, respectively. The structure of 5d is also established by the X-ray single crystal study (Fig. 2). It seems that KOtBu removes the less hindered methyl proton whereas CH3Li prefers abstraction of the highly acidic proton adjacent to the CF3 group.
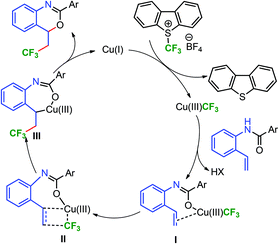
In the mechanistic consideration, copper(I) can form the Cu(III)CF3 intermediate by the reaction of Umemoto's reagent 2 (Scheme 5).10a The formation of the Cu(I)CF3 intermediate is also possible by the disproportionation of Cu(I) into Cu(II) and Cu(0) followed by the reaction of Cu(0) with Umemoto's reagent (please see the ESI, pp. S8–S13† for details).12e,18 Nevertheless, both Cu(I)CF3 and Cu(III)CF3 could act as a trifluoromethylating reagent.12e Ligand exchange with the alkenamide substrate would lead to the substrate–copper intermediate I.19
Intramolecular coordination of an alkene followed by the formation of the Heck-type four-membered ring would generate the transition state II. This could allow the formation of the C–CF3 bond leading to III, subsequent reductive elimination may give benzoxazine, by concomitant release of the catalyst. It seems unlikely that the reaction proceeds via a radical pathway because the reaction mixture failed to give any signal in the EPR spectrum.
The non-reactive nature of ortho-methyl and trifluoromethyl substituted substrates (vide supra, 3o and 3p) and formation of the trifluoromethylated allylic product 4bb could be rationalized based on the proposed intermediates I–III. It seems that allylic trifluoromethylation of the alkene through the allylic C–H bond activation process10a also occurred along with the formation of new C–CF3 and C–O bonds. This may be due to the steric effect of the iso-propyl substituent, therefore, giving a mixture of the C–CF3 and C–O bond forming product 3bb and the trifluoromethylated allylic product 4bb.
In conclusion, we have developed a simple, ligand and base free copper catalyzed method for the construction of trifluoromethylated benzoxazines by using Umemoto's reagent. As the reaction involves mild conditions, trifluoromethylated benzoxazines with functionalities such as nitro and bromo could be constructed which are useful for the later stage modifications. The synthetic utility of trifluoromethylation reaction has also been demonstrated by converting CF3-containing benzoxazines into allylic and (E) vinylic benzamides. The stereoselective synthesis utilizing a chiral ligand and copper catalytic system as well as the biological study of trifluoromethylated benzoxazines are under investigation in our laboratory.
S.K. thanks DRDO, New Delhi and IISER, Bhopal for generous funding. S.J., S.K. and A.V. acknowledge IISER, Bhopal and UGC, New Delhi, respectively, for the fellowship. S.K. also thanks Ch. Durga Prasad for proofreading the manuscript.
Notes and references
- (a) S. J. Hays, B. W. Caprathe, J. L. Gilmore, N. Amin, M. R. Emmerling, W. Michael, R. Nadimpalli, R. Nath, K. J. Raser, D. Stafford, D. Watson, K. Wang and J. C. Jaen, J. Med. Chem., 1998, 41, 1060 CrossRef CAS PubMed; (b) N. Dias, J.-F. Goossens, B. Baldeyrou, A. Lansiaux, P. Colson, A. Di Salvo, J. Bernal, A. Turnbull, D. J. Mincher and C. Bailly, Bioconjugate Chem., 2005, 16, 949 CrossRef CAS PubMed.
- P. Zhang, E. A. Terefenko, A. Fensome, Z. Zhang, Y. Zhu, J. Cohen, R. Winnerker, J. Wrobel and J. Yardley, Bioorg. Med. Chem. Lett., 2002, 12, 787 CrossRef CAS.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320 RSC.
