Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Benzyllithiums bearing aldehyde carbonyl groups. A flash chemistry approach†
Aiichiro
Nagaki
,
Yuta
Tsuchihashi
,
Suguru
Haraki
and
Jun-ichi
Yoshida
*
Department of Synthetic and Biological Chemistry, Graduate School of Engineering, Kyoto University, Nishikyo-ku, Kyoto, 615-8510, Japan. E-mail: yoshida@sbchem.kyoto-u.ac.jp
First published on 9th June 2015
Abstract
Reductive lithiation of benzyl halides bearing aldehyde carbonyl groups followed by reaction with subsequently added electrophiles was successfully accomplished without affecting the carbonyl groups by taking advantage of short residence times in flow microreactors.
Chemoselectivity is one of the central issues in chemistry and chemical synthesis.1 One of the goals in synthetic chemistry is the development of chemoselective transformations without affecting the highly reactive functional groups that are not involved in the desired transformation. We have been interested in organolithium reactions without affecting the aldehyde carbonyl groups as an extreme case of chemoselective transformations.2 According to the textbooks of organic chemistry, organolithiums react with aldehydes very quickly and they are not compatible with each other. On the other hand, aldehyde carbonyl groups are very common functional groups and organolithium reactions are frequently used in organic synthesis.3 Therefore, if we could perform organolithium reactions without affecting the aldehyde carbonyl groups, such reactions would serve as powerful synthetic methods. We took an approach to this challenge based on flash chemistry,4 in which highly unstable reactive species are generated and transferred to another location to be used in the next reaction5 before they decompose by high-resolution residence time control using flow microreactor systems.6–8 In fact, recently, we had already reported the generation and reactions of aryllithiums bearing ketone carbonyl groups.5e However, in general, aldehyde carbonyl groups are more reactive than ketone carbonyl groups. Therefore, faster generation is necessary to solve the more challenging problem of aldehyde cases. Here, we show that flash chemistry enables the generation of benzyllithiums9–12 bearing aldehyde carbonyl groups and their use in the reactions with subsequently added electrophiles without affecting the aldehyde carbonyl groups.
We first examined the generation of simple benzyllithiums by reductive lithiation13 of benzyl halides (Fig. 1). This reaction is problematic because of Wurtz-type coupling, i.e. the coupling of benzyllithiums with starting benzyl halides. It was reported that benzyllithium can be generated from benzyl chloride by using lithium naphthalenide (LiNp) in a mixed solvent (Et2O/THF/light petroleum = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
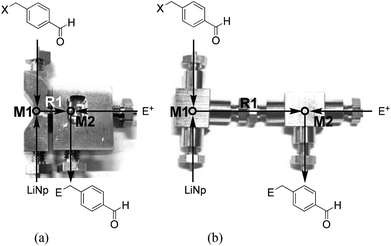
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at −95 °C in a conventional batch reactor.9a However, the reaction in THF and/or at higher temperatures such as −78 °C leads to a dramatic decrease in the yield because of Wurtz-type coupling. We envisioned that extremely fast micromixing is effective to avoid undesired Wurtz-type coupling because it is known that the product selectivity of fast consecutive competitive reactions14 can be dramatically improved by extremely fast micromixing.15 Thus, we examined the reactions of benzyl halides with LiNp in a flow microreactor system, which consists of two T-shaped micromixers M1 (ϕ = 250 μm) and M2 (ϕ = 250 μm) and two microtube reactors R1 (ϕ = 1000 μm, length = 3.5 cm and R2 (ϕ = 1000 μm, length = 50 cm) (see the ESI† for details). For the reactions with very short residence times such as 1.3 ms, a built-in type system as shown in Fig. 2a (R1: ϕ = 250 μm, length = 1.0 cm) was used, whereas a conventional modular type system was used for the reactions with longer residence times (Fig. 2b).
1) at −95 °C in a conventional batch reactor.9a However, the reaction in THF and/or at higher temperatures such as −78 °C leads to a dramatic decrease in the yield because of Wurtz-type coupling. We envisioned that extremely fast micromixing is effective to avoid undesired Wurtz-type coupling because it is known that the product selectivity of fast consecutive competitive reactions14 can be dramatically improved by extremely fast micromixing.15 Thus, we examined the reactions of benzyl halides with LiNp in a flow microreactor system, which consists of two T-shaped micromixers M1 (ϕ = 250 μm) and M2 (ϕ = 250 μm) and two microtube reactors R1 (ϕ = 1000 μm, length = 3.5 cm and R2 (ϕ = 1000 μm, length = 50 cm) (see the ESI† for details). For the reactions with very short residence times such as 1.3 ms, a built-in type system as shown in Fig. 2a (R1: ϕ = 250 μm, length = 1.0 cm) was used, whereas a conventional modular type system was used for the reactions with longer residence times (Fig. 2b).
