Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Enhancement of fluorescent properties of near-infrared dyes using clickable oligoglycerol dendrons†
Orit
Redy-Keisar
a,
Katharina
Huth
b,
Uwe
Vogel
c,
Bernd
Lepenies
c,
Peter H.
Seeberger
c,
Rainer
Haag
b and
Doron
Shabat
*a
aSchool of Chemistry, Raymond and Beverly Sackler Faculty of Exact Sciences, Tel-Aviv University, Tel Aviv, 69978 Israel. E-mail: chdoron@post.tau.ac.il; Fax: +972 (0) 3 640 9293; Tel: +972 (0) 3 640 8340
bInstitute of Chemistry and Biochemistry, Organic Chemistry, Freie Universität Berlin Takustr. 3, 14195 Berlin, Germany
cMax Planck Institute of Colloids and Interfaces, Institute of Chemistry and Biochemistry, Department of Biology, Chemistry, and Pharmacy, Free University Berlin, Arnimallee 22, 14195 Berlin-Dahlem, Germany
First published on 16th March 2015
Abstract
Near-infrared (NIR) fluorescent dyes are gaining increased attention due to their potential to serve as molecular probes for in vivo imaging. Here, we demonstrate that oligoglycerol dendrons effectively enhance the fluorescence properties of an NIR dye by increasing the solubility in water and the prevention of aggregate formation. First- and second-generation oligoglycerol dendrons were conjugated to an NIR dye via a dipolar-cycloaddition (click) reaction. The two new dye conjugates exhibited enhanced NIR fluorescent emission and considerably higher fluorescent quantum yields than the dye alone. The high photostability measured for one of the oligoglycerol-linked dyes, in comparison to commonly used fluorogenic dyes such as Cy5 and Cy7, was validated using fluorescence microscopy of macrophages.
Fluorescence imaging is increasingly used in the chemical, biological, and medical sciences1–3 and drives a growing interest in near-infrared (NIR) fluorophores as imaging probes.4,5 Fluorophores that emit in the NIR region have some advantages for in vivo applications compared to shorter wavelength fluorophores including deeper penetration through organic tissues and higher signal-to-noise ratios due to lower autofluorescence background. To be useful in vivo, fluorophores must be water-soluble and physiologically stable. In addition, they should have a high fluorescent quantum yield, large Stokes shift, and good photostability.
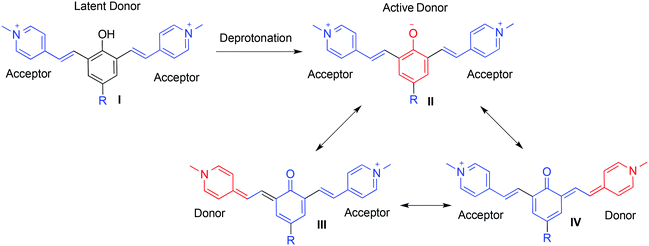
In order to image alterations of fluorescent signals using molecular probes, a turn-ON mechanism is usually employed. In cyanines, a commonly used family of NIR dyes, turn-ON is often achieved using a fluorescence resonance energy transfer (FRET) process. Recently, we reported on a novel strategy for the design of long-wavelength fluorogenic probes with a turn-ON option.6 The design is based on a donor-two-acceptor π-electron cyanine dye that can undergo an intramolecular charge transfer to form a new fluorochrome with an extended π–conjugated system. This strategy was translated to the synthesis of a library of dyes with fluorescence emission in the near-infrared (NIR) region.7,8 A representative example for the design of a donor-two-acceptor dye system is illustrated in Fig. 1. The dye is composed of a protected phenol moiety (I) that functions as a latent donor conjugated with two picolinium acceptors. Deprotonation of the phenol leads to the formation of a phenolate active donor II that is able to donate a pair of π-electrons to either one of the conjugated picolinium acceptors (structures III and IV). This intramolecular charge transfer generates a resonance species with a π-electron pattern similar to that of a cyanine fluorochrome.9 The donor capability of the phenolate species II can be masked by a proton or a specific protecting group. This type of protected phenol can be used as a molecular probe for detection or imaging of an analyte that reacts with the probe to remove the protecting group.10
Based on this design, a series of donor-two acceptor dyes were synthesized and their spectroscopic properties were evaluated.7 Some of the dyes exhibited very good fluorescence properties, whereas others showed relatively low fluorescence in the NIR region. The low fluorescence was attributed to poor water solubility and the formation of aggregates. The Haag oligoglycerol dendrons11–15 enhance water solubility and reduce the aggregation of hydrophobic perylenediimide dyes.16,17 In the present work we sought to study the effect of oligoglycerol dendrons on one of our donor-two-acceptor dyes that exhibits moderate fluorescence properties.