- (a) J. W. Kobzina, Canadian Patent1059125, 1979 Search PubMed; (b) S. D. Young, S. F. Britcher, L. O. Tran, L. S. Payne, W. C. Lumma, T. A. Lyle, J. R. Huff, P. S. Anderson, D. B. Olsen, S. S. Carroll, D. J. Pettibone, J. A. O'Brien, R. G. Ball, S. K. Balani, J. H. Lin, I.-W. Chen, W. A. Schleif, V. V. Sardana, W. J. Long, V. W. Byres and E. A. Emini, Antimicrob. Agents Chemother., 1995, 39, 2602 CrossRef CAS; (c) D. Putman, D. Hongenkamp, O. Dasse, E. R. Whittemore and M. S. Jensan, US Patent20080039453A1, 2008 Search PubMed.
- Recent reviews on trifluoromethylation: (a) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294 CrossRef CAS PubMed; (b) S. Barata-Vallejo, B. Lantaño and A. Postigo, Chem. – Eur. J., 2014, 20, 16806 CrossRef CAS PubMed; (c) J. Xu, X. Lui and Y. Fu, Tetrahedron Lett., 2014, 55, 585 CrossRef CAS PubMed; (d) E. Merino and C. Nevado, Chem. Soc. Rev., 2014, 43, 6598 RSC; (e) T. Koike and M. Akita, J. Fluorine Chem., 2014, 167, 30 CrossRef CAS PubMed; (f) C. Alonso, E. M. D. Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847 CrossRef CAS PubMed; (g) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650 CrossRef CAS PubMed.
- Oxytrifluoromethylation: (a) R. Zhu and S. L. Buchwald, J. Am. Chem. Soc., 2012, 134, 12462 CrossRef CAS PubMed; (b) R. Zhu and S. L. Buchwald, Angew. Chem., Int. Ed., 2013, 52, 12655 CrossRef CAS PubMed; (c) Y.-T. He, H.-L. Li, Y.-F. Yang, Y.-Q. Wang, J.-Y. Luo, X.-Y. Liu and Y.-M. Liang, Chem. Commun., 2013, 49, 5687 RSC; (d) Q. Yu and S. Ma, Chem. – Eur. J., 2013, 19, 13304 CrossRef CAS PubMed; (e) Photoredox-catalyzed: Y. Yasu, Y. Arai, R. Tomita, T. Koike and M. Akita, Org. Lett., 2014, 16, 780 CrossRef CAS PubMed.
- Aminotrifluoromethylation: (a) J.-S. Lin, Y.-P. Xiong, C.-L. Ma, L.-J. Zhao, B. Tan and X.-Y. Liu, Chem. – Eur. J., 2014, 20, 1332 CrossRef CAS PubMed; (b) J.-S. Lin, X.-G. Liu, X.-L. Zhu, B. Tan and X.-Y. Liu, J. Org. Chem., 2014, 79, 7084 CrossRef CAS PubMed; (c) S. Kawamura, H. Egami and M. Sodeoka, J. Am. Chem. Soc., 2015, 137, 4865 CrossRef CAS PubMed; (d) P. Yu, S.-C. Zheng, N.-Y. Yang, B. Tan and X.-Y. Liu, Angew. Chem., Int. Ed., 2015, 54, 4041 CrossRef CAS PubMed.
