Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
1,3-Dipolar cycloadditions of azomethine imines
Carmen
Nájera
*,
José M.
Sansano
and
Miguel
Yus
Departamento de Química Orgánica, Facultad de Ciencias, and Centro de Innovación en Química Avanzada (ORFEO-CINQA), Universidad de Alicante, Apdo. 99, 03080 Alicante, Spain. E-mail: cnajera@ua.es
First published on 24th June 2015
Abstract
Azomethine imines are considered 1,3-dipoles of the aza-allyl type which are transient intermediates and should be generated in situ but can also be stable and isolable compounds. They react with electron-rich and electron-poor olefins as well as with acetylenic compounds and allenoates mainly by a [3 + 2] cycloaddition but they can also take part in [3 + 3], [4 + 3], [3 + 2 + 2] and [5 + 3] with different dipolarophiles. These 1,3-dipolar cycloadditions (1,3-DC) can be performed not only under thermal or microwave conditions but also using metallo- and organocatalytic systems. In recent years enantiocatalyzed 1,3-dipolar cycloadditions have been extensively considered and applied to the synthesis of a great variety of dinitrogenated heterocycles with biological activity. Acyclic azomethine imines derived from mono and disubstituted hydrazones could be generated by prototropy under heating or by using Lewis or Brønsted acids to give, after [3 + 2] cycloadditions, pyrazolidines and pyrazolines. Cyclic azomethine imines, incorporating a C–N bond in a ring, such as isoquinolinium imides are the most widely used dipoles in normal and inverse-electron demand 1,3-DC allowing the synthesis of tetrahydro-, dihydro- and unsaturated pyrazolo[1,5-a]isoquinolines in racemic and enantioenriched forms with interesting biological activity. Pyridinium and quinolinium imides give the corresponding pyrazolopyridines and indazolo[3,2-a]isoquinolines, respectively. In the case of cyclic azomethine imines with an N–N bond incorporated into a ring, N-alkylidene-3-oxo-pyrazolidinium ylides are the most popular stable and isolated dipoles able to form dinitrogen-fused saturated and unsaturated pyrazolopyrazolones as racemic or enantiomerically enriched compounds present in many pharmaceuticals, agrochemicals and other useful chemicals.
1 General introduction
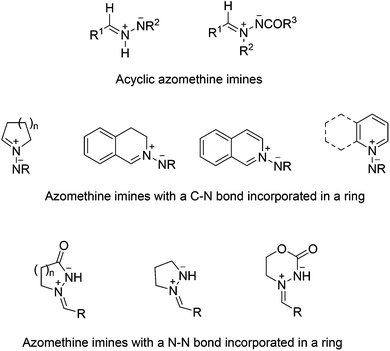
Azomethine imines are 1,3-dipoles of the allylic type, which present two types of resonance structures, iminium imide and diazonium ylide.1–4 They are readily accessible as stable compounds or as intermediates for the synthesis of diverse dinitrogenated heterocycles by 1,3-dipolar cycloadditions (1,3-DC) under thermal or catalyzed conditions.2–12 Numerous types of pharmaceuticals, agrochemicals and other biologically active compounds can be prepared by different types of [3 + 2] cycloadditions, mainly with alkenes and alkynes, but also high order cycloadditions, such as [3 + 3], [4 + 3] and [3 + 2 + 3], have been recently developed. The asymmetric processes have been performed using chiral substrates, chiral metal complexes or organocatalysts.13–15 In this review we have summarized the diverse types of azomethine imines (Scheme 1) which have been used as 1,3-dipoles in the last ten years, not only in racemic, but also in asymmetric processes. They have been classified according to Schantl's review10 covering the literature until 2003.2 Acyclic azomethine imines
These types of dipoles have been postulated as intermediates in [3 + 2] cycloaddition reactions and are derived from hydrazones and carbazates (Scheme 1) leading to pyrazolines and pyrazolidines, and their derivatives. The corresponding precursors can be prepared from monosubstituted and 1,2-disubstituted hydrazines.2.1 Monosubstituted hydrazines
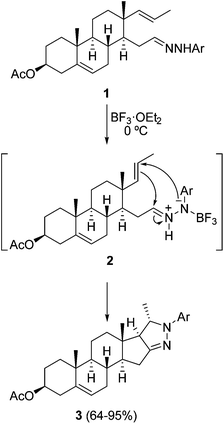
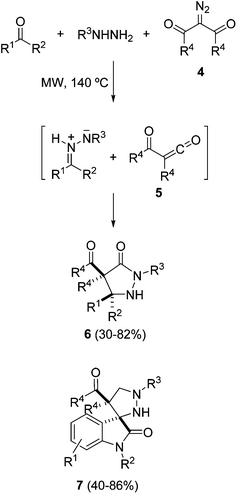
Azomethine imines derived from acyclic hydrazones are generated easily upon 1,2-prototropy either under heating by Lewis acid catalysis or by protonation, and can be trapped with different dipolarophiles to afford five-membered dinitrogenated heterocycles through inter- and intramolecular cycloadditions.16–19 Normally, electron-deficient dipolarophiles are used, but also simple alkenes in the case of intramolecular processes.10 A recent intramolecular process has been applied to the synthesis of androstenoarylpyrazolines 3 using BF3·OEt2 as a Lewis acid, previously used for intermolecular cycloadditions (Scheme 2).20 The reaction takes place stereoselectively at 0 °C in high yields from the corresponding hydrazones 1 by a BF3-promoted formation of intermediate azomethine imines 2.Three-component or consecutive intermolecular 1,3-DC of azomethine imines with α-oxoketenes 5 has been performed under thermal conditions. Both hydrazones and dipolarophiles are generated in situ, affording the corresponding pyrazolidinones 6 in a stereoselective manner (Scheme 3).21a Intermediate dipolarophiles 5 are generated from 2-diazo-1,3-diones 4 under microwave heating. When isatins are used as a carbonyl precursor the corresponding spirooxindoles 7 are obtained in a stereoselective manner (Scheme 3).
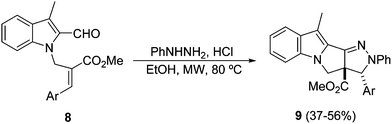
Recently, a microwave-assisted intramolecular 1,3-DC of azomethine imines, in situ generated from indole-2-carboxaldehydes 8 and phenylhydrazine, has been described. This process takes place in the presence of HCl as an additive in ethanol, providing [a]-annelated pyrazolopyrroloindoles 9 in a regio- and stereoselective manner (Scheme 4).21b The reaction in the presence of other additives such as AcOH, BF3·OEt2 or iodine gave either lower yields or no reaction.
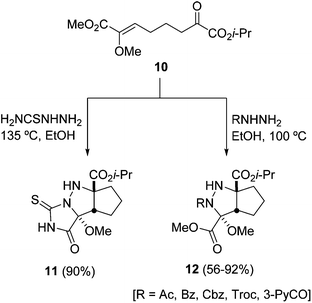
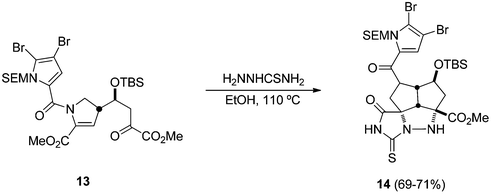
The stereocontrolled synthesis of cis-cyclopentanopyrazolidines has been carried out from the α-methoxy-α,β-unsaturated ester 10 bearing an α-keto ester at the end of the chain (Scheme 5).22 In the case when thiosemicarbazide is used, the intermediate azomethine imine is generated under heating, giving the tricyclic thiohydantoin 11. N-Acyl or N-alkoxycarbonyl hydrazines gave, under thermal conditions, the corresponding cycloadducts 12 in good yields (Scheme 5). This approach was previously described by the same group to prepare potential precursors of palau'amine (Scheme 6).23
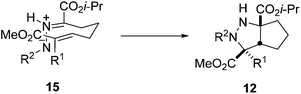
The high stereoselectivity observed in these cycloadditions can be explained by the formation of a chair-like transition state 15, which favors the overlap between the π-orbitals of the dipole and dipolarophile (Scheme 7).
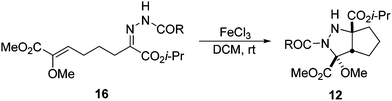
Alternatively, it was possible to prepare the corresponding hydrazones 16 from 10 using a catalytic amount of HCl in ethanol at room temperature, and then the 1,3-DC takes place at ambient temperature in the presence of one equivalent of FeCl3 in dichloromethane, giving products 12 in good yields (Scheme 8).22
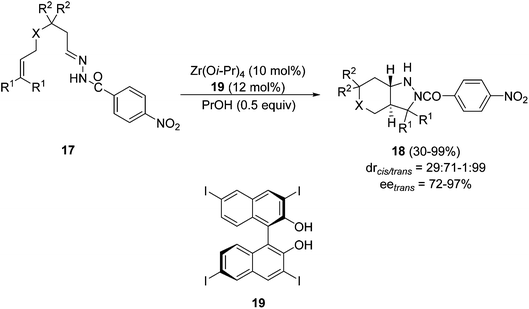
The first example of a catalytic asymmetric intramolecular [3 + 2] cycloaddition of hydrazones with olefins was performed in the presence of a chiral zirconium catalyst.24 Different 4-nitrobenzoylhydrazones 17 gave trans-pyrazolidines 18 with high diastereo- and enantioselectivity in the presence of Zr(Oi-Pr)4 (10 mol%) and the Binol derivative 19 at room temperature in dichloromethane (Scheme 9).
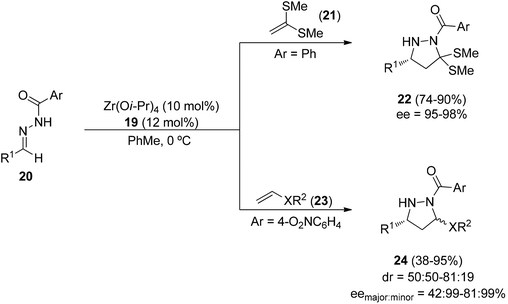
The same chiral catalyst formed by Zr(Oi-Pr)4 and Binol 19 has been used in the intermolecular [3 + 2] cycloaddition of benzoylhydrazones 20 and electron-rich alkenes like the ketene dimethyl dithioacetal 21. The corresponding 3,5-disubstituted pyrazolidines 22 were obtained in good yields and enantioselectivities (Scheme 10).25 In the case of vinyl ethers or thioethers 23, compounds 24 were obtained in low to moderate diastereoselectivity and high enantioselectivity. Samarium diiodide reduction of 24 afforded the corresponding 1,3-diamines, whereas LiAlH4 gave pyrazolidines.
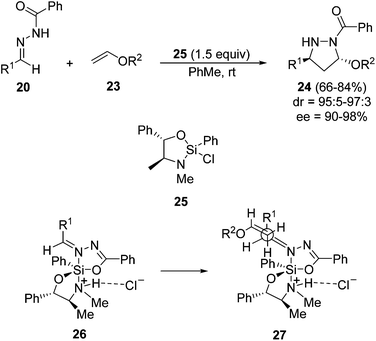
A chiral silicon Lewis acid has been used in the intermolecular 1,3-DC of benzoylhydrazones 20 with vinyl ethers 23 (Scheme 11).26 The process needs 1.5 equivalents of compound 25, derived from pseudoephedrine, to take place, giving the corresponding pyrazolidines 24 at room temperature in high trans-diastereoselectivity and excellent ee. The intermediacy of complex 26, isolated and characterized by X-ray crystallography,27 explains the approach of the ether by the Si face of the hydrazone (27). Samarium diiodide reduction of pyrazolidines 22 gave the corresponding anti-1,3-diamines.
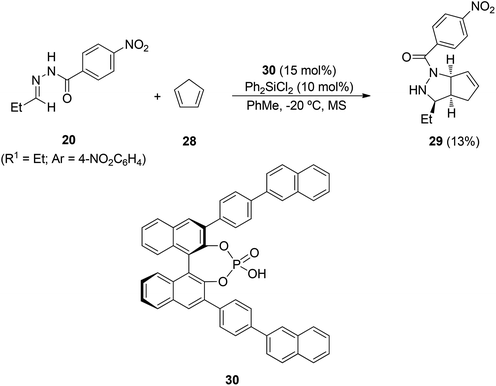
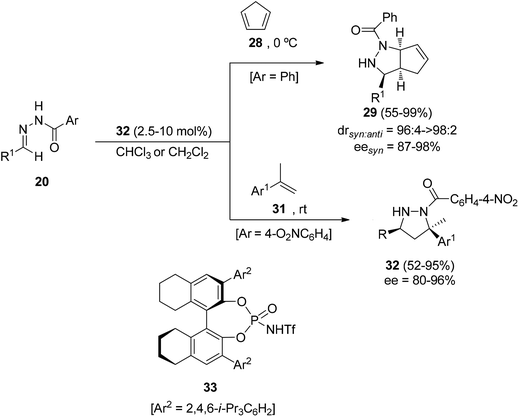
Hydrazones derived from ethyl glyoxylate and aliphatic or aromatic aldehydes react with cyclopentadiene (28) at room temperature in the presence of TMSOTf (10 mol%) as a catalyst. The enantiocatalytic process was next assayed with an in situ generated Binol-phosphate derived silicon Lewis acid from 30 and Ph2SiCl2 (Scheme 12).28 Cycloadduct 29 was obtained in a high syn/anti diastereomeric ratio (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and up to 89% ee, but in a low yield (13%).
5) and up to 89% ee, but in a low yield (13%).
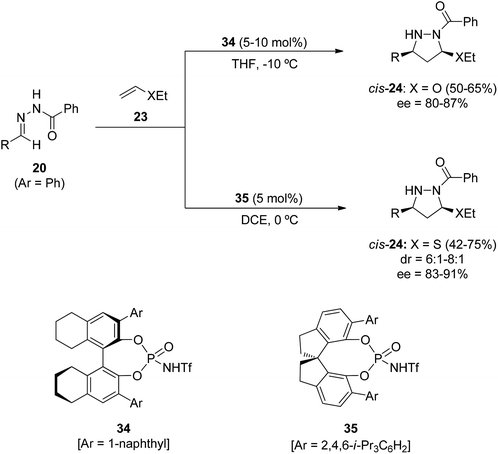
The asymmetric Brønsted acid catalyzed 1,3-DC of benzoylhydrazones 20 could be efficiently performed with cyclopentadiene (28) and α-methylstyrenes 31 as dipolarophiles (Scheme 13).29 Different Binol-derived phosphoric acids (pKa 13–14 in acetonitrile) were initially assayed as organocatalysts giving very low yields. However, the more acidic [H8]-Binol-based N-trifluorophosphoramides 33 (pKa 6–7 in acetonitrile) gave pyrazolidines 29 and 32, respectively, in high yields and enantioselectivities. Cycloadducts 29 were isolated mainly as cis-diastereomers, whereas α-methylstyrene adducts 32 were obtained as single diastereomers. The cycloaddition product 29 with R = t-Bu was transformed by SmI2 reduction into a 1,3-diamine with a core structure similar to that of the influenza drug peramivir.30 By the oxidation of cycloadduct 32 (R = t-Bu) with copper(II) chloride the corresponding pyrazoline was obtained maintaining the ee value.
However, for the [3 + 2] cycloaddition of N-benzoylhydrazones 20 with ethyl vinyl ether 23 (R2 = Et) the [H8]-Binol derived N-triflylphosphoramide 34 was the optimized catalyst. The corresponding cis-pyrazolidines 24 (XR2 = OEt) were obtained in good yields and enantioselectivities (Scheme 14).31 However, for the cycloaddition with ethyl vinyl thioether 23 (XR2 = SEt) the Spinol-derived N-triflylphosphoramide 35 was the best organocatalyst affording pyrazolidines 24 (XR2 = SEt) in good yields, diastereo- and enantioselectivities.
 | ||
| Scheme 14 N-Triflylphosphoramides as chiral organocatalysts in the intermolecular 1,3-DC of benzoylhydrazones 20. | ||
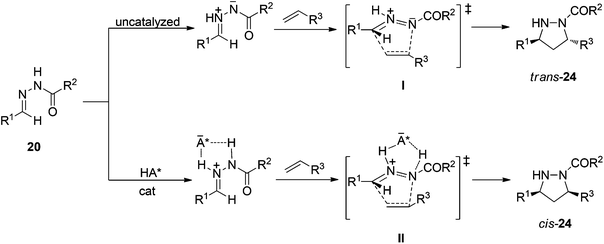
The mechanism of N-triflylphosphoramide-catalyzed asymmetric [3 + 2] cycloadditions was explored using DFT (MO6-2X) calculations.31 Protonation of hydrazones 20 by these Brønsted acids produces ion-pair complexes, which are more reactive than those formed from azomethine imines by 1,2-prototropy of the hydrazone through the transition state I (Scheme 15). These ion-pair hydrazonium–phosphoramide anions are reactive in [3+ + 2] cycloadditions and only small distortions32 of them are required in the transition state II giving in this case the cis-pyrazolidines 24. The origin of enantioselectivities was also explained.