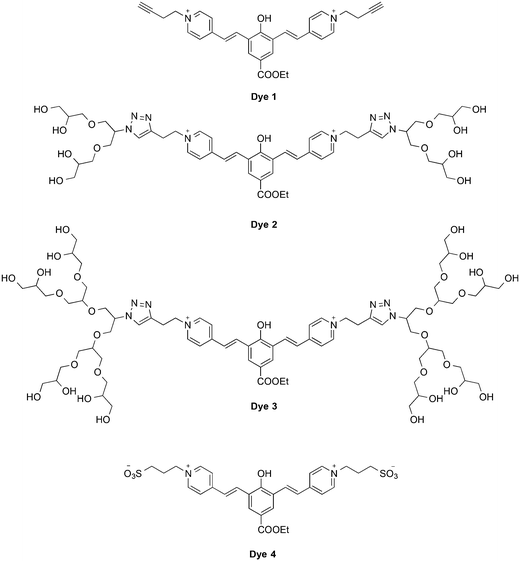
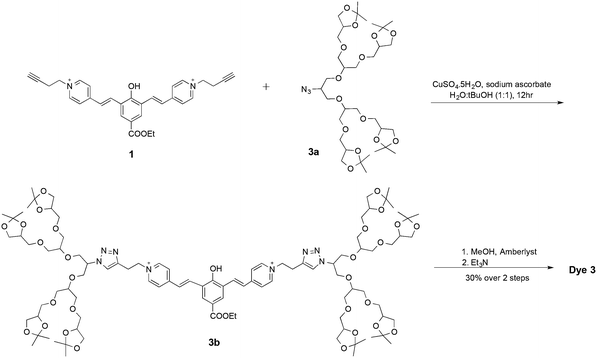
To evaluate the influence of the oligoglycerol dendrons on the fluorescence properties of our cyanine based dyes, we chose dye 1 as a study-model. The dye is equipped with two acetylene handles on the picolinium acceptors, ready for click-conjugation18 with suitable azide derivatives of oligoglycerol-dendrons (Fig. 2). Dye 2 and 3 were obtained following a click reaction with [G1]-oligoglycerol dendron and [G2]-oligoglycerol dendron correspondingly. To better understand the effect of the oligoglycerol dendrons on the hydrophobic dye, we also prepared a sulfonated dye-derivative (dye 4). Since sulfonate groups are commonly incorporated into non-polar compounds to increase their aqueous solubility,19 we were interested in the relative effects of the sulfonate and the oligoglycerol dendrons.
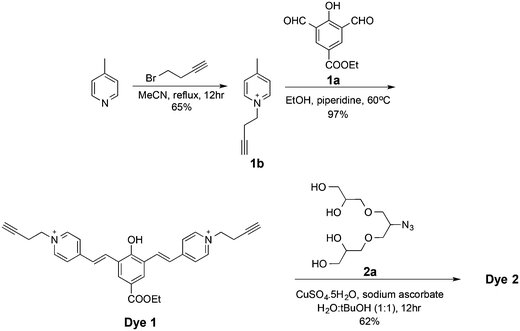
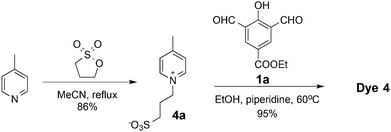
The syntheses of dyes 1, 2, and 3 were achieved as illustrated in Fig. 3 and 4. Alkylation of 4-picoline with 4-bromobut-1-yne gave picolinium 1b, which was then condensed with dialdehyde 1a to afford dye 1. Click reaction of two eq. of oligoglycerol dendron 2a with dye 1 afforded dye 2 (Fig. 3). Similarly, dye 3 was obtained by a click reaction of dye 1 with two eq. of oligoglycerol dendron 3a. A simple deprotection step yielded the desired dye.
