Open Access Article
Open Access ArticleHarmony of CdI2 with CuBr for the one-pot synthesis of optically active α-allenols†
Jiasheng
Zhang
a,
Juntao
Ye
a and
Shengming
Ma
*ab
aState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, P. R. China. E-mail: masm@sioc.ac.cn; Fax: (+86)-21-64167510
bDepartment of Chemistry, Fudan University, 220 Handan Road, Shanghai 200322, P. R. China
First published on 3rd February 2015
Abstract
A highly efficient one-pot synthesis of chiral α-allenols from propargylic alcohols, aldehydes and pyrrolidine induced by CuBr and (R,Ra)-N-PINAP or (R,Sa)-N-PINAP and CdI2 has been developed. Both the yields and enantioselectivities of the allenols of this one-pot procedure are practical. Comparison with ZnI2 control experiments revealed that CdI2 can convert propargylic amine to allene in the presence of CuBr efficiently.
Introduction
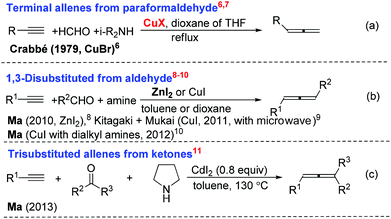
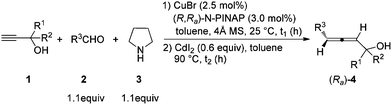
Allenes are becoming more and more important in organic synthesis.1,2 Allene units have also been identified in some biologically active natural products and drugs.3 Furthermore, the potential of their axial-to-central chirality transfer would provide a very appealing and unique route to chiral products.4 Thus, the development of highly efficient approaches to different types of allenes, especially optically active ones, is of current interest with urgency.5 One of the methods that has been attracting our attention is the allenylation of terminal alkynes (ATA), pioneered by Crabbé originally using paraformaldehyde (the Crabbé reaction) (Scheme 1).6–11Enantioselective syntheses of chiral allenes from terminal alkynes and aldehydes have also been developed in a one-pot or two-pot manner using chiral amines (Scheme 2).12–14 In 2012, we also developed a two-step approach by using a catalytic amount of chiral N-PINAP/CuBr and ZnI2 (or ZnI2 together with NaI) (reaction c in Scheme 2).12a Although much progress has been made in allene synthesis, the synthesis of chiral allenols is still fairly complicated.15 We are especially interested in developing one-pot approaches to the chiral allenes with a catalytic amount of a chiral ligand. In reaction c12a of Scheme 2, although the ee is practical, ZnI2 is not able to convert propargylic amine to allene efficiently in the presence of CuBr. In this paper, we wish to report our recent observation on the realization of a one-pot efficient synthesis of optically active α-allenols by an enantioselective ATA (allenylation of terminal alkynes) reaction of terminal propargylic alcohols, in which CdI2(ref. 11) may work in the presence of CuBr to convert the propargylic amine intermediate to allene efficiently.