2.2 1,2-Disubstituted hydrazines
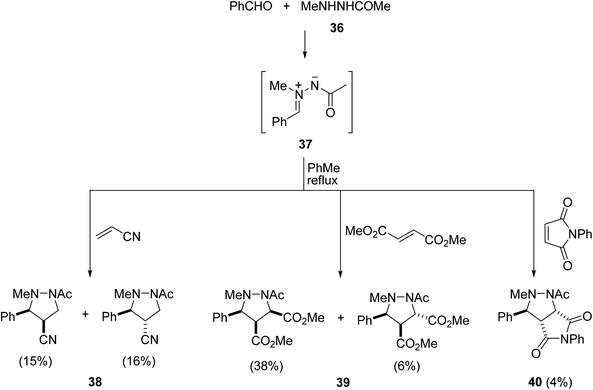
The condensation of 1,2-disubstituted hydrazines and N-substituted carbazates or hydrazides with carbonyl compounds generates in situ directly the corresponding acyclic azomethine imines,33 which can be trapped in situ by dipolarophiles through an inter- or intramolecular [3 + 2] cycloaddition.10 In this case, they react preferentially with electron-deficient dipolarophiles under thermal conditions.Intermolecular 1,3-DC of azomethine imines 37, generated in situ from aldehydes and N1-alkyl-N2-acyl hydrazines 36, takes place with electron-deficient dipolarophiles under refluxing toluene using a Dean–Stark trap (Scheme 16).34 The corresponding 3,4-disubstituted pyrazolidines 38–40, derived from benzaldehyde, were obtained as a mixture of cis/trans diastereomers in low yields.
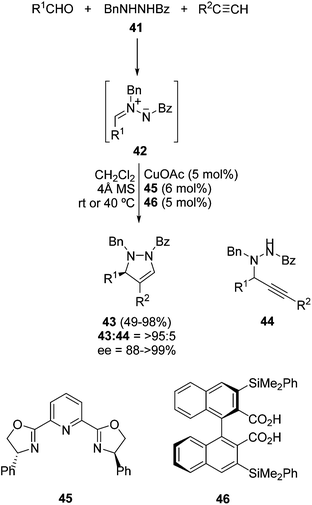
The first and only example of an enantiocatalytic three-component 1,3-DC of aldehydes, hydrazides and alkynes was performed using a PyBox 45/Cu(I) complex as a catalyst and a chiral binaphthyl dicarboxylic acid 46 as a cocatalyst (Scheme 17).35N1-Benzylbenzoylhydrazide 41 was used for the generation of the corresponding azomethine imine intermediates 42, which react with terminal alkynes affording pyrazolines 43 in a chemoselective manner (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); only small amounts of compounds 44 resulting from the nucleophilic addition of copper acetylide to 42 were also obtained. Aromatic and aliphatic aldehydes can be used in the presence of 4 Å MS to eliminate the water formed during the condensation step. Moreover, aromatic and aliphatic alkynes can be used as well, affording the corresponding pyrazolines 43 in high enantioselectivities (Scheme 17).
5); only small amounts of compounds 44 resulting from the nucleophilic addition of copper acetylide to 42 were also obtained. Aromatic and aliphatic aldehydes can be used in the presence of 4 Å MS to eliminate the water formed during the condensation step. Moreover, aromatic and aliphatic alkynes can be used as well, affording the corresponding pyrazolines 43 in high enantioselectivities (Scheme 17).
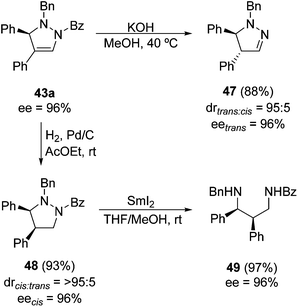
A representative 3,4-disubstituted pyrazoline 43a (with R1 = R2 = Ph) was further transformed into different heterocyclic compounds 47 and 48, as well as the diamine 49 by reduction of the last one with samarium diiodide (Scheme 18).
3 Cyclic azomethine imines incorporating C–N in the ring
Several types of heterocyclic systems with the C–N double bond incorporated into a ring constitute a subclass of azomethine imines (Scheme 1).10 These types of dipoles have been extensively studied for the synthesis of different ring-fused pyrazolidines, pyrazolines, and pyrazoles.3.1 Heterocyclic hydrazone derived azomethine imines
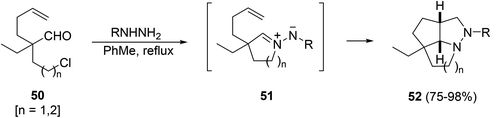
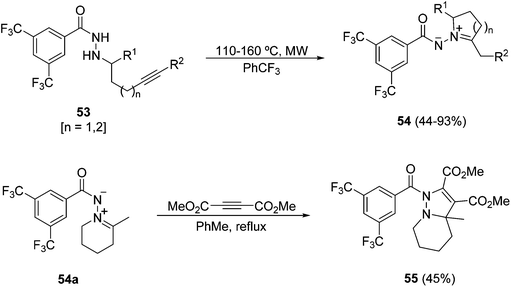
Few examples have been described of using heterocyclic azomethine imines mainly in intramolecular processes. Heterocyclic azomethine imines of the type 51 can be prepared from the corresponding aldehydes 50 bearing a halogen atom at the γ- or δ-position. A cascade of cyclization and 1,3-DC gave all-cis tricyclic compounds 52 in high yields (Scheme 19).36Azomethine imines 54 can be prepared by MW heating of benzoylhydrazides 53 bearing an alkyne in the chain through an intramolecular hydroamination reaction (Scheme 20).37 The reactivity of one example 54a with methyl acetylenedicarboxylate gave the fused pyrazoline 55 in a moderate yield.
3.2 Isoquinolinium-N-aryl imides
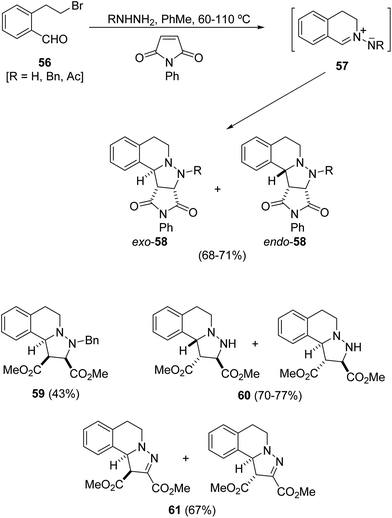
N-Iminoisoquinolin-2-ium ylides 57 are the most recently used cyclic azomethine imines bearing a C–N bond in the ring.10 They have been mainly used in metal-catalyzed [3 + 2] cycloadditions not only with electron-deficient dipolarophiles but also with electron-rich alkenes. In addition, organocatalyzed processes, including asymmetric ones, have also been studied. A direct method to access this type of intermediate is the cascade cyclization reaction of the aldehyde 56 with hydrazines to afford azomethine imines 57,38 which can be trapped in situ with N-phenylmaleimide (NPM) (Scheme 21).36 Cycloadducts 58 were obtained as mixtures of endo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exo diastereomers (2
exo diastereomers (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1–3
1–3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The reaction of the aldehyde 56 with benzylhydrazine in the presence of dimethyl maleate gave the cycloadduct 59 with all-cis relative configuration. The same 3
1). The reaction of the aldehyde 56 with benzylhydrazine in the presence of dimethyl maleate gave the cycloadduct 59 with all-cis relative configuration. The same 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of cycloadducts 60 was obtained by a reaction of the aldehyde 56 with hydrazine hydrate in the presence of dimethyl maleate or fumarate. In the case of dimethyl acetylenedicarboxylate a 5
1 mixture of cycloadducts 60 was obtained by a reaction of the aldehyde 56 with hydrazine hydrate in the presence of dimethyl maleate or fumarate. In the case of dimethyl acetylenedicarboxylate a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomeric fused pyrazolines 61 was isolated under toluene reflux (Scheme 21).
1 mixture of diastereomeric fused pyrazolines 61 was isolated under toluene reflux (Scheme 21).
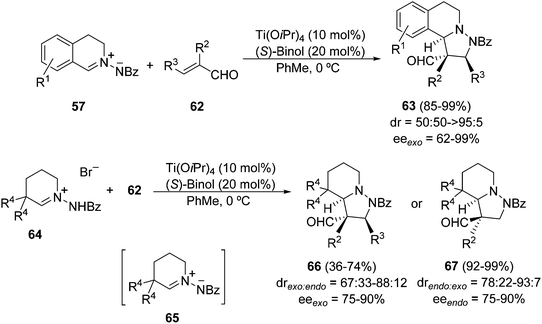
Already prepared C,N-cyclic azomethine imines 57 (with R = Bz) were used for the first time as dipoles in enantiocatalyzed [3 + 2] cycloadditions using enals 62 as dipolarophiles and titanium binolate complexes as catalysts (Scheme 22).38 The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (S)-Binol/Ti(Oi-Pr)4 complex gave at 0 °C the corresponding exo-cycloadducts 63 in high yields, diastereo-, and enantioselectivities. Structurally related C,N-cyclic azomethine imines 65 were prepared in situ from 64 under basic conditions using 2,6-di-tert-butyl-4-methylpyridine (DTMP) as a base compatible with the Lewis acid as a catalyst. The resulting cycloadducts 66 were obtained mainly as exo-adducts with β-substituted enals (62, R2 = H), whereas β-unsubstituted enals (62, R3 = H) gave mainly endo-cycloadducts 67 (Scheme 22).
1 (S)-Binol/Ti(Oi-Pr)4 complex gave at 0 °C the corresponding exo-cycloadducts 63 in high yields, diastereo-, and enantioselectivities. Structurally related C,N-cyclic azomethine imines 65 were prepared in situ from 64 under basic conditions using 2,6-di-tert-butyl-4-methylpyridine (DTMP) as a base compatible with the Lewis acid as a catalyst. The resulting cycloadducts 66 were obtained mainly as exo-adducts with β-substituted enals (62, R2 = H), whereas β-unsubstituted enals (62, R3 = H) gave mainly endo-cycloadducts 67 (Scheme 22).
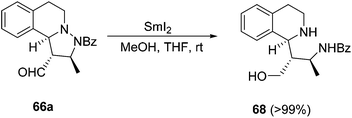
Samarium diiodide-mediated N–N cleavage of the adduct 66a (with R1 = R2 = H, R3 = Me) gave the tetrahydroisoquinoline 68 (Scheme 23).38
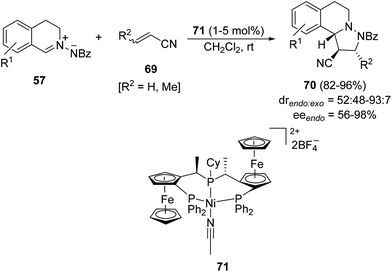
The same type of metal-catalyzed 1,3-DC of the azomethine imine 57 with unsaturated nitriles 69 was performed using a dicationic nickel(II) complex containing bis{(R)-1-[(SP)-2-(diphenylphosphino)ferrocenyl]ethyl}cyclohexylphosphine [(R,SP)-Pigiphos] 71 as the catalyst (Scheme 24).39 The [3 + 2] cycloaddition gave compounds 70 mainly as the endo-diastereomer in good yields and enantioselectivities.
 | ||
| Scheme 24 Ni-Pigiphos-catalyzed 1,3-DC of C,N-cyclic azomethine imines 57 with unsaturated nitriles. | ||
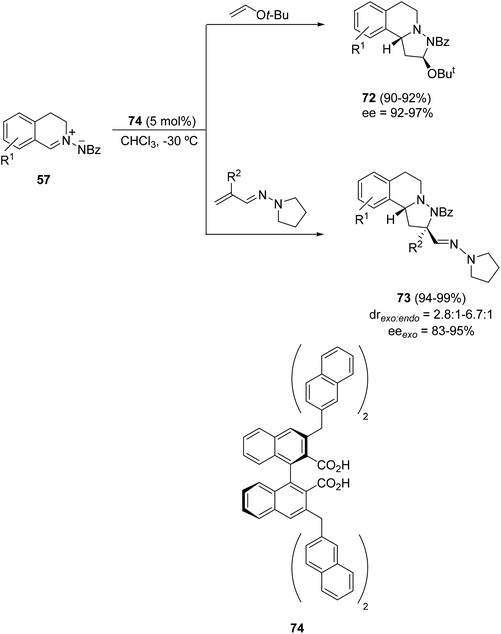
Asymmetric inverse-electron-demand 1,3-DC of C,N-cyclic azomethine imines 57 with tert-butyl vinyl ether could be performed firstly with the chiral dicarboxylic acid 74 as a Brønsted acid (Scheme 25).40 The corresponding adducts 72 were obtained with different regioselectivities by interaction of the LUMO of the dipole with the HOMO of the alkene. Moreover, exo-cycloadducts 72 were obtained in high yields and enantioselectivities. Vinylogous aza-enamines gave mainly exo-cycloadducts 73 in high yields and good enantioselectivities. The hydrazone unit of compound 73 (with R1 = Br, R2 = H) was transformed into the corresponding cyano group by magnesium monoperoxyphthalate in 80% yield.
 | ||
| Scheme 25 Organocatalyzed asymmetric 1,3-DC of cyclic azomethine imine 57 with electron-rich alkenes. | ||
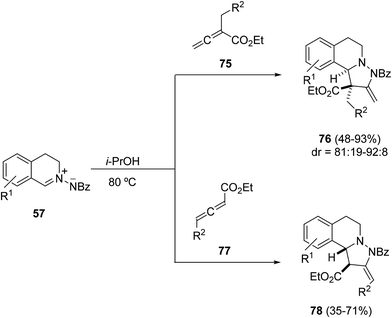
The thermal [3 + 2] cycloaddition reaction of azomethine imines 57 with α-substituted allenoates 75 occurs under mild reaction conditions to provide adducts 76 as a mixture of diastereomers in a highly regioselective manner (Scheme 26).41 The major endo-diastereomer could be separated and isolated by flash chromatography or recrystallization. In the case of γ-substituted allenoates 77 the 1,3-DC takes place in lower yields giving mainly exo-cycloadducts 78.
When the same 1,3-DC was carried out in the presence of a trialkyl phosphine as a catalyst (20 mol%) two different reaction pathways, [3 + 2] and [4 + 3] cyclizations depending on the phosphine and the allenoate were observed. In the case of α-alkyl substituted allenoates, only [3 + 2] cycloaddition products 79 were obtained independently of the phosphine used (Scheme 27).42 However, α-benzyl substituted allenoates gave mainly the diazepine derivatives 80 through a [4 + 3] cycloaddition.
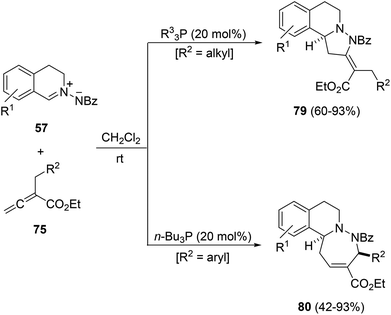 | ||
| Scheme 27 Phosphine-catalyzed [3 + 2] versus [4 + 3] cycloadditions of azomethine imines 57 with allenoates 75. | ||
However, when γ-substituted allenoates 77 were used as dipolarophiles the phosphine-catalyzed 1,3-DC with azomethine imines 57 gave the [3 + 2] cycloaddition. This process has been carried out with the ferrocenyl diphosphine 82 as a chiral catalyst affording tetrahydroisoquinoline derivatives 81 in good yields, high exo-diastereoselectivities and moderate to high enantioselectivities (Scheme 28).43
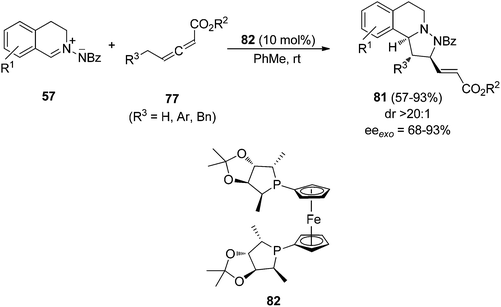 | ||
| Scheme 28 Enantiocatalyzed [3 + 2] cycloaddition of azomethine imines 57 with γ-substituted allenoates 77 by the chiral phosphine 82. | ||
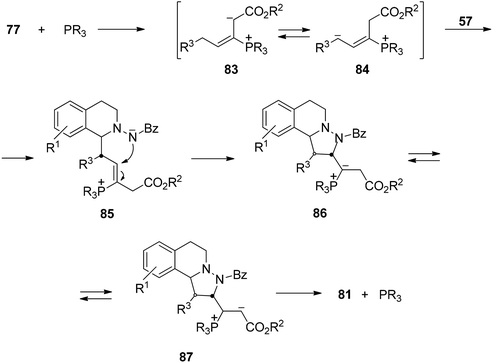
Based on previous experiments about the formation of phosphonium-inner salts by reaction of allenoates with phosphines,44–46 the zwitterionic intermediates 83 and 84 were proposed. Intermediate 84 underwent δ-addition to the azomethine imine 57 to give 85 (Scheme 29).43 Intramolecular Michael addition gave 86, which after [1,2] proton transfer afforded intermediate 87. Final elimination of the phosphine catalyst yielded the cycloadduct 81.