Because it is well known that the mixing speed in a micromixer depends on the inner diameter and the flow rate,16 we examined the reactions by varying the inner diameter of M1 and the flow rates of the solution of benzyl halide and LiNp. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of benzyl halide and LiNp was maintained in all experiments. Methanol was used as an electrophile and the reactions were carried out at 20 °C using a conventional modular type system (Fig. 2b). As summarized in Table 1, the yield of the desired protonated product, toluene, increased with a decrease in the inner diameter. The yield also increased with an increase in the flow rate. Satisfactory yields were obtained with M1 of 250 μm inner diameter and the total flow rate of 9.0 mL min−1 in the case of benzyl chloride. In the case of benzyl bromide, a higher flow rate was necessary to obtain satisfactory yields, presumably benzyl bromide is more reactive toward benzyllithium than benzyl chloride. Anyway, it is noteworthy that the flow microreactor system enables the generation of benzyllithium at 20 °C, although the reactions should be carried out at −95 °C in a conventional batch macro reactor. It is also advantageous that THF can be used instead of the mixed solvent. Furthermore, benzyl bromide can be used as a starting material, although such transformation is impossible in a conventional batch macro reactor. These remarkable features seem to be ascribed to the extremely fast micromixing of benzyl halide and LiNp at 1
1 molar ratio of benzyl halide and LiNp was maintained in all experiments. Methanol was used as an electrophile and the reactions were carried out at 20 °C using a conventional modular type system (Fig. 2b). As summarized in Table 1, the yield of the desired protonated product, toluene, increased with a decrease in the inner diameter. The yield also increased with an increase in the flow rate. Satisfactory yields were obtained with M1 of 250 μm inner diameter and the total flow rate of 9.0 mL min−1 in the case of benzyl chloride. In the case of benzyl bromide, a higher flow rate was necessary to obtain satisfactory yields, presumably benzyl bromide is more reactive toward benzyllithium than benzyl chloride. Anyway, it is noteworthy that the flow microreactor system enables the generation of benzyllithium at 20 °C, although the reactions should be carried out at −95 °C in a conventional batch macro reactor. It is also advantageous that THF can be used instead of the mixed solvent. Furthermore, benzyl bromide can be used as a starting material, although such transformation is impossible in a conventional batch macro reactor. These remarkable features seem to be ascribed to the extremely fast micromixing of benzyl halide and LiNp at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio.
1 molar ratio.
| X | Flow rate (mL min−1) | Inner diameter of M1 (μm) | Yieldb (%) | |||
|---|---|---|---|---|---|---|
| Benzyl halide | LiNp | Total | Toluene | Bibenzyl | ||
| a R1: ϕ = 250 μm, L = 3.5 cm, 20 °C. b Determined by GC using an internal standard. | ||||||
| Cl | 6.0 | 3.0 | 9.0 | 500 | 70 | 13 |
| 6.0 | 3.0 | 9.0 | 250 | 89 | 4 | |
| 3.0 | 1.5 | 4.5 | 250 | 81 | 4 | |
| Br | 6.0 | 3.0 | 9.0 | 800 | 15 | 29 |
| 6.0 | 3.0 | 9.0 | 500 | 38 | 30 | |
| 6.0 | 3.0 | 9.0 | 250 | 77 | 10 | |
| 12 | 6.0 | 18 | 250 | 80 | 8 | |
| 3.0 | 1.5 | 4.5 | 250 | 49 | 24 | |
| 1.5 | 0.75 | 2.25 | 250 | 39 | 30 | |
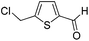
Under the optimized conditions, the reactions of benzyllithium with other electrophiles, such as methyl iodide, aldehydes, ketones, trimethylsilyl chloride, and isocyanates, were examined. As shown in Table 2, the corresponding products were obtained in good yields. Notably, the lithiation of 2-(chloromethyl)thiophene followed by the reaction with an electrophile was successfully carried out without ring-opening, although conventional batch reactions often suffer from this side reaction.17 The productivity of the present method is high enough for laboratory synthesis. In the case of the reaction of benzyllithium with benzophenone, a 15 min operation gave 1.09 g of the desired product (see the ESI† for details).
| Benzyl halides | Electrophile | Product | Yieldb (%) |
|---|---|---|---|
| a R1: ϕ = 250 μm, L = 3.5 cm, 20 °C. Benzyl chloride: total flow rate = 9 ml min−1. Benzyl bromide, 2-(chloromethyl)thiophene: flow rate of benzyl halide = 18 ml min−1. b Isolated yield. c Determined by GC using an internal standard. | |||