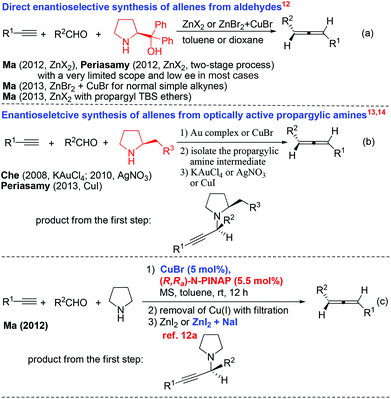 | ||
| Scheme 2 The evolution of syntheses of axially chiral 1,3-disubstituted allenes from terminal alkynes and aldehydes. | ||
Results and discussion
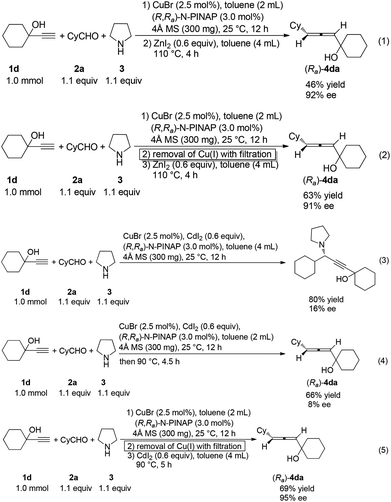
With 2-methyl-3-butyn-2-ol, cyclohexanal and pyrrolidine as the starting point based on our previous report,12a interestingly we observed that CdI2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 may work in harmony with the presence of 2.5 mol% CuBr to produce (Ra)-4aa in practical ee with one-pot operation. After screening some parameters for the second step, we observed that the amount of CdI2 is important to the yields of (Ra)-allenol (entries 1–3, Table 1). The reaction temperature is also critical to the yields (entries 4–6, Table 1). Neither the loading of CdI2 nor the reaction temperature has an obvious effect on the enantioselectivity. Therefore, the standard conditions have been defined as follows (entry 7): a mixture of 2.5 mol% CuBr, 3.0 mol% (R,Ra)-N-PINAP, 1.0 mmol propargylic alcohol, 1.1 mmol aldehyde and 1.1 mmol pyrrolidine was heated in toluene with stirring; after the first step was complete, CdI2 and toluene were added sequentially to the original Schlenk tube without filtration, which was then placed in an oil bath at 90 °C to execute the next step.
11 may work in harmony with the presence of 2.5 mol% CuBr to produce (Ra)-4aa in practical ee with one-pot operation. After screening some parameters for the second step, we observed that the amount of CdI2 is important to the yields of (Ra)-allenol (entries 1–3, Table 1). The reaction temperature is also critical to the yields (entries 4–6, Table 1). Neither the loading of CdI2 nor the reaction temperature has an obvious effect on the enantioselectivity. Therefore, the standard conditions have been defined as follows (entry 7): a mixture of 2.5 mol% CuBr, 3.0 mol% (R,Ra)-N-PINAP, 1.0 mmol propargylic alcohol, 1.1 mmol aldehyde and 1.1 mmol pyrrolidine was heated in toluene with stirring; after the first step was complete, CdI2 and toluene were added sequentially to the original Schlenk tube without filtration, which was then placed in an oil bath at 90 °C to execute the next step.
| Entry | CdI2 (equiv.) | T (°C) | (Ra)-4aa | |
|---|---|---|---|---|
| Yieldb (%) | eec (%) | |||
| a The reactions were carried out with 1a (1.0 mmol), 2a (1.1 mmol), and 3 (1.1 mmol) in 2 mL of toluene, then CdI2 was added to convert propargylic amine to allene. b Isolated yields. c Determined by chiral HPLC analysis. d After CdI2 was added, an additional 2 mL of toluene were added. e The reaction time for the second step was 2 h. | ||||
| 1 | 0.8 | 90 | 41 | 95 |
| 2 | 0.6 | 90 | 53 | 95 |
| 3 | 0.4 | 90 | 45 | 97 |
| 4d | 0.6 | 90 | 66 | 95 |
| 5d | 0.6 | 100 | 45 | 94 |
| 6d | 0.6 | 80 | 44 | 95 |
| 7e | 0.6 | 90 | 68 | 97 |
With the optimized reaction conditions in hand, the generality of the reaction was investigated. Tertiary propargylic alcohols were firstly chosen to react with cyclohexanal and pyrrolidine. Tertiary propargylic alcohols were able to afford the corresponding allenols in moderate to good yields with over 90% ee (entries 1–9, Table 2). The scope of the aldehydes is also quite general: secondary alkyl (entries 1–7, Table 2), normal alkyl (entry 8, Table 2) and aromatic aldehydes (entry 9, Table 2) were all able to be used to afford the products with moderate to good yields and decent enantioselectivities. For the enantioselectivity, in general, the (R,Ra)-N-PINAP ligand (entries 2, 4 and 9, Table 2) is better than (R,Sa)-N-PINAP (entries 10–12, Table 2), which may be caused by the low solubility of the complex formed from CuBr and (R,Sa)-N-PINAP.16 Most of the results are comparable to those of the ZnI2-mediated two-pot approach,12a and some of them are even better in terms of yields and enantioselectivities.
