Open Access Article
Open Access ArticleDivergent synthesis of various iminocyclitols from D-ribose†
Ramu
Petakamsetty
,
Vipin Kumar
Jain
,
Pankaj Kumar
Majhi
and
Ramesh
Ramapanicker
*
Department of Chemistry and Center for Environmental Science and Engineering, Indian Institute of Technology Kanpur, Kanpur, 208016, India. E-mail: rameshr@iitk.ac.in
First published on 30th June 2015
Abstract
A very efficient route to the diastereoselective synthesis of polyhydroxy pyrrolidines, piperidines and azepanes from an aldehyde derivative of ribose is reported. Asymmetric α-amination of aldehydes using proline catalysed hydrazination is the key step in the synthesis. The method utilizes the stereocenters present in ribose and the extra carbon atoms present in the target molecules are incorporated using Wittig reactions. The incorporation of the amino group is carried out asymmetrically to account for additional stereocenters. This synthetic route to iminocyclitols has the potential to be extended for the synthesis of a large class of such compounds starting from other sugar derived aldehydes.
Introduction
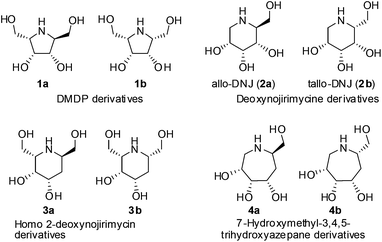
Iminocyclitols are sugar analogues with a secondary or tertiary amino group replacing the oxygen atom in the ring. These compounds, consisting of a 5, 6 or 7 membered polyhydroxy N-heterocyclic frameworks are generally referred to as iminosugars or azasugars (Fig. 1). They are potential inhibitors of various carbohydrate processing enzymes1 and are key to the development of therapeutics for a wide range of diseases such as diabetes, cancer and viral infections including AIDS.2 2,5-Dihydroxymethyl-3,4-dihydroxypyrrolidine (DMDP, 1a) along with its isomers are first among the pyrrolidine class of iminosugars to be isolated from natural sources. They are known to inhibit both α- and β-glycosidases.3 1- and 2-deoxynojirimycin derivatives 2a, 2b, 3a and 3b are among the 6-membered iminosugars, which exhibit inhibitory activity towards α-glucosidases and ceramide glucosyltransferases (CGT).4 Compounds 4a and 4b are polyhydroxyazepane derivatives and are examples of 7-membered iminocyclitols, which have received relatively less attention in terms of synthesis and evaluation of inhibitory activity.5 Stereoselective routes to the synthesis of these compounds are of great interest and significance due to their potential in therapeutics.Incorporation of an amino group stereoselectively into a cyclic or linear polyhydroxy system is the important step in most of the reported syntheses of these compounds. The incorporation of the amino group is achieved by nucleophilic displacement of a leaving group with azide anions or with alkyl amines.6–11 These routes are generally limited to the possibility of synthesizing only one diastereomer of the molecule from a starting compound. It will therefore, be useful to have strategies, where at least two diastereomers of the target molecules can be synthesized from the same intermediate by switching a chiral catalyst to generate new stereocentres with control. The incorporation of additional carbon atoms into a synthetic intermediate is achieved either by Wittig reaction or using organometallic reagents in general. Generation of new stereocentres during or after the incorporation of additional carbon atoms on a synthetic intermediate is often done through the asymmetric induction offered by the existing stereocentres within the intermediate.
We report here a divergent method for the synthesis of various iminocyclitols starting from ribose. The method opens up the possibility of synthesizing target molecules with higher number of carbon atoms and stereocentres than the starting material used. Wittig reactions were used to incorporate additional carbon atoms and organocatalytic asymmetric amination of aldehydes12 was used for the generation of new chiral centres functionalized with nitrogen atoms. The use of L- and D-proline separately as catalysts offers the possibility to generate two different diastereomers with high selectivity.13 Although, proline catalysed asymmetric amination has been used widely in synthesis13d,e,14 it has not been explored to the same extent in carbohydrate chemistry.13a,15
Results and discussion
Our strategy towards the synthesis of these molecules is based on the synthesis of an amino alcohol derivative with a suitably positioned leaving group (mesylate). These amino alcohols are prepared by proline catalyzed asymmetric amination of aldehydes derived from ribose. A nucleophilic substitution of the mesylate group by the free amino group leading to the formation of 5, 6 or 7 membered rings was expected (Scheme 1).The syntheses of the 5-membered iminocyclitols 1a and 1b were achieved from the aldehyde 9, synthesized from the commercially available ribose derivative 5 (Scheme 2). Wittig reaction of 5 using methyltriphenylphosphonium bromide yielded the alkene 6.16 The secondary hydroxyl group in 6 was converted to a mesylate to get 7 in very high yield. Hydroboration/oxidation of 7 gave the primary alcohol 8, which on Swern oxidation yielded the aldehyde 9 (Scheme 2).
The aldehyde 9 was treated with dibenzyl azodicarboxylate in the presence of both L- and D-proline (0.1 equiv.) in separate reactions to get the hydrazino aldehydes, which were reduced to the corresponding primary alcohols 10a and 10b in one-pot. The diastereomeric ratios of these compounds were estimated using chiral HPLC and were established by comparing the chromatograms with that of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the diastereomers obtained through reactions catalyzed by DL-proline.17 The asymmetric α-hydrazination reaction follows a very ordered transition state, which is similar to the one proposed by List and Jorgensen.12 The orientation of the carboxylic acid function of proline decides the outcome of the reaction and thus the stereochemistry of the products. While L-proline prefers the attack on the Re-face, the reaction using D-proline proceeds on the Si-face.12 While asymmetric hydrazination catalyzed by L-proline gave 10a as a single diastereomer, the reaction catalyzed by D-proline yielded 10b and 10a in a 90
1 mixture of the diastereomers obtained through reactions catalyzed by DL-proline.17 The asymmetric α-hydrazination reaction follows a very ordered transition state, which is similar to the one proposed by List and Jorgensen.12 The orientation of the carboxylic acid function of proline decides the outcome of the reaction and thus the stereochemistry of the products. While L-proline prefers the attack on the Re-face, the reaction using D-proline proceeds on the Si-face.12 While asymmetric hydrazination catalyzed by L-proline gave 10a as a single diastereomer, the reaction catalyzed by D-proline yielded 10b and 10a in a 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio. The lower selectivity achieved in one of these α-functionalization reactions is expected and is accounted for based on the influence of the stereochemistry of the β-carbon. Such differences in selectivity during asymmetric hydrazination using L- and D-proline on the same substrate can be more prominent depending on the nature of the substrate.13b,e Similar observations in the case of proline catalyzed aldol reactions have extensively been studied.7k
10 ratio. The lower selectivity achieved in one of these α-functionalization reactions is expected and is accounted for based on the influence of the stereochemistry of the β-carbon. Such differences in selectivity during asymmetric hydrazination using L- and D-proline on the same substrate can be more prominent depending on the nature of the substrate.13b,e Similar observations in the case of proline catalyzed aldol reactions have extensively been studied.7k
Hydrogenolysis of the hydrazino groups in 10a and 10b using freshly prepared RANEY® Ni and H2 yielded the corresponding amino compounds, which underwent immediate cyclization by displacing the mesylate to give the pyrrolidine derivatives 11a and 11b in 78% and 73% yields respectively. The targeted iminocyclitols 1a and 1b were obtained in overall yields of 27% and 21%, respectively by acidolysis of the protecting groups using 4 N HCl in EtOAc (Scheme 3). The absolute stereochemistry of 1a was established by detailed NMR analysis of a dibenzyl derivative prepared from 11a.18 Compound 1b was a meso derivative with no optical activity. The spectral data for 1a and 1b were compared with those available in the literature and found to be matching.6c
The deoxynojirimycin derivatives 2a and 2b were synthesized using a similar strategy as the one used for the synthesis of 1a and 1b through 9. An aldehyde derivative 16 was prepared from 6 by using a different strategy for protecting and activating the hydroxyl groups. Accordingly, the secondary hydroxyl group in 6 was protected using MOMCl to get 12 in 89% yield. The TBDPS group in 12 was removed with TBAF to get the primary alcohol 13, which was treated with MsCl to get 14. Hydroboration/oxidation of 14 gave the primary alcohol 15, which on Swern oxidation yielded the required aldehyde 16 (Scheme 4). Following the strategy used for the synthesis of 1a and 1b from 9, 2a and 2b were synthesized from 16 with overall yields of 19% and 17%, respectively (Scheme 5). The diastereoselectivity of the L-proline catalyzed reaction was very high (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) in favour of 17a. However, the corresponding D-proline catalyzed reaction was only moderately selective. It has to be noted that the DL-proline catalyzed reaction on 16 proceeded to give 17a and 17b as a 60
5) in favour of 17a. However, the corresponding D-proline catalyzed reaction was only moderately selective. It has to be noted that the DL-proline catalyzed reaction on 16 proceeded to give 17a and 17b as a 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 mixture. Although 17a and 17b are not separable by column chromatography, the iminocyclitol derivatives 18a and 18b are separated easily. If 2a and 2b are required to be synthesized, use of DL-proline as a catalyst and separation of 18a and 18b are preferred. The stereochemistry of 2a and 2b was confirmed using NMR studies18 and the spectral data were also found to match with those reported in the literature.7i,10d
40 mixture. Although 17a and 17b are not separable by column chromatography, the iminocyclitol derivatives 18a and 18b are separated easily. If 2a and 2b are required to be synthesized, use of DL-proline as a catalyst and separation of 18a and 18b are preferred. The stereochemistry of 2a and 2b was confirmed using NMR studies18 and the spectral data were also found to match with those reported in the literature.7i,10d
The homonojirimycin derivatives 3a and 3b and the 7-hydroxymethyl-3,4,5-trihydroxyazepane derivatives 4a and 4b were synthesized by increasing the number of carbon atoms in the ribose derivative 5 by two through a Wittig reaction using the stabilized ylide, Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCOOEt. The α,β-unsaturated ester obtained was reduced using Pd/C and H2 to get the hydroxy ester 19 in 85% yield (Scheme 6). The secondary hydroxyl group was converted to a mesylate using MsCl in the presence of triethylamine to get 20. The ester group in 20 was reduced using DIBAL-H to get the aldehyde 21 required for the preparation of 3a and 3b (Scheme 6).
CHCOOEt. The α,β-unsaturated ester obtained was reduced using Pd/C and H2 to get the hydroxy ester 19 in 85% yield (Scheme 6). The secondary hydroxyl group was converted to a mesylate using MsCl in the presence of triethylamine to get 20. The ester group in 20 was reduced using DIBAL-H to get the aldehyde 21 required for the preparation of 3a and 3b (Scheme 6).
Hydrazination of 21 under similar conditions used for the synthesis of 10 from 9, using both L- and D-proline yielded 22a and 22b in good yields. Unlike the α-functionalization of 9 and 16, the reaction of the aldehyde 21 proceeded with very high diastereoselectivity with L- and D-proline. The absence of inherent chirality on the β-carbon atom allows the asymmetric induction to be controlled entirely by proline. The amine generated by hydrogenolysis of 22 with RANEY® Ni did not undergo cyclization in situ unlike the reaction of 17. The crude reaction mixture had to be heated in the presence of triethylamine to displace the mesylate and get the piperidine derivatives 23a and 23b. It may be assumed that the secondary mesylate was difficult to be displaced in the presence of an adjacent O-TBDPS group. The homonojirimycin derivatives 3a and 3b were obtained by acidolysis of 23a and 23b, respectively using 4 N HCl in EtOAc (Scheme 7). Detailed NMR analysis of the benzyl derivatives of 3a and 3b prepared by the dibenzylation of 23 confirmed their structures to be as given in Scheme 7.18
The aldehyde 27 required for the preparation of 4a and 4b was prepared from 19 by using a different protection and activation strategy for the hydroxyl groups. Similar to the preparation of aldehyde 16, the primary hydroxyl group was converted to a mesylate and the adjacent secondary hydroxyl group was protected as a MOM derivative to get 27 (Scheme 8). Asymmetric hydrazination of 27 using L- and D-proline proceeded in good yield and high diastereoselectivity, as in the case of 22a and 22b, to give 28a and 28b, respectively (Scheme 9).17 Hydrogenolysis followed by cyclization using triethylamine yielded the azepane derivatives 29a and 29b from 28a and 28b, respectively. Acidolysis of 29a and 29b gave the target molecules 4a and 4b in overall yields of 20% and 19% (Scheme 9). Dibenzyl derivatives made from 29 were analyzed using NMR spectroscopy to confirm the stereochemistry of 4a and 4b.18
Conclusions
We have developed a divergent strategy for synthesizing various iminocyclitols from ribose and have successfully applied this in the synthesis of 5, 6 and 7 membered iminosugars. The absolute configuration of the stereocenter bearing the imino group is switched by changing the catalyst from L-proline to D-proline. While most of these reactions proceeded with very high diastereoselectivity, induction by the adjacent chiral centres reduced the diastereoselectivity achieved in the reactions catalyzed by D-proline in two of the examples. In comparison with other methods available for the synthesis of similar compounds, the current strategy allows the preparation of target molecules with increased number of carbon atoms and stereocenters to that of the starting compounds. The method reported here has the potential to be a very useful strategy for making a large number of iminocyclitols by varying the sugar unit used as the starting compound. It provides a general method for the synthesis of imino and azasugars from a lower sugar homolog.Experimental section
General experimental methods
All the commercially available reagents were used directly without any further purification. All the reactions were carried out under an inert atmosphere unless otherwise mentioned. Acetonitrile was distilled initially from phosphorus pentoxide and subsequently from calcium hydride; DCM was distilled from calcium hydride. Yields reported are for purified compounds using column chromatography. All the reactions were monitored by analytical thin layer chromatography carried out on 0.25 mm Merck silica gel plates (60F-254) using UV light as a visualizing agent and ninhydrin, 5% H2SO4 as a staining agent. Merck silica gel (particle size 60–120 and 100–200 mesh) was used for column chromatography. All proton NMR spectra were recorded at either 400 or 500 MHz using a Jeol spectrometer and 13C NMR spectra were recorded at 100 or 125 MHz. 1H NMR splitting pattern was designated as singlet (s), doublet (d), doublet of doublets (dd), triplet (t), multiplet (m), and broad singlet (bs). IR spectra were recorded as a thin film for liquids and as KBr pellets for solids. High-resolution mass spectra were recorded using a Waters Q/Tof Premier micromass HAB 213 spectrometer with an ESI source. Optical rotation was measured using a 5.0 ml cell with a 10 dm path length and is reported as [α]25D (c in g per 100 mL solvent). The diastereoselectivity was determined by chiral HPLC analysis using a Daicel chiralpack OD-H column with a 254 nm UV detector and by using a mixture of isopropanol and n-hexane as the eluent at 25 °C.Compound 6. Column chromatography (petroleum ether/EtOAc, 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.76 g, 1.78 mmol, 89%); [α]25D = −7.23 (c 1.3, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.69–7.66 (m, 4H), 7.45–7.37 (m, 6H), 6.01 (ddd, J = 16.6, 10.3, 6.3 Hz, 1H), 5.40 (d, J = 17.1 Hz, 1H), 5.27 (d, J = 10.3 Hz, 1H), 4.69 (t, J = 6.5 Hz, 1H), 4.15 (dd, J = 8.6, 6.3 Hz, 1H), 3.86 (dd, J = 10.3, 2.9 Hz, 1H), 3.82–3.78 (m, 1H), 3.71 (bs, 1H), 2.55 (bs, 1H), 1.39 (s, 3H), 1.34 (s, 3H), 1.07 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 135.6, 134.1, 129.9, 127.8, 117.7, 108.7, 78.9, 77.5, 69.9, 65.3, 27.8, 26.9, 25.5, 19.4 ppm; IR νmax (thin film): 3460, 2030, 1585 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C25H34NaO4Si 449.2124; found 449.2128.