|
MeOH |
|
89c |
| Mel |

|
82c | |
| PhCHO |

|
80 | |
| (CH3)2CO |

|
42c | |
| Ph2CO |

|
93 | |
| Me3SiCl |

|
80c | |

|
MeOH |

|
80c |
| Mel |
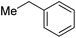
|
82c | |
| PhCHO |

|
75 | |
| Ph2CO |

|
71 | |

|
MeOH |
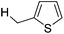
|
97c |
| Mel |
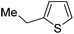
|
72c | |
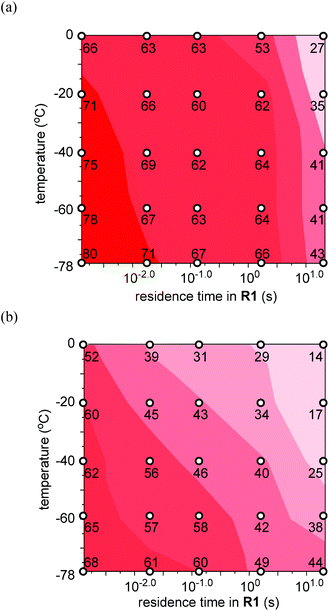
With the successful generation of benzyllithiums by virtue of extremely fast micromixing in hand, we next examined the generation and reactions of benzyllithiums bearing carbonyl groups. In this case the high-resolution residence time control is critical because such benzyllithiums should be transferred extremely quickly to another location to be used in the reaction with electrophiles before they decompose. Temperature–residence time mapping serves as a powerful tool for optimizing the residence time. Fig. 3a shows the contour plots with scattered overlay of the yields of the protonated product for the lithiation of p-propanoylbenzyl chloride, which has a ketone carbonyl group, followed by trapping with methanol. The yield decreases with an increase in the residence time in R1. The yield also decreases with an increase in the temperature although the effect of the temperature is not large. The optimal yield (80%) was obtained with the residence time of 1.3 ms at −78 °C.
The effects of the residence time and the temperature are more significant in the lithiation of p-formylbenzyl chloride, which has an aldehyde carbonyl group (Fig. 3b). As can be seen obviously by comparing Fig. 3a and b, p-formylbenzyllithium is significantly less stable than p-propanoylbenzyllithium. With the residence time of 1.3 ms at −78 °C, however, p-formylbenzyllithium can be generated and used in the subsequent reaction with methanol to give the protonated product in a reasonable yield (68%). This means that the aldehyde carbonyl group can survive in the organolithium reaction.
Under the optimized conditions several benzyllithiums bearing ketone and aldehyde carbonyl groups were generated and reacted with several electrophiles including phenylisocyanate, benzaldehyde, TMSOTf, and MeOTf. The results are summarized in Table 3. Such transformations are very difficult or practically impossible by using conventional batch macro reactors.
| Benzyl halides | Electrophile | Product | Yieldb (%) |
|---|---|---|---|
a R1: ϕ = 250 μm, L = 1.0 cm, −78 °C.
b Isolated yield.
c Determined by GC.
d Diastereomeric ratio = 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 (determined by 1H NMR spectra).
e Diastereomeric ratio = 60 12 (determined by 1H NMR spectra).
e Diastereomeric ratio = 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 (determined by 1H NMR spectra). 40 (determined by 1H NMR spectra).
|
|||