Dye 4 was synthesized starting from alkylation of 4-picoline with 1,3-propane sultone to give picolinium 4a. Condensation of two eq. of picolinium 4a with dialdehyde 1a gave dye 4 (Fig. 5).
With dyes 1–4 in hand, we measured the absorbance and fluorescence spectra (Fig. 6). The spectroscopic data are summarized in Table 1. The absorbance spectra of the four dyes were almost identical with a maximum absorbance wavelength of 500 nm (Fig. 6A). Similarly, all four dyes exhibited fluorescence spectra with an emission wavelength at 700 nm. The fluorescence intensity of dye 3, bearing two [G2]-oligoglycerol dendrons, was significantly higher than that of the other three dyes. The fluorescence intensity of dye 2, bearing two [G1]-oligoglycerol dendrons was lower than that of dye 3, but was still considerably higher than that of core-dye 1. Interestingly, whereas the addition of oligoglycerol dendrons on dye 1 resulted in a dramatic enhancement of fluorescence, the introduction of sulfonate groups on the same core structure (as in dye 4), failed to provide a similar effect (Fig. 6B). Dye 4 showed fluorescence intensity values slightly higher than that of unsubstituted dye 1 but significantly inferior to dendronized dyes 2 and 3. The attachment of [G2]-oligoglycerol dendron to dye 1 resulted in a remarkable increase of the fluorescence quantum yield from 4.9% to 12.7%. In contrast, the addition of the sulfonate groups had little effect on the quantum yield. The advantage of the oligoglycerol dendron to increase the fluorescent quantum yield can be attributed to their bulky volume, which sterically shields the aromatic fluorophore to prevent aggregation. These observations confirm the superiority of the oligoglycerol dendron relative to the sulfonate to enhance fluorescent properties.
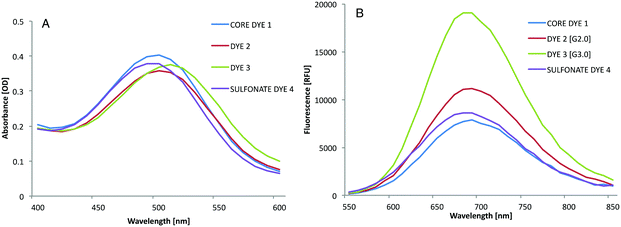 | ||
| Fig. 6 (A) Absorption spectra of dyes 1–4 in PBS (concentration: 50 μM, pH 7.4). (B) NIR fluorescence spectra of dyes 1–4 in PBS (concentration: 50 μM, pH 7.4) (λex = 500 nm). | ||
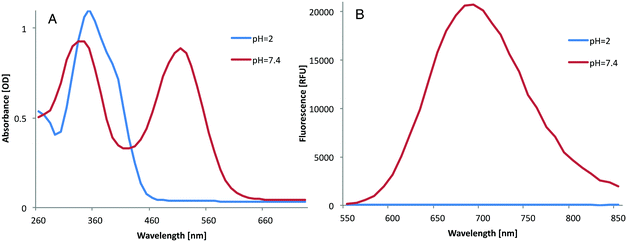
The structural properties of the dye molecule result in a turn-ON fluorescence signal upon deprotonation of the phenolic functional group (Fig. 1). Thus, while the phenol is protonated, the intramolecular charge transfer is blocked and the fluorescence of the dye is OFF. To demonstrate this effect, we focused on dye 3 and measured its UV-Vis and fluorescence spectra under acidic and neutral conditions (Fig. 7).
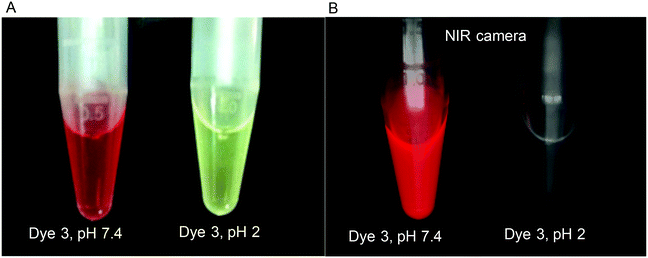
At pH 2, the dye is protonated and showed no emission in the NIR region. However, when the pH was increased to 7.4, the UV-Vis-spectrum was red-shifted and strong NIR fluorescence emission was observed. These color changes between the protonated and deprotonated forms of the dye are clearly shown in Fig. 8A. The yellow color observed at pH 2 indicates that the dye is in its phenol form, whereas the red color observed at pH 7.4 indicates that the dye is in its quinone form. The turn-ON fluorescence signal of the dye produced by the increase of the pH can be imaged under an NIR camera (Fig. 8B).