| Entry | 1 | 2 | 4 | ||
|---|---|---|---|---|---|
| R1, R2 | R3 | t 1/t2 | Yieldb | eec (%) | |
| a The reactions were carried out on a 1.0 mmol scale of 1 in toluene unless otherwise noted. b Isolated yield. c Determined by HPLC analysis. d 10.0 mmol of 1d were used in this reaction. e (R,Sa)-N-PINAP was used as the ligand. | |||||
| 1 | Me, Me (1a) | Cy (2a) | 12/2 | 68 (Ra-4aa) | 97 |
| 2 | Et, Et (1b) | Cy (2a) | 12/12 | 84 (Ra-4ba) | 93 |
| 3 | –(CH2)4– (1c) | Cy (2a) | 12/12 | 56 (Ra-4ca) | 93 |
| 4 | –(CH2)5– (1d) | Cy (2a) | 12/4.5 | 68 (Ra-4da) | 93 |
| 5d | –(CH2)5– (1d) | Cy (2a) | 12/12 | 67 (Ra-4da) | 94 |
| 6 | –(CH2)5– (1d) | i-Pr (2b) | 21.5/6 | 50 (Ra-4db) | 91 |
| 7 | –(CH2)5– (1d) | i-Bu (2c) | 13/4 | 48 (Ra-4dc) | 93 |
| 8 | –(CH2)5– (1d) | n-C7H15 (2d) | 23/3 | 53 (Ra-4dd) | 92 |
| 9 | –(CH2)5– (1d) | Ph (2e) | 19/5.5 | 88 (Ra-4de) | 95 |
| 10e | Et, Et (1b) | Cy (2a) | 12/12 | 80 (Sa-4ba) | 92 |
| 11e | –(CH2)5– (1d) | Cy (2a) | 12/4.5 | 70 (Sa-4da) | 90 |
| 12e | –(CH2)5– (1d) | Ph (2e) | 19/4.5 | 82 (Sa-4de) | 93 |
Control experiments showed that in the ZnI2-mediated reaction, removal of the CuBr complex by filtration has no obvious effect on the enantioselectivity; however, it greatly improves the yield (eqn (1) and (2), Scheme 3). Interestingly, in the CdI2-mediated reaction, there is no obvious effect on either yield or enantioselectivity, indicating the harmony of CdI2 with CuBr and the ligand for allene formation (entry 4 in Table 2 and eqn (5) in Scheme 3). Although CuBr, the ligand and CdI2 could together promote this reaction in one pot at room temperature yielding propargylic amines in 80% yield, the ee is very low (16%) (eqn (3) in Scheme 3), and the same reaction at 90 °C afforded the allene in 66% yield and 8% ee (eqn (4) in Scheme 3), indicating that CdI2 could promote the formation of propargylic amine in the absence of the ligand.
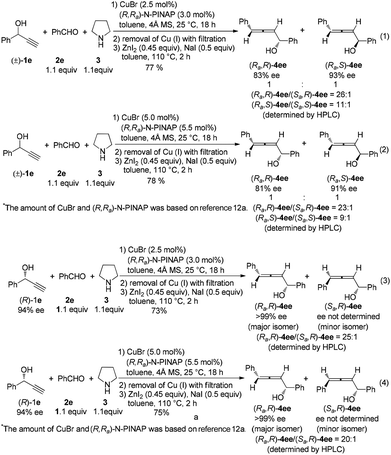
Next, by using optically active (R)- or (S)-1-phenyl-2-propyn-1-ol, (Ra,R)-4ee and (Ra,S)-4ee could be prepared by using (R,Ra)-N-PINAP as the ligand (eqn (1)–(3) in Scheme 4). Similarly, (Sa,R)-4ee and (Sa,R)-4ee were also prepared via the reactions with (R,Sa)-N-PINAP as the ligand (eqn (1)–(3) in Scheme 5).