|
PhNCO |

|
78 |
| PhCHO |

|
88 | |

|
PhNCO |

|
60 |
| PhCHO |
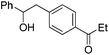
|
64 | |
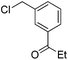
|
Me3SiOTf |
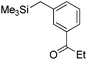
|
68 |
| PhCHO |
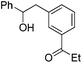
|
67 | |

|
PhCHO |

|
89 |

|
PhNCO |
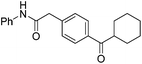
|
77 |
| PhCHO |

|
83 | |

|
PhCHO |
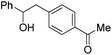
|
85 |
| MeOTf |
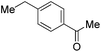
|
41c | |

|
PhCHO |

|
55 |
| Me3SiOTf |

|
58 | |
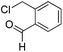
|
PhCHO |
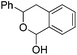
|
59d |

|
PhCHO |

|
76e |
|
PhCHO |

|
77 |

|

|
72 | |
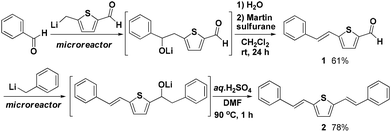
As an application of the present method, we accomplished the synthesis of a π-conjugated system as shown in Fig. 4. The reaction of benzaldehyde with (5-formylthiophen-2-yl)methyllithium followed by elimination with bis[α,α-bis(trifluoromethyl)benzenemethanolato]diphenylsulfur (Martin sulfurane) gave aldehyde 1 in 61% isolated yield. The aldehyde carbonyl group in 1 was used for subsequent reaction with benzyllithium. The subsequent dehydration gave compound 2 (78% isolated yield), in which one thiophene ring and two benzene rings are connected by carbon–carbon double bonds.18
In conclusion, flash chemistry using flow microreactor systems enables the generation and reactions of benzyllithiums bearing aldehyde carbonyl groups. Extremely fast micromixing is responsible for the generation of benzyllithiums avoiding Wurtz-type coupling, and high-resolution residence time control is responsible for survival of aldehyde carbonyl groups. The present findings open a new aspect of protecting-group-free19 organolithium chemistry.
Acknowledgements
This work was partially supported by the Grant-in-Aid for Scientific Research (S) (no. 26220804) and Scientific Research (B) (no. 26288049). We also thank Taiyo Nippon Sanso for providing a low temperature cooling device and partial financial support.References
- (a) R. W. Hoffmann, Synthesis, 2006, 3531 CrossRef CAS PubMed; (b) N. A. Afagh and A. K. Yudin, Angew. Chem., Int. Ed., 2010, 49, 262 CrossRef CAS PubMed.
- (a) J. A. Bajgrowicz, A. El Hallaoui, R. Jacquier, Ch. Pigiere and Ph. Viallefont, Tetrahedron, 1985, 41, 1833 CrossRef CAS; (b) T. Mandai, S. Matsumoto, M. Kohama, M. Kawada and J. Tsuji, J. Org. Chem., 1990, 55, 5671 CrossRef CAS; (c) T. Mandai, T. Murakami, M. Kawada and J. Tsuji, Tetrahedron Lett., 1991, 32, 3399 CrossRef CAS; (d) J. F. Gil, D. J. Ramón and M. Yus, Tetrahedron, 1993, 49, 4923 CrossRef CAS.
- (a) J. E. Baldwin and R. M. Williams, Organolithiums: Selectivity for Synthesis, Pergamon, Amsterdam, 2002 Search PubMed; (b) P. Knochel, Handbook of Functionalized Organometallics, Wiley-VCH, Weinheim, 2005 Search PubMed; (c) R. Luisi and V. Capriati, Lithium Compounds in Organic Synthesis - From Fundamentals to Applications, Wiley-VCH, Weinheim, 2014 Search PubMed.