1); oily liquid (0.76 g, 1.78 mmol, 89%); [α]25D = −7.23 (c 1.3, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.69–7.66 (m, 4H), 7.45–7.37 (m, 6H), 6.01 (ddd, J = 16.6, 10.3, 6.3 Hz, 1H), 5.40 (d, J = 17.1 Hz, 1H), 5.27 (d, J = 10.3 Hz, 1H), 4.69 (t, J = 6.5 Hz, 1H), 4.15 (dd, J = 8.6, 6.3 Hz, 1H), 3.86 (dd, J = 10.3, 2.9 Hz, 1H), 3.82–3.78 (m, 1H), 3.71 (bs, 1H), 2.55 (bs, 1H), 1.39 (s, 3H), 1.34 (s, 3H), 1.07 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 135.6, 134.1, 129.9, 127.8, 117.7, 108.7, 78.9, 77.5, 69.9, 65.3, 27.8, 26.9, 25.5, 19.4 ppm; IR νmax (thin film): 3460, 2030, 1585 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C25H34NaO4Si 449.2124; found 449.2128.
The same procedure was used for the preparation of 14, 20 and 26.
Compound 7. Column chromatography (petroleum ether/EtOAc, 8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); oily liquid (0.96 g, 1.9 mmol, 95%); [α]25D = −0.45 (c 0.20, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.68–7.64 (m, 4H), 7.45–7.37 (m, 6H), 5.79 (ddd, J = 17.1, 8.6, 6.8 Hz, 1H), 5.35 (d, J = 18.6 Hz, 1H), 5.28–5.25 (m, 1H), 4.74–4.71 (m, 1H), 4.68 (t, J = 6.8 Hz, 1H), 4.51–4.48 (m, 1H), 3.98–3.95 (m, 1H), 3.92–3.88 (m, 1H), 3.02 (s, 3H), 1.33 (s, 3H), 1.31 (s, 3H), 1.07 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 135.7, 132.8, 132.7, 132.4, 130.1, 130.0, 127.9, 127.8, 119.5, 109.0, 81.9, 78.0, 76.3, 63.0, 39.3, 27.0, 26.8, 25.0, 19.2 ppm; IR νmax (thin film): 3072, 1589 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C26H36NaO6SSi 527.1900; found 527.1897.
2); oily liquid (0.96 g, 1.9 mmol, 95%); [α]25D = −0.45 (c 0.20, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.68–7.64 (m, 4H), 7.45–7.37 (m, 6H), 5.79 (ddd, J = 17.1, 8.6, 6.8 Hz, 1H), 5.35 (d, J = 18.6 Hz, 1H), 5.28–5.25 (m, 1H), 4.74–4.71 (m, 1H), 4.68 (t, J = 6.8 Hz, 1H), 4.51–4.48 (m, 1H), 3.98–3.95 (m, 1H), 3.92–3.88 (m, 1H), 3.02 (s, 3H), 1.33 (s, 3H), 1.31 (s, 3H), 1.07 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 135.7, 132.8, 132.7, 132.4, 130.1, 130.0, 127.9, 127.8, 119.5, 109.0, 81.9, 78.0, 76.3, 63.0, 39.3, 27.0, 26.8, 25.0, 19.2 ppm; IR νmax (thin film): 3072, 1589 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C26H36NaO6SSi 527.1900; found 527.1897.
Compound 14. Column chromatography (petroleum ether/EtOAc, 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); oily liquid (0.56 g, 1.8 mmol, 90%); [α]25D = +9.34 (c 0.4, CHCl3); 1H NMR (500 MHz, CDCl3): δ 5.91–5.84 (m, 1H), 5.41 (d, J = 17.2 Hz, 1H), 5.26 (d, J = 10.3 Hz, 1H), 4.71 (t, J = 6.5 Hz, 1H), 4.66 (d, J = 6.9 Hz, 1H), 4.64 (d, J = 6.9 Hz, 1H), 4.53 (dd, J = 10.9, 2.3 Hz, 1H), 4.34 (dd, J = 10.8, 4.0 Hz, 1H), 4.26 (dd, J = 7.7, 6.5 Hz, 1H), 3.68–3.69 (m, 1H), 3.39 (s, 3H), 3.02 (s, 3H), 1.45 (s, 3H), 1.35 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 133.1, 118.0, 108.9, 97.3, 78.0, 76.1, 75.2, 70.0, 56.3, 37.3, 27.6, 25.2 ppm; IR νmax (thin film): 2988, 1643, 1546 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C12H22NaO7S 333.0984; found 333.0987.
4); oily liquid (0.56 g, 1.8 mmol, 90%); [α]25D = +9.34 (c 0.4, CHCl3); 1H NMR (500 MHz, CDCl3): δ 5.91–5.84 (m, 1H), 5.41 (d, J = 17.2 Hz, 1H), 5.26 (d, J = 10.3 Hz, 1H), 4.71 (t, J = 6.5 Hz, 1H), 4.66 (d, J = 6.9 Hz, 1H), 4.64 (d, J = 6.9 Hz, 1H), 4.53 (dd, J = 10.9, 2.3 Hz, 1H), 4.34 (dd, J = 10.8, 4.0 Hz, 1H), 4.26 (dd, J = 7.7, 6.5 Hz, 1H), 3.68–3.69 (m, 1H), 3.39 (s, 3H), 3.02 (s, 3H), 1.45 (s, 3H), 1.35 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 133.1, 118.0, 108.9, 97.3, 78.0, 76.1, 75.2, 70.0, 56.3, 37.3, 27.6, 25.2 ppm; IR νmax (thin film): 2988, 1643, 1546 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C12H22NaO7S 333.0984; found 333.0987.
Compound 20. Column chromatography (petroleum ether/EtOAc, 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (1.04 g, 1.8 mmol, 90%); [α]25D = −1.08 (c 0.74, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.70–7.68 (m, 4H), 7.44–7.37 (m, 6H), 4.76 (m, 1H), 4.39 (t, J = 6.6 Hz, 1H), 4.22–4.18 (m, 1H), 4.13 (q, J = 7.4 Hz, 2H), 3.99–3.98 (m, 2H), 3.06 (s, 3H), 2.52–2.38 (m, 2H), 2.03–1.96 (m, 1H), 1.77–1.69 (m, 1H), 1.29 (s, 3H), 1.26–1.23 (m, 6H), 1.07 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 173.1, 135.7, 132.8, 130.0, 129.9, 127.9, 127.8, 108.5, 80.6, 76.2, 75.0, 63.4, 60.4, 39.4, 30.9, 27.6, 26.8, 25.5, 24.9, 19.3, 14.3 ppm; IR νmax (thin film): 3072, 1737, 1589 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C29H42NaO8SSi 601.2267; found 601.2270.
1); oily liquid (1.04 g, 1.8 mmol, 90%); [α]25D = −1.08 (c 0.74, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.70–7.68 (m, 4H), 7.44–7.37 (m, 6H), 4.76 (m, 1H), 4.39 (t, J = 6.6 Hz, 1H), 4.22–4.18 (m, 1H), 4.13 (q, J = 7.4 Hz, 2H), 3.99–3.98 (m, 2H), 3.06 (s, 3H), 2.52–2.38 (m, 2H), 2.03–1.96 (m, 1H), 1.77–1.69 (m, 1H), 1.29 (s, 3H), 1.26–1.23 (m, 6H), 1.07 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 173.1, 135.7, 132.8, 130.0, 129.9, 127.9, 127.8, 108.5, 80.6, 76.2, 75.0, 63.4, 60.4, 39.4, 30.9, 27.6, 26.8, 25.5, 24.9, 19.3, 14.3 ppm; IR νmax (thin film): 3072, 1737, 1589 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C29H42NaO8SSi 601.2267; found 601.2270.
Compound 26. Column chromatography (petroleum ether/EtOAc, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.73 g, 1.9 mmol, 95%); [α]25D = −1.1 (c 0.35, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.76 (d, J = 6.8 Hz, 1H), 4.70 (d, J = 6.8 Hz, 1H), 4.58 (d, J = 11 Hz, 1H), 4.35 (dd, J = 10.9, 3.4 Hz, 1H), 4.21–4.17 (m, 1H), 4.17–4.13 (m, 1H), 4.11 (q, J = 6.8 Hz, 2H), 3.79 (d, J = 7.9 Hz, 1H), 3.40 (s, 3H), 3.03 (s, 3H), 2.56–2.50 (m, 1H), 2.44–2.37 (m, 1H), 1.99–1.92 (m, 1H), 1.80–1.72 (m, 1H), 1.38 (s, 3H), 1.30 (s, 3H), 1.23 (t, J = 6.9 Hz, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 173.2, 108.4, 96.7, 76.5, 75.2, 74.4, 69.7, 60.4, 56.4, 37.3, 31.1, 28.0, 25.6, 24.9, 14.2 ppm; IR νmax (thin film): 1734 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C15H28NaO9S 407.1352; found 407.1351.
1); oily liquid (0.73 g, 1.9 mmol, 95%); [α]25D = −1.1 (c 0.35, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.76 (d, J = 6.8 Hz, 1H), 4.70 (d, J = 6.8 Hz, 1H), 4.58 (d, J = 11 Hz, 1H), 4.35 (dd, J = 10.9, 3.4 Hz, 1H), 4.21–4.17 (m, 1H), 4.17–4.13 (m, 1H), 4.11 (q, J = 6.8 Hz, 2H), 3.79 (d, J = 7.9 Hz, 1H), 3.40 (s, 3H), 3.03 (s, 3H), 2.56–2.50 (m, 1H), 2.44–2.37 (m, 1H), 1.99–1.92 (m, 1H), 1.80–1.72 (m, 1H), 1.38 (s, 3H), 1.30 (s, 3H), 1.23 (t, J = 6.9 Hz, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 173.2, 108.4, 96.7, 76.5, 75.2, 74.4, 69.7, 60.4, 56.4, 37.3, 31.1, 28.0, 25.6, 24.9, 14.2 ppm; IR νmax (thin film): 1734 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C15H28NaO9S 407.1352; found 407.1351.
The same procedure was used for the preparation of 15 from 14.
Compound 8. Column chromatography (petroleum ether/EtOAc, 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); oily liquid (0.75 g, 1.44 mmol, 72%); [α]25D = −0.877 (c 0.23, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.69–7.66 (m, 4H), 7.43–7.37 (m, 6H), 4.77–4.75 (m, 1H), 4.40–4.35 (m, 2H), 4.02–3.95 (m, 2H), 3.79–3.73 (m, 2H), 3.03 (s, 3H), 1.88–1.69 (m, 2H), 1.30 (s, 3H), 1.37 (s, 3H), 1.08 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 135.7, 132.7, 130.1, 130.0, 127.9, 127.8, 108.6, 81.1, 76.2, 75.2, 63.3, 61.0, 39.5, 32.3, 27.5, 26.9, 25.4, 19.3 ppm; IR νmax (thin film): 3486, 3080, 1546 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C26H38NaO7SSi 545.2005; found 545.2009.
3); oily liquid (0.75 g, 1.44 mmol, 72%); [α]25D = −0.877 (c 0.23, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.69–7.66 (m, 4H), 7.43–7.37 (m, 6H), 4.77–4.75 (m, 1H), 4.40–4.35 (m, 2H), 4.02–3.95 (m, 2H), 3.79–3.73 (m, 2H), 3.03 (s, 3H), 1.88–1.69 (m, 2H), 1.30 (s, 3H), 1.37 (s, 3H), 1.08 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 135.7, 132.7, 130.1, 130.0, 127.9, 127.8, 108.6, 81.1, 76.2, 75.2, 63.3, 61.0, 39.5, 32.3, 27.5, 26.9, 25.4, 19.3 ppm; IR νmax (thin film): 3486, 3080, 1546 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C26H38NaO7SSi 545.2005; found 545.2009.