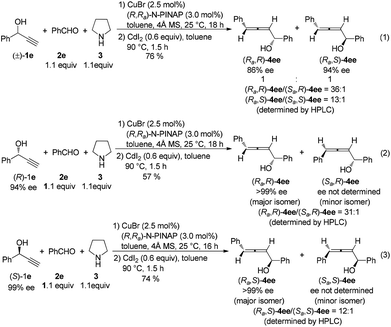 | ||
| Scheme 4 The one-pot approach for the reaction of 1e, benzaldehyde and pyrrolidine with (R,Ra)-N-PINAP as the ligand. | ||
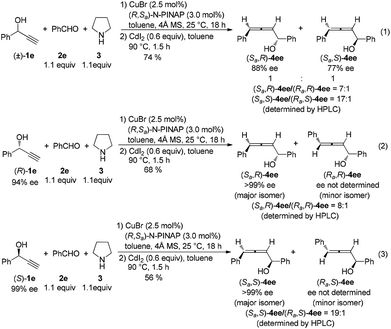 | ||
| Scheme 5 The one-pot approach for the reaction of 1e, benzaldehyde and pyrrolidine with (R,Sa)-N-PINAP as the ligand. | ||
Results of the control experiments involving the ZnI2-mediated reactions of rac- or (R)-1-phenyl-2-propyn-1-ol, cyclohexanal and pyrrolidine12a further show that the results of the current protocol are comparable (eqn (1)–(4) in Scheme 6).
The absolute configurations of the allenols were assigned based on our previous study12a and the Lowe-Brewster rule.17 A model to predict the absolute configuration of the allenols is shown in Scheme 7.
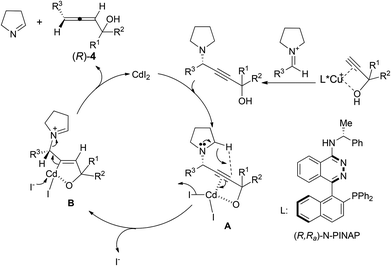 | ||
| Scheme 7 Proposed mechanism and prediction of the absolute configuration of the axial chirality in allenols. | ||
Conclusions
In conclusion, we have observed that CdI2 can work in harmony with CuBr to transform terminal propargylic alcohols and aldehydes to allenols with high yields and enantioselectivities. The difference between ZnI2 and CdI2 is striking since ZnI2 fails to convert the intermediates, propargylic amines, to allenes in high efficiency in the presence of CuBr and the chiral ligand. Due to the fact that the starting materials are commercially available, this protocol with simple operation avoiding filtration will be of great interest to the scientific community. Further studies on the substrate scope, mechanism of the weak interaction and applications of allenols are being carried out in our lab.Experimental
General information
All reactions were carried out in oven-dried Schlenk tubes. All 1H NMR experiments were referenced relative to the signal of tetramethylsilane (0 ppm) in CDCl3 and 13C NMR experiments were referenced with the signal of residual chloroform (77.00 ppm) in CDCl3. IR spectra were recorded on a Bruker Tensor 27 infrared spectrometer. CuBr (98%) was purchased from Acros and CdI2 (99.9%) was purchased from Aladdin and kept in a glove box; ZnI2 was purchased from Alfa Aesar and kept in a glove box; (R,Ra)-N-PINAP (97%) and (R,Sa)-N-PINAP (97%) were purchased from Strem Chemicals and kept in a glove box; 4 Å molecular sieves were purchased from Alfa Aesar and kept in a glove box after activation (heated at 450 °C for 10 h in a Muffle furnace, taken out after cooling to 200 °C and then kept in a glove box and allowed to cool to room temperature). Aldehydes were distilled immediately before use. 2-Methyl-3-butyn-2-ol (1a) and pyrrolidine (3) were redistilled. (R)-1-Phenyl-2-propyn-1-ol and (S)-1-phenyl-2-propyn-1-ol were prepared according to the previous report.18 Toluene was dried over sodium wire with benzophenone as the indicator and distilled freshly before use. Other reagents were used without further treatment. All temperatures refer to the temperature of the oil bath used. Petroleum ether (60–90 °C) for chromatography was distilled before use.Typical procedure I
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford (Ra)-4aa (122.8 mg, 68%) as a low-melting point white solid:12a 97% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR(major) = 10.4 min, tR (minor) = 11.9 min); [α]27D = −99.0 (c = 1.02, CHCl3) (reported value: 97% ee; [α]20D = −99.5 (c = 1.15, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.35 (dd, J1 = 6.2 Hz, J2 = 3.0 Hz, 1 H, one proton from CH