- Flash chemistry is defined as a field of chemical synthesis where extremely fast reactions are conducted in a highly controlled manner to produce the desired compounds with high selectivity: (a) J. Yoshida, Chem. Commun., 2005, 4509 RSC; (b) J. Yoshida, A. Nagaki and T. Yamada, Chem. – Eur. J., 2008, 14, 7450 CrossRef CAS PubMed; (c) P. J. Nieuwland, K. Koch, N. van Harskamp, R. Wehrens, J. C. M. van Hest and F. P. J. T. Rutjes, Chem. – Asian J., 2010, 5, 799 CrossRef CAS PubMed; (d) J. Yoshida, Chem. Rec., 2010, 10, 332 CrossRef CAS PubMed; (e) J. Yoshida, Y. Takahashi and A. Nagaki, Chem. Commun., 2013, 49, 9896 RSC.
- Some examples of generation and reactions of short-lived organolithiums in flow: (a) H. Usutani, Y. Tomida, A. Nagaki, H. Okamoto, T. Nokami and J. Yoshida, J. Am. Chem. Soc., 2007, 129, 3046 CrossRef CAS PubMed; (b) A. Nagaki, E. Takizawa and J. Yoshida, J. Am. Chem. Soc., 2009, 131, 1654 CrossRef CAS PubMed; (c) A. Nagaki, A. Kenmoku, Y. Moriwaki, A. Hayashi and J. Yoshida, Angew. Chem., Int. Ed., 2010, 49, 7543 CrossRef CAS PubMed; (d) Y. Tomida, A. Nagaki and J. Yoshida, J. Am. Chem. Soc., 2011, 133, 3744 CrossRef CAS PubMed; (e) H. Kim, A. Nagaki and J. Yoshida, Nat. Commun., 2011, 2, 264 CrossRef PubMed; (f) A. Nagaki, C. Matsuo, S. Kim, K. Saito, A. Miyazaki and J. Yoshida, Angew. Chem., Int. Ed., 2012, 51, 3245 CrossRef CAS PubMed.
- Books on flow microreactor synthesis: (a) W. Ehrfeld, V. Hessel and H. Löwe, Microreactors, Wiley-VCH, Weinheim, 2000 CrossRef PubMed; (b) V. Hessel, S. Hardt and H. Löwe, Chemical Micro Process Engineering, Wiely-VCH Verlag, Weinheim, 2004 Search PubMed; (c) J. Yoshida, Flash Chemistry. Fast Organic Synthesis in Microsystems, Wiley-Blackwell, 2008 Search PubMed; (d) Micro Precess Engineering, ed. V. Hessel, A. Renken, J. C. Schouten and J. Yoshida, Wiley-VCH Verlag, Weinheim, 2009 Search PubMed; (e) Microreactors in Organic Chemistry and Catalysis, ed. T. Wirth, Wiley-VCH Verlag, Weinheim, 2nd edn, 2013 Search PubMed.
- Reviews on flow microreactor synthesis: (a) K. Jähnisch, V. Hessel, H. Löwe and M. Baerns, Angew. Chem., Int. Ed., 2004, 43, 406 CrossRef PubMed; (b) J. Kobayashi, Y. Mori and S. Kobayashi, Chem. – Asian. J., 2006, 1, 22 CrossRef CAS PubMed; (c) B. P. Mason, K. E. Price, J. L. Steinbacher, A. R. Bogdan and D. T. McQuade, Chem. Rev., 2007, 107, 2300 CrossRef CAS PubMed; (d) B. Ahmed-Omer, J. C. Brandt and T. Wirth, Org. Biomol. Chem., 2007, 5, 733 RSC; (e) T. Fukuyama, M. T. Rahman, M. Sato and I. Ryu, Synlett, 2008, 151 CAS; (f) R. L. Hartman and K. F. Jensen, Lab Chip, 2009, 9, 2495 RSC; (g) J. Yoshida, H. Kim and A. Nagaki, ChemSusChem, 2011, 4, 331 CrossRef CAS PubMed; (h) C. Wiles and P. Watts, Green Chem., 2012, 14, 38 RSC; (i) A. Kirschining, L. Kupracz and J. Hartwig, Chem. Lett., 2012, 41, 562 CrossRef; (j) D. T. McQuade and P. H. Seeberger, J. Org. Chem., 2013, 78, 6384 CrossRef CAS PubMed; (k) J. C. Pastre, D. L. Browne and S. V. Ley, Chem. Soc. Rev., 2013, 42, 8849 RSC; (l) I. R. Baxendale, J. Chem. Technol. Biotechnol., 2013, 88, 519 CrossRef CAS PubMed.
- Some selected recent examples: (a) D. Cantillo, M. Baghbanzadeh and C. O. Kappe, Angew. Chem., Int. Ed., 2012, 51, 10190 CrossRef CAS PubMed; (b) W. Shu and S. L. Buchwald, Angew. Chem., Int. Ed., 2012, 51, 5355 CrossRef CAS PubMed; (c) A. Nagaki, Y. Moriwaki and J. Yoshida, Chem. Commun., 2012, 48, 11211 RSC; (d) F. Lévesque and P. H. Seeberger, Angew. Chem., Int. Ed., 2012, 51, 1706 CrossRef PubMed; (e) K. C. Basavaraju, S. Sharma, R. A. Maurya and D. P. Kim, Angew. Chem., Int. Ed., 2013, 52, 6735 CrossRef CAS PubMed; (f) C. Brancour, T. Fukuyama, Y. Mukai, T. Skrydstrup and I. Ryu, Org. Lett., 2013, 15, 2794 CrossRef CAS PubMed; (g) J. D. Nguyen, B. Reiß, C. Dai and C. R. J. Stephenson, Chem. Commun., 2013, 49, 4352 RSC; (h) C. Battilocchio, J. M. Hawkins and S. V. Ley, Org. Lett., 2013, 15, 2278 CrossRef CAS PubMed; (i) A. S. Kleinke and T. F. Jamison, Org. Lett., 2013, 15, 710 CrossRef CAS PubMed; (j) L. Guetzoyan, N. Nikbin, I. R. Baxendale and S. V. Ley, Chem. Sci., 2013, 4, 764 RSC; (k) S. Fuse, Y. Mifune and T. Takahashi, Angew. Chem., Int. Ed., 2014, 53, 851 CrossRef CAS PubMed; (l) Z. He and T. F. Jamison, Angew. Chem., Int. Ed., 2014, 53, 3353 CrossRef CAS PubMed; (m) A. Nagaki, Y. Takahashi and J. Yoshida, Chem. – Eur. J., 2014, 20, 7931 CrossRef CAS PubMed; (n) A. Nagaki, D. Ichinari and J. Yoshida, J. Am. Chem. Soc., 2014, 136, 12245 CrossRef CAS PubMed; (o) S. Sharma, K. Basavaraju, A. Singh and D. Kim, Org. Lett., 2014, 16, 3974 CrossRef CAS PubMed; (p) S. Umezu, Y. Yoshiiwa, M. Tokeshi and M. Shindo, Tetrahedron Lett., 2014, 55, 1822 CrossRef CAS PubMed.
- Generation of benzyllithiums by reductive lithiation: (a) K. Smith and D. Hou, J. Chem. Soc., Perkin Trans. 1, 1995, 185 RSC; (b) C. Gómez, F. F. Huerta and M. Yus, Tetrahedron, 1997, 53, 13897 CrossRef; (c) C. Gómez, F. F. Huerta and M. Yus, Tetrahedron, 1998, 54, 1853 CrossRef; (d) C. Gómez, S. Ruiz and M. Yus, Tetrahedron, 1999, 55, 7017 CrossRef; (e) D. Guijarro and M. Yus, J. Organomet. Chem., 2001, 624, 53 CrossRef CAS.
- Generation of benzyllithiums by halogen–lithium exchange: (a) W. E. Parham, L. D. Jones and Y. A. Sayed, Org. Chem., 1976, 41, 1184 CrossRef CAS; (b) S. Warren, P. Wyatt, M. McPartlin and T. Woodroffe, Tetrahedron Lett., 1996, 37, 5609 CrossRef CAS; (c) L. Kupracz and A. Kischning, Adv. Synth. Catal., 2013, 355, 3375 CrossRef CAS PubMed.
