Small gold nanoparticles for interfacial Staudinger–Bertozzi ligation†
Abstract
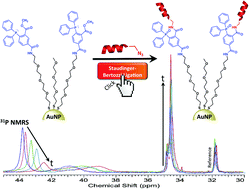
Small gold nanoparticles (AuNPs) that possess interfacial methyl-2-(diphenylphosphino)benzoate moieties have been successfully synthesized (Staudinger-AuNPs) and characterized by multi-nuclear MR spectroscopy, transmission electron microscopy (TEM), UV-Vis spectroscopy, thermogravimetric analysis, and X-ray photoelectron spectroscopy (XPS). In particular, XPS was remarkably sensitive for characterization of the novel nanomaterial, and in furnishing proof of its interfacial reactivity. These Staudinger-AuNPs were found to be stable to the oxidation of the phosphine center. The reaction with benzyl azide in a Staudinger–Bertozzi ligation, as a model system, was investigated using 31P NMR spectroscopy. This demonstrated that the interfacial reaction was clean and quantitative. To showcase the potential utility of these Staudinger-AuNPs in bioorganic chemistry, a AuNP bioconjugate was prepared by reacting the Staudinger-AuNPs with a novel azide-labeled CRGDK peptide. The CRGDK peptide could be covalently attached to the AuNP efficiently, chemoselectively, and with a high loading.

 Please wait while we load your content...
Please wait while we load your content...