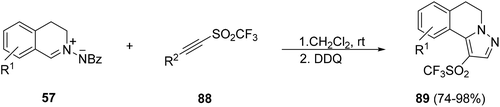
Triflyl alkynes 88 gave 1,3-DC by a reaction with azomethine imines 57 at room temperature, and after oxidative aromatization, pyrazoleisoquinoline triflones 89 were regioselectively obtained (Scheme 30).47
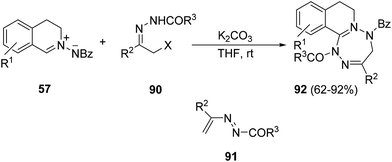
A [4 + 3] cycloaddition has been observed when 1,2-diaza-1,3-dienes 91, generated in situ from the corresponding α-halo hydrazones 90, are allowed to react with C,N-cyclic azomethine imines 57 (Scheme 31).48 These 1,2-diaza-1,3-dienes 91 behave differently compared to azaenamines, which gave [3 + 2] cycloaddition with 57 (Scheme 25).40 In this case, an unprecedented [4 + 3] cycloaddition afforded highly functionalized 1,2,4,5-tetrazepine derivatives 92, which were obtained under mild reaction conditions.
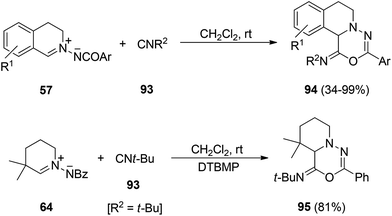
In the case of isocyanides 93 these azomethine imines 57 experimented a [5 + 1] cycloaddition at room temperature leading to the corresponding imino-1,3,4-oxadiazin-6-one derivatives 94 in high yields (Scheme 32).49 Related C,N-cyclic azomethine imine 64 not fused to the aromatic ring also gave this [5 + 1] cycloaddition with tert-butyl isocyanide in the presence of DTBMP as a base, affording product 95 (Scheme 32).49
Amine-catalyzed enantioselective [3 + 2] cycloadditions of aldehydes with azomethine imines 57 led to the formation of adducts 97 (Scheme 33).50 Intermediate enamines formed with the chiral prolinol silyl ether 99 gave intermediate products 96, which after water attack afforded compounds 97. These hemiaminals were reduced in situ with sodium borohydride to 1-substituted tetrahydroisoquinolines 98 in high diastereo- and enantioselectivities.
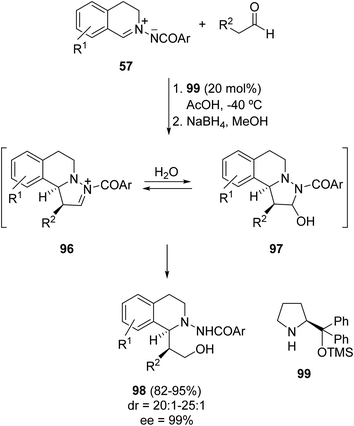 | ||
| Scheme 33 [3 + 2] Cycloaddition of azomethine imines 57 and aldehydes organocatalyzed by the chiral silylated prolinol 99. | ||
The same group performed an enantioselective 1,3-DC using an intermediate dienamine 57 and enals (R2 = aryl), and a silylated prolinol 102 as an organocatalyst. By the subsequent reduction of the aldehyde functionality the corresponding alcohols 100 were isolated in good yields, diastereo- and enantioselectivities (Scheme 34).51 However, when aliphatic enals (R2 = alkyl) were used, regioisomeric derivatives 101 were obtained according to the formation of α,β-unsaturated iminium ions as intermediates. Similar iminium–dienamine reactivity has been reported independently with prolinols 99 and 102 by Alemán and Fraile.52
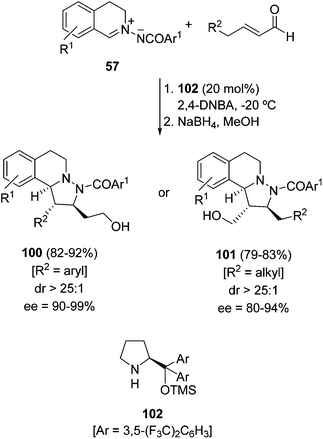 | ||
| Scheme 34 [3 + 2] Cycloaddition of compound 57 with enals organocatalyzed by the chiral silylated prolinol 102. | ||
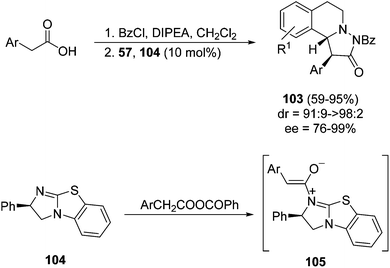
A new type of 1,3-DC has been recently performed with azomethine imines 57 and N-acyliminium ions 105 affording cycloadducts 103 (Scheme 35).53 The chiral Lewis base 104 acted as an organocatalyst forming the corresponding activated intermediates 105 by reaction with mixed anhydrides.
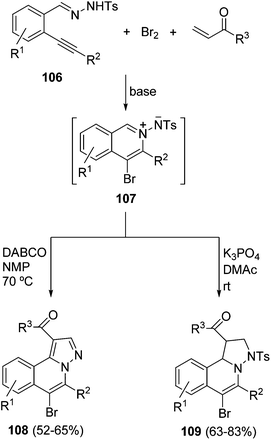
Another family of isoquinolinium ylides are the corresponding unsaturated systems which should be prepared in situ in two- or three-component reactions. Thus, N′-(2-alkynylbenzylidene)hydrazides 106 react with bromine and α,β-unsaturated ketones via a three-component reaction to afford either 6-bromo-4H-pyrazolo[5,1-a]isoquinolines 108 in NMP at 70 °C in the presence of DABCO as a base, or 6-bromo-1,2,3-10b-tetrahydropyrazolo[5,1-a]isoquinolines 109 in DMAc at room temperature in the presence of potassium phosphate as a base (Scheme 36).54 These processes took place by a bromine-promoted 6-endo-cyclization to give the isoquinolinium-2-yl amide 107 followed by a [3 + 2] cycloaddition with the α,β-unsaturated carbonyl compound followed by aromatization. The same group has shown that H-pyrazolo[5,1-a]isoquinolines present promising activity as protein tyrosine phosphatase inhibitors.
When the former process was carried out with 2-alkynyl benzaldehydes 110, p-toluenesulfonyl hydrazide and unsaturated carbonyl compounds in the presence of bromine or iodine, the multicomponent reaction afforded isoquinolines 108 with alkyl groups at the 1 and 5 positions.55 A similar process has been performed using AgOTf as a catalyst, which after a 6-endo–dig cyclization produced the isoquinolinium-2-yl imide 111. The three-component reaction between 2-alkynyl benzaldehydes 110, tosyl hydrazide and α,β-unsaturated carbonyl compounds gave functionalized H-pyrazolo[5,1-a]isoquinoline-1-carboxylates 112 (Scheme 37).56
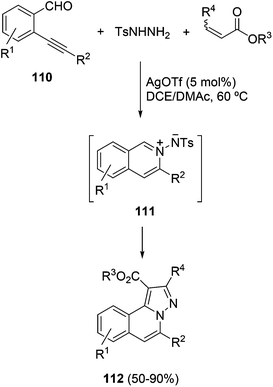 | ||
| Scheme 37 Silver triflate-catalyzed three-component reaction of 2-alkynyl benzaldehydes 110, tosyl hydrazide and α,β-unsaturated esters. | ||
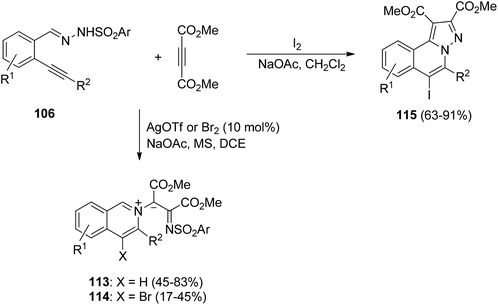
When an acetylenic dipolarophile is used, only a halogen or silver triflate promotes the [3 + 2] cycloaddition. Thus, N′-(2-alkynylbenzylidene)hydrazides 106 react with acetylenes either catalyzed by silver triflate or promoted by bromine or iodine in the presence of NaOAc. In the case of dimethyl acetylenedicarboxylate (DMAD) in the presence of either AgOTf or bromine the fused dihydroisoquinolines undergo a rearrangement involving an N–N homolysis to give compounds 113 or 114, respectively (Scheme 38).57 However, in the presence of iodine the fused 1,2-dihydroisoquinolines 115 are obtained.
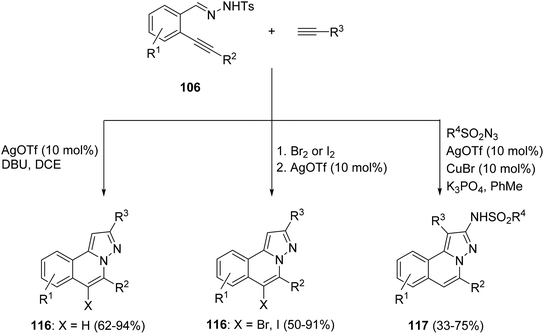
The same process in the presence of terminal acetylenes gave the H-pyrazolo[5,1-a]isoquinolines 116 (X = H)58a in the case of AgOTf or 116 (X = Br, I)58b first by a bromine or iodine promoted cyclization followed by a silver-catalyzed nucleophilic addition of the acetylide to give the isoquinolinium-2-yl imide of the type 117 (Scheme 39). However, when this process is carried out in the presence of tosyl azide with silver triflate and copper(I) bromide as a cocatalyst, 5-sulfonylamine-substituted isoquinolines 117 were obtained (Scheme 39).59
N-Allyl ynamides reacted with N′-(2-alkynylbenzylidene)hydrazides 106 in a process catalyzed by silver triflate and palladium acetate generating 2-amino-H-pyrazolo[5,1-a]isoquinolines 118 in good yields (Scheme 40).60 In this case, the [3 + 2] cycloaddition takes place after the silver-promoted cyclization to give azomethine imines 111, with ynamido-palladium π-allyl complexes 119 affording intermediates 120. Subsequently, an intramolecular [3,3]-sigmatropic rearrangement produces compound 121, which undergoes aromatization releasing a tosyl group.
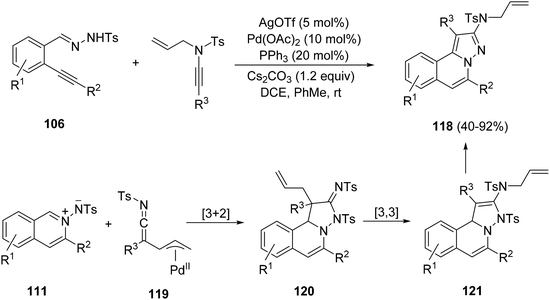 | ||
| Scheme 40 Synthesis of compounds 118 by AgOTf and Pd(OAc)2 catalyzed cascade reactions of hydrazides 106 with N-allyl ynamides. | ||
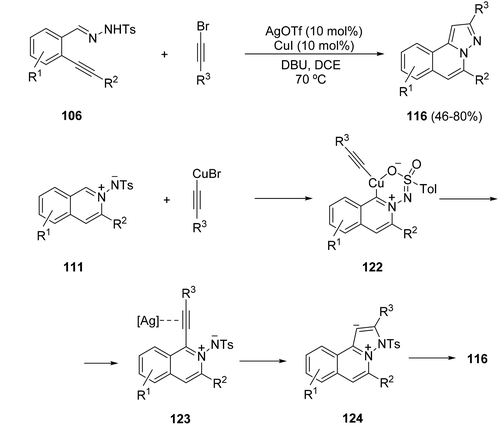
An alternative route to pyrazoloisoquinolines 116 (X = H) used bromoalkynes as dipolarophiles. In this case, the alkynylation of 111, formed by the silver-catalyzed cyclization of 106, takes place by a C–H activation. The bromoalkyne is activated via oxidative addition to CuI, which through a concerted metallation–deprotonation process would give intermediates 122. After reductive elimination, intermediates 123 undergo a 5-endo–dig-cyclization to give 124, followed by subsequent aromatization to form the final H-pyrazolo[5,1-a]isoquinolines 116 (Scheme 41).61
2-Trifluoromethylpyrazolo[5,1-a]isoquinolines 125 can be prepared from N′-(2-alkynylbenzylidene)hydrazides 106 and ethyl 4,4,4-trifluorobut-2-ynoate by means of the tandem silver triflate catalyzed cyclization and [3 + 2] cycloaddition of the corresponding N-iminoisoquinolinium ylides 111 (Scheme 42).62
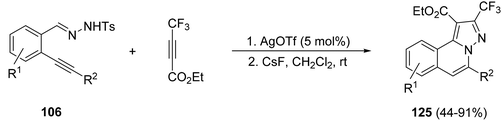 | ||
| Scheme 42 Synthesis of compounds 125 by a [3 + 2] cycloaddition of N-iminoisoquinolinium ylides 111. | ||
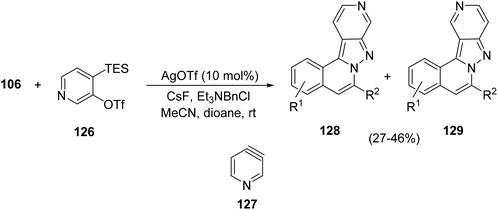
In the case of the silver triflate-catalyzed cyclization of compounds 106 in the presence of the in situ generated pyridyne 127 from 126, the corresponding regioisomeric H-pyrazolo[5,1-a]isoquinolines 128 and 129 were prepared in modest yields (Scheme 43).63a Polyfluoroarenes react with N′-(2-alkynylbenzylidine) hydrazide 106 catalyzed by silver triflate in the presence of cesium carbonate leading to polyfluoroaryl-fused H-pyrazolo[5,1-a]isoquinolines in good yields.63b Recently, the three-component reaction of aldehydes 110, sulfonyl hydrazide and benzyne, affording the corresponding H-pyrazolo[5,1-a]isoquinolines in very good yields (83–98%), has been described.63c
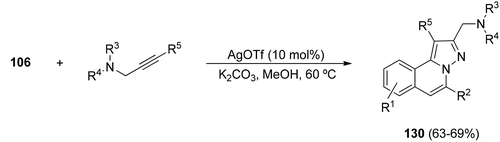
Propargyl amines afford [3 + 2] cycloadditions with N-iminoisoquinolinium ylides 111 generated in situ from hydrazides 106, to give the corresponding H-pyrazolo[5,1-a]isoquinolines 130 bearing an aminomethyl substituent at the 5-position (Scheme 44).64
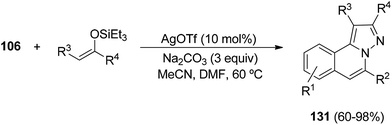
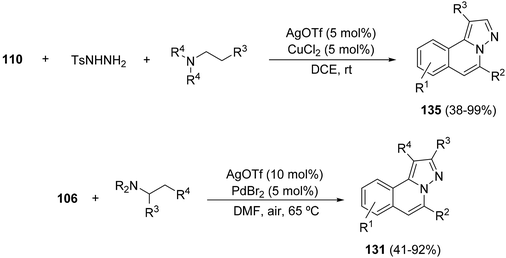
Silyl enol ethers have been used as dipolarophiles with N-iminoisoquinolinium ylides 111, generated in situ from hydrazides 106. Thus, the tandem process affords the 5,6-disubstituted H-pyrazolo[5,1-a]isoquinolines 131 in good yields (Scheme 45).65
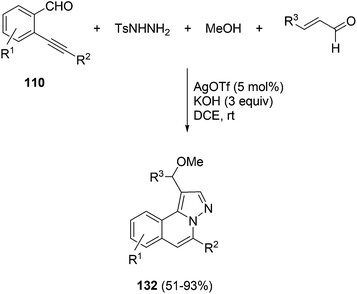
The multicomponent reaction of 2-alkynyl benzaldehydes 110, tosyl hydrazide, methanol and α,β-unsaturated aldehydes catalyzed by silver triflate gave H-pyrazolo[5,1-a]isoquinolines 132 with excellent regioselectivity (Scheme 46).66 Preliminary biological assays of these compounds show their promising activity as CDC25B, TC-PTP, and PTP1B inhibitors.