Compound 15. Column chromatography (petroleum ether/EtOAc, 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6); oily liquid (0.46 g, 1.4 mmol, 70%); [α]25D = −3.16 (c 0.32, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.74 (d, J = 6.8 Hz, 1H), 4.69 (d, J = 6.8 Hz, 1H), 4.58 (dd, J = 10.9, 1.7 Hz, 1H), 4.39–4.30 (m, 2H), 4.17–4.14 (m, 1H), 3.83–3.77 (m, 3H), 3.39 (s, 3H), 3.03 (s, 3H), 1.85–1.77 (m, 2H), 1.40 (s, 3H), 1.32 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 108.6, 96.7, 76.3, 75.3, 74.6, 69.7, 61.0, 56.4, 37.3, 31.6, 27.9, 25.5 ppm; IR νmax (thin film): 3460, 2938, 1455 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C12H24O8S 329.1270; found 329.1273.
6); oily liquid (0.46 g, 1.4 mmol, 70%); [α]25D = −3.16 (c 0.32, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.74 (d, J = 6.8 Hz, 1H), 4.69 (d, J = 6.8 Hz, 1H), 4.58 (dd, J = 10.9, 1.7 Hz, 1H), 4.39–4.30 (m, 2H), 4.17–4.14 (m, 1H), 3.83–3.77 (m, 3H), 3.39 (s, 3H), 3.03 (s, 3H), 1.85–1.77 (m, 2H), 1.40 (s, 3H), 1.32 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 108.6, 96.7, 76.3, 75.3, 74.6, 69.7, 61.0, 56.4, 37.3, 31.6, 27.9, 25.5 ppm; IR νmax (thin film): 3460, 2938, 1455 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C12H24O8S 329.1270; found 329.1273.
The same procedure was used for the preparation of 16 from 15.
Compound 9. Column chromatography (petroleum ether/EtOAc, 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.98 g, 1.9 mmol, 95%); [α]25D = +9.3 (c 0.8, CHCl3); 1H NMR (500 MHz, CDCl3): δ 9.73 (s, 1H), 7.69–7.64 (m, 4H), 7.44–7.37 (m, 6H), 4.75–4.66 (m, 2H), 4.42–4.39 (m, 1H), 4.00–3.95 (m, 2H), 3.02 (s, 3H), 2.88–2.70 (m, 2H), 1.32 (s, 3H), 1.26 (s, 3H), 1.04 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 199.5, 135.7, 132.6, 130.2, 130.0, 128.0, 127.9, 108.8, 80.2, 74.1, 71.8, 63.2, 43.8, 39.5, 27.6, 26.9, 25.4, 19.2 ppm; IR νmax (thin film): 2740, 1728, 1589 cm−1; HRMS (ESI-TOF) m/z [M + NH4]+ calcd for C26H40NO7SSi 538.2295; found 538.2299.
1); oily liquid (0.98 g, 1.9 mmol, 95%); [α]25D = +9.3 (c 0.8, CHCl3); 1H NMR (500 MHz, CDCl3): δ 9.73 (s, 1H), 7.69–7.64 (m, 4H), 7.44–7.37 (m, 6H), 4.75–4.66 (m, 2H), 4.42–4.39 (m, 1H), 4.00–3.95 (m, 2H), 3.02 (s, 3H), 2.88–2.70 (m, 2H), 1.32 (s, 3H), 1.26 (s, 3H), 1.04 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 199.5, 135.7, 132.6, 130.2, 130.0, 128.0, 127.9, 108.8, 80.2, 74.1, 71.8, 63.2, 43.8, 39.5, 27.6, 26.9, 25.4, 19.2 ppm; IR νmax (thin film): 2740, 1728, 1589 cm−1; HRMS (ESI-TOF) m/z [M + NH4]+ calcd for C26H40NO7SSi 538.2295; found 538.2299.
Compound 16. Column chromatography (petroleum ether/EtOAc, 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); oily liquid (0.62 g, 1.92 mmol, 96%); [α]25D = +13.1 (c 0.32, CHCl3); 1H NMR (500 MHz, CDCl3): δ 9.77 (s, 1H), 4.76–4.72 (m, 2H), 4.67 (d, J = 6.8 Hz, 1H), 4.59 (dd, J = 8.7 Hz, 1H), 4.34 (dd, J = 10.9, 3.2 Hz, 1H), 4.21 (dd, J = 5.9, 5.5 Hz, 1H), 3.71 (d, J = 8.7 Hz, 1H), 3.39 (s, 3H), 3.03 (s, 3H), 2.80–2.03 (m, 2H), 1.40 (s, 3H), 1.34 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 199.7, 108.9, 96.5, 74.6, 74.4, 72.1, 69.1, 56.4, 43.8, 37.4, 27.9, 25.4 ppm; IR νmax (thin film): 2937, 2726, 1727, 1455 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C12H23O8S 327.1114; found 327.1118.
3); oily liquid (0.62 g, 1.92 mmol, 96%); [α]25D = +13.1 (c 0.32, CHCl3); 1H NMR (500 MHz, CDCl3): δ 9.77 (s, 1H), 4.76–4.72 (m, 2H), 4.67 (d, J = 6.8 Hz, 1H), 4.59 (dd, J = 8.7 Hz, 1H), 4.34 (dd, J = 10.9, 3.2 Hz, 1H), 4.21 (dd, J = 5.9, 5.5 Hz, 1H), 3.71 (d, J = 8.7 Hz, 1H), 3.39 (s, 3H), 3.03 (s, 3H), 2.80–2.03 (m, 2H), 1.40 (s, 3H), 1.34 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 199.7, 108.9, 96.5, 74.6, 74.4, 72.1, 69.1, 56.4, 43.8, 37.4, 27.9, 25.4 ppm; IR νmax (thin film): 2937, 2726, 1727, 1455 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C12H23O8S 327.1114; found 327.1118.
Note: The hydrazine derivatives give complex NMR spectra at room temperature due to the existence of rotamers.
The same procedure was used for the preparation of 17, 22 and 28.
Compound 10a. Column chromatography (petroleum ether/EtOAc, 8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); oily liquid (1.25 g, 1.52 mmol, 76%); [α]25D = +2.80 (c 0.25, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.74–7.62 (m, 4H), 7.46–7.26 (m, 16H), 5.19–5.09 (m, 3H), 4.84–4.67 (m, 2H), 4.52–4.27 (m, 4H), 4.03–3.91 (m, 2H), 3.80 (d, J = 12 Hz, 1H), 3.57–3.55 (m, 1H), 2.81 (s, 3H), 1.27 (s, 3H), 1.23 (s, 3H), 1.10 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.5, 155.9, 141.0, 135.8, 135.6, 135.1, 132.5, 132.3, 130.3, 130.1, 128.7, 128.6, 128.5, 128.2, 128.1, 127.9, 127.6, 127.0, 109.5, 82.7, 76.7, 73.5, 68.6, 65.3, 62.9, 60.4, 57.6, 38.5, 26.9, 26.5, 25.2 ppm; IR νmax (thin film): 3464, 3378, 1717, 1588 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C42H52N2O11SSi 843.2959; found 843.2962.
2); oily liquid (1.25 g, 1.52 mmol, 76%); [α]25D = +2.80 (c 0.25, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.74–7.62 (m, 4H), 7.46–7.26 (m, 16H), 5.19–5.09 (m, 3H), 4.84–4.67 (m, 2H), 4.52–4.27 (m, 4H), 4.03–3.91 (m, 2H), 3.80 (d, J = 12 Hz, 1H), 3.57–3.55 (m, 1H), 2.81 (s, 3H), 1.27 (s, 3H), 1.23 (s, 3H), 1.10 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.5, 155.9, 141.0, 135.8, 135.6, 135.1, 132.5, 132.3, 130.3, 130.1, 128.7, 128.6, 128.5, 128.2, 128.1, 127.9, 127.6, 127.0, 109.5, 82.7, 76.7, 73.5, 68.6, 65.3, 62.9, 60.4, 57.6, 38.5, 26.9, 26.5, 25.2 ppm; IR νmax (thin film): 3464, 3378, 1717, 1588 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C42H52N2O11SSi 843.2959; found 843.2962.
Compound 10b. Column chromatography (petroleum ether/EtOAc, 8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); oily liquid (1.13 g, 1.38 mmol, 69%); [α]25D = +4.6 (c 0.46, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.72–7.64 (m, 4H), 7.42–7.24 (m, 16H), 6.74 (bs, 1H), 5.30–5.05 (m, 4H), 4.86–4.36 (m, 2H), 4.20–3.90 (m, 4H), 3.73–3.55 (m, 2H), 3.16–3.03 (m, 3H), 2.66 (bs, 1H), 1.19–1.06 (m, 15H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.2, 158.5, 157.2, 156.0, 135.8, 135.6, 135.1, 133.1, 132.9, 132.8, 130.3, 130.1, 129.9, 129.8, 128.8, 128.6, 128.5, 128.4, 128.3, 128.2, 128.0, 127.8, 127.7, 108.4, 108.2, 79.6, 78.5, 74.2, 73.2, 73.6, 73.4, 69.6, 68.8, 68.4, 68.2, 67.8, 64.4, 62.9, 62.6, 60.0, 59.8, 58.7, 53.5, 39.4, 39.1, 26.9, 26.8, 25.4, 25.3, 19.4, 19.3 ppm; IR νmax (thin film): 3464, 3378, 1717, 1588 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C42H52N2O11SSi 843.2959; found 843.2962.
2); oily liquid (1.13 g, 1.38 mmol, 69%); [α]25D = +4.6 (c 0.46, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.72–7.64 (m, 4H), 7.42–7.24 (m, 16H), 6.74 (bs, 1H), 5.30–5.05 (m, 4H), 4.86–4.36 (m, 2H), 4.20–3.90 (m, 4H), 3.73–3.55 (m, 2H), 3.16–3.03 (m, 3H), 2.66 (bs, 1H), 1.19–1.06 (m, 15H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.2, 158.5, 157.2, 156.0, 135.8, 135.6, 135.1, 133.1, 132.9, 132.8, 130.3, 130.1, 129.9, 129.8, 128.8, 128.6, 128.5, 128.4, 128.3, 128.2, 128.0, 127.8, 127.7, 108.4, 108.2, 79.6, 78.5, 74.2, 73.2, 73.6, 73.4, 69.6, 68.8, 68.4, 68.2, 67.8, 64.4, 62.9, 62.6, 60.0, 59.8, 58.7, 53.5, 39.4, 39.1, 26.9, 26.8, 25.4, 25.3, 19.4, 19.3 ppm; IR νmax (thin film): 3464, 3378, 1717, 1588 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C42H52N2O11SSi 843.2959; found 843.2962.
Compound 17a. Column chromatography (petroleum ether/EtOAc, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.91 g, 1.46 mmol, 73%); [α]25D = −22.85 (c 0.14, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.39–7.13 (m, 10H), 5.33–5.05 (m, 4H), 4.53–4.16 (m, 8H), 3.94–3.84 (m, 1H), 3.61 (bs, 1H), 3.28 (s, 3H), 2.99–2.93 (d, 3H), 1.42 (s, 3H), 1.29 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.2, 158.8, 155.8, 155.6, 135.5, 135.4, 135.1, 128.9, 128.7, 128.6, 128.3, 108.8, 96.9, 75.3, 74.7, 74.4, 74.2, 69.9, 69.4, 68.9, 68.6, 60.4, 58.6, 57.6, 56.3, 56.2, 37.1, 27.6, 25.6 ppm; IR νmax (thin film): 3422, 1704, 1720, 1650, 1596 cm−1; HRMS (ESI-TOF) m/z [M + NH4]+ calcd for C28H42N3O12S 644.2489; found 644.2494.
1); oily liquid (0.91 g, 1.46 mmol, 73%); [α]25D = −22.85 (c 0.14, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.39–7.13 (m, 10H), 5.33–5.05 (m, 4H), 4.53–4.16 (m, 8H), 3.94–3.84 (m, 1H), 3.61 (bs, 1H), 3.28 (s, 3H), 2.99–2.93 (d, 3H), 1.42 (s, 3H), 1.29 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.2, 158.8, 155.8, 155.6, 135.5, 135.4, 135.1, 128.9, 128.7, 128.6, 128.3, 108.8, 96.9, 75.3, 74.7, 74.4, 74.2, 69.9, 69.4, 68.9, 68.6, 60.4, 58.6, 57.6, 56.3, 56.2, 37.1, 27.6, 25.6 ppm; IR νmax (thin film): 3422, 1704, 1720, 1650, 1596 cm−1; HRMS (ESI-TOF) m/z [M + NH4]+ calcd for C28H42N3O12S 644.2489; found 644.2494.
Compound 17b. Column chromatography (petroleum ether/EtOAc, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.82 g, 1.32 mmol, 66%); [α]25D = +18.33 (c 0.12, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.37–7.19 (m, 10H), 6.61 (bs, 1H), 5.33–5.06 (m, 4H), 4.68–4.63 (m, 1H), 4.59–4.39 (m, 2H), 4.30–4.26 (m, 2H), 4.02–3.89 (m, 2H), 3.74–3.47 (m, 3H), 3.32–3.28 (m, 3H), 3.03–2.99 (d, 3H), 1.29 (s, 3H), 1.27 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.1, 158.3, 157.4, 156.4, 135.7, 135.5, 135.0, 128.8, 128.7, 128.6, 128.5, 128.4, 128.3, 127.7, 108.8, 108.3, 108.2, 96.9, 96.6, 75.3, 74.9, 74.7, 74.3, 74.2, 74.0, 68.9, 68.7, 68.6, 68.4, 60.1, 59.9, 59.0, 57.7, 56.8, 56.6, 56.3, 56.2, 37.2, 29.7, 29.6, 27.7, 27.6 ppm; IR νmax (thin film): 3422, 1704, 1720, 1650, 1596 cm−1; HRMS (ESI-TOF) m/z [M + NH4]+ calcd for C28H42N3O12S 644.2489; found 644.2494.