The pKa of dye 3 was determined by measuring the emitted fluorescence at different pH values (see ESI†). Dye 3 was found to have a pKa value of 3.9, indicating that under physiological conditions (pH 7.4) and also in the acidic pH of the lysosome (pH ∼5), the dye will exist in its fluorescent quinone form. Since the change in the spectral properties of the dye is reversible and controlled completely by environmental pH, such dyes can be utilized as pH probes in the range of their pKa value. In its deprotonated fluorescence form, the dye exhibits a Stokes shift of 200 nm between absorption and emission peaks. This relatively large Stokes shift is yet another favorable feature as this indicates little potential for self-quenching of the fluorescence signal.
Photostability is an important factor of a dye, especially when it is considered for use as an in vivo imaging probe. Thus, we evaluated the photostability of dye 3 in comparison to that of Cy5 and Cy7, commonly used NIR fluorophores. The dyes were dissolved in PBS buffer and irradiated for 50 minutes. The fluorescence intensity values of the dyes were recorded during the irradiation time and plotted as a function of time (Fig. 9). The fluorescence emission intensity of Cy5 and Cy7 decreased relatively rapidly; dye 3 was significantly more photostable. Although a quantitative comparison is impossible due to the different excitation wavelenghts, this finding clearly demonstrates the high photostability of dye 3.
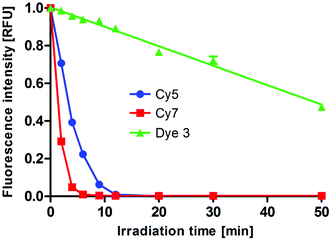 | ||
| Fig. 9 Photobleaching of dye 3, Cy5, and Cy7 in solution: fluorescence intensity [RFU] emitted upon irradiation of 100 μM Dye 3 (λex = 500 nm, λem = 700 nm), Cy5 (λex = 650 nm, λem = 670 nm), and Cy7 (λex = 740 nm, λem = 760 nm) in PBS (concentration: 50 μM, pH 7.4) as a function of time. The compounds were irradiated using a UV crosslinker as described in the ESI.† The fluorescence intensity of each dye was normalized to the intensity at the beginning of the experiment. Data are representative of three independent experiments. | ||
To further explore the photostability of dye 3 and its compatibility with in-cell assays, we compared the photobleaching of dye 3 and of the Cy7 fluorophore after uptake into macrophage cells (Fig. 10). The Cy7 fluorescence decreased with each laser scan. The fluorescence intensity of dye 3 was only slightly reduced after 10 laser scans. This observation provides additional indication for the potential of dye 3 as a suitable fluorescence probe for biological assays.
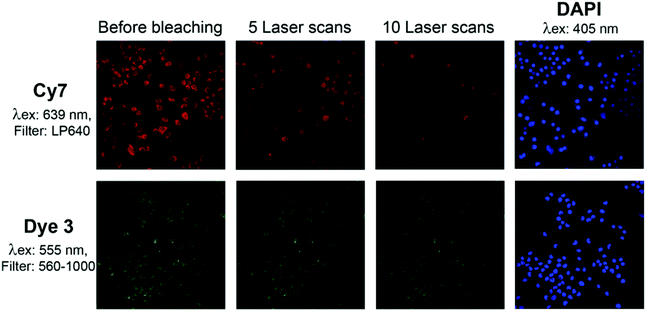 | ||
| Fig. 10 Photobleaching of dye 3 and Cy7 after uptake into macrophages: following incubation with the fluorescent dyes, the cells were fixed with paraformaldehyde, quenched with glycine buffer, and stained with DAPI (4′,6-diamidino-2-phenylindole) as described in the ESI.† Photobleaching was induced by repeated scanning. The following laser/filter combinations were used: DAPI: Laser 405 nm, Filter 420–1000; Dye 3: Laser 555 nm, Filter 560–1000; Cy7: Laser 639 nm, Filter LP 640. | ||
In summary, we have demonstrated that oligoglycerol dendrons can be incorporated into NIR dye molecules to significantly enhance fluorescent properties. Conjugation of the dye to a first-generation dendron resulted in a moderate increase of the fluorescent quantum yield compared to the dye alone; the second-generation dendron conjugation produced a dye derivative with considerably higher fluorescent emission. In addition, the oligoglycerol dendrons exhibited superior ability to prevent aggregate formation of hydrophobic dye when evaluated versus traditional sulfonate functional groups. The oligoglycerol-NIR-dye conjugate showed high photostability in comparison to commonly used fluorogenic dyes such as Cy5 and Cy7. The remarkable stability of the dye to photobleaching was visually demonstrated in imaging of macrophages. We anticipate that the clickable oligoglycerol approach described here will enhance the fluorescent properties other hydrophobic aromatic dyes as well.