Similarly, the bromine-promoted cyclization of hydrazides 106 afforded the brominated N-iminoisoquinolinium ylides 107, which also react with α,β-unsaturated aldehydes in the presence of methanol to give the fused brominated isoquinolines 133 (Scheme 47).67
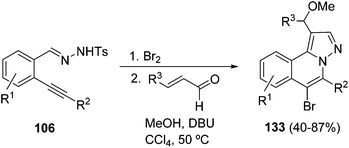 | ||
| Scheme 47 Bromine promoted cyclization of hydrazides 106 and [3 + 2] cycloaddition with α,β-unsaturated aldehydes. | ||
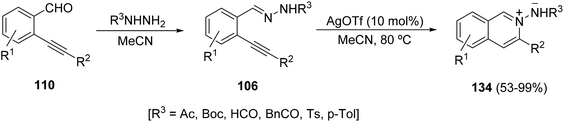
Based on the former methods for the in situ generation of isoquinolinium-2-yl imides 134, these azomethine imines have been recently prepared and isolated by the one-pot reaction of 2-alkynyl benzaldehydes 110, hydrazides and final silver triflate catalyzed cyclization (Scheme 48).68
A silver-catalyzed process involving 2-alkynyl benzaldehydes 110, tosyl hydrazide and carbonyl compounds is a simple and direct strategy for the synthesis of H-pyrazolo[5,1-a]isoquinolines 131 (Scheme 49).69
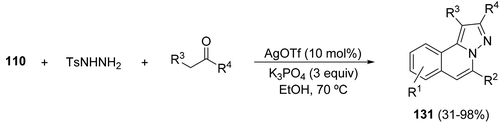 | ||
| Scheme 49 One-pot tandem reaction of 2-alkynyl benzaldehydes, tosylhydrazide and carbonyl compounds. | ||
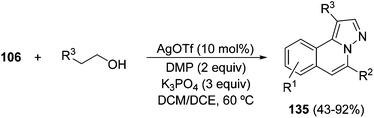
Alternatively, by using primary alcohols and hydrazides 106 instead of aldehydes 110, the presence of the Dess–Martin reagent (DMP) as an oxidant is compulsory to afford 6-monosubstituted H-pyrazolo[5,1-a]isoquinolines 135 (Scheme 50).70a,b
The same transformation can be performed by a silver triflate–palladium chloride cooperative catalysis. The presence of oxygen promotes the palladium-catalyzed oxidation of the alcohol to the corresponding aldehyde or ketone. The in situ generated enolate attacks the isoquinolinium-2-yl imide, followed by condensation and aromatization to afford products 135 in 47–90% yield.70c
Silver triflate–copper(II) acetate cooperative catalysis has been used for the cyclization/[3 + 2] cycloaddition of N′-(2-alkynylbenzylidene) hydrazides 106 with allenoates 77 in the presence of dioxygen to afford H-pyrazolo[5,1-a]isoquinolines 136 (Scheme 51).71a The proposed mechanism involves a peroxy-copper(III) intermediate 138, which evolves to 139 and, after elimination of Cu(II)–OH, generates a carbonyl compound 140. Final aromatization yielded products 136 in moderate to good yields. When this reaction was performed with Ph3P as a catalyst the corresponding isoquinolines 136 were obtained with an R3CH2 group instead of the ketone functionality.71b
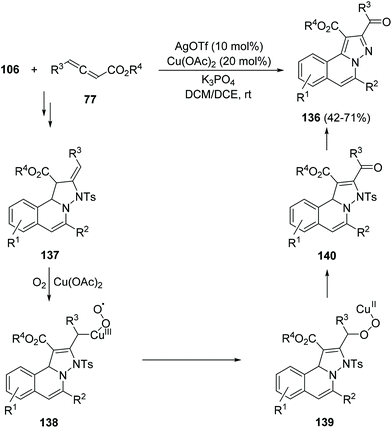 | ||
| Scheme 51 Reaction of hydrazides 106 with allenoates 77 in the presence of dioxygen cocatalyzed by silver triflate and copper(II) acetate. | ||
Silver–rhodium(I) cooperative catalysis has been used for the reaction of hydrazides 106 with cycloprop-2-ene-1,1-dicarboxylate72 or with 2-vinyloxirane73 for the synthesis of the corresponding H-pyrazolo[5,1-a]isoquinolines 141 or 142, respectively (Scheme 52). The use of the Wilkinson catalyst is crucial for the [3 + 2] cycloaddition.
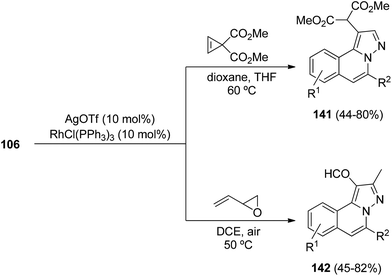 | ||
| Scheme 52 Silver–rhodium(I) cooperative catalysis in the reaction of 106 with cycloprop-ene-1,1-dicarboxylate or 2-vinyloxirane. | ||
When silver triflate and copper(II) chloride are used as cooperative catalysts it is possible to prepare H-pyrazole[5,1-a]isoquinolines 135 through a three-component process. Thus, 2-alkynyl benzaldehydes, tosyl hydrazide and tertiary amines in air gave products 135 by a silver-catalyzed cyclization and copper(II)-catalyzed oxidation of an aliphatic C–H bond of the tertiary amine in air (Scheme 53).74 A related process using palladium dibromide as a cocatalyst gave isoquinolines 131, which has been performed starting from the hydrazides 106 instead of aldehydes 110 (Scheme 53).75a The same transformation has been previously performed using Fe2(CO)9 as a cocatalyst (5 mol%) and tert-butyl hydroperoxide (3 equiv.) affording products 135 in 46–83% yields.75b
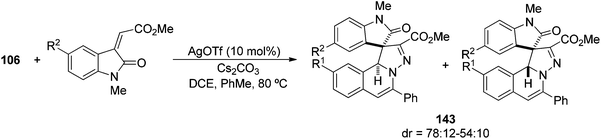
Methylene indolinones have been used as dipolarophiles for the diastereoselective construction of fused H-pyrazolo[3,2-a]isoquinolines 143 as a mixture of diastereomers (Scheme 54).76 In this case, the Wu et al.56 methodology was applied to a process starting from N′-(alkynylbenzylidene) hydrazides 106 under silver-catalyzed 6-endo cyclization to generate the N-iminoisoquinolinium ylide 111.
In general, these unsaturated isoquinolinium imides have been mainly used in [3 + 2] cycloaddition with acetylenic dipolarophiles. The only example of a [3 + 3] cycloaddition of azomethine imines 144 has been performed using cyclopropane diesters and a Ni(ClO4)2 complex with trisoxazoline derivatives 146 as a chiral ligand.77 This process allows the preparation of 6,6,6-tricyclic dihydroisoquinoline derivatives 145, in general with high diastereo- and enantioselectivities (Scheme 55). This reaction is based on the non-asymmetric example described previously by Charette et al.78 with N-benzoyliminoisoquinolinium ylide and methyl 2-phenylcyclopropane-1,1-dicarboxylate catalyzed by Ni(ClO4)2 in a modest yield (21%).
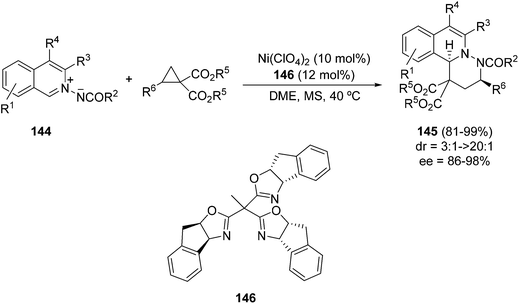 | ||
| Scheme 55 Enantiocatalyzed [3 + 3] cycloaddition of azomethine imines 144 and cyclopropane diesters. | ||
3.3 Pyridinium and quinolinium imides
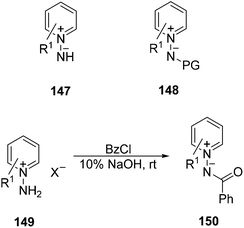
Pyridinium imides 147, also called N-iminopyridinium ylides or pyridin-N-imines, are masked cyclic azomethine imines incorporating C–N into the ring, which react mainly with acetylenic dipolarophiles.10 They are unstable and have to be generated in situ from N-aminopyridinium halides and since the aromaticity of pyridine is broken after the cycloaddition, they have to be oxidized to give the desired product. A greater stability can be achieved by introducing an electron-withdrawing group such as acyl, alkoxycarbonyl, or sulfonyl to afford ylides 148. N-Benzoyliminopyridinium ylides 150 have been the most used imines of this family. They can be prepared by benzoylation of the corresponding N-aminopyridinium salts 149 (Scheme 56) easily accessible by direct N-amination of pyridine using hydroxylamine-O-sulfonic acid.79–81 Alternatively, N-aminopyridinium salts 147 can be prepared using different electrophilic amination reagents, especially efficient being O-(2,4-dinitrophenyl) hydroxylamine, which gave good yields with several types of substituted pyridines, quinolines and isoquinolines.82By using alkynes as dipolarophiles pyrazolopyridines can be prepared83 which exhibit a wide range of biological activities including dopamine D3 receptor antagonist and partial agonist,84 dopamine D4 antagonist,85 as well as adenosine A1 receptor antagonist,86 and antiherpetic87 and antiallergenic88 properties. Consequently, they are applicable in the treatment of neurological disorders such as schizophrenia, attention-deficit disorder, and Parkinson's disease.
Polystyrene-bound alkenes 152 have been used for the solid-phase synthesis of pyrazolopyridines 153 by in situ generation of pyridinium imides 147 from N-aminopyridinium salts 151 followed by TFA cleavage (Scheme 57). Alternatively, by using NaOMe in THF/MeOH the corresponding methyl esters can be isolated.89
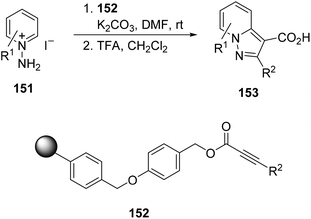 | ||
| Scheme 57 Solid-phase synthesis of pyrazolopyridines 153 by a [3 + 2] cycloaddition of pyridinium imides with polymer-bound propiolates 152. | ||
In the case when arynes are used as dipolarophiles and pyridinium imides 148 with different electron-withdrawing groups on the imide nitrogen, it was found that the pyrido[1,2-b]indazoles 155 are obtained in high yields (Scheme 58) using the tosyl derivatives 154, whereas the benzoyl, pivaloyl, benzyloxycarbonyl, and tert-butyloxycarbonyl ones gave lower results.90 This methodology has been also used with N-tosylisoquinolinium imides to afford indazolo[3,2-a]isoquinolines.
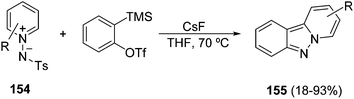 | ||
| Scheme 58 Synthesis of pyrido[1,2-b]indazoles 155 by aryne [3 + 2] cycloaddition with N-tosyl pyridinium imides. | ||
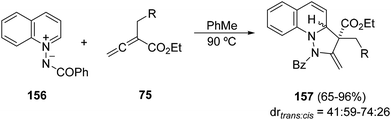
The thermal [3 + 2] cycloaddition of N-benzoylquinolinium imide 156 with allenoates 75 provides products 157 in good yields, albeit with poor diastereoselectivity (Scheme 59).91 This cycloaddition has been carried out with N-benzoylisoquinolinium imides with similar results concerning yield and diastereoselectivity.
A formal [3 + 2] cycloaddition catalyzed by a gold complex 159 between N-benzoyliminopyridinium ylides 158 and N-alkynylsulfonamides gave 2,4,5-trisubstituted oxazoles 160 in high yields (Scheme 60).92
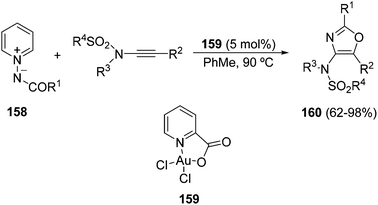 | ||
| Scheme 60 Gold-catalyzed formal [3 + 2] cycloaddition of N-benzoyliminopyridinium ylides 158 with N-alkynyl sulfonamides. | ||
The benzocondensed azomethine imines 156 have been employed in [3 + 3] cycloadditions. Thus, the nickel-catalyzed [3 + 3] cycloaddition of N-benzoylquinolinium imide 156 with 1,1-cyclopropane diesters provided products 161 in modest to good yields and moderate diastereoselectivity (Scheme 61).78 This cycloaddition has also been performed with the N-benzoylisoquinolinium ylide with a modest yield (21%).
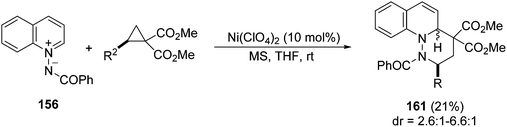 | ||
| Scheme 61 Ni-catalyzed formal [3 + 3] cycloaddition of quinolinium imides and 1,1-cyclopropane diesters. | ||
An enantioselective formal [3 + 3] cycloaddition has been performed also with N-benzoylpyridinium ylides 158 and silylated enol diazoacetates 162 using the rhodium catalysts 164. Bicyclic dearomatized 1,2,3,6-tetrahydropyridazine derivatives 163 were obtained in high yields and enantioselectivities (Scheme 62).93 The reaction is triggered by Rh(II)-catalyzed dinitrogen extrusion with formation of a rhodium carbenoid intermediate followed by addition of the pyridinium ylide.
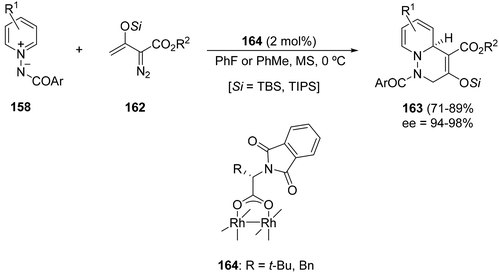 | ||
| Scheme 62 Rh-catalyzed formal [3 + 3] cycloaddition of N-acylpyridinium ylides 158 with silylated enol diazoacetates 162. | ||
4 Cyclic azomethine imines incorporating a N–N bond in a ring
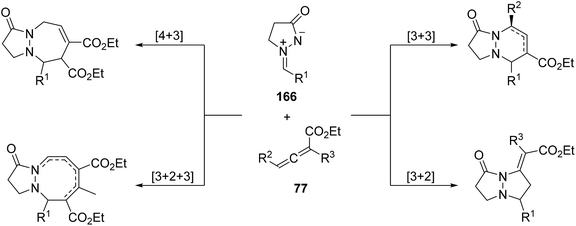
The most studied cyclic azomethine imines incorporating a N–N bond in a ring are N-alkylidene-3-oxopyrazolidinium imides 166, which are stable and readily accessible. They have been employed as 1,3-dipoles in thermal and metallo- or organo-catalyzed cycloadditions, not only [3 + 2] but also [3 + 3], [4 + 3] and [3 + 2 + 3] ones. These annulation reactions gave rise to dinitrogen-fused heterocycles including tetrahydropyrazolo-pyrazolones, -pyridazinones, -diazepinones, and -diazocinones, which are important products or intermediates for the preparation of useful chemicals and diverse bioactive molecules.4.1 N-Alkylidene-3-oxopyrazolidin-1-ium-2-ides
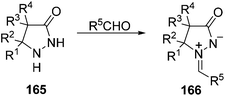
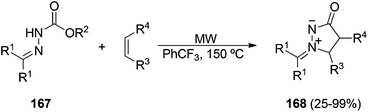
Azomethine imines 166, derived from pyrazolidin-3-ones 165, are usually prepared by condensation with carbonyl compounds.10 They can be isolated, especially in the case of aromatic aldehydes, by heating in anhydrous methanol catalyzed by means of trifluoroacetic acid (Scheme 63).10,94A new route to azomethine imines has recently been described using hydrazones derived from ketones and N-alkoxycarbonylhydrazines 167 and alkenes (Scheme 64).95 Under microwave assisted heating at 150 °C the intermediate isocyanate is formed and through a concerted alkene aminocarbonylation pathway the corresponding azomethine imines 168 are produced in good yields. Several types of acyclic and cyclic alkenes can be used, including vinyl ethers and enamides. With terminal alkenes (R4 = H) a total regioselectivity was observed.
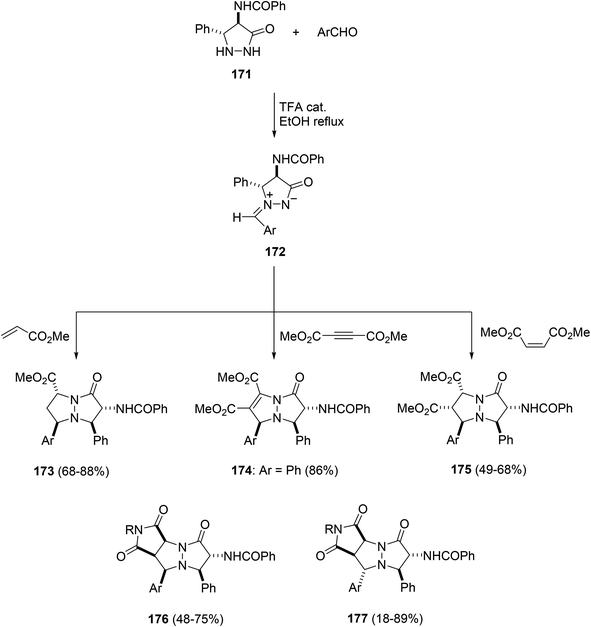
Stereoselective synthesis of fused pyrazolones has been studied with racemic pyrazolidin-3-one 171, which after reaction with benzaldehydes, followed by [3 + 2] cycloadditions of the generated azomethine imines 172 with electron-deficient dipolarophiles such as methyl acrylate, dimethyl acetylene dicarboxylate and dimethyl maleate, gave pyrazolopyrazolone derivatives 173–175 with high stereocontrol (Scheme 66).96,97 The stereoselective cycloaddition of azomethine imines 172 with maleimides provided cycloadducts 176 when the aldehydes had no substituents at the ortho-position. However, with ortho-substituted aldehydes diastereomeric adducts 177 were formed.98,99 Similarly, butyraldehyde and acetone react with pyrazolidinone 171 under an acid-catalyzed process to afford the corresponding azomethine imines, which react under thermal conditions with dimethyl acetylenedicarboxylate, methyl acrylate, methyl maleate or fumarate and N-phenylmaleimide to give the corresponding cycloadducts.100
In the case of the Cu(I)-catalyzed [3 + 2] cycloaddition of azomethine imines 172 (Ar = Ph) it takes place at room temperature in acetonitrile using Hünig's base with methyl propiolate giving the product 178 (Scheme 67).101 When the chiral ynone 179 was allowed to react with 172 a mixture of diastereomeric cycloadducts 180 was obtained.