- Generation of benzyllithiums by other methods: (a) H. Gilman and H. McNinch, J. Org. Chem., 1961, 26, 3723 CrossRef CAS; (b) D. F. Hoeg and D. I. Lusk, J. Organomet. Chem., 1966, 5, 1 CrossRef CAS; (c) C. G. Screttas and M. Micha-Screttas, J. Org. Chem., 1979, 44, 713 CrossRef CAS; (d) M. Schlosser and S. Sven, Tetrahedron Lett., 1984, 25, 741 CrossRef CAS; (e) M. Clarembeau and A. Krief, Tetrahedron Lett., 1985, 26, 1093 CrossRef CAS; (f) T. Hiiro, N. Kambe, A. Ogawa, N. Miyoshi, S. Murai and N. Sonoda, Angew. Chem., Int. Ed. Engl., 1987, 26, 1187 CrossRef PubMed; (g) D. Guijarro, B. Mancheño and M. Yus, Tetrahedron, 1992, 48, 4593 CrossRef CAS; (h) C. Strohmann, K. Lehmen, K. Wild and D. Schildbach, Organometallics, 2002, 21, 3079 CrossRef CAS; (i) M. Casimiro, P. Oña-Burgos, J. Meyer, S. Styra, I. Kuzu, F. Breher and I. Fernández, Chem. – Eur. J., 2013, 19, 691 CrossRef CAS PubMed.
- Generation of other benzylmetallics: (a) A. H. Stoll, A. Krasovskiy and P. Knochel, Angew. Chem., Int. Ed., 2006, 45, 606 CrossRef CAS PubMed; (b) A. Metzger, F. M. Piller and P. Knochel, Chem. Commun., 2008, 5824 RSC; (c) Y.-H. Chen, M. Sun and P. Knochel, Angew. Chem., Int. Ed., 2009, 48, 2236 CrossRef CAS PubMed; (d) C. Duplais, A. Krasovskiy, A. Wattenberg and B. H. Lipshutz, Chem. Commun., 2010, 46, 562 RSC; (e) T. D. Bluemke, K. Groll, K. Karaghiosoff and P. Knochel, Org. Lett., 2011, 13, 6440 CrossRef CAS PubMed; (f) D.-G. Yu, X. Wang, R.-Y. Zhu, S. Luo, X.-B. Zhang, B.-Q. Wang, L. Wang and Z.-J. Shi, J. Am. Chem. Soc., 2012, 134, 14638 CrossRef CAS PubMed; (g) Y. Fu, Y. Liu, Y. Chen, H. M. Hügel, M. Wang, D. Huanga and Y. Hua, Org. Biomol. Chem., 2012, 10, 7669 RSC; (h) G. Dagousset, C. Francois, T. Leon, R. Blanc, E. Sansiaume-Dagousset and P. Knochel, Synthesis, 2014, 3133 CAS.
- N. Chinkov, H. Chechik, S. Majumdar, A. Liard and I. Marek, Synthesis, 2002, 2473 CAS.
- (a) P. Rys, Acc. Chem. Res., 1976, 10, 345 CrossRef; (b) P. Rys, Angew. Chem., Int. Ed. Engl., 1977, 12, 807 CrossRef PubMed.
- (a) A. Nagaki, M. Togai, S. Suga, N. Aoki, K. Mae and J. Yoshida, J. Am. Chem. Soc., 2005, 127, 11666 CrossRef CAS PubMed; (b) J. Yoshida, A. Nagaki, T. Iwasaki and S. Suga, Chem. Eng. Technol., 2005, 28, 259 CrossRef CAS PubMed; (c) A. Nagaki, N. Takabayashi, Y. Tomida and J. Yoshida, Org. Lett., 2008, 18, 3937 CrossRef PubMed; (d) A. Nagaki, D. Ichinari and J. Yoshida, Chem. Commun., 2013, 49, 3242 RSC.
- W. Ehrfeld, K. Golbig, V. Hessel, H. Löwe and T. Richter, Ind. Eng. Chem. Res., 1999, 38, 1075 CrossRef CAS.
- T. Jing, T. Ting, C. Chia, L. Hsing, Z. Jia and T. Sheng, Synthesis, 2010, 4242 Search PubMed.
- (a) Y. He, W. Wu, G. Zhao, Y. Liu and Y. Li, Macromolecules, 2008, 41, 9760 CrossRef CAS; (b) A. H. Younes, L. Zhang, M. W. Davidson and L. Zhu, Org. Biomol. Chem., 2010, 8, 5431 RSC; (c) C. Zhang, J. Sun, R. Li, S. Sun, E. Lafalce and X. Jiang, Macromolecules, 2011, 44, 6389 CrossRef CAS.
- I. S. Young and P. S. Baran, Nat. Chem., 2009, 1, 193 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ob00958h |
| This journal is © The Royal Society of Chemistry 2015 |