1) to afford (Ra)-4aa (122.8 mg, 68%) as a low-melting point white solid:12a 97% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR(major) = 10.4 min, tR (minor) = 11.9 min); [α]27D = −99.0 (c = 1.02, CHCl3) (reported value: 97% ee; [α]20D = −99.5 (c = 1.15, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.35 (dd, J1 = 6.2 Hz, J2 = 3.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.30 (t, J = 6.0 Hz, 1 H, one proton from CH
CH), 5.30 (t, J = 6.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.06–1.92 (m, 2 H, OH and CH from Cy), 1.82–1.59 (m, 5 H, protons from Cy), 1.34 (s, 6 H, 2 × CH3), 1.31–1.01 (m, 5 H, protons from Cy); 13C NMR (100 MHz, CDCl3) δ = 199.1, 102.0, 101.0, 69.5, 37.2, 33.02, 32.99, 30.0, 29.9, 26.04, 26.01, 25.99; MS (EI) m/z (%): 180 (M+, 11.45), 91 (100); IR (neat): v = 3347, 2973, 2923, 2850, 1963, 1446, 1402, 1363, 1153 cm−1, HRMS calcd for C12H20O [M+]: 180.1514, found: 180.1518.
CH), 2.06–1.92 (m, 2 H, OH and CH from Cy), 1.82–1.59 (m, 5 H, protons from Cy), 1.34 (s, 6 H, 2 × CH3), 1.31–1.01 (m, 5 H, protons from Cy); 13C NMR (100 MHz, CDCl3) δ = 199.1, 102.0, 101.0, 69.5, 37.2, 33.02, 32.99, 30.0, 29.9, 26.04, 26.01, 25.99; MS (EI) m/z (%): 180 (M+, 11.45), 91 (100); IR (neat): v = 3347, 2973, 2923, 2850, 1963, 1446, 1402, 1363, 1153 cm−1, HRMS calcd for C12H20O [M+]: 180.1514, found: 180.1518.
The following compounds (Ra)-4ba – (Ra)-4de in Table 2 were prepared according to this Typical Procedure I. All the racemic products were also prepared according to this procedure in the absence of the chiral ligand.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an oil:12a 93% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.7 mL min−1, λ = 214 nm, tR(major) = 7.5 min, tR(minor) = 8.1 min); [α]24D = −108.5 (c = 1.03, CHCl3) (reported value: 96% ee; [α]20D = −85.9 (c = 1.03, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.35 (t, J = 6.2 Hz, 1 H, one proton from CH
1) as an oil:12a 93% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.7 mL min−1, λ = 214 nm, tR(major) = 7.5 min, tR(minor) = 8.1 min); [α]24D = −108.5 (c = 1.03, CHCl3) (reported value: 96% ee; [α]20D = −85.9 (c = 1.03, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.35 (t, J = 6.2 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.16 (dd, J1 = 6.2 Hz, J2 = 3.0 Hz, 1 H, one proton from CH
CH), 5.16 (dd, J1 = 6.2 Hz, J2 = 3.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.06–1.94 (m, 1 H, CH from Cy), 1.81–1.68 (m, 5 H, protons from Cy and C
CH), 2.06–1.94 (m, 1 H, CH from Cy), 1.81–1.68 (m, 5 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2CH3)2), 1.68–1.47 (m, 5 H, protons from Cy and C
CC(OH)(CH2CH3)2), 1.68–1.47 (m, 5 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2CH3)2), 1.35–1.03 (m, 5 H, protons from Cy and C
CC(OH)(CH2CH3)2), 1.35–1.03 (m, 5 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2CH3)2), 0.91 (t, J = 7.4 Hz, 3 H, CH3), 0.89 (t, J = 7.6 Hz, 3 H, CH3); 13C NMR (100 MHz, CDCl3) δ = 199.6, 101.6, 99.6, 73.7, 37.3, 33.10, 33.07, 33.0, 32.9, 26.02, 26.01, 25.98, 8.1, 8.0; MS (EI) m/z (%): 208 (M+, 1.20), 87 (100); IR (neat): v = 3039, 2925, 2851, 1960, 1449, 1350, 1293, 1129, 1024 cm−1.