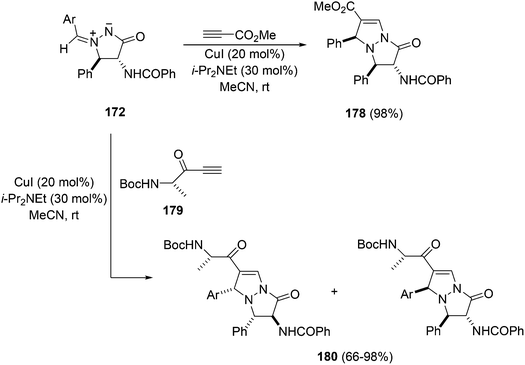 | ||
| Scheme 67 Diastereoselective Cu(I)-catalyzed [3 + 2] cycloaddition of azomethine imine 172 with acetylenic dipolarophiles. | ||
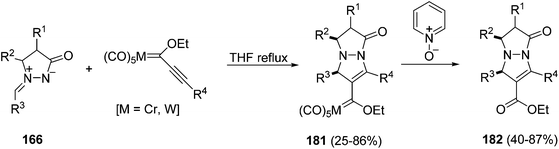
Azomethine imines 166 and 172 derived from unsubstituted 165 and substituted 171 pyrazolidin-3-ones, respectively, have been used as dipoles in the reaction with α-(trifluoromethyl)acrylates affording the corresponding adducts with a moderate diastereoselectivity.102 In the case of alkynyl Fischer carbene complexes a regioselective [3 + 2] cycloaddition takes place giving, after oxidative demetallation, the corresponding functionalized pyrazolopyrazolone derivatives 182 (Scheme 68).103
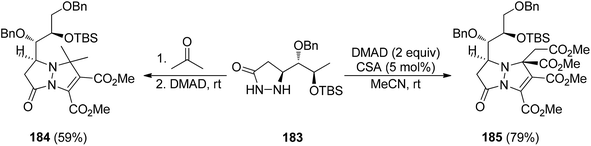
Several pyrazolidin-3-ones bearing a chiral chain have been applied as precursors of chiral azomethine imines in diastereoselective [3 + 3] cycloadditions. Thus, 5-substituted pyrazolidin-3-ones 183 derived from 2,3-unsaturated sugar 1,5-lactones react at room temperature with acetone and then with dimethyl acetylenedicarboxylate (DMAD) to provide either the cycloadduct 184 or two equivalents of DMAD, under camphorsulfonic acid (CSA) catalysis, to give mainly the cycloadduct 185 (Scheme 69).104
The intramolecular [3 + 2] cycloaddition of azomethine imine 187 derived from pyrazolidin-3-one 165 (R1 = R2 = R3 = R4 = H) and the glucose-derived aldehyde 186 gave the diazatriquinane 188 (Scheme 70).105 This methodology has been studied with different sugar-derived hexen-5-als giving the corresponding diazatriquinanes in high yields and total stereocontrol, which were used for biological screening.
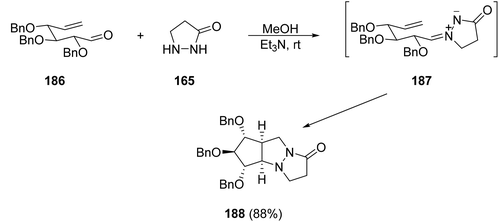 | ||
| Scheme 70 Synthesis of polyoxygenated diazatriquinane 188via intramolecular diastereoselective [3 + 2] cycloaddition. | ||
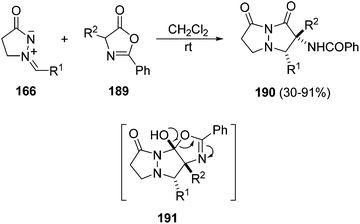
Different types of dipolarophiles have been investigated under thermal conditions with azomethine imines 166 derived from unsubstituted 165, for example, acetylenic sulfones,106 arynes,107 β-nitrostyrenes,108 cyclic vinyl sulfones,109 and trifluoroethylidene malonates.110 In the case when azlactones 189 are used as dipolarophiles, a [3 + 2] cycloaddition, followed by a rearrangement at room temperature, gave the pyrazolopyrazolone derivatives 190 (Scheme 71).111 The cycloadduct intermediates 191 are unstable and undergo a rearrangement affording 190 with high diastereoselectivity (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
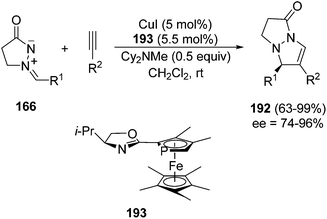 | ||
| Scheme 72 Enantioselective copper-catalyzed [3 + 2] cycloaddition of azomethine imines ylides 166 with terminal alkynes. | ||
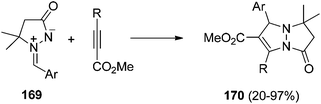
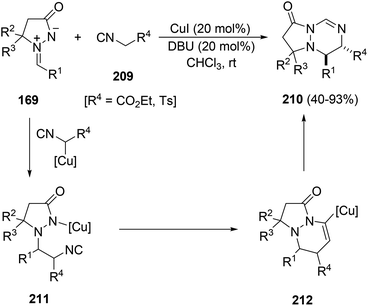
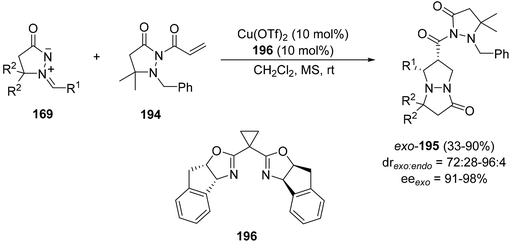
The [3 + 2] cycloaddition of azomethine imines 169 with the N-acryloylpyrazolidinone 194 catalyzed by the chiral complex Cu(OTf)2·bisoxazoline 196 gave regio- and diastereoselectively exo-cycloadducts 195 in good yields (Scheme 74).114 These processes have been performed only with pyrazolidinone 194, which is able to be chelated by the copper complex and different C5-substituted azomethine imines.
 | ||
| Scheme 74 Enantioselective copper-catalyzed [3 + 2] exo-cycloaddition of azomethine imines 169 with the pyrazolidinone 194. | ||
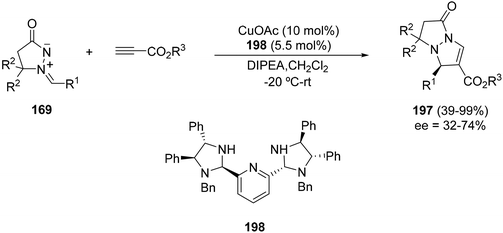
The chiral bis(imidazolidine) 198 CuOAc complex has been used as a catalyst for the [3 + 2] cycloaddition of the azomethine imine 169 with propiolates affording cycloadducts 197 with modest enantioselectivities (Scheme 75).115
 | ||
| Scheme 75 Enantioselective copper-catalyzed [3 + 2] cycloaddition of azomethine imines 169 with propiolates using ligand 198. | ||
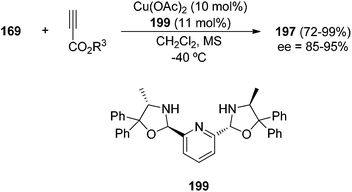
The same group developed a better ligand PyBodine (L-Ala) 199, which is able to perform this cycloaddition with Cu(OAc)2 as a metallic salt in better yields and enantioselectivities (Scheme 76).116
 | ||
| Scheme 76 Enantioselective copper-catalyzed [3 + 2] cycloaddition of 169 with propiolates using ligand 199. | ||
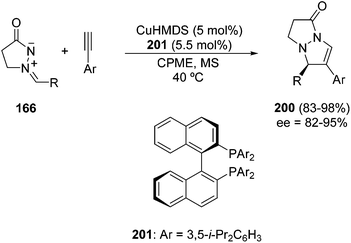
Group 11 metal amides, copper(I) and silver bis(trimethylsilyl)amides (HMDS) in THF catalyzed the same [3 + 2] cycloaddition of 166 with terminal alkynes with opposite regioselectivity. This process has been performed in an enantioselective manner when (S)-DIP-BINAP ligand 201 was used with CuHMDS. Thus, the corresponding 5,7-disubstituted cycloadducts 200 were obtained in good yields and enantioselectivities (Scheme 77).117,118 This regioselectivity is explained by 1,2-addition of the copper acetylide to the iminium moiety followed by intramolecular cyclization.
 | ||
| Scheme 77 Enantioselective copper-catalyzed [3 + 2] cycloaddition of azomethine imines 166 with terminal alkynes. | ||
By using propiolylpyrazoles 202 as acetylenic dipolarophiles, terminal and internal alkynes gave very good enantioselection in the [3 + 2] cycloaddition of azomethine imines 166 catalyzed by a chiral π-cation catalyst 204 (Scheme 78).119 The main difference of this type of copper catalyst compared to the previous ones is that the copper(I) acetylide-mediated cycloaddition of azomethine imines with terminal alkynes is not operating (Method A). Instead, a Lewis acid-catalyzed cycloaddition by coordination with the carbonyl group (Method B) takes place (Scheme 79).
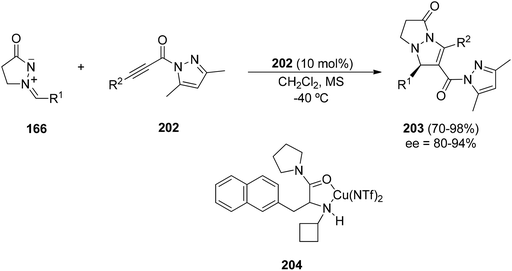 | ||
| Scheme 78 Enantioselective copper-catalyzed [3 + 2] cycloaddition of 166 with propiolylpyrazoles catalyzed by the chiral complex 202. | ||
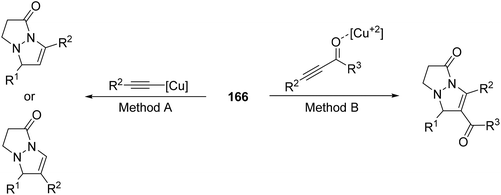 | ||
| Scheme 79 Strategies for the copper-catalyzed [3 + 2] cycloaddition of azomethine imines 166 with alkynes. | ||
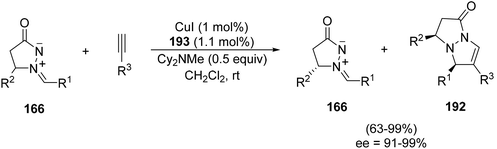
The racemic copper-catalyzed [3 + 2] cycloaddition has been performed not only with CuI but also with Cu(I) zeolites as the heterogeneous ligand-free catalysts.120,121 They are easy to be removed by simple filtration and can be recycled up to six times without decreasing the efficiency. Heterogeneous supported copper hydroxide Cu(OH)x/Al2O3 has also been used as an efficient reusable catalyst.122
The catalytic asymmetric cross-1,3-DC of two different dipoles, azomethine ylides generated from iminoesters 205 and imines 166, gave highly substituted 1,2,4-triazinanes with total diastereo- and enantioselectivity. (S, SP)-t-Bu-Phosferrox 207 as a ligand and AgOAc or Cu(MeCN)BF4 salts have been assayed as chiral catalysts for this [3 + 3] cycloaddition. The best results were obtained with the Cu complex giving the cycloadducts 206 in high yields, diastereo- (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enantioselectivities (Scheme 80).123
1) and enantioselectivities (Scheme 80).123
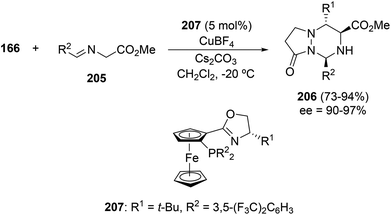 | ||
| Scheme 80 Enantioselective copper-catalyzed [3 + 3] cycloaddition of azomethine ylides and azomethine imines. | ||
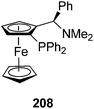
Independently, a similar [3 + 3] cycloaddition has been performed using the ferrocenyl P,N-chiral ligand 208 and the Cu(MeCN)4ClO4 salt as a catalyst. This process takes place giving products 206 with good yields (71–89%), diastereo- (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and enantioselectivities (50–96%).124
1) and enantioselectivities (50–96%).124
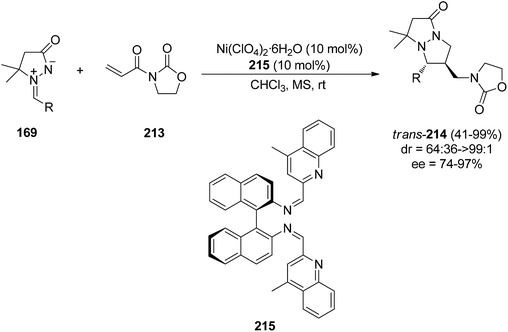 | ||
| Scheme 82 Enantioselective Ni(II)-catalyzed [3 + 2] cycloaddition of azomethine imines 169 and 3-acryloyl-2-oxazolidinone 213. | ||
Recently, a Ni(II)-catalyzed enantioselective [3 + 2] cycloaddition of azomethine imine 166 and alkylidene malonates as dipolarophiles has been described. In this case trans-pyrazolone derivatives 216 have been obtained with total diastereoselectivity and good enantioselectivities by using a chiral N,N′-dioxide 217 as the chiral ligand (Scheme 83).127 The reaction also proceeds by a dipole–HOMO/dipolarophiles–LUMO interaction, the Ni-complex acting as a chiral Lewis acid coordinating the two carbonyl groups of the alkylidene malonate.
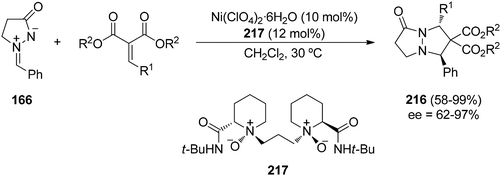 | ||
| Scheme 83 Enantioselective Ni(II)-catalyzed [3 + 2] cycloaddition of the azomethine imine 166 and alkylidene malonates. | ||
The palladium-catalyzed [3 + 3] cycloaddition of trimethylenemethane (TMM) with azomethine imines 166 led to the formation of six-membered cycloadducts 219.128 Starting from [2-(acetoxymethyl)-2-propenyl]trimethylsilane 218, the Pd–TMM complex reported by Trost,129 generated from Pd(PPh3)4 in DCM, gave the best results (Scheme 84). Azomethine imines bearing substituents on the pyrazolidinone ring can also be used in this [3 + 3] cycloaddition giving the hexahydropyridazines in high diastereoselectivity. However, using substituted TMM different products are formed.
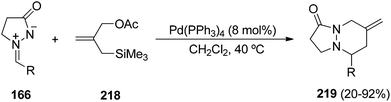 | ||
| Scheme 84 Palladium-catalyzed [3 + 3] cycloaddition of trimethylenemethane with the azomethine imines 166. | ||
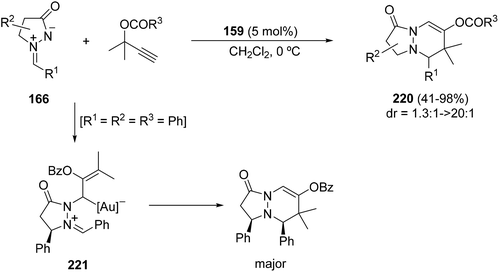
Gold-catalyzed [3 + 3] cycloadditions of azomethine imines 166 and propargyl esters have been observed to proceed by a stepwise mechanism with a gold(III) carbenoid 221 as an intermediate. The reaction takes place in the presence of 5 mol% of picolinate–gold dichloride (159) as a catalyst affording adducts 220 with moderate to high diastereoselectivity (Scheme 85).130
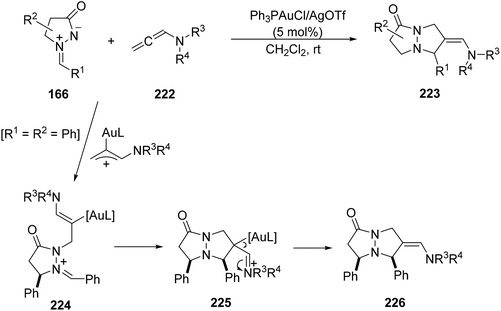
N-Allenyl amides 222 underwent 1,3-DC of azomethine imines 166 under gold(I) catalysis to provide [3 + 2] cycloadducts 223 (Scheme 86).131 This process can occur through a gold allene intermediate, which can give another intermediate 224 by an outer-sphere nucleophilic addition. Subsequent intramolecular cycloaddition of 224 yielded the iminium intermediate 225, which after deauration gave the final cycloadduct 226.