1); oily liquid (0.82 g, 1.32 mmol, 66%); [α]25D = +18.33 (c 0.12, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.37–7.19 (m, 10H), 6.61 (bs, 1H), 5.33–5.06 (m, 4H), 4.68–4.63 (m, 1H), 4.59–4.39 (m, 2H), 4.30–4.26 (m, 2H), 4.02–3.89 (m, 2H), 3.74–3.47 (m, 3H), 3.32–3.28 (m, 3H), 3.03–2.99 (d, 3H), 1.29 (s, 3H), 1.27 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.1, 158.3, 157.4, 156.4, 135.7, 135.5, 135.0, 128.8, 128.7, 128.6, 128.5, 128.4, 128.3, 127.7, 108.8, 108.3, 108.2, 96.9, 96.6, 75.3, 74.9, 74.7, 74.3, 74.2, 74.0, 68.9, 68.7, 68.6, 68.4, 60.1, 59.9, 59.0, 57.7, 56.8, 56.6, 56.3, 56.2, 37.2, 29.7, 29.6, 27.7, 27.6 ppm; IR νmax (thin film): 3422, 1704, 1720, 1650, 1596 cm−1; HRMS (ESI-TOF) m/z [M + NH4]+ calcd for C28H42N3O12S 644.2489; found 644.2494.
Compound 22a. Column chromatography (petroleum ether/EtOAc, 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); oily liquid (1.21 g, 1.46 mmol, 73%); [α]25D = −16.3 (c 0.8, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.69–7.63 (m, 4H), 7.42–7.30 (m, 16H), 6.49–6.44 (m, 1H), 5.25–5.12 (m, 4H), 4.69–4.62 (m, 1H), 4.30–4.19 (m, 2H), 4.14–4.03 (m, 1H), 3.69–3.84 (m, 2H), 1.30 (s, 3H), 3.55–3.39 (m, 2H), 3.04–2.97 (m, 3H), 1.70–1.45 (m, 1H), 1.09–1.02 (m, 14H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.0, 158.3, 156.9, 156.6, 135.9, 135.7, 135.2, 132.6, 130.1, 130.0, 128.7, 128.6, 128.5, 128.2, 127.9, 127.8, 127.7, 127.6, 108.7, 108.5, 81.0, 80.8, 75.5, 75.4, 75.2, 68.6, 68.4, 68.1, 63.5, 63.3, 61.8, 61.6, 59.9, 58.7, 39.6, 39.5, 39.4, 27.2, 27.1, 26.9, 24.8, 24.6, 19.3 ppm; IR νmax (thin film): 3473, 1721, 1588 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C43H54N2NaO11SSi 857.3115; found 857.3115.
3); oily liquid (1.21 g, 1.46 mmol, 73%); [α]25D = −16.3 (c 0.8, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.69–7.63 (m, 4H), 7.42–7.30 (m, 16H), 6.49–6.44 (m, 1H), 5.25–5.12 (m, 4H), 4.69–4.62 (m, 1H), 4.30–4.19 (m, 2H), 4.14–4.03 (m, 1H), 3.69–3.84 (m, 2H), 1.30 (s, 3H), 3.55–3.39 (m, 2H), 3.04–2.97 (m, 3H), 1.70–1.45 (m, 1H), 1.09–1.02 (m, 14H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.0, 158.3, 156.9, 156.6, 135.9, 135.7, 135.2, 132.6, 130.1, 130.0, 128.7, 128.6, 128.5, 128.2, 127.9, 127.8, 127.7, 127.6, 108.7, 108.5, 81.0, 80.8, 75.5, 75.4, 75.2, 68.6, 68.4, 68.1, 63.5, 63.3, 61.8, 61.6, 59.9, 58.7, 39.6, 39.5, 39.4, 27.2, 27.1, 26.9, 24.8, 24.6, 19.3 ppm; IR νmax (thin film): 3473, 1721, 1588 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C43H54N2NaO11SSi 857.3115; found 857.3115.
Compound 22b. Column chromatography (petroleum ether/EtOAc, 7
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); oily liquid (1.25 g, 1.5 mmol, 75%); [α]25D = +23.5 (c 1.6, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.68–7.65 (m, 4H), 7.44–7.30 (m, 16H), 7.11–7.03 (m, 1H), 5.22–5.12 (m, 4H), 4.70–4.64 (m, 1H), 4.29–4.20 (m, 2H), 4.14–4.05 (m, 1H), 3.96–3.88 (m, 2H), 3.50–3.42 (m, 2H), 3.01–2.97 (m, 3H), 1.76–1.47 (m, 2H), 1.32–1.23 (m, 6H), 1.06 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.1, 158.7, 156.9, 156.2, 136.0, 135.7, 135.6, 135.5, 135.3, 132.7, 132.6, 130.2, 130.0, 128.7, 128.6, 128.5, 128.4, 128.3, 128.2, 128.1, 127.9, 127.7, 127.6, 109.1, 108.7, 108.5, 81.0, 80.8, 80.6, 80.2, 75.5, 75.4, 75.2, 74.3, 74.1, 73.6, 68.6, 68.4, 68.3, 68.2, 68.1, 67.9, 63.7, 63.6, 63.5, 63.3, 62.3, 62.2, 39.6, 39.4, 28.2, 28.1, 27.1, 26.9, 25.9, 25.8, 24.8, 24.6, 19.3 ppm; IR νmax (thin film): 3317, 1722, 1586 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C43H55N2O11SSi 835.3296; found 835.3292.
3); oily liquid (1.25 g, 1.5 mmol, 75%); [α]25D = +23.5 (c 1.6, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.68–7.65 (m, 4H), 7.44–7.30 (m, 16H), 7.11–7.03 (m, 1H), 5.22–5.12 (m, 4H), 4.70–4.64 (m, 1H), 4.29–4.20 (m, 2H), 4.14–4.05 (m, 1H), 3.96–3.88 (m, 2H), 3.50–3.42 (m, 2H), 3.01–2.97 (m, 3H), 1.76–1.47 (m, 2H), 1.32–1.23 (m, 6H), 1.06 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.1, 158.7, 156.9, 156.2, 136.0, 135.7, 135.6, 135.5, 135.3, 132.7, 132.6, 130.2, 130.0, 128.7, 128.6, 128.5, 128.4, 128.3, 128.2, 128.1, 127.9, 127.7, 127.6, 109.1, 108.7, 108.5, 81.0, 80.8, 80.6, 80.2, 75.5, 75.4, 75.2, 74.3, 74.1, 73.6, 68.6, 68.4, 68.3, 68.2, 68.1, 67.9, 63.7, 63.6, 63.5, 63.3, 62.3, 62.2, 39.6, 39.4, 28.2, 28.1, 27.1, 26.9, 25.9, 25.8, 24.8, 24.6, 19.3 ppm; IR νmax (thin film): 3317, 1722, 1586 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C43H55N2O11SSi 835.3296; found 835.3292.
Compound 28a. Column chromatography (petroleum ether/EtOAc, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.98 g, 1.53 mmol, 77%); [α]25D = −33.93 (c 0.28, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.37–7.26 (m, 10H), 6.60 (bs, 1H), 5.26–5.10 (m, 4H), 4.74–4.70 (m, 1H), 4.65–4.48 (m, 3H), 4.34–4.28 (m, 2H), 4.16–4.00 (m, 2H), 3.69–3.67 (m, 1H), 3.66–3.41 (m, 1H), 3.36 (s, 3H), 3.00 (s, 3H), 1.89–1.79 (m, 1H), 1.50–1.46 (m, 1H), 1.34–1.30 (m, 3H), 1.10–1.09 (m, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.0, 158.4, 156.9, 156.8, 135.9, 135.6, 135.3, 128.7, 128.6, 128.5, 128.4, 128.2, 127.6, 108.6, 108.5, 96.5, 75.8, 75.5, 75.3, 74.5, 69.2, 68.6, 68.5, 68.4, 68.1, 61.9, 61.8, 59.8, 58.6, 65.5, 37.4, 29.7, 27.7, 26.9, 24.9, 24.8 ppm; IR νmax (thin film): 3463, 1719 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C29H41N2O12S 641.2380; found 641.2388.
1); oily liquid (0.98 g, 1.53 mmol, 77%); [α]25D = −33.93 (c 0.28, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.37–7.26 (m, 10H), 6.60 (bs, 1H), 5.26–5.10 (m, 4H), 4.74–4.70 (m, 1H), 4.65–4.48 (m, 3H), 4.34–4.28 (m, 2H), 4.16–4.00 (m, 2H), 3.69–3.67 (m, 1H), 3.66–3.41 (m, 1H), 3.36 (s, 3H), 3.00 (s, 3H), 1.89–1.79 (m, 1H), 1.50–1.46 (m, 1H), 1.34–1.30 (m, 3H), 1.10–1.09 (m, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.0, 158.4, 156.9, 156.8, 135.9, 135.6, 135.3, 128.7, 128.6, 128.5, 128.4, 128.2, 127.6, 108.6, 108.5, 96.5, 75.8, 75.5, 75.3, 74.5, 69.2, 68.6, 68.5, 68.4, 68.1, 61.9, 61.8, 59.8, 58.6, 65.5, 37.4, 29.7, 27.7, 26.9, 24.9, 24.8 ppm; IR νmax (thin film): 3463, 1719 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C29H41N2O12S 641.2380; found 641.2388.
Compound 28b. Column chromatography (petroleum ether/EtOAc, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (1.00 g, 1.56 mmol, 79%); [α]25D = +22.1 (c 0.16, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.88–7.27 (m, 10H), 6.63–6.59 (m, 1H), 5.27–5.10 (m, 4H), 4.74–4.71 (m, 1H), 4.66–4.62 (m, 1H), 4.55–4.49 (m, 1H), 4.35–4.28 (m, 2H), 4.16–4.04 (m, 2H), 3.70–3.66 (m, 1H), 3.52–3.41 (m, 2H), 3.38–3.35 (m, 3H), 3.01–3.00 (m, 3H), 1.90–1.79 (m, 1H), 1.55–1.47 (m, 1H), 1.34–1.31 (m, 3H), 1.11–1.10 (m, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.3, 158.6, 157.2, 157.0, 136.1, 135.9, 135.5, 129.0, 128.9, 128.8, 128.7, 128.6, 128.5, 127.8, 108.8, 108.7, 96.8, 76.0, 75.7, 75.6, 74.8, 69.4, 68.9, 68.8, 68.6, 68.4, 62.2, 62.1, 60.1, 58.9, 56.8, 37.6, 27.9, 25.1 ppm; IR νmax (thin film): 3463, 1719 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C29H41N2O12S 641.2380; found 641.2388.
1); oily liquid (1.00 g, 1.56 mmol, 79%); [α]25D = +22.1 (c 0.16, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.88–7.27 (m, 10H), 6.63–6.59 (m, 1H), 5.27–5.10 (m, 4H), 4.74–4.71 (m, 1H), 4.66–4.62 (m, 1H), 4.55–4.49 (m, 1H), 4.35–4.28 (m, 2H), 4.16–4.04 (m, 2H), 3.70–3.66 (m, 1H), 3.52–3.41 (m, 2H), 3.38–3.35 (m, 3H), 3.01–3.00 (m, 3H), 1.90–1.79 (m, 1H), 1.55–1.47 (m, 1H), 1.34–1.31 (m, 3H), 1.11–1.10 (m, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ 159.3, 158.6, 157.2, 157.0, 136.1, 135.9, 135.5, 129.0, 128.9, 128.8, 128.7, 128.6, 128.5, 127.8, 108.8, 108.7, 96.8, 76.0, 75.7, 75.6, 74.8, 69.4, 68.9, 68.8, 68.6, 68.4, 62.2, 62.1, 60.1, 58.9, 56.8, 37.6, 27.9, 25.1 ppm; IR νmax (thin film): 3463, 1719 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C29H41N2O12S 641.2380; found 641.2388.
The same procedure was used for the preparation of 18 from 17. Compounds 23 and 29 were also prepared using a similar procedure, however refluxing with triethylamine (2 equiv.) was required for the desired cyclization to occur.
Compound 11a. Column chromatography (petroleum ether/EtOAc, 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6); oily liquid (0.69 g, 1.56 mmol, 78%); [α]25D = +12.3 (c 0.31, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.68–7.66 (m, 4H, Ar–H), 7.40–7.32 (m, 6H, Ar–H), 4.75–4.71 (m, 1H, C3H), 4.59–4.52 (m, 1H, C2H), 3.99–3.54 (m, 6H, C1H, C4H, C5H, C6H), 1.44 (s, 3H, C7H), 1.25 (s, 3H, C8H), 1.02 (s, 9H, (CH3)3C–Si) ppm; 13C NMR (100 MHz, CDCl3): δ 135.7 (C–Ar), 133.0 (C–Ar), 132.9 (C–Ar), 129.8 (C–Ar), 127.8 (C–Ar), 127.7 (C–Ar), 112.3 (C-9), 81.8 (C-3), 79.4 (C-2), 66.3 (C-5), 62.8 (C-6), 60.2 (C-1), 60.0 (C-4), 26.8 ((CH3)3C–Si), 26.2 (C-8), 24.3 (C-7), 19.2 (C–Si) ppm; IR νmax (thin film): 3407, 3470, 1669 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C24H36NO4Si 442.2414; found 442.2417.
6); oily liquid (0.69 g, 1.56 mmol, 78%); [α]25D = +12.3 (c 0.31, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.68–7.66 (m, 4H, Ar–H), 7.40–7.32 (m, 6H, Ar–H), 4.75–4.71 (m, 1H, C3H), 4.59–4.52 (m, 1H, C2H), 3.99–3.54 (m, 6H, C1H, C4H, C5H, C6H), 1.44 (s, 3H, C7H), 1.25 (s, 3H, C8H), 1.02 (s, 9H, (CH3)3C–Si) ppm; 13C NMR (100 MHz, CDCl3): δ 135.7 (C–Ar), 133.0 (C–Ar), 132.9 (C–Ar), 129.8 (C–Ar), 127.8 (C–Ar), 127.7 (C–Ar), 112.3 (C-9), 81.8 (C-3), 79.4 (C-2), 66.3 (C-5), 62.8 (C-6), 60.2 (C-1), 60.0 (C-4), 26.8 ((CH3)3C–Si), 26.2 (C-8), 24.3 (C-7), 19.2 (C–Si) ppm; IR νmax (thin film): 3407, 3470, 1669 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C24H36NO4Si 442.2414; found 442.2417.