CC(OH)(CH2CH3)2), 0.91 (t, J = 7.4 Hz, 3 H, CH3), 0.89 (t, J = 7.6 Hz, 3 H, CH3); 13C NMR (100 MHz, CDCl3) δ = 199.6, 101.6, 99.6, 73.7, 37.3, 33.10, 33.07, 33.0, 32.9, 26.02, 26.01, 25.98, 8.1, 8.0; MS (EI) m/z (%): 208 (M+, 1.20), 87 (100); IR (neat): v = 3039, 2925, 2851, 1960, 1449, 1350, 1293, 1129, 1024 cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an oil:12a 93% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.7 mL min−1, λ = 214 nm, tR(major) = 9.5 min, tR(minor) = 10.2 min); [α]23D = −90.8 (c = 1.00, CHCl3) (reported value: 95% ee; [α]20D = −101.8 (c = 0.68, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.41 (dd, J1 = 6.2 Hz, J2 = 3.0 Hz, 1 H, one proton from CH
1) as an oil:12a 93% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.7 mL min−1, λ = 214 nm, tR(major) = 9.5 min, tR(minor) = 10.2 min); [α]23D = −90.8 (c = 1.00, CHCl3) (reported value: 95% ee; [α]20D = −101.8 (c = 0.68, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.41 (dd, J1 = 6.2 Hz, J2 = 3.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.31 (t, J = 6.2 Hz, 1 H, one proton from CH
CH), 5.31 (t, J = 6.2 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.05–1.94 (m, 1 H, CH from Cy), 1.92–1.58 (m, 14 H, protons from Cy and C
CH), 2.05–1.94 (m, 1 H, CH from Cy), 1.92–1.58 (m, 14 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)4), 1.35–1.01 (m, 5 H, protons from Cy and C
CC(OH)(CH2)4), 1.35–1.01 (m, 5 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)4); 13C NMR (100 MHz, CDCl3) δ = 199.3, 100.9, 100.2, 79.9, 40.32, 40.26, 37.2, 33.02, 33.00, 26.02, 25.98, 25.96, 23.6; MS (EI) m/z (%): 206 (M+, 3.57), 85 (100); IR (neat): v = 3345, 2923, 2851, 1960, 1446, 1384, 1318, 1292, 1186, 1072 cm−1.
CC(OH)(CH2)4); 13C NMR (100 MHz, CDCl3) δ = 199.3, 100.9, 100.2, 79.9, 40.32, 40.26, 37.2, 33.02, 33.00, 26.02, 25.98, 25.96, 23.6; MS (EI) m/z (%): 206 (M+, 3.57), 85 (100); IR (neat): v = 3345, 2923, 2851, 1960, 1446, 1384, 1318, 1292, 1186, 1072 cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (m.p.: 47–49 °C, we were not able to obtain the crystal from the solvent tested, the m.p. was determined by using the solid after evaporation of the eluent):12a 93% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR(major) = 11.1 min, tR(minor) = 11.8 min); [α]28D = −99.4 (c = 1.02, CHCl3) (reported value: 96% ee; [α]20D = −108.6 (c = 0.98, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.33–5.29 (m, 2 H, CH
1) as a white solid (m.p.: 47–49 °C, we were not able to obtain the crystal from the solvent tested, the m.p. was determined by using the solid after evaporation of the eluent):12a 93% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR(major) = 11.1 min, tR(minor) = 11.8 min); [α]28D = −99.4 (c = 1.02, CHCl3) (reported value: 96% ee; [α]20D = −108.6 (c = 0.98, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.33–5.29 (m, 2 H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.05–1.93 (m, 1 H, CH from Cy), 1.80–1.43 (m, 15 H, protons from Cy and C
CH), 2.05–1.93 (m, 1 H, CH from Cy), 1.80–1.43 (m, 15 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5), 1.38–1.02 (m, 6 H, protons from Cy and C
CC(OH)(CH2)5), 1.38–1.02 (m, 6 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5); 13C NMR (100 MHz, CDCl3) δ = 199.9, 101.3, 100.7, 70.4, 38.3, 38.2, 37.2, 33.05, 32.97, 25.97, 25.95, 25.5, 22.42, 22.41; MS (EI) m/z (%): 220 (M+, 1.36), 99 (100); IR (neat): v = 3310, 2921, 2848, 1961, 1444, 1398, 1352, 1267, 1248, 1141, 1061, 1034 cm−1.