The asymmetric 1,3-DC of azomethine imines 166 to allyl alcohol was possible using stoichiometric amounts of a strong Lewis acid formed by diisopropyl (R,R)-tartrate (DIPT) and an excess (3 equiv.) of butylmagnesium bromide necessary for the deprotonation of allyl alcohol to form the intermediate 228. The reaction proceeds at 80 °C in acetonitrile affording only the corresponding trans-pyrazolinonepyrazolidines 227 (Scheme 87).132,133
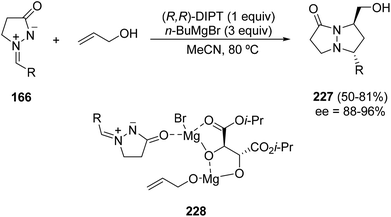 | ||
| Scheme 87 Enantioselective [3 + 2] cycloaddition of azomethine imines 166 with allyl alcohol catalyzed by magnesium diisopropyl (R,R)-tartrate. | ||
Under similar reaction conditions homoallylic alcohols have been used for this type of [3 + 2] cycloaddition. In this case only 20 mol% of DIPT, one equivalent of MgBr2 and 1.5 equivalents of n-BuMgCl were used providing also the trans-cycloadducts 230 in 23–93% yield and 63–93% ee.133,134 In the proposed transition state 229, the azomethine imine is coordinated to magnesium by the nitrogen and the carbonyl group to afford pyrazolidinones 230 (Scheme 88). The same group has developed a desymmetrization of 1,4-pentadien-3-ol by the asymmetric 1,3-DC of azomethine imines using magnesium diisopropyl tartrate as a chiral Lewis acid in up to 98% ee.135
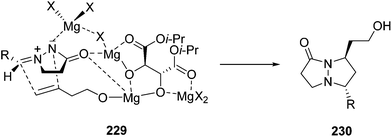 | ||
| Scheme 88 Enantioselective [3 + 2] cycloaddition of azomethine imines 166 and homoallyl alcohol catalyzed by magnesium diisopropyl tartrate. | ||
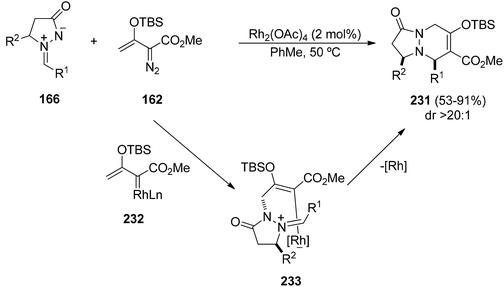
Doyle et al. have studied enol diazoacetate 162 as a dipolarophile for the [3 + 2] cycloaddition with azomethine imines 166 catalyzed by Sc(OTf)3 or In(OTf)3 as Lewis acids.136 The corresponding cycloadducts 232 are obtained diastereoselectively in good yields. Selective 1,2-C→C and N→C migrations catalyzed by rhodium(II) salts or CuPF6 were observed to give six membered rings. However, using rhodium(II) acetate the corresponding [3 + 3] annulation products cis-231 were regio- and diastereoselectively obtained (Scheme 89).137 The azomethine imine attacks the vinylogous position of the Rh(II)-vinyl carbine 232 to give the intermediate 233, which after subsequent ring formation followed by extrusion of the catalyst gives the fused bicyclic pyrazolidinones 231.
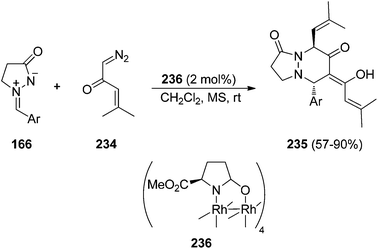
When a diazoketone 234 was used as a dipolarophile a formal [3 + 2 + 1] annulation with azomethine imines 166 was observed (Scheme 90).138 In this case, a similar intermediate metal carbine 232 (Scheme 89) is trapped by another molecule of the diazoketone 234 to give diastereoselectively products 235 by means of the chiral dirhodium(II) carboxamidate 236.
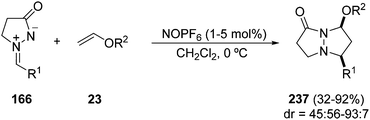
For electron-rich alkenes, such as vinyl ethers 23, the highly reactive nitrosonium hexafluorophosphate must be used as a catalyst for the [3 + 2] cycloaddition of azomethine imines 166 (Scheme 91). The corresponding fused pyridazinones 237 were obtained with low to good cis/trans diastereoselectivity.139
The first organocatalyzed asymmetric [3 + 2] cycloaddition of α,β-unsaturated aldehydes to azomethine imines 116 was carried out using the α,α-diarylprolinol salt 239. The enal activation takes place by iminium formation giving the corresponding fused pyrazolidinones 238 mainly with exo-selectivity and high enantioselectivities (Scheme 92).140 The possible reaction models for this 1,3-DC are illustrated in Scheme 92: both transition states A and B with s-cis and s-trans conformations, respectively, would afford the exo-cycloadducts.
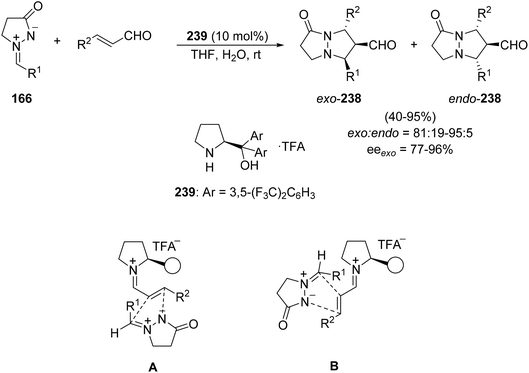 | ||
| Scheme 92 Organocatalyzed enantioselective [3 + 2] cycloaddition of azomethine imines 166 with enals. | ||
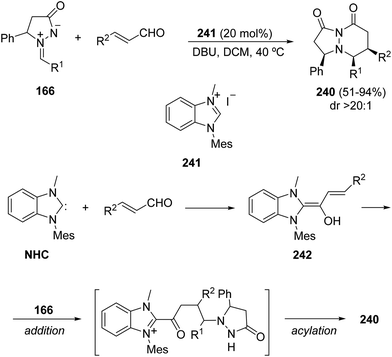
When this reaction is catalyzed by N-heterocyclic carbenes, a highly stereoselective formal [3 + 3] cycloaddition takes place to provide pyridazinones 240 (Scheme 93).141 The addition of the N-mesitylbenzimidazolyl carbene, generated from the benzimidazolium iodide 242, by an addition/acylation sequence with 166 affords the final bicyclic heterocycles 240.
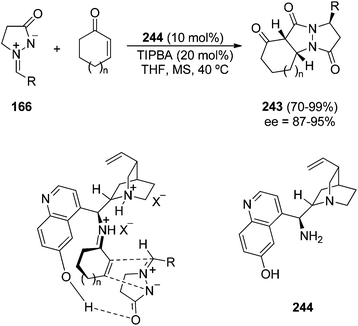
The enantioselective [3 + 2] cycloaddition of cyclic enones and azomethine imines 166 has been performed in the presence of the chiral primary amine 9-amino-9-deoxyepiquinine 244 and 2,4,6-triisopropylbenzenesulfonic acid (TIPBA) as a catalyst (Scheme 94).142 The corresponding tricyclic pyrazolidinones 243 were obtained in good yields, diastereo- and enantioselectivities. The Cinchona derived catalyst activates the enone forming a ketiminium cation and an additional hydrogen bonding between the OH and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups to produce the endo and Re-face selectivities in the final cycloadducts.
O groups to produce the endo and Re-face selectivities in the final cycloadducts.
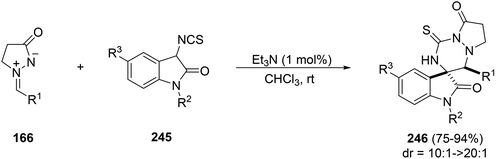
The base-catalyzed diastereoselective [3 + 3] annulations of 3-isothiocyanatooxindoles 245 to azomethine imines 166 gave 3,3′-triazinyl spirooxindoles 246 (Scheme 95).143 Using 1 mol% of triethylamine the reaction takes place in only five minutes at room temperature with high yields and diastereoselectivities.
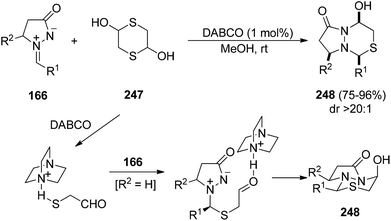
Another example of a base-catalyzed [3 + 3] cycloaddition of azomethine imines 166 takes place with 1,4-dithiane-2,5-diol 247. DABCO catalyzes this process (Scheme 96) in methanol giving products 248 resulting from the attack of the base to mercaptoacetaldehyde followed by addition to the azomethine imine and subsequent intramolecular cyclization, the diastereoselectivity being controlled by the anomeric effect.144 5-Methyl and 5-phenyl substituted azomethines 166 gave the all cis-cycloadducts 248.
The multicomponent synthesis of pyrazolidinones 249 has been performed starting from 165, the aldehyde and Meldrum's acid. This process was organocatalyzed by (DHQ)2PHAL 250 acting as a chiral base. A domino Knoevenagel-aza-Michael-cyclocondensation reaction gave the resulting cycloadducts 249 in good yields and enantioselectivities (Scheme 97).145
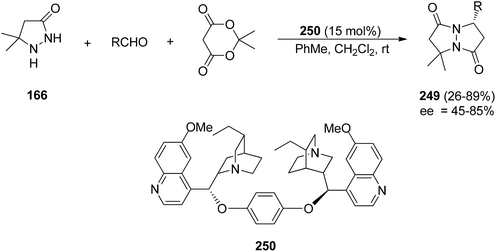 | ||
| Scheme 97 Multicomponent enantioselective [3 + 2] cycloaddition of pyrazolidinones 165 with aldehydes and Meldrum's acid. | ||
Nucleophilic phosphine catalysis has been used for different types of [3 + n] cycloadditions of azomethine imines 166 with allenoates 77 (Scheme 98). These reactions take place by the formation of various zwitterionic intermediates by the addition of a phosphine as a Lewis base to the β-carbon of the α-allenic ester, affording five -, six-, seven-, and eight-membered dinitrogen containing heterocycles. These types of cycloadditions have also been studied with C,N-cyclic azomethine imines 57 (Scheme 27).42
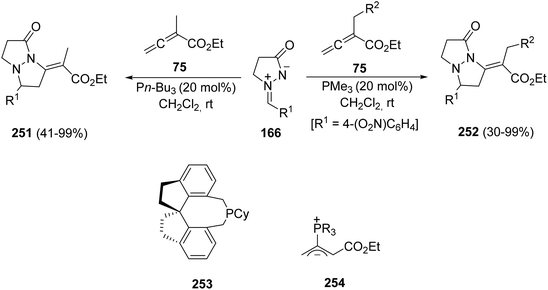
Ethyl 2-methylbuta-2,3-dienoate 75 reacted with azomethine imines 166 under tri-n-butylphosphine-catalyzed [3 + 2] cycloaddition to afford the exocyclic alkylidene adducts 251 as single isomers (Scheme 99).146 By using a chiral phosphine 253, the product 251 (with R1 = Ph) was obtained in 56% yield and 89% ee. When azomethine imine 166 [with R = 4-(O2N)C6H4] was allowed to react with other α-alkyl allenoates 75, trimethylphosphine was the catalyst of choice to prepare products 252. These 1,3-DC take place by the formation of the corresponding 1,3-zwitterionic intermediate 254.
The reaction involving diethyl 2-vinylidene succinate 255 was more complicated giving mixtures of five-, six-, and seven-membered rings either with tri-n-butyl- or trimethylphosphine (Scheme 100).146 It has been proposed that zwitterionic intermediates A and B gave the five- and the six- or seven-membered ring, respectively.
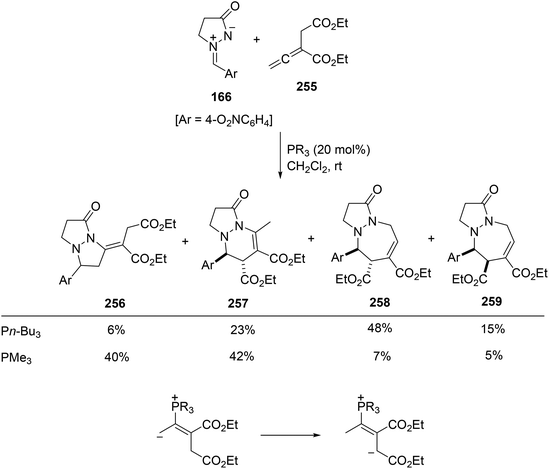 | ||
| Scheme 100 Phosphine-catalyzed [3 + 2], [3 + 3] and [4 + 3] cycloadditions of azomethine imine 166 and diethyl 2-vinylidene succinate 255. | ||
For unsubstituted ethyl 2,3-butadienoate 260, a mixture of [3 + 2] and [3 + 3] cycloadditions 261 and 262 is formed in different proportions depending on the phosphine used as a catalyst. Trimethylphosphine favors the formation of the tetrahydropyrazolopyrazolone 261 and tri-n-butylphosphine the tetrahydropyrazolopyridazinone 262 by the addition of the γ-carbon of the allenoate and the α-carbon, respectively (Scheme 101).146
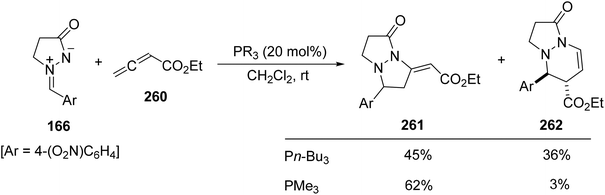 | ||
| Scheme 101 Phosphine-catalyzed [3 + 2] and [3 + 3] cycloaddition of azomethine imine 166 and ethyl 2,3-butadienoate 260. | ||
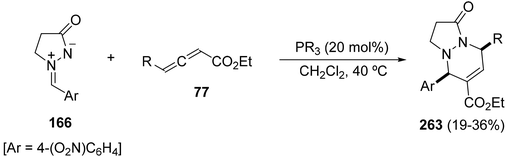
When γ-substituted allenoates 77 are allowed to react with azomethine imine 166 [Ar = 4-(O2N)C6H4], only the tetrahydropyrazolopyridazinones 263 were obtained in modest yields and with total diastereoselectivity, among other non-isolated products (Scheme 102).146
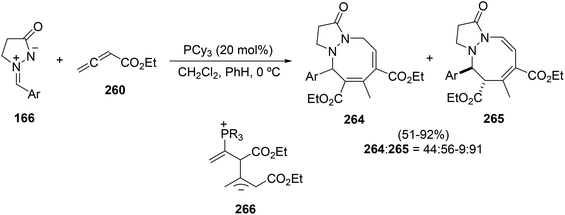
The reaction of ethyl 2,3-butadienoate 260 with different azomethine imines 166 was studied in more detail in order to determine the structure of secondary products. It was found that the formation of [3 + 2 + 3] products, such as 1-oxo-2,3,5,6-tetrahydro-1H-pyrazolo[1,2-a][1,2]diazines 264 and 265, took place mainly when tricyclohexylphosphine was used as a catalyst (Scheme 103).146 In this case, experimental and theoretical studies support the participation of 1,5-zwitterionic intermediates 266, in order to explain the formation of the eight- and seven-membered rings through [5 + 3] and [5 + 2] cycloadditions, respectively.147
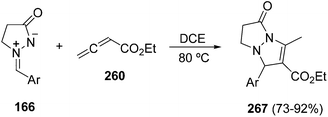
However, under thermal conditions, only the [3 + 2] cycloaddition products 267 were formed in high yields (Scheme 104),41 whereas with α-, and γ-substituted allenoates a complex mixture of products was obtained. When ethyl 2-butynoate was used instead of ethyl 2,3-butadienoate (260), the tri-n-butylphosphine-promoted cyclization with azomethine imines 166 also afforded a mixture of products 261 and 262 by intermediacy of the same 1,3-zwitterionic intermediate 254.148
Electron-deficient alkenes such as the (Z)-1,2-bis(phenylsulfonyl)ethylene 268 gave, under Ph2PMe-catalysis at room temperature, the corresponding [3 + 2] cycloaddition products 269 in the reaction with azomethine imines 166 (Scheme 105).149 Products 269 were obtained with high diastereoselectivity and the relative configuration was the same when using (E)-268. In this case, the participation of the zwitterionic intermediate 270 has been proposed, which attacks the azomethine imine followed by intramolecular cyclization regenerating the phosphine. The same [3 + 2] cycloaddition has been observed with C,N-cyclic azomethine imines 57, as well as with 144 and 156.
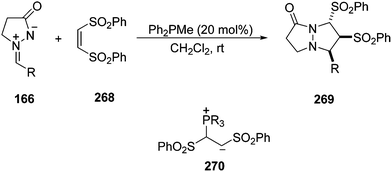 | ||
| Scheme 105 Phosphine-catalyzed [3 + 2] cycloaddition of 166 with (Z)-bis-1,2-bis(phenylsulfonyl)ethylene 268. | ||
Chiral bis-phosphoric acid 272 has been used as the Brønsted acid catalyst for the 1,3-DC of alkylideindolinones 271 with azomethine imines 166 to afford spiro pyrazolidin-3,3′-oxindoles 273 (Scheme 106).150 By MS and DFT calculation experiments the best transition state has been established in which both the alkylideneindolines and the azomethine imines are hydrogen bound with the OH group of both phosphoric acid moieties.