Compound 11b. Chromatography (petroleum ether/EtOAc, 4
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6); oily liquid (0.65 g, 1.46 mmol, 73%); [α]25D = +10.5 (c 0.2, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.71–7.67 (m, 4H, Ar–H), 7.43–7.33 (m, 6H, Ar–H), 4.67–4.64 (m, 2H, C2H, C3H), 3.98–3.83 (m, 4H, C5H, C6H), 3.11–3.08 (m, 1H, C1H), 2.96–2.93 (m, 1H, C4H), 1.41 (s, 3H, C7H), 1.27 (s, 3H, C8H), 1.04 (s, 9H, (CH3)3C–Si) ppm; 13C NMR (100 MHz, CDCl3): δ 135.7 (C–Ar), 135.6 (C–Ar), 133.6 (C–Ar), 133.4 (C–Ar), 129.7 (C–Ar), 127.7 (C–Ar), 127.6 (C–Ar), 111.4 (C-9), 82.3 (C-3), 81.2 (C-2), 63.6 (C-5), 62.7 (C-6), 61.7 (C-1), 60.9 (C-4), 26.9 ((CH3)3C–Si), 25.6 (C-8), 23.9 (C-7), 19.3 (C–Si) ppm; IR νmax (thin film): 3407, 3470, 1669 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C25H36NO4Si 441.2414; found 442.2416.
6); oily liquid (0.65 g, 1.46 mmol, 73%); [α]25D = +10.5 (c 0.2, CHCl3); 1H NMR (400 MHz, CDCl3): δ 7.71–7.67 (m, 4H, Ar–H), 7.43–7.33 (m, 6H, Ar–H), 4.67–4.64 (m, 2H, C2H, C3H), 3.98–3.83 (m, 4H, C5H, C6H), 3.11–3.08 (m, 1H, C1H), 2.96–2.93 (m, 1H, C4H), 1.41 (s, 3H, C7H), 1.27 (s, 3H, C8H), 1.04 (s, 9H, (CH3)3C–Si) ppm; 13C NMR (100 MHz, CDCl3): δ 135.7 (C–Ar), 135.6 (C–Ar), 133.6 (C–Ar), 133.4 (C–Ar), 129.7 (C–Ar), 127.7 (C–Ar), 127.6 (C–Ar), 111.4 (C-9), 82.3 (C-3), 81.2 (C-2), 63.6 (C-5), 62.7 (C-6), 61.7 (C-1), 60.9 (C-4), 26.9 ((CH3)3C–Si), 25.6 (C-8), 23.9 (C-7), 19.3 (C–Si) ppm; IR νmax (thin film): 3407, 3470, 1669 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C25H36NO4Si 441.2414; found 442.2416.
Compound 18a. Column chromatography (DCM/MeOH, 95
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); oily liquid (0.37 g, 1.5 mmol, 75%); [α]25D = −16.25 (c 0.24, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.76 (s, 2H, –O–CH2–O–), 4.46 (dd, J = 4, 3.4 Hz, 1H, C3H), 3.92–3.87 (m, 3H, C2H, C4H), 3.57 (dd, J = 10.9, 6.3 Hz, 1H, C5H), 3.40 (s, 3H, –OCH3), 3.15 (dd, J = 11.4, 5.1 Hz, 1H, C1H), 2.89 (dd, J = 11.4, 11.4 Hz, 1H, C1H), 2.76 (bs, 1H, –OH), 1.53 (s, 3H, C8H), 1.38 (s, 3H, C7H) ppm; 13C NMR (125 MHz, CDCl3): δ 110.2 (C-9), 96.5 (–O–CH2–O–), 74.5 (C-2), 74.0 (C-3), 72.5 (C-4), 62.7 (C-6), 58.9 (–OCH3), 55.7 (C-5), 44.2 (C-1), 28.3 (C-8), 26.4 (C-7) ppm; IR νmax (thin film): 3486 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C11H22NO5 248.1498; found 248.1499.
5); oily liquid (0.37 g, 1.5 mmol, 75%); [α]25D = −16.25 (c 0.24, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.76 (s, 2H, –O–CH2–O–), 4.46 (dd, J = 4, 3.4 Hz, 1H, C3H), 3.92–3.87 (m, 3H, C2H, C4H), 3.57 (dd, J = 10.9, 6.3 Hz, 1H, C5H), 3.40 (s, 3H, –OCH3), 3.15 (dd, J = 11.4, 5.1 Hz, 1H, C1H), 2.89 (dd, J = 11.4, 11.4 Hz, 1H, C1H), 2.76 (bs, 1H, –OH), 1.53 (s, 3H, C8H), 1.38 (s, 3H, C7H) ppm; 13C NMR (125 MHz, CDCl3): δ 110.2 (C-9), 96.5 (–O–CH2–O–), 74.5 (C-2), 74.0 (C-3), 72.5 (C-4), 62.7 (C-6), 58.9 (–OCH3), 55.7 (C-5), 44.2 (C-1), 28.3 (C-8), 26.4 (C-7) ppm; IR νmax (thin film): 3486 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C11H22NO5 248.1498; found 248.1499.
Compound 18b. Column chromatography (DCM/MeOH, 95
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); oily liquid (0.37 g, 1.52 mmol, 76%); [α]25D = −12.1 (c 0.22, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.66 (d, J = 6.9 Hz, 1H, –O–CH2–O–), 4.60 (d, J = 6.8 Hz, 1H, –O–CH2–O–), 4.57 (dd, J = 8.0, 2.8 Hz, 1H, C3H), 4.42 (dd, J = 7.4, 3.4 Hz, 1H, C2H), 3.80 (dd, J = 9.7, 6.9 Hz, 1H, C4H), 3.76–3.71 (m, 2H, C6H), 3.33 (s, 3H, –OCH3), 2.98 (dd, J = 10.9, 10.8 Hz, 1H, C5H), 2.92–2.89 (m, 1H, C1H), 2.44 (dd, J = 15.0, 5.0 Hz, 1H, C1H), 1.56 (s, 3H, C7H), 1.37 (s, 3H, C8H) ppm; 13C NMR (125 MHz, CDCl3) δ 109.1 (C-9), 95.2 (–O–CH2–O–), 75.4 (C-2), 72.9 (C-3), 69.3 (C-4), 59.2 (C-6), 57.5 (–OCH3), 55.5 (C-5), 46.3 (C-1), 26.2 (C-8), 24.4 (C-8) ppm; IR νmax (thin film): 3486 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C11H22NO5 248.1498; found 248.1499.
5); oily liquid (0.37 g, 1.52 mmol, 76%); [α]25D = −12.1 (c 0.22, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.66 (d, J = 6.9 Hz, 1H, –O–CH2–O–), 4.60 (d, J = 6.8 Hz, 1H, –O–CH2–O–), 4.57 (dd, J = 8.0, 2.8 Hz, 1H, C3H), 4.42 (dd, J = 7.4, 3.4 Hz, 1H, C2H), 3.80 (dd, J = 9.7, 6.9 Hz, 1H, C4H), 3.76–3.71 (m, 2H, C6H), 3.33 (s, 3H, –OCH3), 2.98 (dd, J = 10.9, 10.8 Hz, 1H, C5H), 2.92–2.89 (m, 1H, C1H), 2.44 (dd, J = 15.0, 5.0 Hz, 1H, C1H), 1.56 (s, 3H, C7H), 1.37 (s, 3H, C8H) ppm; 13C NMR (125 MHz, CDCl3) δ 109.1 (C-9), 95.2 (–O–CH2–O–), 75.4 (C-2), 72.9 (C-3), 69.3 (C-4), 59.2 (C-6), 57.5 (–OCH3), 55.5 (C-5), 46.3 (C-1), 26.2 (C-8), 24.4 (C-8) ppm; IR νmax (thin film): 3486 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C11H22NO5 248.1498; found 248.1499.
Compound 23a. Column chromatography (petroleum ether/EtOAc, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.63 g, 1.38 mmol, 69%); [α]25D = +12.6 (c 0.1, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.70–7.67 (m, 4H, Ar–H), 7.43–7.35 (m, 6H, Ar–H), 4.52–4.49 (m, 1H, C3H), 4.36–4.34 (m, 1H, C2H), 3.65–3.63 (m, 2H, C7H), 3.41–3.36 (m, 1H, C1H), 3.19–3.13 (m, 2H, C6H), 2.77–2.74 (m, 1H, C5H), 1.84–1.79 (m, 2H, C4H), 1.44 (s, 3H, C10H), 1.35 (s, 3H, C11H), 1.05 (s, 9H, (CH3)3C–Si) ppm; 13C NMR (125 MHz, CDCl3): δ 135.7 (C–Ar), 135.6 (C–Ar), 133.7 (C–Ar), 133.5 (C–Ar), 129.8 (C–Ar), 129.7 (C–Ar), 127.7 (C–Ar), 127.6 (C–Ar), 127.4 (C–Ar), 107.8 (C-12), 71.9 (C-3), 71.7 (C-2), 64.8 (C-7), 63.9 (C-6), 52.0 (C-1), 47.9 (C-5), 29.2 (C-4), 26.9 (C-12), 26.3 ((CH3)3C–Si), 26.0 (C-11), 19.3 (C–Si) ppm; IR νmax (thin film): 3408, 3071, 1670 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C26H38NO4Si 456.2570; found 456.2572.
1); oily liquid (0.63 g, 1.38 mmol, 69%); [α]25D = +12.6 (c 0.1, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.70–7.67 (m, 4H, Ar–H), 7.43–7.35 (m, 6H, Ar–H), 4.52–4.49 (m, 1H, C3H), 4.36–4.34 (m, 1H, C2H), 3.65–3.63 (m, 2H, C7H), 3.41–3.36 (m, 1H, C1H), 3.19–3.13 (m, 2H, C6H), 2.77–2.74 (m, 1H, C5H), 1.84–1.79 (m, 2H, C4H), 1.44 (s, 3H, C10H), 1.35 (s, 3H, C11H), 1.05 (s, 9H, (CH3)3C–Si) ppm; 13C NMR (125 MHz, CDCl3): δ 135.7 (C–Ar), 135.6 (C–Ar), 133.7 (C–Ar), 133.5 (C–Ar), 129.8 (C–Ar), 129.7 (C–Ar), 127.7 (C–Ar), 127.6 (C–Ar), 127.4 (C–Ar), 107.8 (C-12), 71.9 (C-3), 71.7 (C-2), 64.8 (C-7), 63.9 (C-6), 52.0 (C-1), 47.9 (C-5), 29.2 (C-4), 26.9 (C-12), 26.3 ((CH3)3C–Si), 26.0 (C-11), 19.3 (C–Si) ppm; IR νmax (thin film): 3408, 3071, 1670 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C26H38NO4Si 456.2570; found 456.2572.
Compound 23b. Column chromatography (petroleum ether/EtOAc, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.63 g, 1.4 mmol, 70%); [α]25D = +14.8 (c 0.6, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.68–7.65 (m, 4H, Ar–H), 7.41–7.33 (m, 6H, Ar–H), 4.50–4.48 (m, 1H, C3H), 4.35–4.33 (m, 1H, C2H), 3.64–3.60 (m, 2H, C7H), 3.41–3.36 (m, 1H, C1H), 3.18–3.12 (m, 2H, C6H), 2.76–2.74 (m, 1H, C5H), 2.02–1.99 (m, 1H, C4H), 1.81–1.77 (m, 1H, C4H), 1.42 (s, 3H, C10H), 1.34–1.28 (s, 3H, C11H), 1.03 (s, 9H, (CH3)3C–Si) ppm; 13C NMR (125 MHz, CDCl3): δ 134.2 (C–Ar), 134.1 (C–Ar), 132.1 (C–Ar), 131.9 (C–Ar), 128.2 (C–Ar), 126.2 (C–Ar), 126.1 (C–Ar), 106.3 (C-12), 70.3 (C-3), 70.1 (C-2), 63.1 (C-7), 62.2 (C-6), 50.5 (C-1), 46.4 (C-5), 30.5 (C-4), 28.2 (C-12), 27.9 ((CH3)3C–Si), 25.3 (C-11), 20.0 (C–Si) ppm; IR νmax (thin film): 3408, 3071, 1670 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C26H38NO4Si 456.2570; found 456.2572.
1); oily liquid (0.63 g, 1.4 mmol, 70%); [α]25D = +14.8 (c 0.6, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.68–7.65 (m, 4H, Ar–H), 7.41–7.33 (m, 6H, Ar–H), 4.50–4.48 (m, 1H, C3H), 4.35–4.33 (m, 1H, C2H), 3.64–3.60 (m, 2H, C7H), 3.41–3.36 (m, 1H, C1H), 3.18–3.12 (m, 2H, C6H), 2.76–2.74 (m, 1H, C5H), 2.02–1.99 (m, 1H, C4H), 1.81–1.77 (m, 1H, C4H), 1.42 (s, 3H, C10H), 1.34–1.28 (s, 3H, C11H), 1.03 (s, 9H, (CH3)3C–Si) ppm; 13C NMR (125 MHz, CDCl3): δ 134.2 (C–Ar), 134.1 (C–Ar), 132.1 (C–Ar), 131.9 (C–Ar), 128.2 (C–Ar), 126.2 (C–Ar), 126.1 (C–Ar), 106.3 (C-12), 70.3 (C-3), 70.1 (C-2), 63.1 (C-7), 62.2 (C-6), 50.5 (C-1), 46.4 (C-5), 30.5 (C-4), 28.2 (C-12), 27.9 ((CH3)3C–Si), 25.3 (C-11), 20.0 (C–Si) ppm; IR νmax (thin film): 3408, 3071, 1670 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C26H38NO4Si 456.2570; found 456.2572.
Compound 29a. Column chromatography (DCM/MeOH, 9.5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5); oily liquid (0.35 g, 1.34 mmol, 67%); [α]25D = −10.37 (c 0.27, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.82 (d, J = 6.9 Hz, 1H, –O–CH2–O–), 4.71 (d, J = 6.8 Hz, 1H, –O–CH2–O–), 4.56 (d, J = 8.5 Hz, 1H, C4H), 4.48–4.45 (m, 1H, C3H), 4.20–4.18 (m, 1H, C5H), 3.77 (dd, J = 11.4, 2.8 Hz, 1H, C7H), 3.62 (dd, J = 10.9, 8.6 Hz, 1H, C7H), 3.39 (s, 3H, (–OCH3)), 3.38–3.30 (m, 3H, C1H, C6H), 2.07–1.94 (m, 2H, C2H), 1.54 (s, 3H, C10H), 1.37 (s, 3H, C11H) ppm; 13C NMR (125 MHz, CDCl3): δ 108.3 (C-12), 96.0 (–O–CH2–O–), 78.4 (C-4), 74.0 (C-3), 71.8 (C-5), 64.0 (C-7), 55.9 (–OCH3), 55.2 (C-1), 45.1 (C-6), 32.5 (C-2), 25.7 (C-11), 23.5 (C-10) ppm; IR νmax (thin film): 3439 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C12H24NO5 262.1654; found 262.1651.
0.5); oily liquid (0.35 g, 1.34 mmol, 67%); [α]25D = −10.37 (c 0.27, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.82 (d, J = 6.9 Hz, 1H, –O–CH2–O–), 4.71 (d, J = 6.8 Hz, 1H, –O–CH2–O–), 4.56 (d, J = 8.5 Hz, 1H, C4H), 4.48–4.45 (m, 1H, C3H), 4.20–4.18 (m, 1H, C5H), 3.77 (dd, J = 11.4, 2.8 Hz, 1H, C7H), 3.62 (dd, J = 10.9, 8.6 Hz, 1H, C7H), 3.39 (s, 3H, (–OCH3)), 3.38–3.30 (m, 3H, C1H, C6H), 2.07–1.94 (m, 2H, C2H), 1.54 (s, 3H, C10H), 1.37 (s, 3H, C11H) ppm; 13C NMR (125 MHz, CDCl3): δ 108.3 (C-12), 96.0 (–O–CH2–O–), 78.4 (C-4), 74.0 (C-3), 71.8 (C-5), 64.0 (C-7), 55.9 (–OCH3), 55.2 (C-1), 45.1 (C-6), 32.5 (C-2), 25.7 (C-11), 23.5 (C-10) ppm; IR νmax (thin film): 3439 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C12H24NO5 262.1654; found 262.1651.
Compound 29b. Column chromatography (DCM/MeOH, 95
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5); oily liquid (0.33 g, 1.28 mmol, 64%); [α]25D = +18.6 (c 0.22, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.84 (d, J = 6.8 Hz, 1H, –O–CH2–O–), 4.71 (d, J = 6.3 Hz, 1H, –O–CH2–O–), 4.41 (q, J = 8.0 Hz, 1H, C4H), 4.19 (dd, J = 8.0, 2.8 Hz, 1H, C3H), 3.91 (dd, J = 5.7, 2.2 Hz, 1H, C5H), 3.51 (dd, J = 10.3, 4.5 Hz, 1H, C1H), 3.38 (s, 3H, (–OCH3)), 3.32–3.23 (m, 2H, C7H), 2.64 (d, J = 14.8 Hz, 1H, C6H), 2.56–2.52 (m, 1H, C6H), 1.91–1.88 (m, 2H, C2H), 1.47 (s, 3H, C10H), 1.33 (s, 3H, C11H) ppm; 13C NMR (125 MHz, CDCl3): δ 109.8 (C-12), 97.5 (–O–CH2–O–), 80.6 (C-4), 76.3 (C-3), 76.1 (C-5), 65.7 (C-7), 56.3 (–OCH3), 55.7 (C-1), 48.3 (C-6), 35.8 (C-2), 26.6 (C-11), 24.0 (C-10), ppm; IR νmax (thin film): 3401 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C12H24NO5 262.1654; found 262.1655.
5); oily liquid (0.33 g, 1.28 mmol, 64%); [α]25D = +18.6 (c 0.22, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.84 (d, J = 6.8 Hz, 1H, –O–CH2–O–), 4.71 (d, J = 6.3 Hz, 1H, –O–CH2–O–), 4.41 (q, J = 8.0 Hz, 1H, C4H), 4.19 (dd, J = 8.0, 2.8 Hz, 1H, C3H), 3.91 (dd, J = 5.7, 2.2 Hz, 1H, C5H), 3.51 (dd, J = 10.3, 4.5 Hz, 1H, C1H), 3.38 (s, 3H, (–OCH3)), 3.32–3.23 (m, 2H, C7H), 2.64 (d, J = 14.8 Hz, 1H, C6H), 2.56–2.52 (m, 1H, C6H), 1.91–1.88 (m, 2H, C2H), 1.47 (s, 3H, C10H), 1.33 (s, 3H, C11H) ppm; 13C NMR (125 MHz, CDCl3): δ 109.8 (C-12), 97.5 (–O–CH2–O–), 80.6 (C-4), 76.3 (C-3), 76.1 (C-5), 65.7 (C-7), 56.3 (–OCH3), 55.7 (C-1), 48.3 (C-6), 35.8 (C-2), 26.6 (C-11), 24.0 (C-10), ppm; IR νmax (thin film): 3401 cm−1; HRMS (ESI-TOF) m/z [M + H]+ calcd for C12H24NO5 262.1654; found 262.1655.
The same procedure was used for the preparation of 2, 3 and 4 from 18, 23 and 29, respectively.
Compound 1a. White solid; (0.161 g, 0.81 mmol, 81%); [α]25D = −23.1 (c 0.4, H2O) [lit.6b [α]25D −25.5 (c 0.90, H2O)]; mp 122–125 °C; 1H NMR (500 MHz, D2O): δ 4.25–4.23 (m, 1H, C3H), 4.17 (dd, J = 9.1, 4.0 Hz, 1H, C4H), 3.91–3.85 (m, 2H, C6H), 3.82–3.73 (m, 2H, C7H), 3.68–3.65 (m, 1H, C2H), 3.56–3.53 (m, 1H, C5H) ppm; 13C NMR (125 MHz, D2O): δ 71.2 (C-3), 70.1 (C-4), 62.2 (C-7), 61.8 (C-6), 58.2 (C-2), 57.5 (C-5) ppm; IR νmax (KBr): 3356 cm−1; HRMS (ESI-TOF) m/z [(M + H) − (HCl)]+ calcd for C6H14NO4 164.0923; found 164.0923.
Compound 1b. White solid (0.165 g, 0.83 mmol, 83%); [α]25D = 0.00 (c 0.39, MeOH); mp 138–139 °C; 1H NMR (400 MHz, D2O): δ 4.34–4.28 (m, 2H, C3H), 3.92–3.86 (m, 4H, C4H), 3.61–3.56 (m, 2H, C2H) ppm; 13C NMR (100 MHz, D2O): δ 70.2 (C-3), 61.9 (C-4), 57.9 (C-2) ppm; IR νmax (KBr): 3356 cm−1; HRMS (ESI-TOF) m/z [(M + H) − (HCl)]+ calcd for C6H14NO4 164.0923; found 164.0923.
Compound 2a. White solid (0.08 g, 0.41 mmol, 83%); [α]25D = −35.2 (c 0.43, MeOH) [lit.7i [α]25D–37.7 (c 1.00, MeOH)]; mp 152–153 °C; 1H NMR (500 MHz, D2O): δ 4.01 (bs, 1H, C3H), 3.86–3.85 (m, 1H, C4H), 3.77–3.69 (m, 3H, C5H, C7H), 3.19–3.11 (m, 1H, C2H), 3.12–3.10 (m, 1H, C6H), 2.98–2.96 (m, 1H, C6H) ppm; 13C NMR (125 MHz, D2O): δ 69.9 (C-3), 65.4 (C-4), 64.6 (C-5), 57.7 (C-7), 54.7 (C-2), 41.6 (C-6) ppm; IR νmax (KBr): 3436 cm−1; HRMS (ESI-TOF) m/z [(M + H) − (HCl)]+ calcd for C6H14NO4 164.0923; found 164.0923.
Compound 2b. White solid (0.08 g, 0.42 mmol, 84%); [α]25D = −23.4 (c 0.4, MeOH), [lit.10d [α]23D −21.7 (c 0.8, MeOH)]; mp 153–155 °C; 1H NMR (400 MHz, D2O): δ 4.10–4.09 (m, 1H, C3H), 4.02–4.01 (m, 1H, C4H), 3.74 (m, 2H, C5H, C2H), 3.71 (t, J = 3.2 Hz, 1H, C7H), 3.37 (dd, J = 13.7, 3.0 Hz, 1H, C7H), 3.26 (dt, J = 1.3, 6.8 Hz, 1H, C6H), 3.13 (dd, J = 13.7, 1.8 Hz, 1H, C6H) ppm; 13C NMR (100 MHz, D2O): δ 69.8 (C-3), 65.2 (C-4), 64.5 (C-5), 57.5 (C-7), 54.6 (C-2), 41.4 (C-6) ppm; IR νmax (KBr): 3436 cm−1; HRMS (ESI-TOF) m/z [(M + H) − (HCl)]+ calcd for C6H14NO4 164.0923; found 164.0924.
Compound 3a. White solid (0.10 g, 0.5 mmol, 80%); [α]25D = +3.8 (c 0.22, MeOH); mp 162–163 °C; 1H NMR (500 MHz, CD3OD): δ 3.94–3.90 (m, 2H, C3H, C4H), 3.88–3.83 (m, 2H, C8H), 3.81–3.74 (m, 2H, C7H), 3.60–3.55 (m, 1H, C6H), 3.46–3.43 (m, 1H, C2H), 2.13–2.05 (m, 2H, C5H) ppm; 13C NMR (125 MHz, CD3OD): δ 66.4 (C-3), 64.9 (C-4), 58.8 (C-7), 58.7 (C-8), 56.2 (C-2), 52.7 (C-6), 26.7 (C-5) ppm; IR νmax (KBr): 3353 cm−1; HRMS (ESI-TOF) m/z [(M + H) − (HCl)]+ calcd for C7H16NO4 178.1079; found 178.1080.
Compound 3b. In a similar manner as described for the molecule 1a, white solid (0.10 g, 0.5 mmol, 81%); [α]25D = +6.3 (c 0.18, MeOH); mp 158–159 °C; 1H NMR (400 MHz, D2O): δ 3.95 (bs, 1H, C3H), 3.91 (dd, J = 6.3, 3.4 Hz, 1H, C4H), 3.87 (dd, J = 5.9, 3.2 Hz, 1H, C7H), 3.80–3.78 (m, 2H, C8H), 3.72 (dd, J = 16, 4.5 Hz, 1H, C7H), 3.65–3.59 (m, 1H, C6H), 3.40 (dt, J = 8.5, 2.3 Hz, 1H, C2H), 1.78–1.73 (m, 2H, C4H) ppm; 13C NMR (100 MHz, D2O): δ 66.0 (C-3), 64.5 (C-4), 58.9 (C-7), 58.1 (C-8), 55.3 (C-2), 52.8 (C-6), 25.5 (C-5) ppm; IR νmax (KBr): 3456 cm−1; HRMS (ESI-TOF) m/z [(M + H) − (HCl)]+ calcd for C7H16NO4 178.1079; found 178.1078.
Compound 4a. White solid (0.10 g, 0.5 mmol, 85%); [α]25D = −9.6 (c 0.64, H2O); mp 145–146 °C; 1H NMR (400 MHz, D2O): δ 4.10–4.06 (m, 2H, C4H, C5H), 3.73–3.62 (m, 3H, C8H, C6H), 3.49 (dd, J = 12.3, 8.2 Hz, 1H, C2H), 3.21–3.17 (m, 2H, C7H), 1.99 (ddd, 15.6, 7.8, 4.3 Hz, 1H, C3H), 1.72 (dd, 15.5, 10.5 Hz, 1H, C3H) ppm; 13C NMR (100 MHz, D2O): δ 74.1 (C-4), 68.8 (C-5), 67.8 (C-6), 61.4 (C-8), 54.4 (C-2), 43.3 (C-7), 28.1 (C-3) ppm; IR νmax (KBr): 3436 cm−1; HRMS (ESI-TOF) m/z [(M + H) − (HCl)]+ calcd for C7H16NO4 178.1079; found 178.1080.
Compound 4b. White solid (0.10 g, 0.5 mmol, 84%); [α]25D = +6.0 (c 0.82, H2O); mp 149–150 °C; 1H NMR (400 MHz, D2O): 4.10 (dd, J = 8.6, 3.2 Hz, 1H, C4H), 4.00 (bs, 1H, C5H), 3.90 (dt, 10.9, 2.9 Hz, 1H, C6H), 3.69 (dd, J = 12.3, 4.1 Hz, 1H, C8H), 3.49 (dd, J = 11.8, 8.2 Hz, 1H, C8H), 3.37–3.28 (m, 2H, C7H, C2H), 3.17 (dd, 13.7, 4.1 Hz, 1H, C7H), 1.98–1.88 (m, 1H, C3H), 1.75–1.71 (m, 1H, C3H) ppm; 13C NMR (100 MHz, D2O): δ 75.4 (C-4), 69.5 (C-5), 65.7 (C-6), 62.1 (C-8), 55.7 (C-2), 44.5 (C-7), 29.4 (C-3) ppm; IR νmax (KBr): 3456 cm−1; HRMS (ESI-TOF) m/z [(M + H) − (HCl)]+ calcd for C7H16NO4 178.1079; found 178.1080.
The same procedure was used for the preparation of 24 from 19.
Compound 12. Column chromatography (petroleum ether/EtOAc, 9
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.84 g, 1.78 mmol, 89%); [α]25D = −9.4 (c 0.32, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.72–7.36 (m, 10H), 5.86–5.83 (m, 1H), 5.32 (d, J = 17.1 Hz, 1H), 5.20 (d, J = 10.4 Hz, 1H), 4.76 (d, J = 6.7 Hz, 1H), 4.68 (d, J = 6.7 Hz, 1H), 4.66 (d, J = 6.7 Hz, 1H), 4.45 (t, J = 7.0 Hz, 1H), 3.92 (dd, J = 11.0, 2.45 Hz, 1H), 3.87 (dd, J = 11.0, 4.25 Hz, 1H), 3.71–3.61 (m, 1H), 3.34 (s, 3H), 1.39 (s, 3H), 1.36 (s, 3H), 1.07 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 135.8, 135.7, 134.3, 133.6, 133.4, 129.7, 129.6, 127.7, 127.6, 117.9, 108.4, 96.9, 78.7, 77.4, 76.5, 64.0, 56.0, 27.7, 26.9, 25.3, 19.3 ppm; IR νmax (thin film): 3072, 3049, 1589, 1472 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C27H38NaO5Si 493.2386; found 493.2381.
1); oily liquid (0.84 g, 1.78 mmol, 89%); [α]25D = −9.4 (c 0.32, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.72–7.36 (m, 10H), 5.86–5.83 (m, 1H), 5.32 (d, J = 17.1 Hz, 1H), 5.20 (d, J = 10.4 Hz, 1H), 4.76 (d, J = 6.7 Hz, 1H), 4.68 (d, J = 6.7 Hz, 1H), 4.66 (d, J = 6.7 Hz, 1H), 4.45 (t, J = 7.0 Hz, 1H), 3.92 (dd, J = 11.0, 2.45 Hz, 1H), 3.87 (dd, J = 11.0, 4.25 Hz, 1H), 3.71–3.61 (m, 1H), 3.34 (s, 3H), 1.39 (s, 3H), 1.36 (s, 3H), 1.07 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 135.8, 135.7, 134.3, 133.6, 133.4, 129.7, 129.6, 127.7, 127.6, 117.9, 108.4, 96.9, 78.7, 77.4, 76.5, 64.0, 56.0, 27.7, 26.9, 25.3, 19.3 ppm; IR νmax (thin film): 3072, 3049, 1589, 1472 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C27H38NaO5Si 493.2386; found 493.2381.
Compound 24. Column chromatography (petroleum ether/EtOAc, 9.5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5); oily liquid (0.92 g, 1.7 mmol, 85%); [α]25D = −8.6 (c 0.18, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.71–7.68 (m, 4H), 7.41–7.35 (m, 6H), 4.85 (d, J = 6.9 Hz, 1H), 4.69 (d, J = 6.9 Hz, 1H), 4.30 (t, J = 7.1 Hz, 1H), 4.19–4.15 (m, 1H), 4.12 (q, J = 7.4 Hz, 2H), 3.98–3.94 (m, 1H), 3.87–3.83 (m, 1H), 3.72–3.70 (m, 1H), 3.36 (s, 3H), 2.56–2.49 (m, 1H), 2.42–2.36 (m, 1H), 2.01–1.94 (m, 1H), 1.79–1.70 (m, 1H), 1.33 (s, 3H), 1.30 (s, 3H), 1.24 (t, J = 7.4 Hz, 3H), 1.03 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 173.5, 135.8, 135.7, 133.5, 133.4, 129.7, 129.6, 127.7, 127.6, 108.0, 96.2, 76.8, 76.4, 75.7, 63.9, 60.3, 56.2, 31.1, 28.1, 26.9, 25.8, 25.4, 19.3, 14.1 ppm; IR νmax (thin film): 3049, 1735, 1587 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C30H44NaO7Si 567.2754; found 567.2751.
0.5); oily liquid (0.92 g, 1.7 mmol, 85%); [α]25D = −8.6 (c 0.18, CHCl3); 1H NMR (500 MHz, CDCl3): δ 7.71–7.68 (m, 4H), 7.41–7.35 (m, 6H), 4.85 (d, J = 6.9 Hz, 1H), 4.69 (d, J = 6.9 Hz, 1H), 4.30 (t, J = 7.1 Hz, 1H), 4.19–4.15 (m, 1H), 4.12 (q, J = 7.4 Hz, 2H), 3.98–3.94 (m, 1H), 3.87–3.83 (m, 1H), 3.72–3.70 (m, 1H), 3.36 (s, 3H), 2.56–2.49 (m, 1H), 2.42–2.36 (m, 1H), 2.01–1.94 (m, 1H), 1.79–1.70 (m, 1H), 1.33 (s, 3H), 1.30 (s, 3H), 1.24 (t, J = 7.4 Hz, 3H), 1.03 (s, 9H) ppm; 13C NMR (125 MHz, CDCl3): δ 173.5, 135.8, 135.7, 133.5, 133.4, 129.7, 129.6, 127.7, 127.6, 108.0, 96.2, 76.8, 76.4, 75.7, 63.9, 60.3, 56.2, 31.1, 28.1, 26.9, 25.8, 25.4, 19.3, 14.1 ppm; IR νmax (thin film): 3049, 1735, 1587 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C30H44NaO7Si 567.2754; found 567.2751.
The same procedure was used for the preparation of 25 from 24.
Compound 13. Column chromatography (petroleum ether/EtOAc, 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4); oily liquid (0.42 g, 1.8 mmol, 90%); [α]25D = +7.33 (c 2.4, CHCl3); 1H NMR (500 MHz, CDCl3): δ 5.89–5.82 (m, 1H), 5.35 (d, J = 17.1 Hz, 1H), 5.22 (d, J = 10.4 Hz, 1H), 4.65 (t, J = 6.7 Hz, 1H), 4.60 (q, J = 6.7 Hz, 1H), 4.12 (t, J = 7.3 Hz, 1H), 3.83 (d, J = 10.4 Hz, 1H), 3.62 (q, J = 6.4 Hz, 1H), 3.51 (t, J = 6.4 Hz, 1H), 3.40 (s, 3H), 3.70–3.33 (m, 1H), 1.45 (s, 3H), 1.34 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 133.8, 117.7, 108.9, 97.7, 81.4, 78.7, 77.1, 63.9, 56.0, 27.7, 25.3 ppm; IR νmax (thin film): 3460, 1590 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C11H20NaO5 255.1208; found 255.1201.
4); oily liquid (0.42 g, 1.8 mmol, 90%); [α]25D = +7.33 (c 2.4, CHCl3); 1H NMR (500 MHz, CDCl3): δ 5.89–5.82 (m, 1H), 5.35 (d, J = 17.1 Hz, 1H), 5.22 (d, J = 10.4 Hz, 1H), 4.65 (t, J = 6.7 Hz, 1H), 4.60 (q, J = 6.7 Hz, 1H), 4.12 (t, J = 7.3 Hz, 1H), 3.83 (d, J = 10.4 Hz, 1H), 3.62 (q, J = 6.4 Hz, 1H), 3.51 (t, J = 6.4 Hz, 1H), 3.40 (s, 3H), 3.70–3.33 (m, 1H), 1.45 (s, 3H), 1.34 (s, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 133.8, 117.7, 108.9, 97.7, 81.4, 78.7, 77.1, 63.9, 56.0, 27.7, 25.3 ppm; IR νmax (thin film): 3460, 1590 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C11H20NaO5 255.1208; found 255.1201.
Compound 25. Column chromatography (petroleum ether/EtOAc, 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1); oily liquid (0.56 g, 1.84 mmol, 92%); [α]25D = +13.1 (c 0.42, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.72 (d, J = 6.8 Hz, 1H), 4.68 (d, J = 6.8 Hz, 1H), 4.17–4.14 (m, 1H), 4.11 (q, J = 6.8 Hz, 2H), 4.03 (dd, J = 8.0, 5.7 Hz, 1H), 3.86 (dd, J = 11.5, 2.8 Hz, 1H), 3.66–3.58 (m, 2H), 3.41 (s, 3H), 2.55–2.49 (m, 1H), 2.42–2.36 (m, 1H), 1.92–1.86 (m, 1H), 1.79–1.71 (m, 1H), 1.38 (s, 3H), 1.29 (s, 3H), 1.23 (t, J = 7.4 Hz, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 173.4, 108.3, 97.1, 80.3, 76.7, 76.3, 63.8, 60.4, 56.1, 31.1, 28.0, 25.6, 25.2, 14.2 ppm; IR νmax (thin film): 3472, 1735 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C14H26NaO7 329.1576; found 329.1571.
1); oily liquid (0.56 g, 1.84 mmol, 92%); [α]25D = +13.1 (c 0.42, CHCl3); 1H NMR (500 MHz, CDCl3): δ 4.72 (d, J = 6.8 Hz, 1H), 4.68 (d, J = 6.8 Hz, 1H), 4.17–4.14 (m, 1H), 4.11 (q, J = 6.8 Hz, 2H), 4.03 (dd, J = 8.0, 5.7 Hz, 1H), 3.86 (dd, J = 11.5, 2.8 Hz, 1H), 3.66–3.58 (m, 2H), 3.41 (s, 3H), 2.55–2.49 (m, 1H), 2.42–2.36 (m, 1H), 1.92–1.86 (m, 1H), 1.79–1.71 (m, 1H), 1.38 (s, 3H), 1.29 (s, 3H), 1.23 (t, J = 7.4 Hz, 3H) ppm; 13C NMR (125 MHz, CDCl3): δ 173.4, 108.3, 97.1, 80.3, 76.7, 76.3, 63.8, 60.4, 56.1, 31.1, 28.0, 25.6, 25.2, 14.2 ppm; IR νmax (thin film): 3472, 1735 cm−1; HRMS (ESI-TOF) m/z [M + Na]+ calcd for C14H26NaO7 329.1576; found 329.1571.
Acknowledgements
The authors thank Department of Science and Technology, Ministry of Science and Technology for financial support (grant number: SR/FT/CS-12/2011) and R. P. thanks University Grants Commission for a senior research fellowship and V. K. J. thanks CSIR India for a JRF.Notes and references
- (a) G. Horne and R. Storer, Drug Discovery Today, 2011, 16, 107 CrossRef CAS PubMed; (b) A. E. Stutz, Iminosugars as Glycosidase Inhibitors, Nojirimycin and Beyond, Wiley-VCH, Weinheim, 1999 Search PubMed; (c) P. E. Compain and O. R. Martin, Iminosugars, From Synthesis to Therapeutic Applications, Wiley-VCH, Weinheim, 2007 Search PubMed.
- (a) B. Andersen, A. Rassov, N. Westergaard and K. Lundgren, Biochem. J., 1999, 342, 545 CrossRef CAS PubMed; (b) R. J. Bernack and W. Korytnyk, Cancer Metastasis Rev., 1985, 4, 81 CrossRef; (c) T. D. Butters, R. A. Dwek and F. M. Platt, Chem. Rev., 2000, 100, 4683 CrossRef CAS PubMed; (d) F. Chery, L. Cronin, J. L. O'Brien and P. V. Murphy, Tetrahedron, 2004, 60, 6597 CrossRef CAS; (e) B. D. Walker, M. Kowalski, W. C. Goh, K. Kozarsky, M. Krieger, C. Rosen, L. Rohrshneider, W. A. Haseltine and J. Sodroski, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 8120 CrossRef CAS PubMed; (f) H. Paulson and I. Brockhausen, Glycoconjugate J., 2001, 18, 867 CrossRef.
- (a) A. Welter, J. Jadot, G. Dardenne, M. Marlier and J. Casimir, Phytochemistry, 1976, 15, 747 CrossRef CAS; (b) S. Takayama, R. Martin, J. Wu, K. Laslo, G. Siuzdak and C.-H. Wong, J. Am. Chem. Soc., 1997, 119, 8146 CrossRef CAS; (c) A. Schäfer, G. Klich, M. Schreiber, H. Paulsen and J. Thiem, Carbohydr. Res., 1998, 313, 107 CrossRef.
- (a) C. Boucheron, V. Desvergnes, P. Compain, O. Martin, R. A. Lavi, M. Mackeen, M. R. Wormald, D. A. Dwek and T. D. Butters, Tetrahedron: Asymmetry, 2005, 16, 1747 CrossRef CAS; (b) T. M. Jespersen, W. Dong, M. R. Sierks, T. Skrydstrup, I. Lundt and M. Bols, Angew. Chem., Int. Ed. Engl., 1994, 1779 Search PubMed; (c) N. Asano, H. Kizu, K. Oseki, E. Tomioka, K. Matsui, M. Okamoto and M. Baba, J. Med. Chem., 1995, 38, 2349 CrossRef CAS PubMed.
- (a) F. Moris-Varas, X.-H. Qian and C.-H. Wong, J. Am. Chem. Soc., 1996, 118, 764 CrossRef; (b) G. F. Painter, P. J. Eldridge and A. Falshaw, Bioorg. Med. Chem., 2004, 12, 225 CrossRef CAS PubMed; (c) F. Popowycz, S. Gerber-Lemaire, C. Schütz and P. Vogel, Helv. Chim. Acta, 2004, 87, 800 CrossRef CAS.
- (a) M. E. Bouillon and S. G. Pyne, Tetrahedron Lett., 2014, 55, 475 CrossRef CAS; (b) A. A. Ansari and Y. D. Vankar, J. Org. Chem., 2013, 78, 9383 CrossRef CAS PubMed; (c) B. J. Ayers, N. Ngo, S. F. Jenkinson, R. F. Martínez, Y. Shimada, I. Adachi, A. C. Weymouth-Wilson, A. Kato and G. W. J. Fleet, J. Org. Chem., 2012, 77, 7777 CrossRef CAS PubMed; (d) Z. Hong, L. Liu, M. Fu, Y. Sugiyama and C. H. Wong, J. Am. Chem. Soc., 2009, 131, 8352 CrossRef CAS PubMed; (e) E. L. Tsou, Y. T. Yeh, P. H. Liang and W. C. Cheng, Tetrahedron, 2009, 65, 93 CrossRef CAS; (f) L. Lay, F. Nicotra, A. Paganani, C. Pangrazio and L. Panza, Tetrahedron Lett., 1993, 34, 4555 CrossRef CAS; (g) J. B. Behr and G. Guillerm, Tetrahedron Lett., 2007, 48, 2369 CrossRef CAS; (h) A. Dondoni and D. Perrone, Tetrahedron Lett., 1999, 40, 9375 CrossRef CAS; (i) V. P. Vyavahare, S. Chattopadhyay, V. G. Puranic and D. D. Dhavale, Synlett, 2007, 559 CAS; (j) M. A. González-Castro, D. L. Poole, J. C. Estévez, G. W. J. Fleet and R. J. Estévez, Tetrahedron: Asymmetry, 2015, 26, 320 CrossRef.