CC(OH)(CH2)5); 13C NMR (100 MHz, CDCl3) δ = 199.9, 101.3, 100.7, 70.4, 38.3, 38.2, 37.2, 33.05, 32.97, 25.97, 25.95, 25.5, 22.42, 22.41; MS (EI) m/z (%): 220 (M+, 1.36), 99 (100); IR (neat): v = 3310, 2921, 2848, 1961, 1444, 1398, 1352, 1267, 1248, 1141, 1061, 1034 cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid:12a 94% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR(major) = 10.7 min, tR(minor) = 11.4 min); [α]27D = −105.5 (c = 1.01, CHCl3) (reported value: 96% ee; [α]20D = −108.6 (c = 0.98, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.35–5.25 (m, 2 H, CH
1) as a white solid:12a 94% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR(major) = 10.7 min, tR(minor) = 11.4 min); [α]27D = −105.5 (c = 1.01, CHCl3) (reported value: 96% ee; [α]20D = −108.6 (c = 0.98, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.35–5.25 (m, 2 H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.10–1.92 (m, 1 H, CH from Cy), 1.84–1.40 (m, 15 H, protons from Cy and C
CH), 2.10–1.92 (m, 1 H, CH from Cy), 1.84–1.40 (m, 15 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5), 1.38–1.01 (m, 6 H, protons from Cy and C
CC(OH)(CH2)5), 1.38–1.01 (m, 6 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5); 13C NMR (100 MHz, CDCl3) δ = 199.9, 101.2, 100.7, 70.4, 38.3, 38.1, 37.2, 33.03, 32.95, 25.96, 25.93, 25.4, 22.39, 22.38; MS (EI) m/z (%): 220 (M+, 1.38), 99 (100); IR (neat): v = 3308, 2921, 2848, 1961, 1443, 1398, 1351, 1267, 1252, 1140, 1061, 1034 cm−1.
CC(OH)(CH2)5); 13C NMR (100 MHz, CDCl3) δ = 199.9, 101.2, 100.7, 70.4, 38.3, 38.1, 37.2, 33.03, 32.95, 25.96, 25.93, 25.4, 22.39, 22.38; MS (EI) m/z (%): 220 (M+, 1.38), 99 (100); IR (neat): v = 3308, 2921, 2848, 1961, 1443, 1398, 1351, 1267, 1252, 1140, 1061, 1034 cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a liquid:12a 91% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.7 mL min−1, λ = 214 nm, tR(major) = 8.2 min, tR(minor) = 8.9 min); [α]26D = −87.6 (c = 1.03, CHCl3) (reported value: 95% ee; [α]20D = −79.7 (c = 0.52, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.36–5.30 (m, J = 4.8 Hz, 2 H, CH
1) as a liquid:12a 91% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.7 mL min−1, λ = 214 nm, tR(major) = 8.2 min, tR(minor) = 8.9 min); [α]26D = −87.6 (c = 1.03, CHCl3) (reported value: 95% ee; [α]20D = −79.7 (c = 0.52, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.36–5.30 (m, J = 4.8 Hz, 2 H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.40–2.26 (m, 1 H, CH from iPr), 1.74–1.24 (m, 11 H, protons from C
CH), 2.40–2.26 (m, 1 H, CH from iPr), 1.74–1.24 (m, 11 H, protons from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5), 1.03 (d, J = 6.8 Hz, 3 H, CH3), 1.025 (d, J = 6.8 Hz, 3 H, CH3); 13C NMR (100 MHz, CDCl3) δ = 199.6, 102.1, 101.6, 70.5, 38.3, 38.2, 27.9, 25.5, 22.47, 22.43, 22.39; MS (EI) m/z (%): 180 (M+, 3.22), 99 (100); IR (neat): v = 3344, 2959, 2928, 2860, 1961, 1462, 1445, 1410, 1381, 1359, 1346, 1318, 1296, 1245, 1192, 1145, 1057 cm−1.