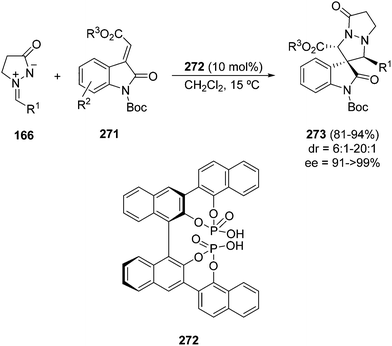 | ||
| Scheme 106 Phosphoric acid-catalyzed enantioselective [3 + 2] cycloaddition of 166 with alkylideneindolinones. | ||
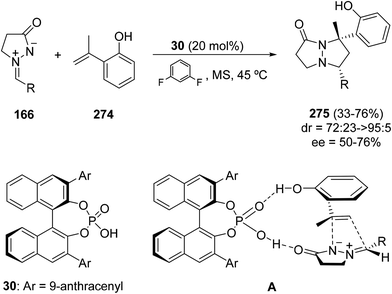
The phosphoric acid 30 (Ar = 9-anthracenyl) has shown good diastereo- and moderate enantioselectivities in the organocatalyzed enantioselective inverse-electron-demanding 1,3-DC of azomethine imines 166 with o-hydroxy-α-methylstyrene 274. Thus, [3 + 2] cycloaddition takes place in 1,3-difluorobenzene giving mainly cycloadducts 275 through a two-step mechanism. The presence of the hydroxy group at the ortho position is crucial for the reaction to occur. A dual activation mode by hydrogen bonding interaction between the two substrates and the catalyst together with the conjugative effect initiated by the o-hydroxy group played an essential role in the proposed transition state A (Scheme 107).151
4.2 N-Alkylidenepyrazolidin-1-ium-2-ides
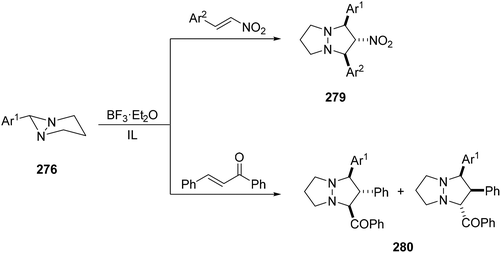
Azomethine imines 277 can be generated from the opening of the diaziridine ring in 1,5-diazabicyclo[3.1.0]hexane 276 either by thermolysis152–154 or by means of scandium triflate and trapped by dipolarophiles to give products 278 (Scheme 108).155,156 This ring opening can be performed in ionic liquids (ILs) in the presence of BF3·Et2O and the resulting unstable azomethine imines can be trapped by nitrostyrenes or chalcone to give the corresponding [3 + 2] cycloadducts 278 and 280, respectively (Scheme 109).157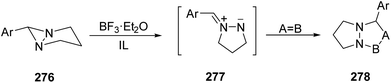 | ||
| Scheme 108 [3 + 2] Cycloadditions of azomethine imines 277 generated from 1,5-diazabicyclo[3.1.0]hexanes 276. | ||
When acrylonitrile or 4-nitrophenyl vinyl sulfone was used as dipolarophiles the corresponding cycloadducts 281 or 282 were obtained with modest diastereoselectivity and with opposite regioselectivity in the first case (Scheme 110).158,159
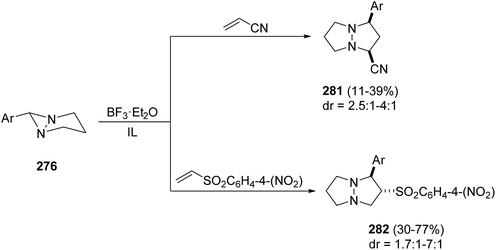 | ||
| Scheme 110 [3 + 2] Cycloaddition of azomethine imines 276 with acrylonitrile and 4-nitrophenyl vinyl sulfone. | ||
4.3 N-Alkylidene 3-oxodiazolidin-1-ium-2-ides
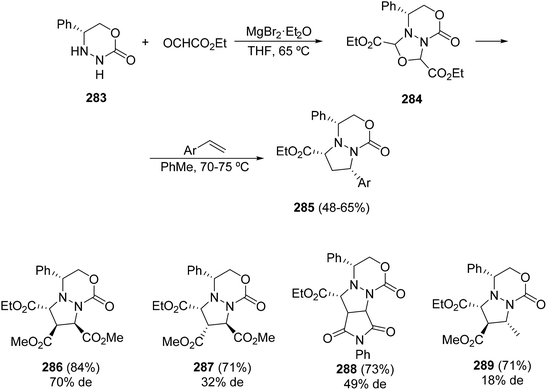
These types of azomethine imines have been less studied than the pyrazolidinium imides. Only glyoxylic azomethine imines derived from 283 have been investigated. These chiral six-membered hydrazides 283 react with aliphatic and aromatic aldehydes to give the corresponding azomethine imines, which react with diethyl acetylene dicarboxylate and olefinic dipolarophiles to provide pyrazolo[1,2-a]pyridazin-5(6H)-ones.10,160,161 When ethyl glyoxylate is used as a carbonyl component in the presence of an excess of magnesium bromide etherate, the corresponding oxadiazolidine 284 is formed, which undergoes cycloreversion–cycloaddition in the presence of various electron-poor dipolarophiles such as styrenes giving cycloadducts 285 (Scheme 111).162 Methyl maleate, fumarate and crotonate as well as N-phenylmaleimide gave cycloadducts 286–289 with modest diastereoselectivities and good yields.5. Conclusions
In the last 10 years, the chemistry of acyclic and especially cyclic azomethine imines has experienced a renaissance in synthesis of heterocycles of wide structural diversity, such as pyrazolidines, pyrazoloisoquinolines and pyrazolopyrazolones, among others. Their reactivity in 1,3-dipolar cycloadditions (1,3-DC) with a great variety of dipolarophiles in a highly regio- and diastereoselective manner has found many applications in the synthesis of dinitrogen heterocycles. Depending on the dipolarophile partially or totally saturated heterocycles can be prepared generally by a [3 + 2] cycloaddition but also by higher order cycloadditions. Most of the methodologies recently studied are in the field of asymmetric synthesis using chiral Lewis bases and Brønsted acids as organocatalysts depending on the dipolarophile and metal complexes bearing chiral ligands. The study of asymmetric catalytic methods has just started and further synthetic applications to be developed in this field would be important in the near future.Abbreviations
| Ac | Acetyl |
| Bn | Benzyl |
| Bz | Benzoyl |
| Cbz | Benzyloxycarbonyl |
| CSA | Camphorsulfonic acid |
| DABCO | Diazabicyclo[2.2.2]octane |
| DBU | 1,8-Diazabicyclo[5.4.0]undec-7-ene |
| 1,3-DC | 1,3-Dipolar cycloaddition |
| DCE | 1,2-Dichloroethane |
| DCM | Dichloromethane |
| DDQ | 1,3-Dichloro-5,6-dicyano-1,4-benzoquinone |
| DFT | Density functional theory |
| DIPEA | Diisopropyl ethyl amine |
| DIPT | Diisopropyl tartrate |
| DMAc | N,N-Dimethylacetamide |
| DMAD | Dimethyl acetylenedicarboxylate |
| DMF | N,N-Dimethylformamide |
| DMP | Dess–Martin reagent |
| 2,4-DNBA | 2,6-Dinitrobenzoic acid |
| DTBMP | 2,6-Di-tert-butyl-4-methylpyridine |
| HDMS | Hexamethyldisilazane |
| ILs | Ionic liquids |
| MS | Mass spectrometry |
| NMP | N-Methylpyrrolidone |
| NPM | N-Phenylmaleimide |
| Py | Pyridine |
| rt | Room temperature |
| SEM | Scanning electron microscopy |
| TBS | tert-Butyldimethylsilyl |
| TIPBA | 2,4,6-Triisopropylbenzenesulfonic acid |
| TIPS | Triisopropylsilyl |
| TMM | Trimethylenemethane |
| TMSOTf | Trimethylsilyl triflate |
| Troc | 2,2,2-Trichloroethoxycarbonyl |
| Ts | p-Toluenesulfonyl |
Acknowledgements
We acknowledge continued financial support from the Ministerio de Ciencia e Innovación (MICINN) (projects CTQ2007-62771/BQU, CTQ2010-20387, CONSOLIDER INGENIO 2010-CDS2007-00006, CTQ2011-24151, and CTQ2011-24165), the Ministerio de Economía y Competitividad (MINECO) (projects CTQ2013-43446-P, CTQ2014-51912-REDC, and CTQ2014-53695-P), FEDER, the Generalitat Valenciana (PROMETEO 2009/039 and PROMETEOII/2014/017), and the University of Alicante.References
- W. O. Gotfredsen and S. Vangedal, Acta Chem. Scand., 1995, 9, 1498 CrossRef PubMed.
- R. Huisgen, R. Grashey, P. Laur and H. Leitermann, Angew. Chem., 1960, 72, 416 CrossRef CAS PubMed.
- H. Dorn and A. Otto, Chem. Ber., 1968, 101, 3287 CrossRef CAS PubMed.
- H. Dorn and A. Otto, Angew. Chem., Int. Ed. Engl., 1968, 7, 214 CrossRef CAS PubMed.
- H. J. Timpe, Adv. Heterocycl. Chem., 1974, 17, 213 CrossRef CAS.
- 1,3-Dipolar Cycloaddition Chemistry, ed. A. Padwa, Wiley, New York, 1984 Search PubMed.
- R. Grashey , in ref. 6, pp. 733–817.
- G. C. Newton and C. A. Ramsden, Tetrahedron, 1982, 38, 2965 CrossRef.
- L. S. Rodina, A. Kolberg and B. Schulze, Heterocycles, 1998, 49, 587 CrossRef CAS.
- J. G. Schantl, Azomethine Imines, in Science of Synthesis, ed. A. Padwa, G. Thieme Verlag KG, Stuttgart, 2004, vol. 27, pp. 731–738 Search PubMed.
- V. Nair and T. D. Suja, Tetrahedron, 2007, 63, 12247 CrossRef CAS PubMed.
- Methods and Applications of Cycloaddition Reactions in Organic Synthesis, ed. N. Nishiwaki, John Wiley & Sons Inc., Hoboken, 2014 Search PubMed.
- L. M. Stanley and M. P. Sibi, Chem. Rev., 2008, 108, 2887 CrossRef CAS PubMed.
- H. Pellissier, Tetrahedron, 2012, 68, 2197 CrossRef CAS PubMed.
- H. Suga and K. Itoh, in ref. 12, ch. 7.
- R. Grigg, J. Kemp and N. Thompson, Tetrahedron Lett., 1978, 19, 2827 CrossRef.
- G. Le Fevre and J. Hamelin, Tetrahedron Lett., 1979, 20, 1757 CrossRef.
- A. Arrieta, J. R. Carrillo, F. P. Cossío, A. Díaz-Ortiz, M. J. Gómez-Escalonilla, A. de la Hoz, F. Langa and A. Moreno, Tetrahedron, 1998, 54, 13167 CrossRef CAS.
- E. Frank, Z. Kardos, J. Wölfling and G. Schneider, Synlett, 2007, 1311 CrossRef CAS PubMed.
- S. Kobayashi, R. Hirabayashi, H. Shimizu, H. Ishitani and Y. Yamashita, Tetrahedron Lett., 2003, 44, 3351 CrossRef CAS.
- (a) M. Presset, K. Mohanan, M. Hamann, Y. Coquerel and J. Rodriguez, Org. Lett., 2011, 13, 4124 CrossRef CAS PubMed; (b) A. H. Shinde, S. Vidyacharan and D. S. Sharada, Tetrahedron Lett., 2014, 55, 3064 CrossRef CAS PubMed.
- J. Gergely, J. B. Morgan and L. E. Overman, J. Org. Chem., 2006, 71, 9144 CrossRef CAS PubMed.
- J. D. Katz and L. E. Overman, Tetrahedron, 2004, 60, 9559 CrossRef CAS PubMed.
- S. Kobayashi, H. Shimizu, Y. Yamashita, H. Ishitani and J. Kobayashi, J. Am. Chem. Soc., 2002, 124, 13678 CrossRef CAS PubMed.
- Y. Yamashita and S. Kobayashi, J. Am. Chem. Soc., 2004, 126, 11279 CrossRef CAS PubMed.
- S. Shirakawa, P. J. Lombardi and J. L. Leighton, J. Am. Chem. Soc., 2005, 127, 9974 CrossRef CAS PubMed.
- R. Berger, K. Duff and J. L. Leighton, J. Am. Chem. Soc., 2004, 126, 5686 CrossRef CAS PubMed.
- A. Zamfir and S. B. Tsogoeva, Synthesis, 2011, 1988 CAS.
- M. Rueping, M. S. Maji, H. B. Küçük and I. Atodiresei, Angew. Chem., Int. Ed., 2012, 51, 12864 CrossRef CAS PubMed.
- M. J. Burk and J. E. Feaster, J. Am. Chem. Soc., 1992, 114, 6266 CrossRef CAS.
- X. Hong, H. B. Küçuk, M. S. Maji, Y.-F. Yang, M. Rueping and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 13769 CrossRef CAS PubMed.
- D. H. Ess and K. N. Houk, J. Am. Chem. Soc., 2008, 130, 10187 CrossRef CAS PubMed.
- W. Oppolzer, Tetrahedron Lett., 1970, 2199 CrossRef CAS.
- R. C. F. Jones, S. J. Hollis and J. N. Iley, ARKIVOC, 2007,(v), 152 CAS.
- T. Hashimoto, Y. Takiguchi and K. Maruoka, J. Am. Chem. Soc., 2013, 135, 11473 CrossRef CAS PubMed.
- H. D. S. Guerrand, H. Adams and I. Coldham, Org. Biomol. Chem., 2011, 9, 7921 CAS.
- A. D. Hunt, I. Dion, N. das Neves, S. Taing and A. M. Beauchemin, J. Org. Chem., 2013, 78, 8847 CrossRef CAS PubMed.
- T. Hashimoto, Y. Maeda, M. Omote, H. Nakatsu and K. Maruoka, J. Am. Chem. Soc., 2010, 132, 4076 CrossRef CAS PubMed.
- S. Milosevic and A. Togni, J. Org. Chem., 2013, 78, 9638 CrossRef CAS PubMed.
- T. Hashimoto, M. Omote and K. Maruoka, Angew. Chem., Int. Ed., 2011, 50, 3489 CrossRef CAS PubMed.
- R. Na, H. Liu, Z. Li, B. Wang, J. Liu, M.-A. Wang, M. Wang, J. Zhong and H. Guo, Tetrahedron, 2012, 68, 2349 CrossRef CAS PubMed.
- C. Jing, R. Na, B. Wang, H. Liu, L. Zhang, J. Liu, M. Wang, J. Zhong, O. Kwon and H. Guo, Adv. Synth. Catal., 2012, 354, 1023 CrossRef CAS PubMed.
- D. Wang, Y. Liu, Y. Wei and M. Shi, Chem. – Eur. J., 2014, 20, 15325 CrossRef CAS PubMed.
- E. Li, Y. Huang, L. Liang and P. Xie, Org. Lett., 2013, 15, 3138 CrossRef CAS PubMed.
- M. Gicquel, C. Gómez, P. Retailleau, A. Voituriez and A. Marinetti, Org. Lett., 2013, 15, 4002 CrossRef CAS PubMed.
- E. Li and Y. Huang, Chem. Commun., 2014, 50, 998 Search PubMed.
- H. Kawai, Z. Yuan, E. Tokunaga and N. Shibata, Org. Lett., 2012, 14, 5330 CrossRef CAS PubMed.
- X.-Q. Hu, J.-R. Chen, S. Gao, B. Feng, L.-Q. Lu and W.-J. Xiao, Chem. Commun., 2013, 49, 7905 RSC.
- T. Soeta, K. Tamura and Y. Ukaji, Org. Lett., 2012, 14, 1226 CrossRef CAS PubMed.
- W. Li, Q. Jia, Z. Du, K. Zhang and J. Wang, Chem. – Eur. J., 2014, 20, 4559 CrossRef CAS PubMed.
- W. Li, J. Wei, Q. Jia, Z. Du, K. Zhang and J. Wang, Chem. – Eur. J., 2014, 20, 6592 CrossRef CAS PubMed.
- C. Izquierdo, F. Esteban, A. Parra, R. Alfaro, J. Alemán, A. Fraile and J. L. García-Ruano, J. Org. Chem., 2014, 78, 10417 CrossRef PubMed.
- L. Hesping, A. Biswas, C. G. Daniluc, C. Mück-Lichtenfeld and A. Studer, Chem. Sci., 2015, 6, 1252 RSC.
- (a) Q. Ding, Z. Chen, X. Yu, Y. Peng and J. Wu, Tetrahedron Lett., 2009, 50, 340 CrossRef CAS PubMed; (b) H. Ren, S. Ye, F. Liu and J. Wu, Tetrahedron, 2010, 66, 8242 CrossRef CAS PubMed.
- X. Yu, X. Pan and J. Wu, Tetrahedron, 2011, 67, 1145 CrossRef CAS PubMed.
- S. Ye, X. Yang and J. Wu, Chem. Commun., 2010, 46, 5238 RSC.
- Z. Chen, Q. Ding, X. Yu and J. Wu, Adv. Synth. Catal., 2009, 351, 1692 CrossRef CAS PubMed.
- (a) Z. Chen, X. Yang and J. Wu, Chem. Commun., 2009, 3469 RSC; (b) Z. Chen, M. Su, X. Yu and J. Wu, Org. Biomol. Chem., 2009, 7, 4641 RSC.
- S. Li, Y. Luo and J. Wu, Org. Lett., 2011, 13, 4312 CrossRef CAS PubMed.
- P. Huang, Z. Chen, Q. Yang and Y. Peng, Org. Lett., 2012, 14, 2790 CrossRef CAS PubMed.
- P. Huang, Q. Yang, Z. Chen, Q. Ding, J. Xu and Y. Peng, J. Org. Chem., 2012, 77, 8092 CrossRef CAS PubMed.