- Carbotrifluoromethylation: (a) H. Egami, R. Shimizu, S. Kawamura and M. Sodeoka, Angew. Chem., Int. Ed., 2013, 52, 4000 CrossRef CAS PubMed; (b) P. Gao, X.-B. Yan, T. Tao, F. Yang, T. He, X.-R. Song, X.-Y. Liu and Y.-M. Liang, Chem. – Eur. J., 2013, 19, 14420 CrossRef CAS PubMed; (c) P. Xu, J. Xie, Q. Xue, C. Pan, Y. Change and C. Zhu, Chem. – Eur. J., 2013, 19, 14039 CrossRef CAS PubMed; (d) H. Egami, R. Shimizu and M. Sodeoka, J. Fluorine Chem., 2013, 152, 51 CrossRef CAS PubMed; (e) L. Li, M. Deng, S.-C. Zheng, Y.-P. Xiong, B. Tan and X.-Y. Yuan, Org. Lett., 2014, 16, 504 CrossRef CAS PubMed; (f) G. Han, Y. Liu and Q. Wang, Org. Lett., 2014, 16, 3188 CrossRef CAS PubMed; (g) G. Hang, Q. Wang, Y. Liu and Q. Wang, Org. Lett., 2014, 16, 5914 CrossRef PubMed; (h) Y.-T. He, L.-H. Li, Z.-Z. Zhou, H.-L. Hua, Y.-F. Qiu, X.-Y. Liu and Y.-M. Liang, Org. Lett., 2014, 16, 3896 CrossRef CAS PubMed; (i) J. Xu, Y.-L. Wang, T.-J. Gong, B. Xio and Y. Fu, Chem. Commun., 2014, 50, 12915 RSC; (j) Y. Wang, M. Jiang and J.-T. Liu, Chem. – Eur. J., 2014, 20, 15315 CrossRef CAS PubMed; (k) L. Hung, J.-S. Lin, B. Tan and X.-Y. Liu, ACS Catal., 2015, 5, 2826 CrossRef; (l) H.-L. Hua, Y.-T. He, Y.-F. Qiu, Y.-X. Li, B. Song, P. Gao, X.-R. Song, D.-H. Guo, X.-Y. Liu and Y.-M. Liang, Chem. – Eur. J., 2015, 21, 1468 CrossRef CAS PubMed; (m) L. Huang, S.-C. Zheng, B. Tan and X.-Y. Liu, Chem. – Eur. J., 2015, 21, 6718 CrossRef CAS PubMed.
- J. Yu, H. Yang and H. Fu, Adv. Synth. Catal., 2014, 356, 3669 CrossRef CAS PubMed.
- (a) J. Xu, Y. Fu, D.-F. Luo, Y.-Y. Jiang, B. Xiao, Z.-J. Liu, T.-J. Gong and L. Liu, J. Am. Chem. Soc., 2011, 133, 15300 CrossRef CAS PubMed; (b) D.-F. Luo, J. Xu, Y. Fu and Q.-X. Guo, Tetrahedron Lett., 2012, 53, 2769 CrossRef CAS PubMed; (c) C.-T. Yang, Z.-Q. Zhang, H. Tajuddin, C.-C. Wu, J. Liang, J.-H. Liu, Y. Fu, M. Czyzewska, P. G. Steel, T. B. Marder and L. Liu, Angew. Chem., Int. Ed., 2012, 51, 528 CrossRef CAS PubMed; (d) J. Xu, B. Xiao, C.-Q. Xie, D.-F. Luo, L. Liu and Y. Fu, Angew. Chem., Int. Ed., 2012, 51, 12551 CrossRef CAS PubMed; (e) J.-J. Dai, C. Fang, B. Xiao, J. Yi, J. Xu, Z.-J. Liu, X. Lu, L. Liu and Y. Fu, J. Am. Chem. Soc., 2013, 135, 8436 CrossRef CAS PubMed; (f) Q. Lu, H. Yu and Y. Fu, Chem. Commun., 2013, 49, 10847 RSC.
- (a) G. G. Dubinina, H. Furutachi and D. A. Vicic, J. Am. Chem. Soc., 2008, 130, 8600 CrossRef CAS PubMed; (b) C.-P. Zhang, Z.-L. Wang, Q.-Y. Chen, C.-T. Zhang, Y.-C. Gu and J.-C. Xiao, Angew. Chem., Int. Ed., 2011, 50, 1896 CrossRef CAS PubMed.
- (a) T. Umemoto and S. Ishihara, Tetrahedron Lett., 1990, 31, 3579 CrossRef CAS; (b) T. Umemoto and S. Ishihara, J. Am. Chem. Soc., 1993, 115, 2156 CrossRef CAS; (c) T. Umemoto, S. Ishihara and K. Adachi, J. Fluorine Chem., 1995, 74, 77 CrossRef CAS; (d) T. Umemoto and S. Ishihara, J. Fluorine Chem., 1999, 98, 75 CrossRef CAS; (e) C. Zhange, Org. Biomol. Chem., 2014, 12, 6580 RSC.
- (a) Synthesis of an oxazoline moiety: L. Engman, J. Org. Chem., 1991, 56, 3425 CrossRef CAS; (b) A. Jaganathan, A. Garzan, D. C. Whitehead, R. J. Staples and B. Borhan, Angew. Chem., Int. Ed., 2011, 50, 2593 CrossRef CAS PubMed; (c) Y.-M. Wang, J. Wu, C. Hoong, V. Rauniyar and F. D. Toste, J. Am. Chem. Soc., 2012, 134, 12928 CrossRef CAS PubMed; (d) J. Wu, Y.-M. Wang, A. Drljevic, V. Rauniyar, R. J. Phipps and F. D. Toste, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 13729 CrossRef CAS PubMed; (e) A. Jaganathan, R. J. Staples and B. Borhan, J. Am. Chem. Soc., 2013, 135, 14806 CrossRef CAS PubMed; (f) Q. Yin and S.-L. You, Org. Lett., 2013, 15, 4266 CrossRef CAS PubMed.
- H. Yang, X.-H. Duan, J.-F. Zhao and L.-N. Guo, Org. Lett., 2015, 17, 1998 CrossRef CAS PubMed.
- (a) F.-L. Liu, J.-R. Chen, B. Feng, X.-Q. Hu, L.-H. Ye, L.-Q. Lu and W.-J. Xiao, Org. Biomol. Chem., 2014, 12, 1057 RSC; (b) X.-Q. Hu, J.-R. Chen, Q. Wei, F.-L. Liu, Q.-H. Deng, A. M. Beauchemin and W.-J. Xiao, Angew. Chem., Int. Ed., 2014, 53, 12163 CrossRef CAS PubMed; (c) X.-Q. Hu, G. Feng, J.-R. Chen, D.-M. Yan, Q.-Q. Zhao, Q. Wei and W.-J. Xiao, Org. Biomol. Chem., 2015, 13, 3457 RSC; (d) Q.-H. Deng, J.-R. Chem, Q. Wei, Q.-Q. Zhao, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2015, 51, 3537 RSC.
- Recent review on Cu-catalyzed functionalization of C–C multiple bonds: Y. Shimizu and M. Kanai, Tetrahedron Lett., 2014, 55, 3727 CrossRef CAS PubMed.
- (a) J. Lu, Y. Jin, H. Liu, Y. Jaing and H. Fu, Org. Lett., 2011, 13, 3694 CrossRef CAS PubMed; (b) S. Majima, Y. Shimizu and M. Kanai, Tetrahedron Lett., 2012, 53, 4381 CrossRef CAS PubMed; (c) X. Wang, Y. Jin, Y. Zhao, L. Zhu and H. Fu, Org. Lett., 2012, 14, 452 CrossRef CAS PubMed; (d) J. Kawai, P. K. Chikkade, Y. Shimizu and M. Kanai, Angew. Chem., Int. Ed., 2013, 52, 7177 CrossRef CAS PubMed; (e) K. Kaneko, T. Yoshino, S. Matsunaga and M. Kanai, Org. Lett., 2013, 15, 2502 CrossRef CAS PubMed; (f) T. Itoh, Y. Shimizu and M. Kanai, Org. Lett., 2014, 16, 2736 CrossRef CAS PubMed.
- 19F NMR of the reaction mixture of 2 and CuI indicates a signal at −32.7 ppm which is attributed to Cu(I)CF3. The reported value of Cu(I)CF3 is δ −33.9 ppm; see ref. 11b.
- For the coordination of Cu with oxygen over alkene in related substrates, please see: B. Chen, X.-L. Hou, Y.-X. Li and Y.-D. Wu, J. Am. Chem. Soc., 2011, 133, 7668 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, spectra, crystal data. CCDC 1063690 (3f), 1402832 (5d) and 1402833 (3z). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ob01196e |
| This journal is © The Royal Society of Chemistry 2015 |