Acknowledgements
D. S. thanks the Israel Science Foundation (ISF), and the German Israeli Foundation (GIF) for financial support. This work was partially supported by grants from the Israeli National Nanotechnology Initiative (INNI), Focal Technology Area (FTA) program: Nanomedicine for Personalized Theranostics, and by The Leona M. and Harry B. Helmsley Nanotechnology Research Fund. P. H. S. acknowledges gratefully generous funding by the Max-Planck Society. Special thanks go to Shiran Farber and Ronit Satchi-Fainaro for assistance to produce the NIR image in Fig. 8.References
- M. C. Chang, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2004, 126, 15392–15393 CrossRef CAS PubMed.
- X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed.
- R. Weissleder, M. Nahrendorf and M. J. Pittet, Nat. Mater., 2014, 13, 125–138 CrossRef CAS PubMed.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- E. Kisin-Finfer, S. Ferber, R. Blau, R. Satchi-Fainaro and D. Shabat, Bioorg. Med. Chem. Lett., 2014, 24, 2453–2458 CrossRef CAS PubMed.
- N. Karton-Lifshin, E. Segal, L. Omer, M. Portnoy, R. Satchi-Fainaro and D. Shabat, J. Am. Chem. Soc., 2011, 133, 10960–10965 CrossRef CAS PubMed.
- N. Karton-Lifshin, L. Albertazzi, M. Bendikov, P. S. Baran and D. Shabat, J. Am. Chem. Soc., 2012, 134, 20412–20420 CrossRef CAS PubMed.
- E. Kisin-Finfer and D. Shabat, Bioorg. Med. Chem., 2013, 21, 3602–3608 CrossRef CAS PubMed.
- S. Gnaim and D. Shabat, Acc. Chem. Res., 2014, 47, 2970–2984 CrossRef CAS PubMed.
- O. Redy-Keisar, E. Kisin-Finfer, S. Ferber, R. Satchi-Fainaro and D. Shabat, Nat. Protocols, 2014, 9, 27–36 CAS.
- M. Wyszogrodzka and R. Haag, Chem. – Eur. J., 2008, 14, 9202–9214 CrossRef CAS PubMed.
- M. Wyszogrodzka, K. Mows, S. Kamlage, J. Wodzifiska, B. Plietker and R. Haag, Eur. J. Org. Chem., 2008, 53–63 CrossRef CAS.
- S. K. Yang, X. H. Shi, S. Park, S. Doganay, T. Ha and S. C. Zimmerman, J. Am. Chem. Soc., 2011, 133, 9964–9967 CrossRef CAS PubMed.
- S. K. Yang, X. H. Shi, S. Park, T. Ha and S. C. Zimmerman, Nat. Chem., 2013, 5, 692–697 CrossRef CAS PubMed.
- A. T. Zill, K. Licha, R. Haag and S. C. Zimmerman, New J. Chem., 2012, 36, 419–427 RSC.
- T. Heek, C. Fasting, C. Rest, X. Zhang, F. Wurthner and R. Haag, Chem. Commun., 2010, 46, 1884–1886 RSC.
- T. Heek, F. Wurthner and R. Haag, Chem. – Eur. J., 2013, 19, 10911–10921 CrossRef CAS PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- R. B. Mujumdar, L. A. Ernst, S. R. Mujumdar, C. J. Lewis and A. S. Waggoner, Bioconjugate Chem., 1993, 4, 105–111 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ob00299k |
| This journal is © The Royal Society of Chemistry 2015 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 586
586