- J. Zhou, M. Liu, P. Luo, Y. Lai, T. Yang and Q. Ding, Beilstein J. Org. Chem., 2014, 10, 2286 CrossRef PubMed.
- (a) L. Jiang, X. Yu, B. Fang and J. Wu, Org. Biomol. Chem., 2012, 10, 8102 RSC; (b) L. Zhang, Q. Xiao, S. Ye and J. Wu, Chem. – Asian J., 2012, 7, 1909 CrossRef CAS PubMed; (c) J. Yang, X. Yu and J. Wu, Synthesis, 2014, 1362 Search PubMed.
- H. Liu, Z. Wang, S. Pu and G. Liu, Synthesis, 2014, 600 Search PubMed.
- X. Yu, Z. Chen, X. Yang and J. Wu, J. Comb. Chem., 2010, 12, 374 CrossRef CAS PubMed.
- Z. Chen and J. Wu, Org. Lett., 2010, 12, 4856 CrossRef CAS PubMed.
- Z. Chen, X. Pan and J. Wu, Synlett, 2011, 964 CAS.
- V. A. Peshkov, O. P. Pereshivko, S. Van Hove, D. S. Ermolat'cv and E. V. Van der Eycken, Synthesis, 2011, 3371 CAS.
- X. Yu, S. Ye and J. Wu, Adv. Synth. Catal., 2010, 352, 2050 CrossRef CAS PubMed.
- (a) W. Hao, T. Zhang and M. Cai, Tetrahedron, 2013, 69, 9219 CrossRef CAS PubMed; (b) X. Yu, S. Ye and J. Wu, Adv. Synth. Catal., 2010, 352, 2050 CrossRef CAS PubMed; (c) Q. Xiao, J. Sheng, Q. Ding and J. Wu, Adv. Synth. Catal., 2013, 355, 2321 CrossRef CAS PubMed.
- (a) Z. Chen, L. Gao, S. Ye, Q. Ding and J. Wu, Chem. Commun., 2012, 48, 3975 RSC; (b) L. Gao, S. Ye, Q. Ding, Z. Chen and J. Wu, Tetrahedron, 2012, 68, 2765 CrossRef CAS PubMed.
- L. Yao, X. Yu, C. Mo and J. Wu, Org. Biomol. Chem., 2012, 10, 9447 CAS.
- H. Liu, G. Liu, G. Qiu, S. Pu and J. Wu, Tetrahedron, 2013, 69, 1476 CrossRef CAS PubMed.
- S. Li and J. Wu, Org. Lett., 2011, 13, 712 CrossRef CAS PubMed.
- (a) J. Sheng, Y. Guo and J. Wu, Tetrahedron, 2013, 69, 6495 CrossRef CAS PubMed; (b) C. Ye, X. Yu, G. Qiu and J. Wu, RSC Adv., 2012, 2, 5961 RSC.
- P. Yuvaraj and B. S. R. Reddy, Tetrahedron Lett., 2014, 55, 806 CrossRef CAS PubMed.
- Y.-Y. Zhou, J. Li, L. Ling, S.-H. Liao, X.-L. Sun, Y.-X. Li, L. J. Wang and Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 1452 CrossRef CAS PubMed.
- C. Perreault, S. R. Goudreau, L. E. Zimmer and A. B. Charette, Org. Lett., 2008, 10, 689 CrossRef CAS PubMed.
- R. Gösl and A. Meuwsen, Chem. Ber., 1959, 92, 2521 CrossRef PubMed.
- R. Gösl and A. Meuwsen, Org. Synth. Coll. Vol. V, 1973, 43 Search PubMed.
- R. G. Wallace, Aldrichimica Acta, 1980, 13, 3 CAS.
- C. Legault and A. B. Charette, J. Org. Chem., 2003, 68, 7119 CrossRef CAS PubMed.
- R. Huisgen, R. Grashey and R. Ksischke, Tetrahedron Lett., 1962, 387 CrossRef CAS.
- L. Bettinetti, K. Schlotter, H. Hübner and P. Gmeiner, J. Med. Chem., 2002, 45, 4594 CrossRef CAS PubMed.
- S. Löber, H. Hübner, W. Utz and P. Gmeiner, J. Med. Chem., 2001, 44, 2691 CrossRef PubMed.
- S. Kuroda, A. Akahane, H. Itani, S. Nishimura, K. Durkin, Y. Tenda and K. Sakane, Bioorg. Med. Chem., 2000, 8, 55 CrossRef CAS.
- B. A. Johns, K. S. Gudmundsson, E. M. Turner, S. H. Allen, V. A. Samano, J. A. Ray, G. A. Freeman, F. L. Boyd, C. J. Sexton, D. W. Selleseth, K. L. Creech and K. R. Moniri, Bioorg. Med. Chem., 2005, 13, 2397 CrossRef CAS PubMed.
- T. Irikura, K. Nishino, S. Suzue and T. Ikeda, Eur. Pat. ApplEP0118916, 1984 Search PubMed.
- K. Harju, I. Kylänlahti, T. Paananen, M. Polamo, J. Nielsen and J. Yli-Kauhaluoma, J. Comb. Chem., 2006, 8, 344 CrossRef CAS PubMed.
- J. Zhao, P. Li, C. Wu, H. Chen, W. Ai, R. Sun, H. Ren, R. C. Larock and F. Shi, Org. Biomol. Chem., 2012, 10, 1922 CAS.
- L. Zhang, C. Jing, H. Liu, B. Wang, Z. Li, H. Jiang, H. Yu and H. Guo, Synthesis, 2013, 53 Search PubMed.
- P. W. Davies, A. Cremonesi and L. Dumitrescu, Angew. Chem., Int. Ed., 2011, 50, 8931 CrossRef CAS PubMed.
- X. Xu, P. Y. Zavalij and M. P. Doyle, Angew. Chem., Int. Ed., 2013, 52, 12664 CrossRef CAS PubMed.
- C. Turk, J. Svete, B. Stanovnik, L. Golič, S. Golič-Grdadolnik, A. Golobič and L. Selič, Helv. Chim. Acta, 2001, 84, 146 CrossRef CAS.
- C. Clavette, W. Gan, A. Bongers, T. Markiewicz, A. B. Toderian, S. I. Gorelsky and A. M. Beauchemin, J. Am. Chem. Soc., 2012, 134, 16111 CrossRef CAS PubMed.
- L. Pezdirc, V. Jovanovski, D. Bevk, R. Jakše, S. Pirc, A. Meden, B. Stanovnik and J. Svete, Tetrahedron, 2005, 61, 3977 CrossRef CAS PubMed.
- J. Svete, ARKIVOC, 2006,(vii), 35 CAS.
- L. Pezdirc, J. Cerkovnik, S. Pirc, B. Stanovnik and J. Svete, J. Comb. Chem., 2008, 45, 181 CAS.
- L. Pezdirc, U. Groscly, A. Meden, B. Stanovnik and J. Svete, J. Comb. Chem., 2007, 9, 717 CrossRef CAS PubMed.
- L. Pezdirc, U. Groscly, A. Meden, B. Sranovnik and J. Svete, J. Heterocycl. Chem., 2008, 45, 181 CrossRef CAS PubMed.
- E. Pusavec, J. Mirnic, L. Šenica, U. Grošely, B. Stanovnik and J. Svete, Z. Naturforsch., B: Chem. Sci., 2014, 69, 615 CrossRef CAS.
- S. Ogawa, T. Nishimine, E. Tokunaga and N. Shibata, Synthesis, 2010, 3274 CAS ; corrigendum Synthesis, 2010, 3274.
- N. Luo, Z. Zheng and Z. Yu, Org. Lett., 2011, 13, 3384 CrossRef CAS PubMed.
- I. Panfil, Z. Urbanczyk-Lipkowska, K. Suwinska, K. J. Solecka and M. Chimielewski, Tetrahedron, 2002, 58, 1199 CrossRef CAS.
- Y. Li, Y. Meng, X. Meng and Z. Li, Tetrahedron, 2011, 67, 4002 CrossRef CAS PubMed.
- D. Gao, H. Zhai, M. Parvez and T. G. Back, J. Org. Chem., 2008, 73, 8057 CrossRef CAS PubMed.
- F. Shi, R. Mancuso and R. C. Larock, Tetrahedron Lett., 2009, 50, 4067 CrossRef CAS PubMed.
- D. Yang, M. Fan, H. Zhu, Y. Guo and J. Guo, Synthesis, 2013, 1325 CrossRef PubMed.
- S. S. Y. Wong, M. G. Brant, C. Barr, A. G. Oliver and J. E. Wulff, Beilstein J. Org. Chem., 2013, 9, 1419 CrossRef PubMed.
- D. Wang, H.-P. Deng, Y. Wei, Q. Xu and M. Shi, Eur. J. Org. Chem., 2013, 401 CrossRef CAS PubMed.
- W. Liu, Y. Xu, X. Sun, D. Lu and L. Guo, Synlett, 2014, 1093 Search PubMed.
- R. Shintani and G. C. Fu, J. Am. Chem. Soc., 2003, 125, 10778 CrossRef CAS PubMed.
- A. Suárez, C. W. Downey and G. C. Fu, J. Am. Chem. Soc., 2005, 127, 11244 CrossRef PubMed.
- M. P. Sibi, D. Rane, L. M. Stanley and T. Soeta, Org. Lett., 2008, 10, 2971 CrossRef CAS PubMed.
- T. Arai and Y. Ogino, Molecules, 2012, 17, 6170 CrossRef CAS PubMed.
- T. Arai, Y. Ogino and T. Sato, Chem. Commun., 2013, 49, 7776 RSC.
- T. Imaizumi, Y. Yamashita and S. Kobayashi, J. Am. Chem. Soc., 2012, 134, 20049 CrossRef CAS PubMed.
- Y. Yamashita and S. Kobayashi, Chem. – Eur. J., 2013, 19, 9420 CrossRef CAS PubMed.
- M. Hori, A. Sakakura and K. Ishihara, J. Am. Chem. Soc., 2014, 136, 13198 CrossRef CAS PubMed.
- M. Keller, A. S. S. Sido, P. Pale and J. Sommer, Chem. – Eur. J., 2009, 15, 2810 CrossRef CAS PubMed.
- S. Chassaing, A. Alix, T. Boningari, K. S. S. Sido, M. Keller, P. Kuhn, B. Louis, J. Sommer and P. Pale, Synthesis, 2010, 1557 CAS.
- K. Yoshimura, T. Oishi, K. Yamaguchi and N. Mizuno, Chem. – Eur. J., 2011, 17, 3827 CrossRef CAS PubMed.
- M.-C. Tong, X. Chen, H.-Y. Tao and C.-J. Wang, Angew. Chem., Int. Ed., 2013, 52, 12377 CrossRef CAS PubMed.
- H. Guo, H. Liu, F.-L. Zhu, R. Na, H. Jiang, Y. Wu, L. Zhang, Z. Li, H. Yu, B. Wang, Y. Xiao, X.-P. Hu and M. Wang, Angew. Chem., Int. Ed., 2013, 52, 12641 CrossRef CAS PubMed.
- J. Du, X. Xu, Y. Li, L. Pan and Q. Liu, Org. Lett., 2014, 16, 4004 CrossRef CAS PubMed.
- H. Suga, A. Funyu and A. Kakehi, Org. Lett., 2007, 9, 97 CrossRef CAS PubMed.
- J. Li, X. Lian, X. Liu, L. Lin and X. Feng, Chem. – Eur. J., 2013, 19, 5134 CrossRef CAS PubMed.
- R. Shintani and T. Hayashi, J. Am. Chem. Soc., 2006, 128, 6330 CrossRef CAS PubMed.
- B. M. Trost and D. M. T. Chan, J. Am. Chem. Soc., 1979, 101, 6429 CrossRef CAS.
- N. D. Shapiro, Y. Shi and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 11654 CrossRef CAS PubMed.
- W. Zhou, X.-X. Li, G.-H. Li, Y. Wu and Z. Chen, Chem. Commun., 2013, 49, 3552 RSC.
- T. Kato, S. Fujinami, Y. Ukaji and K. Inomata, Chem. Lett., 2008, 342 CrossRef CAS.
- Y. Ukaji and K. Inomata, Chem. Rec., 2010, 10, 173 CrossRef CAS PubMed.
- K. Tanaka, T. Kato, S. Fujinami, Y. Ukaji and K. Inomata, Chem. Lett., 2010, 39, 1036 CrossRef CAS.
- M. Yoshida, N. Sassa, T. Kato, S. Fujinami, T. Soeta, K. Inomata and Y. Ukaji, Chem. – Eur. J., 2014, 20, 2058 CrossRef CAS PubMed.
- X. Xu, Y. Qian, P. Y. Zavalij and M. P. Doyle, J. Am. Chem. Soc., 2013, 135, 1244 CrossRef CAS PubMed.
- Y. Qian, P. Y. Zavalij, W. Hu and M. P. Doyle, Org. Lett., 2013, 15, 1564 CrossRef CAS PubMed.
- X. Xu, X. Xu, P. Y. Zavalij and M. P. Doyle, Chem. Commun., 2013, 49, 2762 RSC.
- Y. Yamashita and S. Kobayashi, Chem. Lett., 2009, 678 CrossRef CAS.
- W. Chen, X.-H. Yuan, R. Li, W. Du, Y. Wu, L.-S. Ding and Y.-C. Chen, Adv. Synth. Catal., 2006, 348, 1818 CrossRef CAS PubMed.
- A. Chan and K. A. Scheidt, J. Am. Chem. Soc., 2007, 129, 5334 CrossRef CAS PubMed.
- W. Chen, W. Du, Y.-Z. Duan, Y. Wu, S.-Y. Yang and Y.-C. Chen, Angew. Chem., Int. Ed., 2007, 46, 7667 CrossRef CAS PubMed.
- G. Zhu, W. Sun, C. Wu, G. Li, L. Hong and R. Wang, Org. Lett., 2013, 15, 4988 CrossRef CAS PubMed.
- X. Fang, J. Li, H.-Y. Tao and C. J. Wang, Org. Lett., 2013, 15, 5554 CrossRef CAS PubMed.
- E. Pair, C. Berini, R. Noël, M. Sanselme, V. Levacher and J.-F. Brère, Chem. Commun., 2014, 50, 10218 RSC.
- R. Na, C. Jing, Q. Xu, H. Jiang, X. Wu, J. Shi, J. Zhong, M. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard III, H. Guo and O. Kwon, J. Am. Chem. Soc., 2011, 133, 13337 CrossRef CAS PubMed.
- W. Meng, H.-T. Zhao, J. Mie, Y. Zheng, A. Fu and J.-A. Ma, Chem. Sci., 2012, 3, 3053 RSC.
- J. Liu, H. Liu, R. Na, G. Wang, Z. Li, H. Yu, M. Wang, J. Zhong and H. Guo, Chem. Lett., 2012, 41, 218 CrossRef CAS.
- Z. Li, H. Yu, H. Liu, L. Zhang, H. Jiang, B. Wang and H. Guo, Chem. – Eur. J., 2014, 20, 1731 CrossRef CAS PubMed.
- L. Hong, M. Kai, C. Wu, W. Sun, G. Zhu, G. Li, X. Yao and R. Wang, Chem. Commun., 2013, 49, 6713 RSC.
- R.-Y. Zhu, C.-S. Wang, J. Zheng, F. Shi and S.-J. Tu, J. Org. Chem., 2014, 79, 9305 CrossRef CAS PubMed.
- Y. B. Koptelov, M. K. Kim, A. P. Molchanov and R. R. Kostikov, Russ. J. Org. Chem., 1999, 35, 110 CAS.
- A. P. Molchanov, D. I. Sipkin, Y. B. Koptelov, B. Yu and R. R. Kostikov, Russ. J. Org. Chem., 2001, 37, 841 CrossRef CAS.
- A. P. Molchanov, D. I. Sipkin, Y. B. Koptelov, J. Kopf and R. R. Kostekov, Russ. J. Org. Chem., 2003, 39, 1338 CrossRef CAS.
- M. Nakawaga and M. Kawahara, Org. Lett., 2000, 2, 953 CrossRef.
- J. S. Yadav, B. V. S. Reddy, S. K. Pandey, P. P. Srihari and I. Prarhap, Tetrahedron Lett., 2001, 42, 9089 CrossRef CAS.
- S. G. Zlotin and N. N. Makhova, Mendeleev Commun., 2010, 20, 63 CrossRef CAS PubMed.
- M. I. Pleshchev, V. V. Kachala, A. S. Goloveshkin, I. S. Bushmarinov, V. V. Kuznetsov, D. V. Khakimov and N. N. Makhova, Mendeleev Commun., 2013, 23, 271 CrossRef CAS PubMed.
- N. N. Makhova, M. I. Pleshchev, M. A. Epishina and A. S. Kulikov, Chem. Heterocycl. Compd., 2014, 50, 634 CrossRef CAS PubMed.
- F. Rousi, M. Bonin, A. Chiaroni, L. Micouin, C. Riche and H. Husson, Tetrahedron Lett., 1999, 40, 3727 CrossRef.
- F. Rousi, A. Chauveau, M. Bonin, L. Micouin and H.-P. Husson, Synthesis, 2000, 1170 CrossRef PubMed.
- F. Chung, A. Chauveau, M. Seltki, M. Bonin and L. Micouin, Tetrahedron Lett., 2004, 45, 3127 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |