Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of pyrazole containing α-amino acids via a highly regioselective condensation/aza-Michael reaction of β-aryl α,β-unsaturated ketones†
Lynne
Gilfillan
a,
Raik
Artschwager
a,
Alexander H.
Harkiss
a,
Rob M. J.
Liskamp
ab and
Andrew
Sutherland
*a
aWestCHEM, School of Chemistry, The Joseph Black Building, University of Glasgow, Glasgow, G12 8QQ, UK. E-mail: Andrew.Sutherland@glasgow.ac.uk; Tel: +44 (0)141 330 5936
bDepartment of Medicinal Chemistry and Chemical Biology, Faculty of Science, Utrecht University, P. O. Box 80082, 3508 TB, Utrecht, The Netherlands
First published on 10th March 2015
Abstract
A synthetic approach for the preparation of a new class of highly conjugated unnatural α-amino acids bearing a 5-arylpyrazole side-chain has been developed. Horner–Wadsworth–Emmons reaction of an aspartic acid derived β-keto phosphonate ester with a range of aromatic aldehydes gave β-aryl α,β-unsaturated ketones. Treatment of these with phenyl hydrazine followed by oxidation allowed the regioselective synthesis of pyrazole derived α-amino acids. As well as evaluating the fluorescent properties of the α-amino acids, their synthetic utility was also explored with the preparation of a sulfonyl fluoride derivative, a potential probe for serine proteases.
Introduction
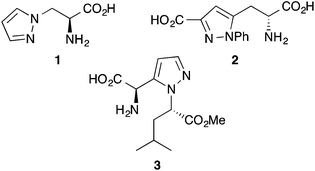
With continued advances in chemical biology and biomedical and life sciences, non-proteinogenic α-amino acids are being increasingly used as probes to study the mechanism of biological processes, investigate the bioactive conformations of proteins and facilitate the discovery of new pharmacologically active compounds.1,2 Furthermore, in the field of organic synthesis, many unnatural α-amino acids have been used as chiral building blocks, ligands and catalysts.3 Due to this increasing range of applications, novel classes of non-proteinogenic α-amino acids and methods for their synthesis are still very much in demand.2,3In recent years, there has been much interest in developing the synthesis and applications of unnatural α-amino acids with heteroaryl containing side-chains.2,4 For example, following the isolation and discovery of a naturally occurring pyrazole containing α-amino acid, (S)-β-pyrazolylalanine (1) (Fig. 1) from Citrullus vulgaris,5 various biological applications of novel pyrazole containing α-amino acids have been reported. Pyrazole analogues of ibotenic acid were shown to be selective antagonists of the metabotropic glutamate receptor 2 (mGluR2),6 while a number of 3-carboxypyrazole α-amino acids (e.g.2) are antagonists of the N-methyl-D-aspartic acid (NMDA) receptor.7 The antitumor activity of ferrocenyl derived α-amino acids linked via a pyrazole moiety has also been demonstrated.8 Other applications include using the rigidity of the pyrazole moiety to generate conformationally restricted peptidomimetics such as α-amino acid 3, which mimics the cis-amide bond.9 Despite exhibiting a range of interesting properties and applications, flexible and general synthetic routes for the preparation of optically active pyrazole containing α-amino acids are still relatively limited.5b,7,9–11 Noteworthy approaches include the cyclocondensation of α-hydrazinoamino acids with 3-trimethylsilanylpropynones or enamino ketones.9 More recently, Conti and co-workers prepared 3-carboxypyrazole derivatives via a 1,3-dipolar cycloaddition of allylglycine with hydrazine derived nitrilimines,7 while Wei and Lubell used the nucleophilic ring-opening of serine derived cyclic sulfamidates for the preparation of β-diketone α-amino acids which were then converted to the corresponding pyrazole by condensation with hydrazine.10a
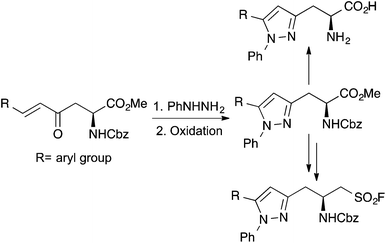
Recently, we reported an efficient approach for the preparation of a rare class of α-amino acid with enone side chains.12 In exploring the reactivity and application of these amino acids, we discovered that these could undergo selective 6-endo-trig cyclisations for the synthesis of 4-oxopipecolic acids,13 and had potential as biological probes with the preparation of a highly fluorescent 4-dimethylamino-1-naphthyl enone analogue.12 Despite these advances, we found that on long-term storage, the enones were prone to decomposition. In an effort to further explore the reactivity of these compounds and produce more robust biologically functional probes, we proposed to investigate heterocyclisation reactions of the enone derived α-amino acids. We now report the development of a highly regioselective condensation/aza-Michael reaction as the key step for the preparation of a new class of α-amino acids bearing 5-arylpyrazole side-chains (Scheme 1). As well as exploring selective deprotection strategies to the parent α-amino acids and measuring their fluorescent properties, the application of one of these compounds for the preparation of a sulfonyl fluoride, well-established serine protease inhibitors is also described.
Results and discussion
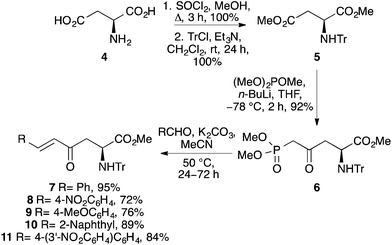
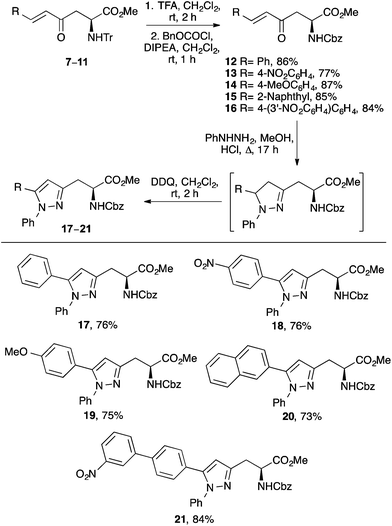
The project began with the preparation of a series of enone derived α-amino acids (Scheme 2).12,13 Reaction of L-aspartic acid (4) with thionyl chloride in methanol followed by N-trityl protection under basic conditions gave N-trityl L-aspartate dimethyl ester 5 in quantitative yield.14 Using the N-trityl group to block the α-methyl ester allowed the highly regioselective reaction of 5 with the lithium anion of dimethyl methylphosphonate. This gave β-ketophosphonate ester 6 as the sole product in 92% yield. To generate stable, highly conjugated heterocycle derived α-amino acids with the potential to act as fluorescent probes, enones with β-aryl side chains were prepared. Under mild conditions, Horner–Wadsworth–Emmons reaction of 6 with various aryl aldehydes gave the corresponding β-aryl E-enones 7–11 in high yields (72–95%). As expected, the reactions of the electron deficient aldehydes were complete after 24 h, while the electron rich and more conjugated analogues required extended reaction times (36–72 h). While other methods have recently been reported for the preparation of these types of enone derived α-amino acids,15 the approach described in Scheme 2 is particularly robust, allowing the multi-gram synthesis of these compounds in excellent overall yields from L-aspartic acid.Methods for formation of the pyrazoles via 2-pyrazolines by reaction of enones 7–11 with phenyl hydrazine were next investigated. Under neutral or acidic conditions, 2-pyrazolines can be prepared by the reaction of enones and hydrazines through a two-stage process involving imine condensation to form a hydrazone, followed by an aza-Michael reaction to complete the cyclisation.16 Initially, reaction of 7 with phenyl hydrazine was attempted under neutral conditions. However, analysis by 1H NMR spectroscopy showed that the reaction had not gone to completion forming only the hydrazone intermediate. As more forcing, acidic conditions were necessary, the acid-labile N-trityl protected α-amino acids 7–11 were converted to the N-Cbz derivatives (Scheme 3). Amino acids 7–11 were easily deprotected using TFA under mild conditions. Without purification, the resulting amines were treated with benzyl chloroformate in the presence of Hünig's base, which gave the Cbz-derivatives 12–16 in high yields over the two-steps (77–87%). Reaction of N-Cbz protected enone 12 with phenyl hydrazine in the presence of HCl was then investigated. Under reflux conditions, this produced the corresponding 2-pyrazoline cleanly, as a single regioisomer. While not important for the synthesis of pyrazoles, it was noted using NMR spectroscopy that the aza-Michael step proceeded without any asymmetric bias from the chiral α-position, forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of diastereomers. Without purification, the 2-pyrazoline was oxidised using DDQ under mild conditions and this gave pyrazole 17 in 76% yield over the two-steps.17 With an optimised approach in hand, the scope of this process was then explored using the other enones with electron-rich, electron-deficient and highly conjugated side-chains. With all the examples, the corresponding pyrazoles were formed as a single regioisomer in high yields (73–84%).
1 mixture of diastereomers. Without purification, the 2-pyrazoline was oxidised using DDQ under mild conditions and this gave pyrazole 17 in 76% yield over the two-steps.17 With an optimised approach in hand, the scope of this process was then explored using the other enones with electron-rich, electron-deficient and highly conjugated side-chains. With all the examples, the corresponding pyrazoles were formed as a single regioisomer in high yields (73–84%).
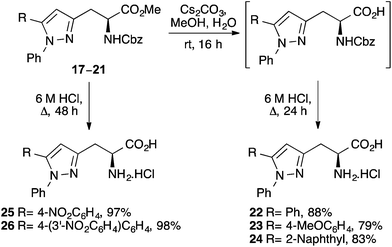
To access the parent α-amino acids, various strategies were explored. We wanted to demonstrate that the α-carboxylic acid position could be selectively deprotected under mild conditions to give N-Cbz protected α-amino acids that could have direct application in peptide synthesis. Using compounds 17, 19 and 20, hydrolysis of the ester moiety with cesium carbonate at room temperature gave the N-Cbz protected α-amino acids cleanly (Scheme 4). Without purification, removal of the amino protecting group under acidic conditions then gave α-amino acids 22–24 in 79–88% yields over the two steps. Using the nitro-substituted α-amino acids, a one-step procedure was also developed. Acid mediated deprotection of compounds 18 and 21 using 6 M HCl resulted in the removal of both protecting groups, allowing the isolation of α-amino acids 25 and 26 in essentially quantitative yields. Overall, this approach for the preparation of α-amino acids bearing 5-arylpyrazole side-chains was general and efficient, allowing the synthesis of the target α-amino acids in 36–53% overall yield from L-aspartic acid.
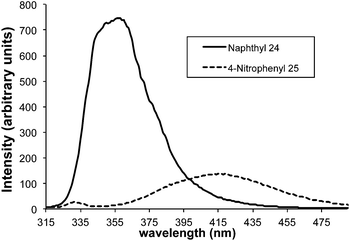
The specific use of aryl substituents for the pyrazoles was to generate α-amino acids with highly conjugated side-chains that would possess fluorescent properties.18 Therefore, following the synthesis of compounds 22–26, the absorption and fluorescence spectra were recorded (Table 1). The absorption and emission maxima showed some correlation with the electronic nature of the aryl substituents. For example, the electron deficient groups generally absorbed and emitted at longer wavelengths. While compounds 22–26 all showed some degree of fluorescence, the naphthyl and 4-nitrophenyl analogues 24 and 25, in particular showed strong fluorescence with maxima at 356 and 415 nm, respectively (Fig. 2).
| Compound | λ Abs (nm) | λ Em (nm) | ε (cm−1 M−1) |
|---|---|---|---|
| a The spectra were recorded in methanol at a concentration of 1 × 10–5 M. | |||
| 22 | 250 | 367 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 320 320 |
| 23 | 254 | 348 | 6170 |
| 24 | 249 | 356 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 650 |
| 25 | 303 | 415 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
| 26 | 270 | 434 | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940 940 |
The emission maxima for the naturally occurring α-amino acid, tryptophan occurs at similar wavelength (λEm = 352 nm) to naphthyl analogue 24.19 This would obviously limit applications of 24 when incorporated into proteins and peptides containing tryptophan. So despite showing weaker fluorescence, it was decided to investigate applications of 4-nitrophenyl analogue 25, which has an emission maximum at higher wavelength compared to proteinogenic α-amino acids.20
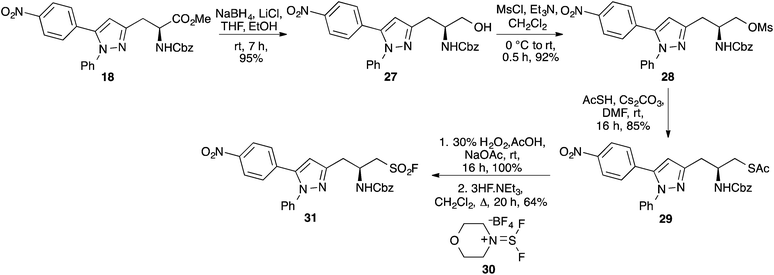
Sulfonyl fluorides derived from α-amino acids and peptides have been synthesised and shown to act as electrophilic traps for the potent inhibition of protease enzymes.21,22 In particular, a N-Cbz protected tetrapeptide incorporating a sulfonyl fluoride unit has demonstrated broad-spectrum antimalarial activity.23 In this project, we wished to investigate whether a sulfonyl fluoride unit could be incorporated into 5-(4′-nitrophenyl)pyrazole α-amino acid 18. The product of this process would generate a multi-functional compound that would have potential as both a protease inhibitor and could undergo further transformations such as peptide synthesis for development as a biological probe. The ester moiety of 18 was initially reduced under mild conditions using sodium borohydride and lithium chloride (Scheme 5). This gave alcohol 27 in 95% yield. The hydroxyl group was activated as the mesylate under standard conditions, and this was then displaced by in situ generated cesium thioacetate forming 29 in high yield. Oxidation of thioacetate 29 with aqueous hydrogen peroxide in the presence of sodium acetate gave the corresponding sodium sulfonate salt in quantitative yield. Sodium sulfonates can be converted to sulfonyl fluorides by treatment with oxalyl chloride and then reaction of the resulting sulfonyl chloride with potassium fluoride, or directly using DAST.21,22a For the preparation of sulfonyl fluoride 31, it was found that direct reaction of the sodium sulfonate salt with triethylamine trihydrofluoride and XtalFluor-M® 30 gave the target compound very cleanly, in 64% yield. The synthesis of sulfonyl fluoride 31 in 48% overall yield from 18 demonstrates the synthetic utility of pyrazole derived α-amino acids and their use for the synthesis of potential functionalised biological probes.
Conclusions
In summary, a series of novel pyrazole derived α-amino acids have been prepared from L-aspartic acid using highly regioselective transformations such as a Horner–Wadsworth–Emmons reaction and a one-pot condensation/aza-Michael process. The synthetic utility of these compounds was explored with the efficient transformation of 5-(4′-nitrophenyl)pyrazole α-amino acid 18 to sulfonyl fluoride derivative 31, a potential serine protease inhibitor. The absorption and emission properties of the pyrazole derived α-amino acids were also recorded with compounds 24 and 25 showing particularly strong fluorescence. This part of the study has revealed the structural features and level of conjugation required for the generation of heterocyclic derived α-amino acids with appropriate emission maxima for application as fluorescent probes. Using this information, the development of new heterocyclisation reactions of enone derived α-amino acids are currently underway. Exploration of the use of sulfonyl fluorides such as 31 as components of peptide-based inhibitors is also being studied. The results of these investigations will be reported in due course.Experimental
Reactions were performed in flame-dried glassware under a positive atmosphere of argon. All reagents and starting materials were obtained from commercial sources and used as received. Dry solvents were purified using a PureSolv 500 MD solvent purification system. Flash column chromatography was carried out using Fisher matrix silica 60. Macherey–Nagel aluminium-backed plates pre-coated with silica gel 60 (UV254) were used for thin layer chromatography and were visualised by staining with KMnO4. 1H NMR and 13C NMR spectra were recorded on a Bruker DPX 400 spectrometer or Bruker 500 spectrometer with chemical shift values in ppm relative to TMS (δH 0.00 and δC 0.0) or residual chloroform (δH 7.28 and δC 77.2) as standard. Assignment of 1H and 13C NMR signals are based on two-dimensional COSY and DEPT experiments, respectively. Mass spectra were obtained using a JEOL JMS-700 spectrometer. Infrared spectra were recorded using Golden Gate apparatus on a JASCO FTIR 410. Melting points were determined on a Reichert platform melting point apparatus. Optical rotations were determined as solutions irradiating with the sodium D line (λ = 589 nm) using an Auto pol V polarimeter. [α]D values are given in units 10−1 deg cm2 g−1.Methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-4-oxo-6-phenylhex-5-enoate (12)
To a solution of methyl (2S,5E)-4-oxo-6-phenyl-2-(tritylamino)hex-5-enoate (7) (1.06 g, 2.23 mmol) in dichloromethane (45 mL) was added trifluoroacetic acid (0.33 mL, 4.50 mmol). The reaction mixture was stirred at room temperature for 2 h before concentrating in vacuo. The resulting residue was dissolved in chloroform (5 mL) and petroleum ether was added until an orange oil formed. The solvent was then decanted off, and the remaining oil was dissolved in dichloromethane (30 mL) followed by the addition of N,N-diisopropylethylamine (0.97 mL, 5.58 mmol) and benzyl chloroformate (0.48 mL, 3.35 mmol). The reaction mixture was stirred at room temperature for 1 h before diluting with water (50 mL). The mixture was then extracted with dichloromethane (4 × 50 mL), dried (MgSO4) and concentrated in vacuo. The resulting residue was purified by column chromatography (elution with 50% ethyl acetate in petroleum ether) to give methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-4-oxo-6-phenylhex-5-enoate (12) as a white solid (0.70 g, 86%). Mp 77–78 °C; νmax/cm−1 (neat) 3333 (NH), 3059, 2951 (CH), 1734 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1688 (C
O), 1688 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1533 (C
O), 1533 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1435, 1343, 1254, 1090, 980, 748; [α]26D +26.5 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.26 (1H, dd, J 17.9, 4.2 Hz, 3-HH), 3.48 (1H, dd, J 17.9, 4.2 Hz, 3-HH), 3.75 (3H, s, OCH3), 4.68 (1H, dt, J 8.4, 4.2 Hz, 2-H), 5.12 (2H, s, OCH2Ph), 5.85 (1H, d, J 8.4 Hz, NH), 6.69 (1H, d, J 16.2 Hz, 5-H), 7.26–7.43 (8H, m, ArH), 7.51–7.59 (3H, m, 6-H and ArH); δC (101 MHz, CDCl3) 42.4 (CH2), 50.2 (CH), 52.9 (CH3) 67.2 (CH2), 125.6 (CH), 128.2 (2 × CH), 128.3 (CH), 128.6 (4 × CH), 129.2 (2 × CH), 131.0 (CH), 134.2 (C), 136.4 (C), 144.2 (CH), 156.2 (C), 171.7 (C), 197.6 (C); m/z (CI) 368.1506 (MH+. C21H22NO5 requires 368.1498), 326 (8%), 260 (23), 234 (21), 219 (18), 181 (6), 147 (17), 107 (16), 85 (100).
C), 1435, 1343, 1254, 1090, 980, 748; [α]26D +26.5 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.26 (1H, dd, J 17.9, 4.2 Hz, 3-HH), 3.48 (1H, dd, J 17.9, 4.2 Hz, 3-HH), 3.75 (3H, s, OCH3), 4.68 (1H, dt, J 8.4, 4.2 Hz, 2-H), 5.12 (2H, s, OCH2Ph), 5.85 (1H, d, J 8.4 Hz, NH), 6.69 (1H, d, J 16.2 Hz, 5-H), 7.26–7.43 (8H, m, ArH), 7.51–7.59 (3H, m, 6-H and ArH); δC (101 MHz, CDCl3) 42.4 (CH2), 50.2 (CH), 52.9 (CH3) 67.2 (CH2), 125.6 (CH), 128.2 (2 × CH), 128.3 (CH), 128.6 (4 × CH), 129.2 (2 × CH), 131.0 (CH), 134.2 (C), 136.4 (C), 144.2 (CH), 156.2 (C), 171.7 (C), 197.6 (C); m/z (CI) 368.1506 (MH+. C21H22NO5 requires 368.1498), 326 (8%), 260 (23), 234 (21), 219 (18), 181 (6), 147 (17), 107 (16), 85 (100).
Methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(4′-nitrophenyl)-4-oxohex-5-enoate (13)
The reactions were performed as described for 7 using methyl (2S,5E)-6-(4′-nitrophenyl)-4-oxo-2-(tritylamino)hex-5-enoate (8) (0.39 g, 0.75 mmol). This gave methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(4′-nitrophenyl)-4-oxohex-5-enoate (13) as a yellow solid (0.24 g, 77%). Mp 73–74 °C; νmax/cm−1 (neat) 3331 (NH), 2953 (CH), 1730 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1686 (C
O), 1686 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1512 (C
O), 1512 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1343, 1202, 1059, 978, 860; [α]28D +20.6 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.30 (1H, dd, J 18.1, 4.2 Hz, 3-HH), 3.48 (1H, dd, J 18.1, 4.2 Hz, 3-HH), 3.76 (3H, s, OCH3), 4.70 (1H, dt, J 8.4, 4.2 Hz, 2-H), 5.12 (2H, s, OCH2Ph), 5.82 (1H, d, J 8.4 Hz, NH), 6.80 (1H, d, J 16.2 Hz, 5-H), 7.29–7.37 (5H, m, Ph), 7.58 (1H, d, J 16.2 Hz, 6-H), 7.69 (2H, d, J 8.8 Hz, 2′-H and 6′-H), 8.26 (2H, d, J 8.8 Hz, 3′-H and 5′-H); δC (101 MHz, CDCl3) 43.0 (CH2), 50.2 (CH), 53.0 (CH3), 67.3 (CH2), 124.4 (2 × CH), 128.2 (2 × CH), 128.4 (CH), 128.7 (2 × CH), 128.9 (CH), 129.1 (2 × CH), 136.3 (C), 140.4 (C), 140.9 (CH), 149.0 (C), 156.2 (C), 171.5 (C), 197.0 (C); m/z (CI) 413.1352 (MH+. C21H21N2O7 requires 413.1349), 383 (42%), 348 (38), 305 (30), 275 (30), 257 (23), 137 (68), 91 (68), 69 (100).
C), 1343, 1202, 1059, 978, 860; [α]28D +20.6 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.30 (1H, dd, J 18.1, 4.2 Hz, 3-HH), 3.48 (1H, dd, J 18.1, 4.2 Hz, 3-HH), 3.76 (3H, s, OCH3), 4.70 (1H, dt, J 8.4, 4.2 Hz, 2-H), 5.12 (2H, s, OCH2Ph), 5.82 (1H, d, J 8.4 Hz, NH), 6.80 (1H, d, J 16.2 Hz, 5-H), 7.29–7.37 (5H, m, Ph), 7.58 (1H, d, J 16.2 Hz, 6-H), 7.69 (2H, d, J 8.8 Hz, 2′-H and 6′-H), 8.26 (2H, d, J 8.8 Hz, 3′-H and 5′-H); δC (101 MHz, CDCl3) 43.0 (CH2), 50.2 (CH), 53.0 (CH3), 67.3 (CH2), 124.4 (2 × CH), 128.2 (2 × CH), 128.4 (CH), 128.7 (2 × CH), 128.9 (CH), 129.1 (2 × CH), 136.3 (C), 140.4 (C), 140.9 (CH), 149.0 (C), 156.2 (C), 171.5 (C), 197.0 (C); m/z (CI) 413.1352 (MH+. C21H21N2O7 requires 413.1349), 383 (42%), 348 (38), 305 (30), 275 (30), 257 (23), 137 (68), 91 (68), 69 (100).
Methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(4′-methoxyphenyl)-4-oxohex-5-enoate (14)
The reactions were performed as described for 7 using methyl (2S,5E)-6-(4′-methoxyphenyl)-4-oxo-2-(tritylamino)hex-5-enoate (9) (0.60 g, 1.19 mmol). This gave methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(4′-methoxyphenyl)-4-oxohex-5-enoate (14) as a colourless oil (0.41 g, 87%). νmax/cm−1 (neat) 3347 (NH), 2953 (CH), 1717 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1655 (C
O), 1655 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597 (C
O), 1597 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1510 (C
C), 1510 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1248, 1208, 1169, 1026, 816; [α]29D +30.3 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.23 (1H, dd, J 17.9, 4.2 Hz, 3-HH), 3.47 (1H, dd, J 17.9, 4.2 Hz, 3-HH), 3.75 (3H, s, OCH3), 3.85 (3H, s, OCH3), 4.67 (1H, dt, J 8.5, 4.2 Hz, 2-H), 5.12 (2H, s, OCH2Ph), 5.88 (1H, d, J 8.5 Hz, NH), 6.58 (1H, d, J 16.2 Hz, 5-H), 6.92 (2H, d, J 8.8 Hz, 3′-H and 5′-H), 7.27–7.38 (5H, m, Ph), 7.49 (2H, d, J 8.8 Hz, 2′-H and 6′-H), 7.52 (1H, d, J 16.2 Hz, 6-H); δC (101 MHz, CDCl3) 42.3 (CH2), 50.3 (CH), 52.8 (CH3), 55.6 (CH3), 67.1 (CH2), 114.6 (2 × CH), 123.4 (CH), 126.9 (C), 128.1 (2 × CH), 128.2 (CH), 128.6 (2 × CH), 130.4 (2 × CH), 136.4 (C), 144.0 (CH), 156.2 (C), 162.1 (C), 171.8 (C), 197.4 (C); m/z (EI) 397.1526 (M+. C22H23NO6 requires 397.1525), 336 (10%), 289 (19), 262 (19), 243 (45), 182 (34), 161 (100), 91 (32).
C), 1248, 1208, 1169, 1026, 816; [α]29D +30.3 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.23 (1H, dd, J 17.9, 4.2 Hz, 3-HH), 3.47 (1H, dd, J 17.9, 4.2 Hz, 3-HH), 3.75 (3H, s, OCH3), 3.85 (3H, s, OCH3), 4.67 (1H, dt, J 8.5, 4.2 Hz, 2-H), 5.12 (2H, s, OCH2Ph), 5.88 (1H, d, J 8.5 Hz, NH), 6.58 (1H, d, J 16.2 Hz, 5-H), 6.92 (2H, d, J 8.8 Hz, 3′-H and 5′-H), 7.27–7.38 (5H, m, Ph), 7.49 (2H, d, J 8.8 Hz, 2′-H and 6′-H), 7.52 (1H, d, J 16.2 Hz, 6-H); δC (101 MHz, CDCl3) 42.3 (CH2), 50.3 (CH), 52.8 (CH3), 55.6 (CH3), 67.1 (CH2), 114.6 (2 × CH), 123.4 (CH), 126.9 (C), 128.1 (2 × CH), 128.2 (CH), 128.6 (2 × CH), 130.4 (2 × CH), 136.4 (C), 144.0 (CH), 156.2 (C), 162.1 (C), 171.8 (C), 197.4 (C); m/z (EI) 397.1526 (M+. C22H23NO6 requires 397.1525), 336 (10%), 289 (19), 262 (19), 243 (45), 182 (34), 161 (100), 91 (32).
Methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(naphthalen-2′-yl)-4-oxohex-5-enoate (15)
The reactions were performed as described for 7 using methyl (2S,5E)-6-(naphthalen-2′-yl)-4-oxo-2-(tritylamino)hex-5-enoate (10) (0.56 g, 1.07 mmol). This gave methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(naphthalen-2′-yl)-4-oxohex-5-enoate (15) as a colourless oil (0.38 g, 85%). νmax/cm−1 (neat) 3339 (NH), 2951 (CH), 1717 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1659 (C
O), 1659 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1505 (C
O), 1505 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1207, 1057, 976, 812; [α]28D +27.5 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.31 (1H, dd, J 18.0, 4.2 Hz, 3-HH), 3.54 (1H, dd, J 18.0, 4.2 Hz, 3-HH), 3.77 (3H, s, OCH3), 4.71 (1H, dt, J 8.6, 4.2 Hz, 2-H), 5.13 (2H, s, OCH2Ph), 5.89 (1H, d, J 8.6 Hz, NH), 6.82 (1H, d, J 16.2 Hz, 5-H), 7.28–7.39 (5H, m, Ph), 7.51–7.58 (2H, m, 6′-H and 7′-H), 7.67 (1H, dd, J 8.6, 1.5 Hz, 3′-H), 7.73 (1H, d, J 16.2 Hz, 6-H), 7.82–7.90 (3H, m, 4′-H, 5′-H and 8′-H), 7.96 (1H, br s, 1′-H); δC (101 MHz, CDCl3) 42.5 (CH2), 50.2 (CH), 52.9 (CH3), 67.2 (CH2), 123.6 (CH), 125.7 (CH), 127.0 (CH), 127.7 (CH), 127.9 (CH), 128.1 (2 × CH), 128.3 (CH), 128.6 (2 × CH), 128.8 (CH), 129.0 (CH), 130.9 (CH), 131.7 (C), 133.4 (C), 134.6 (C), 136.4 (C), 144.3 (CH), 156.2 (C), 171.7 (C), 197.5 (C); m/z (CI) 418.1653 (MH+. C25H24NO5 requires 418.1654), 383 (32%), 310 (48), 275 (20), 147 (30), 107 (29).
C), 1207, 1057, 976, 812; [α]28D +27.5 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.31 (1H, dd, J 18.0, 4.2 Hz, 3-HH), 3.54 (1H, dd, J 18.0, 4.2 Hz, 3-HH), 3.77 (3H, s, OCH3), 4.71 (1H, dt, J 8.6, 4.2 Hz, 2-H), 5.13 (2H, s, OCH2Ph), 5.89 (1H, d, J 8.6 Hz, NH), 6.82 (1H, d, J 16.2 Hz, 5-H), 7.28–7.39 (5H, m, Ph), 7.51–7.58 (2H, m, 6′-H and 7′-H), 7.67 (1H, dd, J 8.6, 1.5 Hz, 3′-H), 7.73 (1H, d, J 16.2 Hz, 6-H), 7.82–7.90 (3H, m, 4′-H, 5′-H and 8′-H), 7.96 (1H, br s, 1′-H); δC (101 MHz, CDCl3) 42.5 (CH2), 50.2 (CH), 52.9 (CH3), 67.2 (CH2), 123.6 (CH), 125.7 (CH), 127.0 (CH), 127.7 (CH), 127.9 (CH), 128.1 (2 × CH), 128.3 (CH), 128.6 (2 × CH), 128.8 (CH), 129.0 (CH), 130.9 (CH), 131.7 (C), 133.4 (C), 134.6 (C), 136.4 (C), 144.3 (CH), 156.2 (C), 171.7 (C), 197.5 (C); m/z (CI) 418.1653 (MH+. C25H24NO5 requires 418.1654), 383 (32%), 310 (48), 275 (20), 147 (30), 107 (29).
Methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(3′′-nitrobiphen-4′-yl)-4-oxohex-5-enoate (16)
The reactions were performed as described for 7 using methyl (2S,5E)-6-(3′′-nitrobiphen-4′-yl)-4-oxo-2-(tritylamino)hex-5-enoate (11) (0.55 g, 0.92 mmol). This gave methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(3′′-nitrobiphen-4′-yl)-4-oxohex-5-enoate (16) as a pale yellow solid (0.38 g, 84%). Mp 102–104 °C; νmax/cm−1 (neat) 3325 (NH), 2953 (CH), 1742 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1683 (C
O), 1683 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1664 (C
O), 1664 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1529 (C
O), 1529 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1342, 1179, 1057, 970, 802; [α]27D +17.2 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.29 (1H, dd, J 18.0, 4.2 Hz, 3-HH), 3.51 (1H, dd, J 18.0, 4.2 Hz, 3-HH), 3.77 (3H, s, OCH3), 4.71 (1H, dt, J 8.5, 4.2 Hz, 2-H), 5.13 (2H, s, OCH2Ph), 5.87 (1H, d, J 8.5 Hz, NH), 6.77 (1H, d, J 16.2 Hz, 5-H), 7.28–7.39 (5H, m, Ph), 7.61 (1H, d, J 16.2 Hz, 6-H), 7.64–7.70 (5H, m, 2′-H, 3′-H, 5′-H, 6′-H and 5′′-H), 7.94 (1H, ddd, J 7.8, 2.0, 1.0 Hz, 6′′-H), 8.24 (1H, ddd, J 8.2, 2.0, 1.0 Hz, 4′′-H), 8.48 (1H, t, J 2.0 Hz, 2′′-H); δC (101 MHz, CDCl3) 42.6 (CH2), 50.2 (CH), 52.9 (CH3), 67.2 (CH2), 122.0 (CH), 122.8 (CH), 126.2 (CH), 127.9 (2 × CH), 128.2 (2 × CH), 128.3 (CH), 128.7 (2 × CH), 129.4 (2 × CH), 130.1 (CH), 133.0 (CH), 134.5 (C), 136.4 (C), 141.0 (C), 141.8 (C), 143.1 (CH), 149.0 (C), 156.2 (C), 171.7 (C), 197.4 (C); m/z (CI) 489.1664 (MH+. C27H25N2O7 requires 489.1662), 459 (8%), 418 (10), 381 (42), 351 (23), 338 (32), 310 (19), 275 (10), 181 (15), 147 (26), 91 (100).
C), 1342, 1179, 1057, 970, 802; [α]27D +17.2 (c 1.0, CHCl3); δH (400 MHz, CDCl3) 3.29 (1H, dd, J 18.0, 4.2 Hz, 3-HH), 3.51 (1H, dd, J 18.0, 4.2 Hz, 3-HH), 3.77 (3H, s, OCH3), 4.71 (1H, dt, J 8.5, 4.2 Hz, 2-H), 5.13 (2H, s, OCH2Ph), 5.87 (1H, d, J 8.5 Hz, NH), 6.77 (1H, d, J 16.2 Hz, 5-H), 7.28–7.39 (5H, m, Ph), 7.61 (1H, d, J 16.2 Hz, 6-H), 7.64–7.70 (5H, m, 2′-H, 3′-H, 5′-H, 6′-H and 5′′-H), 7.94 (1H, ddd, J 7.8, 2.0, 1.0 Hz, 6′′-H), 8.24 (1H, ddd, J 8.2, 2.0, 1.0 Hz, 4′′-H), 8.48 (1H, t, J 2.0 Hz, 2′′-H); δC (101 MHz, CDCl3) 42.6 (CH2), 50.2 (CH), 52.9 (CH3), 67.2 (CH2), 122.0 (CH), 122.8 (CH), 126.2 (CH), 127.9 (2 × CH), 128.2 (2 × CH), 128.3 (CH), 128.7 (2 × CH), 129.4 (2 × CH), 130.1 (CH), 133.0 (CH), 134.5 (C), 136.4 (C), 141.0 (C), 141.8 (C), 143.1 (CH), 149.0 (C), 156.2 (C), 171.7 (C), 197.4 (C); m/z (CI) 489.1664 (MH+. C27H25N2O7 requires 489.1662), 459 (8%), 418 (10), 381 (42), 351 (23), 338 (32), 310 (19), 275 (10), 181 (15), 147 (26), 91 (100).
Methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-(1′,5′-diphenyl-1′H-pyrazol-3′-yl)propanoate (17)
To a solution of methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-4-oxo-6-phenylhex-5-enoate (12) (0.17 g, 0.46 mmol) in methanol (4 mL) was added phenylhydrazine (0.05 mL, 0.46 mmol) and 37% aqueous hydrochloric acid (0.02 mL). The reaction mixture was stirred under reflux for 17 h, cooled to room temperature and concentrated in vacuo. The resulting material was diluted with ethyl acetate (10 mL) and a saturated aqueous solution of sodium hydrogen carbonate (10 mL), extracted with ethyl acetate (3 × 10 mL), dried (MgSO4) and concentrated in vacuo. The resulting material was then dissolved in dichloromethane (30 mL) and 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (0.10 g, 0.46 mmol) was added. After stirring at room temperature for 2 h, the reaction mixture was concentrated in vacuo and the resulting solid purified by column chromatography (elution with 20–30% ethyl acetate in petroleum ether), followed by triturating with chloroform to give methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-(1′,5′-diphenyl-1′H-pyrazol-3′-yl)propanoate (17) as a colourless oil (0.16 g, 76%). νmax/cm−1 (neat) 3379 (NH), 2951 (CH), 1717 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1503 (C
O), 1503 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1375, 1207, 1051, 912, 761; [α]23D +29.2 (c 0.3, CHCl3); δH (400 MHz, CDCl3) 3.23 (1H, dd, J 14.9, 4.9 Hz, 3-HH), 3.30 (1H, dd, J 14.9, 5.8 Hz, 3-HH), 3.77 (3H, s, OCH3), 4.71–4.79 (1H, m, 2-H), 5.10 (1H, d, J 12.2 Hz, OCHHPh), 5.14 (1H, d, J 12.2 Hz, OCHHPh), 5.77 (1H, d, J 8.4 Hz, NH), 6.27 (1H, s, 4′-H), 7.15–7.36 (15H, m, 3 × Ph); δC (101 MHz, CDCl3) 30.7 (CH2), 52.5 (CH3), 53.6 (CH), 67.0 (CH2), 107.6 (CH), 125.2 (2 × CH), 127.4 (CH), 128.2 (3 × CH), 128.4 (CH), 128.5 (2 × CH), 128.6 (2 × CH), 128.8 (2 × CH), 128.9 (2 × CH), 130.5 (C), 136.5 (C), 140.0 (C), 144.0 (C), 148.3 (C), 156.1 (C), 172.1 (C); m/z (CI) 456.1925 (MH+. C27H26N3O4 requires 456.1923), 368 (9%), 348 (100), 305 (11), 257 (23), 137 (59).
C), 1375, 1207, 1051, 912, 761; [α]23D +29.2 (c 0.3, CHCl3); δH (400 MHz, CDCl3) 3.23 (1H, dd, J 14.9, 4.9 Hz, 3-HH), 3.30 (1H, dd, J 14.9, 5.8 Hz, 3-HH), 3.77 (3H, s, OCH3), 4.71–4.79 (1H, m, 2-H), 5.10 (1H, d, J 12.2 Hz, OCHHPh), 5.14 (1H, d, J 12.2 Hz, OCHHPh), 5.77 (1H, d, J 8.4 Hz, NH), 6.27 (1H, s, 4′-H), 7.15–7.36 (15H, m, 3 × Ph); δC (101 MHz, CDCl3) 30.7 (CH2), 52.5 (CH3), 53.6 (CH), 67.0 (CH2), 107.6 (CH), 125.2 (2 × CH), 127.4 (CH), 128.2 (3 × CH), 128.4 (CH), 128.5 (2 × CH), 128.6 (2 × CH), 128.8 (2 × CH), 128.9 (2 × CH), 130.5 (C), 136.5 (C), 140.0 (C), 144.0 (C), 148.3 (C), 156.1 (C), 172.1 (C); m/z (CI) 456.1925 (MH+. C27H26N3O4 requires 456.1923), 368 (9%), 348 (100), 305 (11), 257 (23), 137 (59).
Methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (18)
The reactions were carried out as described for 12 using methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(4′-nitrophenyl)-4-oxohex-5-enoate (13) (0.15 g, 0.36 mmol). Purification by column chromatography (elution with 20–40% ethyl acetate in petroleum ether) gave methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (18) as a yellow oil (0.14 g, 76%). νmax/cm−1 (neat) 3350 (NH), 2951 (CH), 1717 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597, 1502 (C
O), 1597, 1502 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1346, 1207, 1051, 972, 853; [α]23D +30.0 (c 0.3, CHCl3); δH (400 MHz, CDCl3) 3.21–3.34 (2H, m, 3-H2), 3.77 (3H, s, OCH3), 4.76 (1H, dt, J 8.2, 5.6 Hz, 2-H), 5.09 (1H, d, J 12.2 Hz, OCHHPh), 5.14 (1H, d, J 12.2 Hz, OCHHPh), 5.72 (1H, d, J 8.2 Hz, NH), 6.41 (1H, s, 4′-H), 7.18–7.23 (2H, m, ArH), 7.29–7.38 (10H, m, 2′′-H, 6′′-H and ArH), 8.14 (2H, d, J 8.7 Hz, 3′′-H and 5′′-H); δC (101 MHz, CDCl3) 30.7 (CH2), 52.7 (CH3), 53.5 (CH), 67.1 (CH2), 108.8 (CH), 123.9 (2 × CH), 125.3 (2 × CH), 128.2 (CH), 128.3 (CH), 128.3 (2 × CH), 128.6 (2 × CH), 129.4 (4 × CH), 136.4 (C), 136.6 (C), 139.5 (C), 141.6 (C), 147.5 (C), 148.9 (C), 156.1 (C), 172.0 (C); m/z (EI) 500.1695 (M+. C27H24N4O6 requires 500.1696), 441 (10%), 392 (15), 349 (100), 278 (68), 232 (22), 91 (47).
C), 1346, 1207, 1051, 972, 853; [α]23D +30.0 (c 0.3, CHCl3); δH (400 MHz, CDCl3) 3.21–3.34 (2H, m, 3-H2), 3.77 (3H, s, OCH3), 4.76 (1H, dt, J 8.2, 5.6 Hz, 2-H), 5.09 (1H, d, J 12.2 Hz, OCHHPh), 5.14 (1H, d, J 12.2 Hz, OCHHPh), 5.72 (1H, d, J 8.2 Hz, NH), 6.41 (1H, s, 4′-H), 7.18–7.23 (2H, m, ArH), 7.29–7.38 (10H, m, 2′′-H, 6′′-H and ArH), 8.14 (2H, d, J 8.7 Hz, 3′′-H and 5′′-H); δC (101 MHz, CDCl3) 30.7 (CH2), 52.7 (CH3), 53.5 (CH), 67.1 (CH2), 108.8 (CH), 123.9 (2 × CH), 125.3 (2 × CH), 128.2 (CH), 128.3 (CH), 128.3 (2 × CH), 128.6 (2 × CH), 129.4 (4 × CH), 136.4 (C), 136.6 (C), 139.5 (C), 141.6 (C), 147.5 (C), 148.9 (C), 156.1 (C), 172.0 (C); m/z (EI) 500.1695 (M+. C27H24N4O6 requires 500.1696), 441 (10%), 392 (15), 349 (100), 278 (68), 232 (22), 91 (47).
Methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-methoxyphenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (19)
The reactions were carried out as described for 12 using methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(4′-methoxyphenyl)-4-oxohex-5-enoate (14). Purification by column chromatography (elution with 20–30% ethyl acetate in petroleum ether) gave methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-methoxyphenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (19) as a colourless oil (0.07 g, 75%). νmax/cm−1 (neat) 3339 (NH), 2951 (CH), 1717 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1506 (C
O), 1506 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1498, 1436, 1248, 1176, 1027, 835; [α]26D +10.6 (c 0.5, CHCl3); δH (400 MHz, CDCl3) 3.23 (1H, dd, J 14.8, 4.9 Hz, 3-HH), 3.30 (1H, dd, J 14.8, 5.7 Hz, 3-HH), 3.78 (3H, s, OCH3), 3.81 (3H, s, OCH3), 4.72–4.78 (1H, m, 2-H), 5.11 (1H, d, J 12.3 Hz, OCHHPh), 5.15 (1H, d, J 12.3 Hz, OCHHPh), 5.80 (1H, d, J 8.3 Hz, NH), 6.22 (1H, s, 4′-H), 6.82 (2H, d, J 8.8 Hz, 3′′-H and 5′′-H), 7.11 (2H, d, J 8.8 Hz, 2′′-H and 6′′-H), 7.22–7.38 (10H, m, 2 × Ph); δC (101 MHz, CDCl3) 30.8 (CH2), 52.6 (CH3), 53.6 (CH), 55.4 (CH3), 67.0 (CH2), 107.1 (CH), 114.0 (2 × CH), 122.9 (C), 125.2 (2 × CH), 127.3 (CH), 128.2 (3 × CH), 128.6 (2 × CH), 128.9 (2 × CH), 130.1 (2 × CH), 136.5 (C), 140.1 (C), 143.9 (C), 148.2 (C), 156.1 (C), 159.7 (C), 172.2 (C); m/z (EI) 485.1961 (M+. C28H27N3O5 requires 485.1951), 426 (31%), 377 (27), 334 (100), 318 (17), 263 (61), 199 (18), 91 (33).
C), 1498, 1436, 1248, 1176, 1027, 835; [α]26D +10.6 (c 0.5, CHCl3); δH (400 MHz, CDCl3) 3.23 (1H, dd, J 14.8, 4.9 Hz, 3-HH), 3.30 (1H, dd, J 14.8, 5.7 Hz, 3-HH), 3.78 (3H, s, OCH3), 3.81 (3H, s, OCH3), 4.72–4.78 (1H, m, 2-H), 5.11 (1H, d, J 12.3 Hz, OCHHPh), 5.15 (1H, d, J 12.3 Hz, OCHHPh), 5.80 (1H, d, J 8.3 Hz, NH), 6.22 (1H, s, 4′-H), 6.82 (2H, d, J 8.8 Hz, 3′′-H and 5′′-H), 7.11 (2H, d, J 8.8 Hz, 2′′-H and 6′′-H), 7.22–7.38 (10H, m, 2 × Ph); δC (101 MHz, CDCl3) 30.8 (CH2), 52.6 (CH3), 53.6 (CH), 55.4 (CH3), 67.0 (CH2), 107.1 (CH), 114.0 (2 × CH), 122.9 (C), 125.2 (2 × CH), 127.3 (CH), 128.2 (3 × CH), 128.6 (2 × CH), 128.9 (2 × CH), 130.1 (2 × CH), 136.5 (C), 140.1 (C), 143.9 (C), 148.2 (C), 156.1 (C), 159.7 (C), 172.2 (C); m/z (EI) 485.1961 (M+. C28H27N3O5 requires 485.1951), 426 (31%), 377 (27), 334 (100), 318 (17), 263 (61), 199 (18), 91 (33).
Methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(naphthalen-2′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (20)
The reactions were carried out as described for 12 using methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(naphthalen-2′-yl)-4-oxohex-5-enoate (15) (0.15 g, 0.36 mmol). Purification by column chromatography (elution with 20% ethyl acetate in petroleum ether) gave methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(naphthalen-2′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (20) as a white solid (0.13 g, 73%). Mp 66–67 °C; νmax/cm−1 (neat) 3341 (NH), 2924 (CH), 1719 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597, 1499 (C
O), 1597, 1499 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1207, 1047, 818, 748; [α]25D +14.0 (c 0.2, CHCl3); δH (400 MHz, CDCl3) 3.27 (1H, dd, J 15.0, 4.7 Hz, 3-HH), 3.33 (1H, dd, J 15.0, 5.7 Hz, 3-HH), 3.79 (3H, s, OCH3), 4.73–4.82 (1H, m, 2-H), 5.10 (1H, d, J 12.3 Hz, OCHHPh), 5.15 (1H, d, J 12.3 Hz, OCHHPh), 5.81 (1H, d, J 8.1 Hz, NH), 6.38 (1H, s, 4′-H), 7.19 (1H, dd, J 8.5, 1.6 Hz, 3′′-H), 7.24–7.38 (10H, m, 2 × Ph), 7.46–7.53 (2H, m, 6′′-H and 7′′-H), 7.69–7.84 (4H, m, 1′′-H, 4′′-H, 5′′-H and 8′′-H); δC (101 MHz, CDCl3) 30.8 (CH2), 52.7 (CH3), 53.6 (CH), 67.1 (CH2), 108.0 (CH), 125.2 (2 × CH), 126.4 (CH), 126.7 (CH), 126.8 (CH), 127.5 (CH), 127.9 (CH), 127.9 (C), 128.1 (CH), 128.2 (CH), 128.2 (3 × CH), 128.3 (CH), 128.6 (2 × CH), 129.1 (2 × CH), 132.9 (C), 133.2 (C), 136.5 (C), 141.1 (C), 144.0 (C), 148.5 (C), 156.2 (C), 172.1 (C); m/z (EI) 505.2012 (M+. C31H27N3O4 requires 505.2002), 446 (10%), 397 (55), 354 (45), 338 (20), 283 (100), 215 (10), 108 (13), 91 (27).
C), 1207, 1047, 818, 748; [α]25D +14.0 (c 0.2, CHCl3); δH (400 MHz, CDCl3) 3.27 (1H, dd, J 15.0, 4.7 Hz, 3-HH), 3.33 (1H, dd, J 15.0, 5.7 Hz, 3-HH), 3.79 (3H, s, OCH3), 4.73–4.82 (1H, m, 2-H), 5.10 (1H, d, J 12.3 Hz, OCHHPh), 5.15 (1H, d, J 12.3 Hz, OCHHPh), 5.81 (1H, d, J 8.1 Hz, NH), 6.38 (1H, s, 4′-H), 7.19 (1H, dd, J 8.5, 1.6 Hz, 3′′-H), 7.24–7.38 (10H, m, 2 × Ph), 7.46–7.53 (2H, m, 6′′-H and 7′′-H), 7.69–7.84 (4H, m, 1′′-H, 4′′-H, 5′′-H and 8′′-H); δC (101 MHz, CDCl3) 30.8 (CH2), 52.7 (CH3), 53.6 (CH), 67.1 (CH2), 108.0 (CH), 125.2 (2 × CH), 126.4 (CH), 126.7 (CH), 126.8 (CH), 127.5 (CH), 127.9 (CH), 127.9 (C), 128.1 (CH), 128.2 (CH), 128.2 (3 × CH), 128.3 (CH), 128.6 (2 × CH), 129.1 (2 × CH), 132.9 (C), 133.2 (C), 136.5 (C), 141.1 (C), 144.0 (C), 148.5 (C), 156.2 (C), 172.1 (C); m/z (EI) 505.2012 (M+. C31H27N3O4 requires 505.2002), 446 (10%), 397 (55), 354 (45), 338 (20), 283 (100), 215 (10), 108 (13), 91 (27).
Methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(3′′′-nitrobiphen-4′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (21)
The reactions were carried out as described for 12 using methyl (2S,5E)-2-[(benzyloxycarbonyl)amino]-6-(3′′-nitrobiphen-4′-yl)-4-oxohex-5-enoate (16) (0.20 g, 0.41 mmol). Purification by column chromatography (elution with 20% ethyl acetate in petroleum ether) gave methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(3′′′-nitrobiphen-4′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (21) as a yellow oil (0.20 g, 84%). νmax/cm−1 (neat) 3359 (NH), 2922 (CH), 1716 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1508 (C
O), 1508 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1500, 1348, 1207, 1052, 803, 727; [α]23D +20.5 (c 0.4, CHCl3); δH (400 MHz, CDCl3) 3.26 (1H, dd, J 15.0, 4.9 Hz, 3-HH), 3.32 (1H, dd, J 15.0, 5.6 Hz, 3-HH), 3.79 (3H, s, OCH3), 4.73–4.81 (1H, m, 2-H), 5.11 (1H, d, J 12.3 Hz, OCHHPh), 5.15 (1H, d, J 12.3 Hz, OCHHPh), 5.78 (1H, d, J 8.3 Hz, NH), 6.36 (1H, s, 4′-H), 7.26–7.39 (12H, m, 2′′-H, 6′′-H and 2 × Ph), 7.56 (2H, d, J 8.3 Hz, 3′′-H and 5′′-H), 7.62 (1H, t, J 8.0 Hz, 5′′′-H), 7.88–7.93 (1H, m, 6′′′-H), 8.21 (1H, ddd, J 8.0, 1.9, 0.8 Hz, 4′′′-H), 8.44 (1H, t, J 1.9 Hz, 2′′′-H); δC (101 MHz, CDCl3) 30.8 (CH2), 52.7 (CH3), 53.6 (CH), 67.1 (CH2), 107.9 (CH), 122.0 (CH), 122.5 (CH), 125.3 (2 × CH), 127.3 (2 × CH), 127.8 (CH), 128.3 (3 × CH), 128.7 (2 × CH), 129.2 (2 × CH), 129.5 (2 × CH), 130.0 (CH), 130.7 (C), 133.0 (CH), 136.5 (C), 138.6 (C), 140.1 (C), 142.0 (C), 143.3 (C), 148.5 (C), 148.9 (C), 156.1 (C), 172.2 (C); m/z (EI) 576.2007 (M+. C33H28N4O6 requires 576.2009), 468 (45%), 425 (15), 409 (26), 381 (17), 354 (100), 308 (17), 202 (6), 159 (9), 77 (18).
C), 1500, 1348, 1207, 1052, 803, 727; [α]23D +20.5 (c 0.4, CHCl3); δH (400 MHz, CDCl3) 3.26 (1H, dd, J 15.0, 4.9 Hz, 3-HH), 3.32 (1H, dd, J 15.0, 5.6 Hz, 3-HH), 3.79 (3H, s, OCH3), 4.73–4.81 (1H, m, 2-H), 5.11 (1H, d, J 12.3 Hz, OCHHPh), 5.15 (1H, d, J 12.3 Hz, OCHHPh), 5.78 (1H, d, J 8.3 Hz, NH), 6.36 (1H, s, 4′-H), 7.26–7.39 (12H, m, 2′′-H, 6′′-H and 2 × Ph), 7.56 (2H, d, J 8.3 Hz, 3′′-H and 5′′-H), 7.62 (1H, t, J 8.0 Hz, 5′′′-H), 7.88–7.93 (1H, m, 6′′′-H), 8.21 (1H, ddd, J 8.0, 1.9, 0.8 Hz, 4′′′-H), 8.44 (1H, t, J 1.9 Hz, 2′′′-H); δC (101 MHz, CDCl3) 30.8 (CH2), 52.7 (CH3), 53.6 (CH), 67.1 (CH2), 107.9 (CH), 122.0 (CH), 122.5 (CH), 125.3 (2 × CH), 127.3 (2 × CH), 127.8 (CH), 128.3 (3 × CH), 128.7 (2 × CH), 129.2 (2 × CH), 129.5 (2 × CH), 130.0 (CH), 130.7 (C), 133.0 (CH), 136.5 (C), 138.6 (C), 140.1 (C), 142.0 (C), 143.3 (C), 148.5 (C), 148.9 (C), 156.1 (C), 172.2 (C); m/z (EI) 576.2007 (M+. C33H28N4O6 requires 576.2009), 468 (45%), 425 (15), 409 (26), 381 (17), 354 (100), 308 (17), 202 (6), 159 (9), 77 (18).
(2S)-2-Amino-3-(1′,5′-diphenyl-1′H-pyrazol-3′-yl)propanoic acid hydrochloride (22)
To a solution of methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-(1′,5′-diphenyl-1′H-pyrazol-3′-yl)propanoate (17) (0.09 g, 0.19 mmol) in methanol and water (5 mL, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was added cesium carbonate (0.08 g, 0.25 mmol). The reaction mixture was stirred at room temperature for 16 h before concentrating in vacuo. The resulting residue was dissolved in water (10 mL), acidified to pH 1 with 1.0 M aqueous hydrochloric acid, extracted with dichloromethane (3 × 10 mL), dried (MgSO4) and concentrated in vacuo. The resulting residue was suspended in 6.0 M aqueous hydrochloric acid (5 mL) and the mixture stirred under reflux for 24 h. The mixture was cooled and concentrated in vacuo. Trituration with diethyl ether gave (2S)-2-amino-3-(1′,5′-diphenyl-1′H-pyrazol-3′-yl)propanoic acid hydrochloride (22) as a white foam (0.05 g, 88%). νmax/cm−1 (neat) 3380 (NH), 3058 (CH), 1631 (C
3) was added cesium carbonate (0.08 g, 0.25 mmol). The reaction mixture was stirred at room temperature for 16 h before concentrating in vacuo. The resulting residue was dissolved in water (10 mL), acidified to pH 1 with 1.0 M aqueous hydrochloric acid, extracted with dichloromethane (3 × 10 mL), dried (MgSO4) and concentrated in vacuo. The resulting residue was suspended in 6.0 M aqueous hydrochloric acid (5 mL) and the mixture stirred under reflux for 24 h. The mixture was cooled and concentrated in vacuo. Trituration with diethyl ether gave (2S)-2-amino-3-(1′,5′-diphenyl-1′H-pyrazol-3′-yl)propanoic acid hydrochloride (22) as a white foam (0.05 g, 88%). νmax/cm−1 (neat) 3380 (NH), 3058 (CH), 1631 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1596, 1503 (C
O), 1596, 1503 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1424, 1375, 1112, 761; [α]28D −25.8 (c 0.4, MeOH); δH (400 MHz, CD3OD) 3.20–3.45 (2H, m, 3-H2), 4.02 (1H, br s, 2-H), 6.53 (1H, s, 4′-H), 7.17–7.40 (10H, m, 2 × Ph); δH (126 MHz, CD3OD) 30.3 (CH2), 55.7 (CH), 108.7 (CH), 126.7 (2 × CH), 129.0 (CH), 129.6 (3 × CH), 129.8 (2 × CH), 130.1 (2 × CH), 131.5 (C), 141.3 (C), 145.9 (C), 150.0 (C), 173.8 (C); m/z (ESI) 308.1382 (MH+. C18H18N3O2 requires 308.1394).
C), 1424, 1375, 1112, 761; [α]28D −25.8 (c 0.4, MeOH); δH (400 MHz, CD3OD) 3.20–3.45 (2H, m, 3-H2), 4.02 (1H, br s, 2-H), 6.53 (1H, s, 4′-H), 7.17–7.40 (10H, m, 2 × Ph); δH (126 MHz, CD3OD) 30.3 (CH2), 55.7 (CH), 108.7 (CH), 126.7 (2 × CH), 129.0 (CH), 129.6 (3 × CH), 129.8 (2 × CH), 130.1 (2 × CH), 131.5 (C), 141.3 (C), 145.9 (C), 150.0 (C), 173.8 (C); m/z (ESI) 308.1382 (MH+. C18H18N3O2 requires 308.1394).
(2S)-2-Amino-3-[5′-(4′′-methoxyphenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoic acid hydrochloride (23)
The reactions were carried out as described for 17 using methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-methoxyphenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (19) (0.07 g, 0.14 mmol). This gave (2S)-2-amino-3-[5′-(4′′-methoxyphenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoic acid hydrochloride (23) as a white foam (0.04 g, 79%). νmax/cm−1 (neat) 3359 (NH), 2921 (CH), 1729 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1611, 1506 (C
O), 1611, 1506 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1499, 1437, 1151, 834; [α]28D −25.0 (c 0.2, CHCl3); δH (500 MHz, CD3OD) 3.32 (1H, dd, J 16.0, 7.5 Hz, 3-HH), 3.42 (1H, dd, J 16.0, 4.4 Hz, 3-HH), 3.78 (3H, s, OCH3), 4.38–4.43 (1H, m, 2-H), 6.46 (1H, s, 4′-H), 6.86 (2H, d, J 8.7 Hz, 3′′-H and 5′′-H), 7.14 (2H, d, J 8.7 Hz, 2′′-H and 6′′-H), 7.27–7.42 (5H, m, Ph); δC (126 MHz, CD3OD) 29.6 (CH2), 53.7 (CH), 55.9 (CH3), 108.0 (CH), 115.1 (2 × CH), 123.6 (C), 126.6 (2 × CH), 129.0 (CH), 130.1 (2 × CH), 131.2 (2 × CH), 141.3 (C), 146.3 (C), 148.3 (C), 161.6 (C), 171.0 (C); m/z (ESI) 338.1489 (MH+. C19H20N3O3 requires 338.1499).
C), 1499, 1437, 1151, 834; [α]28D −25.0 (c 0.2, CHCl3); δH (500 MHz, CD3OD) 3.32 (1H, dd, J 16.0, 7.5 Hz, 3-HH), 3.42 (1H, dd, J 16.0, 4.4 Hz, 3-HH), 3.78 (3H, s, OCH3), 4.38–4.43 (1H, m, 2-H), 6.46 (1H, s, 4′-H), 6.86 (2H, d, J 8.7 Hz, 3′′-H and 5′′-H), 7.14 (2H, d, J 8.7 Hz, 2′′-H and 6′′-H), 7.27–7.42 (5H, m, Ph); δC (126 MHz, CD3OD) 29.6 (CH2), 53.7 (CH), 55.9 (CH3), 108.0 (CH), 115.1 (2 × CH), 123.6 (C), 126.6 (2 × CH), 129.0 (CH), 130.1 (2 × CH), 131.2 (2 × CH), 141.3 (C), 146.3 (C), 148.3 (C), 161.6 (C), 171.0 (C); m/z (ESI) 338.1489 (MH+. C19H20N3O3 requires 338.1499).
(2S)-2-Amino-3-[5′-(naphthalen-2′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoic acid hydrochloride (24)
The reactions were carried out as described for 17 using (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(naphthalen-2′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (20) (0.03 g, 0.06 mmol). This gave (2S)-2-amino-3-[5′-(naphthalen-2′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoic acid hydrochloride (24) as an off-white foam (0.02 g, 83%). νmax/cm−1 (neat) 3366 (NH), 2922 (CH), 1739 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1596 (C
O), 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1498, 1205, 1081, 819, 752; [α]28D −33.7 (c 0.3, MeOH); δH (400 MHz, CD3OD) 3.40 (1H, dd, J 15.8, 7.3 Hz, 3-HH), 3.49 (1H, br d, J 15.8 Hz, 3-HH), 4.44–4.51 (1H, m, 2-H), 6.70 (1H, s, 4′-H), 7.25 (1H, br d, J 8.2 Hz, 3′′-H), 7.32–7.42 (5H, m, Ph), 7.46–7.54 (2H, m, 6′′-H and 7′′-H), 7.72–7.87 (4H, m, 1′′-H, 4′′-H, 5′′-H and 8′′-H); δC (101 MHz, CD3OD) 29.6 (CH2), 53.5 (CH), 109.0 (CH), 126.7 (2 × CH), 127.0 (CH), 127.8 (CH), 128.1 (CH), 128.5 (C), 128.7 (CH), 129.2 (CH), 129.3 (2 × CH), 129.3 (CH) 130.2 (2 × CH), 134.4 (2 × C), 140.9 (C), 146.3 (C), 148.4 (C), 171.0 (C); m/z (ESI) 358.1541 (MH+. C22H20N3O2 requires 358.1550).
C), 1498, 1205, 1081, 819, 752; [α]28D −33.7 (c 0.3, MeOH); δH (400 MHz, CD3OD) 3.40 (1H, dd, J 15.8, 7.3 Hz, 3-HH), 3.49 (1H, br d, J 15.8 Hz, 3-HH), 4.44–4.51 (1H, m, 2-H), 6.70 (1H, s, 4′-H), 7.25 (1H, br d, J 8.2 Hz, 3′′-H), 7.32–7.42 (5H, m, Ph), 7.46–7.54 (2H, m, 6′′-H and 7′′-H), 7.72–7.87 (4H, m, 1′′-H, 4′′-H, 5′′-H and 8′′-H); δC (101 MHz, CD3OD) 29.6 (CH2), 53.5 (CH), 109.0 (CH), 126.7 (2 × CH), 127.0 (CH), 127.8 (CH), 128.1 (CH), 128.5 (C), 128.7 (CH), 129.2 (CH), 129.3 (2 × CH), 129.3 (CH) 130.2 (2 × CH), 134.4 (2 × C), 140.9 (C), 146.3 (C), 148.4 (C), 171.0 (C); m/z (ESI) 358.1541 (MH+. C22H20N3O2 requires 358.1550).
(2S)-2-Amino-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoic acid hydrochloride (25)
To a solution of methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (18) (0.08 g, 0.16 mmol) in methanol (0.5 mL) was added 6.0 M aqueous hydrochloric acid (4.5 mL). The reaction mixture was then stirred under reflux for 48 h. After cooling to room temperature, the mixture was concentrated in vacuo and triturated with diethyl ether to give (2S)-2-amino-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoic acid hydrochloride (25) as a pale yellow foam (0.06 g, 97%). νmax/cm−1 (neat) 3400 (NH), 2918 (CH), 2850, 1744 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1596 (C
O), 1596 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1515, 1502, 1344, 1255, 1080, 854; [α]27D −16.0 (c 0.3, MeOH); δH (400 MHz, CD3OD) 3.33–3.51 (2H, m, 3-H2), 4.41–4.48 (1H, m, 2-H), 6.73 (1H, s, 4′-H), 7.29–7.46 (5H, m, Ph), 7.48 (2H, d, J 7.7 Hz, 2′′-H and 6′′-H), 8.19 (2H, d, J 7.7 Hz, 3′′-H and 5′′-H); δC (101 MHz, CD3OD) 29.6 (CH2), 53.5 (CH), 109.9 (CH), 124.7 (2 × CH), 126.8 (2 × CH), 129.6 (CH), 130.4 (2 × CH), 130.8 (2 × CH), 137.5 (C), 140.6 (C), 143.8 (C), 148.7 (C), 148.9 (C), 170.9 (C); m/z (ESI) 351.1082 ([M − H]−. C18H15N4O4 requires 351.1099).
C), 1515, 1502, 1344, 1255, 1080, 854; [α]27D −16.0 (c 0.3, MeOH); δH (400 MHz, CD3OD) 3.33–3.51 (2H, m, 3-H2), 4.41–4.48 (1H, m, 2-H), 6.73 (1H, s, 4′-H), 7.29–7.46 (5H, m, Ph), 7.48 (2H, d, J 7.7 Hz, 2′′-H and 6′′-H), 8.19 (2H, d, J 7.7 Hz, 3′′-H and 5′′-H); δC (101 MHz, CD3OD) 29.6 (CH2), 53.5 (CH), 109.9 (CH), 124.7 (2 × CH), 126.8 (2 × CH), 129.6 (CH), 130.4 (2 × CH), 130.8 (2 × CH), 137.5 (C), 140.6 (C), 143.8 (C), 148.7 (C), 148.9 (C), 170.9 (C); m/z (ESI) 351.1082 ([M − H]−. C18H15N4O4 requires 351.1099).
(2S)-2-Amino-3-[5′-(3′′′-nitrobiphen-4′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoic acid hydrochloride (26)
The reaction was carried out as described for 18 using methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(3′′′-nitrobiphen-4′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (21) (0.15 g, 0.26 mmol). This gave (2S)-2-amino-3-[5′-(3′′′-nitrobiphen-4′′-yl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoic acid hydrochloride (26) as an off-white foam (0.12 g, 98%). νmax/cm−1 (neat) 3026 (NH), 2861 (CH), 1731 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1526 (C
O), 1526 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1500, 1347, 1198, 973, 803, 726; [α]28D −38.3 (c 0.3, MeOH); δH (400 MHz, CD3OD) 3.37 (1H, dd, J 15.8, 7.5 Hz, 3-HH), 3.46 (1H, dd, J 15.8, 4.6 Hz, 3-HH), 4.45 (1H, dd, J 7.5, 4.6 Hz, 2-H), 6.64 (1H, s, 4′-H), 7.33–7.45 (7H, m, 2′′-H, 6′′-H and Ph), 7.66–7.73 (3H, m, 3′′-H, 5′′-H and 5′′′-H), 8.04 (1H, ddd, J 7.8, 2.0, 0.9 Hz, 6′′′-H), 8.23 (1H, ddd, J 8.2, 2.0, 0.9 Hz, 4′′′-H), 8.46 (1H, t, J 2.0 Hz, 2′′′-H); δC (101 MHz, CD3OD) 29.6 (CH2), 53.6 (CH), 108.8 (CH), 122.5 (CH), 123.4 (CH), 126.8 (2 × CH), 128.4 (2 × CH), 129.3 (CH), 130.3 (2 × CH), 130.6 (2 × CH), 131.3 (CH), 131.5 (C), 134.1 (CH), 140.2 (C), 141.1 (C), 143.0 (C), 145.5 (C), 148.5 (C), 150.3 (C), 171.0 (C); m/z (ESI) 429.1545 (MH+. C24H21N4O4 requires 429.1557).
C), 1500, 1347, 1198, 973, 803, 726; [α]28D −38.3 (c 0.3, MeOH); δH (400 MHz, CD3OD) 3.37 (1H, dd, J 15.8, 7.5 Hz, 3-HH), 3.46 (1H, dd, J 15.8, 4.6 Hz, 3-HH), 4.45 (1H, dd, J 7.5, 4.6 Hz, 2-H), 6.64 (1H, s, 4′-H), 7.33–7.45 (7H, m, 2′′-H, 6′′-H and Ph), 7.66–7.73 (3H, m, 3′′-H, 5′′-H and 5′′′-H), 8.04 (1H, ddd, J 7.8, 2.0, 0.9 Hz, 6′′′-H), 8.23 (1H, ddd, J 8.2, 2.0, 0.9 Hz, 4′′′-H), 8.46 (1H, t, J 2.0 Hz, 2′′′-H); δC (101 MHz, CD3OD) 29.6 (CH2), 53.6 (CH), 108.8 (CH), 122.5 (CH), 123.4 (CH), 126.8 (2 × CH), 128.4 (2 × CH), 129.3 (CH), 130.3 (2 × CH), 130.6 (2 × CH), 131.3 (CH), 131.5 (C), 134.1 (CH), 140.2 (C), 141.1 (C), 143.0 (C), 145.5 (C), 148.5 (C), 150.3 (C), 171.0 (C); m/z (ESI) 429.1545 (MH+. C24H21N4O4 requires 429.1557).
(2S)-2-[(Benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propan-1-ol (27)
Methyl (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propanoate (18) (2.90 g, 5.79 mmol) was dissolved in THF (25 mL) under an atmosphere of argon at room temperature. Lithium chloride (0.61 g, 14.5 mmol) was added, followed by sodium borohydride (0.55 g, 14.5 mmol, 2.50 eq.) and the mixture was stirred for 0.1 h. Ethanol (34 mL) was added and the cloudy mixture stirred for 7 h at room temperature. The reaction mixture was cooled to 0 °C and quenched with a saturated solution of ammonium chloride (28 mL) and water (81 mL). The solution was extracted with ethyl acetate (3 × 100 mL). The combined organic phases were dried (MgSO4) and the solvent removed in vacuo. Purification by column chromatography (gradient elution with ethyl acetate in petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 7
1 to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) gave (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propan-1-ol (27) as a colourless solid (2.60 g, 95%). νmax/cm−1 (neat) 3335 (OH), 2934 (CH), 1697 (C
3) gave (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propan-1-ol (27) as a colourless solid (2.60 g, 95%). νmax/cm−1 (neat) 3335 (OH), 2934 (CH), 1697 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597, 1518, 1502, 1346, 1219, 853, 752, 696; [α]21D +11.5 (c 0.9, CDCl3); δH (500 MHz, CDCl3) 2.94 (1H, dd, J 14.8, 6.1 Hz, 3-HH), 3.00 (1H, dd, J 14.8, 6.6 Hz, 3-HH), 3.06 (1H, br s, OH), 3.62–3.76 (2H, m, 1-H2), 3.96–4.06 (1H, m, 2-H), 5.02 (1H, d, J 12.3 Hz, OCHHPh), 5.05 (1H, d, J 12.3 Hz, OCHHPh), 5.37 (1H, d, J 7.0 Hz, NH), 6.42 (1H, s, 4′-H), 7.12–7.17 (2H, m, 2′′-H and 6′′-H), 7.20–7.33 (10H, m, 2 × Ph), 8.05–8.09 (2H, m, 3′′-H and 5′′-H); δC (126 MHz, CDCl3) 30.1 (CH2), 52.2 (CH), 64.6 (CH2), 66.8 (CH2), 109.1 (CH), 123.8 (2 × CH), 125.3 (2 × CH), 128.1 (CH), 128.2 (CH), 128.3 (2 × CH), 128.5 (2 × CH), 129.2 (2 × CH), 129.4 (2 × CH), 136.4 (C), 136.4 (C), 139.2 (C), 141.6 (C), 147.4 (C), 150.2 (C), 156.5 (C); m/z (ESI) 495.1632 (MNa+. C26H24N4NaO5 requires 495.1639).
O), 1597, 1518, 1502, 1346, 1219, 853, 752, 696; [α]21D +11.5 (c 0.9, CDCl3); δH (500 MHz, CDCl3) 2.94 (1H, dd, J 14.8, 6.1 Hz, 3-HH), 3.00 (1H, dd, J 14.8, 6.6 Hz, 3-HH), 3.06 (1H, br s, OH), 3.62–3.76 (2H, m, 1-H2), 3.96–4.06 (1H, m, 2-H), 5.02 (1H, d, J 12.3 Hz, OCHHPh), 5.05 (1H, d, J 12.3 Hz, OCHHPh), 5.37 (1H, d, J 7.0 Hz, NH), 6.42 (1H, s, 4′-H), 7.12–7.17 (2H, m, 2′′-H and 6′′-H), 7.20–7.33 (10H, m, 2 × Ph), 8.05–8.09 (2H, m, 3′′-H and 5′′-H); δC (126 MHz, CDCl3) 30.1 (CH2), 52.2 (CH), 64.6 (CH2), 66.8 (CH2), 109.1 (CH), 123.8 (2 × CH), 125.3 (2 × CH), 128.1 (CH), 128.2 (CH), 128.3 (2 × CH), 128.5 (2 × CH), 129.2 (2 × CH), 129.4 (2 × CH), 136.4 (C), 136.4 (C), 139.2 (C), 141.6 (C), 147.4 (C), 150.2 (C), 156.5 (C); m/z (ESI) 495.1632 (MNa+. C26H24N4NaO5 requires 495.1639).
(2S)-2-[(Benzyloxycarbonyl)amino]-1-methanesulfonate-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propane (28)
(2S)-2-[(Benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propan-1-ol (27) (2.56 g, 5.42 mmol) was dissolved in dichloromethane (64 mL) and cooled to 0 °C. Methanesulfonyl chloride (0.63 mL, 8.13 mmol) and triethylamine (1.21 mL, 8.67 mmol) were added dropwise and the reaction mixture stirred at room temperature for 0.5 h. Dichloromethane (50 mL) was added to the reaction mixture, which was then washed with 1 M aqueous potassium hydrogensulfonate (100 mL), water (100 mL), brine (100 mL) and dried (MgSO4). The solvent was removed in vacuo and gave 28 as a colourless solid (2.72 g, 92%). νmax/cm−1 (neat) 3270 (NH), 3028 (CH), 1713 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597, 1518, 1502, 1346, 1172, 963, 750, 696; [α]23D −7.1 (c 0.8, CDCl3); δH (400 MHz, CDCl3) 2.94 (3H, s, SO2CH3), 3.00 (2H, d, J 5.1 Hz, 3-H2), 4.22–4.33 (3H, m, 1-H2 and 2-H), 5.04 (2H, s, OCH2Ph), 5.41 (1H, br s, NH), 6.44 (1H, s, 4′-H), 7.13–7.18 (2H, m, 2′′-H and 6′′-H), 7.21–7.33 (10H, m, 2 × Ph), 8.05–8.12 (2H, m, 3′′-H and 5′′-H); δC (101 MHz, CDCl3) 29.4 (CH2), 37.4 (CH3), 49.8 (CH), 67.0 (CH2), 69.6 (CH2), 109.0 (CH), 123.8 (2 × CH), 125.3 (2 × CH), 128.1 (2 × CH), 128.3 (2 × CH), 128.6 (2 × CH), 129.3 (2 × CH), 129.3 (2 × CH), 136.3 (C), 136.4 (C), 139.3 (C), 141.7 (C), 147.4 (C), 149.0 (C), 155.8 (C); m/z (ESI) 573.1389 (MNa+. C27H26N4NaO7S requires 573.1414).
O), 1597, 1518, 1502, 1346, 1172, 963, 750, 696; [α]23D −7.1 (c 0.8, CDCl3); δH (400 MHz, CDCl3) 2.94 (3H, s, SO2CH3), 3.00 (2H, d, J 5.1 Hz, 3-H2), 4.22–4.33 (3H, m, 1-H2 and 2-H), 5.04 (2H, s, OCH2Ph), 5.41 (1H, br s, NH), 6.44 (1H, s, 4′-H), 7.13–7.18 (2H, m, 2′′-H and 6′′-H), 7.21–7.33 (10H, m, 2 × Ph), 8.05–8.12 (2H, m, 3′′-H and 5′′-H); δC (101 MHz, CDCl3) 29.4 (CH2), 37.4 (CH3), 49.8 (CH), 67.0 (CH2), 69.6 (CH2), 109.0 (CH), 123.8 (2 × CH), 125.3 (2 × CH), 128.1 (2 × CH), 128.3 (2 × CH), 128.6 (2 × CH), 129.3 (2 × CH), 129.3 (2 × CH), 136.3 (C), 136.4 (C), 139.3 (C), 141.7 (C), 147.4 (C), 149.0 (C), 155.8 (C); m/z (ESI) 573.1389 (MNa+. C27H26N4NaO7S requires 573.1414).
(2S)-2-[(Benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]-1-thioacetylpropane (29)
Thioacetic acid (0.78 mL, 10.9 mmol) was added to a suspension of cesium carbonate (1.77 g, 5.43 mmol) in DMF (5 mL). This solution was added to (2S)-2-[(benzyloxycarbonyl)amino]-1-methanesulfonate-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propane (28) (2.72 g, 4.94 mmol) in DMF (10 mL) under an atmosphere of argon. The reaction mixture was stirred overnight in the dark at room temperature. Ethyl acetate (50 mL) and water (50 mL) were added and the organic layer was washed with 1 M aqueous potassium hydrogensulfate (100 mL), 1 M aqueous sodium hydrogencarbonate (100 mL), brine (100 mL) and dried (MgSO4). The solvents were evaporated and the crude product purified by column chromatography (30% ethyl acetate in petroleum ether). (2S)-2-[(Benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]-1-thioacetylpropane (29) was obtained as a slightly orange-brownish solid (2.22 g, 85%). νmax/cm−1 (neat) 3355 (NH), 3075 (CH), 1694 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597, 1518, 1503, 1347, 1252, 1133, 853, 754; [α]21D −3.2 (c 0.7, CDCl3); δH (500 MHz, CDCl3) 2.27 (3H, s, COCH3), 2.94 (2H, d, J 6.2 Hz, 3-H2), 3.06–3.18 (2H, m, 1-H2), 4.07–4.18 (1H, m, 2-H), 5.01 (2H, s, OCH2Ph), 5.41 (1H, d, J 8.2 Hz, NH), 6.42 (1H, s, 4′-H), 7.14–7.18 (2H, m, 2′′-H and 6′′-H), 7.20–7.33 (10H, m, 2 × Ph), 8.04–8.10 (2H, m, 3′′-H and 5′′-H); δC (101 MHz, CDCl3) 30.6 (CH3), 32.5 (CH2), 33.1 (CH2), 51.0 (CH), 66.6 (CH2), 108.9 (CH), 123.8 (2 × CH), 125.3 (2 × CH), 128.0 (CH), 128.1 (CH), 128.1 (2 × CH), 128.5 (2 × CH), 129.2 (2 × CH), 129.3 (2 × CH), 136.6 (C), 139.4 (C), 141.5 (2 × C), 147.3 (C), 149.7 (C), 156.0 (C), 195.7 (C); m/z (ESI) 553.1502 (MNa+. C28H26N4NaO5S requires 553.1516).
O), 1597, 1518, 1503, 1347, 1252, 1133, 853, 754; [α]21D −3.2 (c 0.7, CDCl3); δH (500 MHz, CDCl3) 2.27 (3H, s, COCH3), 2.94 (2H, d, J 6.2 Hz, 3-H2), 3.06–3.18 (2H, m, 1-H2), 4.07–4.18 (1H, m, 2-H), 5.01 (2H, s, OCH2Ph), 5.41 (1H, d, J 8.2 Hz, NH), 6.42 (1H, s, 4′-H), 7.14–7.18 (2H, m, 2′′-H and 6′′-H), 7.20–7.33 (10H, m, 2 × Ph), 8.04–8.10 (2H, m, 3′′-H and 5′′-H); δC (101 MHz, CDCl3) 30.6 (CH3), 32.5 (CH2), 33.1 (CH2), 51.0 (CH), 66.6 (CH2), 108.9 (CH), 123.8 (2 × CH), 125.3 (2 × CH), 128.0 (CH), 128.1 (CH), 128.1 (2 × CH), 128.5 (2 × CH), 129.2 (2 × CH), 129.3 (2 × CH), 136.6 (C), 139.4 (C), 141.5 (2 × C), 147.3 (C), 149.7 (C), 156.0 (C), 195.7 (C); m/z (ESI) 553.1502 (MNa+. C28H26N4NaO5S requires 553.1516).
(2S)-2-[(Benzyloxycarbonyl)amino]-1-fluorosulfonyl-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propane (31)
(2S)-2-[(Benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]-1-thioacetylpropane (29) (2.22 g, 4.18 mmol) was dissolved in acetic acid (15 mL) and 30% hydrogen peroxide (4.8 mL) was added and stirred overnight at room temperature. Sodium acetate (0.34 g, 4.18 mmol) was added and the mixture stirred for 1 h. DMF (5 mL) was added and the solution was partially concentrated in vacuo. This procedure was repeated three more times until the DMF was removed completely. After co-evaporation twice with water and three times with toluene, sodium (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propane-1-sulfonate was obtained as a yellow solid (2.37 g, 100%). A quantity of (2S)-2-[(benzyloxycarbonyl)amino]-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propane-1-sulfonate (0.20 g, 0.36 mmol) was dissolved in dichloromethane (15 mL) under an atmosphere of argon and XtalFluor-M® (30) (0.16 g, 644 μmol) and triethylamine trihydrofluoride (2.5 μL, 15.4 μmol) were added. The reaction mixture was heated under reflux for 20 h. Silica was added to quench the reaction and the solvent removed in vacuo. Purification by column chromatography (40% ethyl acetate in petroleum ether) gave (2S)-2-[(benzyloxycarbonyl)amino]-1-fluorosulfonyl-3-[5′-(4′′-nitrophenyl)-1′-phenyl-1′H-pyrazol-3′-yl]propane (31) as a yellow solid (0.123 g, 64%). νmax/cm−1 (neat) 3337 (NH), 2930 (CH), 1699 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598, 1538, 1515, 1407, 1388, 1347, 1265, 1195, 1050, 973, 854; [α]20D +5.0 (c 1.0, CDCl3); δH (400 MHz, CDCl3) 3.10–3.24 (2H, m, 3-H2), 3.70 (1H, dt, J 14.6, 5.7 Hz, 1-HH), 3.91 (1H, dd, J 14.6, 5.7 Hz, 1-HH), 4.52–4.63 (1H, m, 2-H), 5.06 (2H, s, OCH2Ph), 5.73 (1H, d, J 8.0 Hz, NH), 6.43 (1H, s, 4′-H), 7.13–7.18 (2H, m, 2′′-H and 6′′-H), 7.23–7.37 (10H, m, 2 × Ph), 8.06–8.12 (2H, m, 3′′-H and 5′′-H); δC (126 MHz, CDCl3) 31.2 (CH2), 46.9 (CH), 53.2 (CH2, d, JC–S–F 15.1 Hz), 67.2 (CH2), 109.3 (CH), 123.8 (2 × CH), 125.2 (2 × CH), 128.2 (2 × CH), 128.3 (CH), 128.4 (CH), 128.6 (2 × CH), 129.3 (2 × CH), 129.4 (2 × CH), 136.0 (C), 136.1 (C), 139.1 (C), 141.8 (C), 147.5 (C), 148.4 (C), 155.4 (C); δF (377 MHz, CDCl3) 60.2 (1F, s, SO2F); m/z (ESI) 561.1192 (MNa+. C26H23FN4NaO6S requires 561.1215).
O), 1598, 1538, 1515, 1407, 1388, 1347, 1265, 1195, 1050, 973, 854; [α]20D +5.0 (c 1.0, CDCl3); δH (400 MHz, CDCl3) 3.10–3.24 (2H, m, 3-H2), 3.70 (1H, dt, J 14.6, 5.7 Hz, 1-HH), 3.91 (1H, dd, J 14.6, 5.7 Hz, 1-HH), 4.52–4.63 (1H, m, 2-H), 5.06 (2H, s, OCH2Ph), 5.73 (1H, d, J 8.0 Hz, NH), 6.43 (1H, s, 4′-H), 7.13–7.18 (2H, m, 2′′-H and 6′′-H), 7.23–7.37 (10H, m, 2 × Ph), 8.06–8.12 (2H, m, 3′′-H and 5′′-H); δC (126 MHz, CDCl3) 31.2 (CH2), 46.9 (CH), 53.2 (CH2, d, JC–S–F 15.1 Hz), 67.2 (CH2), 109.3 (CH), 123.8 (2 × CH), 125.2 (2 × CH), 128.2 (2 × CH), 128.3 (CH), 128.4 (CH), 128.6 (2 × CH), 129.3 (2 × CH), 129.4 (2 × CH), 136.0 (C), 136.1 (C), 139.1 (C), 141.8 (C), 147.5 (C), 148.4 (C), 155.4 (C); δF (377 MHz, CDCl3) 60.2 (1F, s, SO2F); m/z (ESI) 561.1192 (MNa+. C26H23FN4NaO6S requires 561.1215).
Acknowledgements
Financial support from SINAPSE (studentship to LG), the University of Glasgow (studentship to RA) and the EPSRC (studentship to AHH, EP/K503058) is gratefully acknowledged.Notes and references
- For reviews see: (a) I. Wagner and H. Musso, Angew. Chem., Int. Ed. Engl., 1983, 22, 816 CrossRef; (b) J. P. Greenstein and M. Winitz, in Chemistry of the Amino Acids, R. E. Krieger, FL, 1984, vol. 1–3 Search PubMed; (c) Chemistry and Biochemistry of the Amino Acids, ed. G. C. Barrett, Chapman and Hall, London, 1985 Search PubMed; (d) M. J. O'Donnell, α-Amino Acid Synthesis, Tetrahedron, 1988, 44, 5253 CrossRef; (e) R. M. Williams, Synthesis of Optically Active α-Amino Acids, Pergamon Press, Oxford, 1989, vol. 7 Search PubMed.
- (a) Y. Ohfune, Acc. Chem. Res., 1992, 25, 360 CrossRef CAS; (b) A. Sutherland and C. L. Willis, Nat. Prod. Rep., 2000, 17, 621 RSC; (c) D. A. Dougherty, Curr. Opin. Chem. Biol., 2000, 4, 645 CrossRef CAS; (d) Y. Ohfune and T. Shinada, Eur. J. Org. Chem., 2005, 5127 CrossRef CAS; (e) H. Vogt and S. Bräse, Org. Biomol. Chem., 2007, 5, 406 RSC; (f) R. Smits, C. D. Cadicamo, K. Burger and B. Koksch, Chem. Soc. Rev., 2008, 37, 1727 RSC; (g) M. Mortensen, R. Husmann, E. Veri and C. Bolm, Chem. Soc. Rev., 2009, 38, 1002 RSC; (h) K. Lang and J. W. Chin, Chem. Rev., 2014, 114, 4764 CrossRef CAS PubMed.
- For example, see: (a) C. J. Easton, Chem. Rev., 1997, 97, 53 CrossRef CAS PubMed; (b) C. Nájera and J. M. Sansano, Chem. Rev., 2007, 107, 4584 CrossRef PubMed; (c) A. F. M. Noisier and M. A. Brimble, Chem. Rev., 2014, 114, 8775 CrossRef CAS PubMed.
- (a) P. Kolar, A. Petrič and M. Tišler, J. Heterocycl. Chem., 1997, 34, 1067 CrossRef CAS; (b) P. Singh, K. Samanta, S. K. Das and G. Panda, Org. Biomol. Chem., 2014, 12, 6297 RSC.
- (a) S. Shinano and T. Kaya, J. Agric. Chem. Soc. Jpn., 1957, 31, 759 CAS; (b) C. N. Farthing, J. E. Baldwin, A. T. Russell, C. J. Schofield and A. C. Spivey, Tetrahedron Lett., 1996, 37, 5225 CrossRef CAS.
- C. G. Jørgensen, H. Bräuner-Osborne, B. Nielsen, J. Kehler, R. P. Clausen, P. Krogsgaard-Larsen and U. Madsen, Bioorg. Med. Chem., 2007, 15, 3524 CrossRef PubMed.
- (a) P. Conti, G. Grazioso, S. J. Di Ventimiglia, A. Pinto, G. Roda, U. Madsen, H. Bräuner-Osborne, B. Nielsen, C. Costagli and A. Galli, Chem. Biodiversity, 2005, 2, 748 CrossRef CAS PubMed; (b) P. Conti, A. Pinto, L. Tamborini, U. Madsen, B. Nielsen, H. Bräuner-Osborne, K. B. Hansen, E. Landucci, D. E. Pellegrini-Giampietro, G. De Sarro, E. D. Di Paola and C. De Micheli, ChemMedChem, 2010, 5, 1465 CrossRef CAS PubMed.
- M. D. Joksović, V. Marković, Z. D. Juranić, T. Stanojković, L. S. Jovanović, I. S. Damljanović, K. M. Szécsényi, N. Todorović, S. Trifunović and R. D. Vukićević, J. Organomet. Chem., 2009, 694, 3935 CrossRef PubMed.
- (a) L. De Luca, M. Falorni, G. Giacomelli and A. Porcheddu, Tetrahedron Lett., 1999, 40, 8701 CrossRef CAS; (b) L. De Luca, G. Giacomelli, A. Porcheddu, A. M. Spanedda and M. Falorni, Synthesis, 2000, 1295 CrossRef CAS PubMed.
- For the synthesis of optically active pyrazole containing α-amino acids, see: (a) L. Wei and W. D. Lubell, Can. J. Chem., 2001, 79, 94 CrossRef CAS; (b) U. Ursic, D. Bevk, S. Pirc, L. Pezdirc, B. Stanovnik and J. Svete, Synthesis, 2006, 2376 CAS.
- For the synthesis of racemic α-amino acids bearing a pyrazole side-chain, see: (a) A. Prešeren, J. Svete and B. Stanovnik, J. Heterocycl. Chem., 1999, 36, 799 CrossRef; (b) A. de la Hoz, A. Díaz-Ortiz, M. V. Gómez, J. A. Mayoral, A. Moreno, A. M. Sánchez-Migallón and E. Vázquez, Tetrahedron, 2001, 57, 5421 CrossRef CAS; (c) P. Calí and M. Begtrup, Tetrahedron, 2002, 58, 1595 CrossRef; (d) L. Vraničar, F. Požgan, S. Polanc and M. Kočevar, Amino Acids, 2003, 24, 273 CrossRef PubMed; (e) A. Heim-Riether, Synthesis, 2008, 883 CrossRef CAS PubMed.
- L. S. Fowler, D. Ellis and A. Sutherland, Org. Biomol. Chem., 2009, 7, 4309 CAS.
- (a) L. S. Fowler, L. H. Thomas, D. Ellis and A. Sutherland, Chem. Commun., 2011, 47, 6569 RSC; (b) M. Daly, A. A. Cant, L. S. Fowler, G. L. Simpson, H. M. Senn and A. Sutherland, J. Org. Chem., 2012, 77, 10001 CrossRef CAS PubMed.
- D. E. Rudisill and J. P. Whitten, Synthesis, 1994, 851 CrossRef CAS PubMed.
- C. M. Reid, K. N. Fanning, L. S. Fowler and A. Sutherland, Tetrahedron, 2015, 71, 245 CrossRef CAS PubMed.
- For reviews of 2-pyrazoline formation from enones, see: (a) A. Lévai, J. Heterocycl. Chem., 2002, 39, 1 CrossRef; (b) A. Lévai, J. Heterocycl. Chem., 2004, 41, 299 CrossRef.
- S. Duggineni, D. Sawant, B. Saha and B. Kundu, Tetrahedron, 2006, 62, 3228 CrossRef CAS PubMed.
- For reviews on fluorescent α-amino acids, see: (a) A. R. Katritzky and T. Narindoshvili, Org. Biomol. Chem., 2009, 7, 627 RSC; (b) M. S. T. Gonçalves, Chem. Rev., 2009, 109, 190 CrossRef PubMed.
- G. D. Fasman, Handbook of Biochemistry and Molecular Biology, Proteins, CRC, Boca Raton, Florida, 1976, vol. I Search PubMed.
- The 3-nitrobiphenyl analogue 26 was found to have a emission maximum at 434 nm, which is potentially most suitable for application in proteins and peptides containing fluorescent proteinogenic α-amino acids such as tyrosine and tryptophan. However, 26 produced the weakest emission maximum of the five pyrazole α-amino acids and so was not considered further.
- A. J. Brouwer, T. Ceylan, T. van der Linden and R. M. J. Liskamp, Tetrahedron Lett., 2009, 50, 3391 CrossRef CAS PubMed.
- (a) A. J. Brouwer, T. Ceylan, A. M. Jonker, T. van der Linden and R. M. J. Liskamp, Bioorg. Med. Chem., 2011, 19, 2397 CrossRef CAS PubMed; (b) A. J. Brouwer, A. Jonker, P. Werkhoven, E. Kuo, N. Li, N. Gallasteugi, J. Kemmink, B. I. Florea, M. Groll, H. S. Overkleeft and R. M. J. Liskamp, J. Med. Chem., 2012, 55, 10995 CrossRef CAS PubMed; (c) C. Dubiella, H. Cui, M. Gersch, A. J. Brouwer, S. A. Sieber, A. Krüger, R. M. J. Liskamp and M. Groll, Angew. Chem., Int. Ed., 2014, 53, 11969 CrossRef CAS PubMed; (d) O. Vosyka, K. R. Vinothkumar, E. V. Wolf, A. J. Brouwer, R. M. J. Liskamp and S. H. L. Verhelst, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 2472 CrossRef CAS PubMed.
- S. Tschan, A. J. Brouwer, P. R. Werkhoven, A. M. Jonker, L. Wagner, S. Knittel, M. N. Aminake, G. Pradel, F. Joanny, R. M. J. Liskamp and B. Mordmüller, Antimicrob. Agents Chemother., 2013, 57, 3576 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and data for all known compounds and NMR spectra for all new compounds. See DOI: 10.1039/c5ob00364d |
| This journal is © The Royal Society of Chemistry 2015 |