An iodine catalyzed metal free domino process for the stereoselective synthesis of oxygen bridged bicyclic ethers†
Abstract
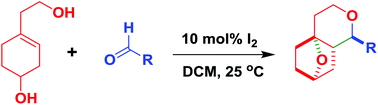
A domino reaction has been developed for the synthesis of oxygen bridged bicyclic ethers through the coupling of 4-(2-hydroxyethyl)cyclohex-3-enols with aldehydes in the presence of 10 mol% of molecular iodine in dichloromethane at 25 °C. This method is highly diastereoselective affording the corresponding bicyclic ethers, i.e. octahydro-4a,7-epoxyisochromenes in good yields with high selectivity. It is the first report on the synthesis of oxygen bridged bicyclic ethers using a domino Prins strategy.

 Please wait while we load your content...
Please wait while we load your content...