- (a) A. Dondoni and A. Nuzzi, J. Org. Chem., 2006, 71, 7574 CrossRef CAS PubMed; (b) G. L. Bouc, C. Thomassigny and C. Greck, Tetrahedron: Asymmetry, 2006, 17, 2006 CrossRef; (c) D. Kalch, N. D. Rycke, X. Moreau and C. Greck, Tetrahedron Lett., 2009, 50, 492 CrossRef CAS; (d) H.-J. Altenbach and K. Himmeldirk, Tetrahedron: Asymmetry, 1995, 6, 1077 CrossRef CAS; (e) N. Ikota, J.-i. Hirano, R. Gamage, H. Nakagawa and H. Hama-Inaba, Heterocycles, 1997, 46, 637 CrossRef CAS; (f) X.-D. Wu, S.-K. Khim, X. Zhang, E. Cederstrom and P. S. Mariano, J. Org. Chem., 1998, 63, 841 CrossRef CAS PubMed; (g) B.-C. Hong, Z.-Y. Chen, A. Nagarajan, R. Kottani, V. Chavan, W.-H. Chen, Y.-F. Jiang, S.-C. Zhang, J.-H. Liao and S. Sarshar, Carbohydr. Res., 2005, 340, 2457 CrossRef CAS PubMed; (h) S. Ghosh, J. Shashidhar and S. K. Dutta, Tetrahedron Lett., 2006, 47, 6041 CrossRef CAS; (i) A. Guaragna, S. D'Errico, D. D'Alonzo, S. Pedatella and G. Palumbo, Org. Lett., 2007, 9, 3473 CrossRef CAS PubMed; (j) E. G. Doyagüez, F. Calderon, F. Sánchez and A. Fernándéz-Mayoralas, J. Org. Chem., 2007, 72, 9353 CrossRef PubMed; (k) F. Calderon, E. G. Doyagúez, P. H.-Y. Cheong, A. Fernandéz-Mayoralas and K. N. Houk, J. Org. Chem., 2008, 73, 7916 CrossRef CAS PubMed; (l) E. V. Crabtree, R. F. Martínez, S. Nakagawa, I. Adachi, T. D. Butters, A. Kato, G. W. J. Fleet and A. F. G. Glawar, Org. Biomol. Chem., 2014, 12, 3932 RSC.
- (a) H. Hashimoto and M. Hayakawa, Chem. Lett., 1989, 1881 CrossRef CAS; (b) C. C. Joseph, H. Regeling, B. Zwanenburg and G. J. F. Chittenden, Carbohydr. Res., 2002, 337, 1083 CrossRef CAS PubMed; (c) C. R. Johnson, A. Golebiowski, E. Schoffers, H. Sundram and M. P. Braun, Synlett, 1995, 313 CrossRef CAS; (d) K. K.-C. Liu, T. Kajimoto, L. Chen, Z. Zhong, Y. Ichikawa and C.-H. Wong, J. Org. Chem., 1991, 56, 6280 CrossRef CAS; (e) W. J. Lees and G. M. Whitesides, Bioorg. Chem., 1992, 20, 173 CrossRef CAS; (f) L.-X. Liao, Z.-M. Wang, H.-X. Zhang and W.-S. Zhou, Tetrahedron: Asymmetry, 1999, 10, 3649 CrossRef CAS; (g) B. J. Ayers, A. F. G. Glawar, R. F. Martínez, N. Ngo, Z. Liu, G. W. J. Fleet, T. D. Butters, R. J. Nash, C.-Y. Yu, M. R. Wormald, S. Nakagawa, I. Adachi, A. Kato and S. F. Jenkinson, J. Org. Chem., 2014, 79, 3398 CrossRef CAS PubMed.
- (a) G.-L. Zhang, X.-J. Zheng, L.-H. Zhang and X.-S. Ye, MedChemComm., 2011, 2, 909 RSC; (b) A. F. G. Glawar, S. F. Jenkinsoni, S. J. Newberry, A. L. Thompson, S. Nakagawa, A. Yoshihara, K. Akimitsu, K. Izumori, T. D. Butters, A. Kato and G. W. J. Fleet, Org. Biomol. Chem., 2013, 11, 6886 RSC; (c) C. Matassini, S. Mirabella, X. Ferhati, C. Faggi, I. Robina, A. Goti, E. Moreno-Clavijo, A. J. Moreno-Vargas and F. Cardona, Eur. J. Org. Chem., 2014, 5419 CrossRef CAS; (d) G. M. J. Lenagh-Snow, S. F. Jenkinson, S. J. Newberry, A. Kato, S. Nakagawa, I. Adachi, M. R. Wormald, A. Yoshihara, K. Morimoto, K. Akimitsu, K. Izumori and G. W. J. Fleet, Org. Lett., 2012, 14, 2050 CrossRef CAS PubMed; (e) N. Asano, M. Nishida, A. Kato, H. Kizu, K. Matsui, Y. Shimada, T. Itoh, M. Baba, A. A. Watson, R. J. Nash, P. M. Lilly, Q. de, D. J. Watkin and G. W. J. Fleet, J. Med. Chem., 1998, 41, 2565 CrossRef CAS PubMed; (f) I. Bruce, G. W. J. Fleet, I. C. di Bello and B. Winchester, Tetrahedron Lett., 1989, 30, 7257 CrossRef CAS; (g) I. Bruce, G. W. J. Fleet, I. C. di Bello and B. Winchester, Tetrahedron, 1992, 48, 10191 CrossRef CAS; (h) K. E. Holt, F. J. Leeper and S. Handa, J. Chem. Soc., Perkin Trans. 1, 1994, 231 RSC; (i) O. M. Saavedra and O. R. Martin, J. Org. Chem., 1996, 61, 6987 CrossRef CAS PubMed; (j) O. R. Martin, O. M. Saavedra, F. Xie, L. Liu, S. Picasso, P. Vogel, H. Kizu and N. Asano, Bioorg. Med. Chem., 2001, 9, 1269 CrossRef CAS PubMed; (k) J.-Y. Goujon, D. Gueyrard, P. Compain, O. R. Martin, K. A. Ikeda, A. Kato and N. Asano, Bioorg. Med. Chem., 2005, 13, 2313 CrossRef CAS PubMed; (l) T. Liu, Y. Zhang and Y. Bleriot, Synlett, 2007, 905 CAS.
- (a) O. Karjalainen, M. Passiniemi and A. M. Koskinen, Org. Lett., 2010, 12, 1145 CrossRef CAS PubMed; (b) S. F. Jenkinson, G. W. J. Fleet, R. J. Nash, Y. Koike, I. Adachi, A. Yoshihara, K. Morimoto, K. Izumori and A. Kato, Org. Lett., 2011, 13, 4064 CrossRef CAS PubMed; (c) L. Cipolla, B. La Ferla and F. Nicotra, Curr. Top. Med. Chem., 2003, 3, 485 CrossRef CAS PubMed; (d) M. Ruiz, T. M. Ruanovai, O. Blanco, F. Nunez, C. Patoi and V. Ojea, J. Org. Chem., 2008, 73, 2240 CrossRef CAS PubMed. (references therein); (e) G. M. J. Lenagh-Snow, N. Araujo, S. F. Jenkinson, C. Rutherford, S. Nakagawa, A. Kato, C.-Y. Yu, A. C. Weymouth-Wilson and G. W. J. Fleet, Org. Lett., 2011, 13, 5834 CrossRef CAS PubMed; (f) M. Mondon, N. Fontelle, J. Désiré, F. Lecornué, J. Guillard, J. Marrot and Y. Blériot, Org. Lett., 2012, 14, 870 CrossRef CAS PubMed; (g) R. G. Soengas, M. I. Simone, S. Hunter, R. J. E. L. NashEvinson and G. W. J. Fleet, Eur. J. Org. Chem., 2012, 2394 CrossRef CAS; (h) G. Danoun, J. Ceccon, A. E. Greene and J.-F. Poisson, Eur. J. Org. Chem., 2009, 4221 CrossRef CAS; (i) A. Lumbroso, I. Beaudet, L. Toupet, E. L. Grognec and J.-P. Quintard, Org. Lett., 2013, 15, 160 CrossRef CAS PubMed.
- (a) H. Paulsen and K. Todt, Chem. Ber., 1967, 100, 512 CrossRef CAS PubMed; (b) L. Poitout, Y. L. Merrer and J.-C. Depezay, Tetrahedron Lett., 1994, 35, 3293 CrossRef CAS; (c) B. B. Lohray, Y. Jayamma and M. Chatterjee, J. Org. Chem., 1995, 60, 5958 CrossRef CAS; (d) H. Li, Y. Blériot, C. Chantereau, J.-M. Mallet, M. Sollogoub, Y. Zhang, E. Rodríguez-García, P. Vogel, J. Jiménez-Barbero and P. Sinay, Org. Biomol. Chem., 2004, 2, 1492 RSC; (e) H. A. Johnson and N. R. Thomas, Bioorg. Med. Chem. Lett., 2002, 12, 237 CrossRef CAS PubMed; (f) L. Gauzy, Y. L. Merrer and J.-C. Depezay, Tetrahedron Lett., 1999, 40, 6005 CrossRef CAS; (g) S. M. Andersen, C. Ekhart, I. Lundt and A. E. Stütz, Carbohydr. Res., 2000, 326, 22 CrossRef CAS PubMed; (h) G. F. Painter and A. Falshaw, J. Chem. Soc., Perkin Trans. 1, 2000, 1157 RSC; (i) D. D. Dhavale, S. D. Markad, N. S. Karanjule and J. Prakasha Reddy, J. Org. Chem., 2004, 69, 4760 CrossRef CAS PubMed; (j) W.-B. Zhao, S. Nakagawa, A. Kato, I. Adachi, Y.-M. Jia, X.-G. Hu, G. W. J. Fleet, F. X. Wilson, G. Horne, A. Yoshihara, K. Izumori and C.-Y. Yu, J. Org. Chem., 2013, 78, 3208 CrossRef CAS PubMed; (k) V. Jha, S. V. Kauloorkar and P. Kumar, Eur. J. Org. Chem., 2014, 4897 CrossRef CAS.
- (a) B. List, J. Am. Chem. Soc., 2002, 124, 5656 CrossRef CAS PubMed; (b) N. K. Kumaragurubharan, A. Juhl, A. Bogevig and K. A. Jorgensen, J. Am. Chem. Soc., 2002, 124, 6254 CrossRef; (c) A. Bogevig, K. Juhl, N. Kumaragurubharan, W. Zhuang and K. A. Jorgensen, Angew. Chem., Int. Ed., 2002, 41, 1790 CrossRef CAS; (d) R. O. Duthaler, Angew. Chem., Int. Ed., 2003, 42, 975 CrossRef CAS PubMed.
- (a) A. Nuzzi, A. Massi and A. Dondoni, Org. Lett., 2008, 10, 4485 CrossRef CAS PubMed; (b) R. Ait-Youcef, K. Sbargoud, X. Moreau and C. Greck, Synlett, 2009, 3007 CAS; (c) Y. Nishikawa, M. Kitajima, N. Kogure and H. Takayama, Tetrahedron, 2009, 65, 1608 CrossRef CAS; (d) R. Ait-Youcef, X. Moreau and C. Greck, J. Org. Chem., 2010, 75, 5312 CrossRef CAS PubMed; (e) R. Petakamsetty, R. P. Das and R. Ramapanicker, Tetrahedron, 2014, 70, 9554 CrossRef CAS.
- (a) P. Kumar, V. Jha and R. G. Gonnade, J. Org. Chem., 2013, 78, 11756 CrossRef CAS PubMed; (b) V. Jha, N. B. Kondekar and P. Kumar, Org. Lett., 2010, 12, 2762 CrossRef CAS PubMed; (c) A. Desmarchelier, V. Coeffard, X. Moreau and C. Greck, Tetrahedron, 2014, 70, 2491 CrossRef CAS.
- (a) V. Jha, S. V. Kauloorkar and P. Kumar, Eur. J. Org. Chem., 2014, 4897 CrossRef CAS; (b) S. V. Kauloorkar, V. Jha, G. Jogdandb and P. Kumar, Org. Biomol. Chem., 2014, 12, 4454 RSC; (c) V. Jha and P. Kumar, RSC Adv., 2014, 4, 3238 RSC; (d) S. P. Kotkar, V. B. Chavan and A. Sudalai, Org. Lett., 2008, 9, 1001 CrossRef PubMed.
- W. J. Choi, H. R. Moon, H. O. Kim, B. N. Yoo, J. A. Lee, D. H. Shin and L. S. Jeong, J. Org. Chem., 2004, 69, 2634 CrossRef CAS PubMed.
- The diastereomeric ratio of these compounds was estimated using chiral HPLC and was established by comparing the chromatograms with that of a 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the diastereomers obtained through reactions catalyzed by DL-proline (given in the ESI†).
1 mixture of the diastereomers obtained through reactions catalyzed by DL-proline (given in the ESI†). - Synthesis of the benzyl derivatives and the NMR spectral analysis are provided in the ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of 1H and 13C NMR spectra of all the compounds, 2D NMR spectra of the final compounds and HPLC data. See DOI: 10.1039/c5ob01042j |
| This journal is © The Royal Society of Chemistry 2015 |