CC(OH)(CH2)5), 1.03 (d, J = 6.8 Hz, 3 H, CH3), 1.025 (d, J = 6.8 Hz, 3 H, CH3); 13C NMR (100 MHz, CDCl3) δ = 199.6, 102.1, 101.6, 70.5, 38.3, 38.2, 27.9, 25.5, 22.47, 22.43, 22.39; MS (EI) m/z (%): 180 (M+, 3.22), 99 (100); IR (neat): v = 3344, 2959, 2928, 2860, 1961, 1462, 1445, 1410, 1381, 1359, 1346, 1318, 1296, 1245, 1192, 1145, 1057 cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a liquid:12a 93% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 100/1, 0.7 mL min−1, λ = 214 nm, tR(major) = 16.3 min, tR(minor) = 17.8 min); [α]28D = −76.1 (c = 1.04, CHCl3) (reported value: 90% ee; [α]20D = −82.1 (c = 1.04, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.30–5.21 (m, 2 H, CH
1) as a liquid:12a 93% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 100/1, 0.7 mL min−1, λ = 214 nm, tR(major) = 16.3 min, tR(minor) = 17.8 min); [α]28D = −76.1 (c = 1.04, CHCl3) (reported value: 90% ee; [α]20D = −82.1 (c = 1.04, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.30–5.21 (m, 2 H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.02–1.87 (m, 2 H, CH2C
CH), 2.02–1.87 (m, 2 H, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1.74–1.30 (m, 12 H, protons from iBu and C
C), 1.74–1.30 (m, 12 H, protons from iBu and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5, 0.93 (d, J = 6.8 Hz, 6 H, 2 × CH3); 13C NMR (100 MHz, CDCl3) δ = 201.8, 99.5, 93.2, 70.7, 38.4, 38.32, 38.30, 28.5, 25.5, 22.60, 22.55, 22.22, 22.18; MS (EI) m/z (%): 194 (M+, 13.66), 99 (100); IR (neat): v = 3358, 2929, 1962, 1463, 1448, 1383, 1367, 1343, 1247, 1146, 1056, 1035 cm−1, HRMS calcd for C13H22O [M+]: 194.1671, found: 194.1676.
CC(OH)(CH2)5, 0.93 (d, J = 6.8 Hz, 6 H, 2 × CH3); 13C NMR (100 MHz, CDCl3) δ = 201.8, 99.5, 93.2, 70.7, 38.4, 38.32, 38.30, 28.5, 25.5, 22.60, 22.55, 22.22, 22.18; MS (EI) m/z (%): 194 (M+, 13.66), 99 (100); IR (neat): v = 3358, 2929, 1962, 1463, 1448, 1383, 1367, 1343, 1247, 1146, 1056, 1035 cm−1, HRMS calcd for C13H22O [M+]: 194.1671, found: 194.1676.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) as an oil:12a 92% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 200/1, 1.0 mL min−1, λ = 214 nm, tR(major) = 15.6 min, tR(minor) = 16.9 min); 1H NMR (400 MHz, CDCl3) δ = 5.34–5.21 (m, 2 H, CH
3) as an oil:12a 92% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 200/1, 1.0 mL min−1, λ = 214 nm, tR(major) = 15.6 min, tR(minor) = 16.9 min); 1H NMR (400 MHz, CDCl3) δ = 5.34–5.21 (m, 2 H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.08–1.97 (m, 2 H, CH2C
CH), 2.08–1.97 (m, 2 H, CH2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 1.76 (s, 1 H, OH), 1.72–1.20 (m, 20 H, 10 CH2), 0.88 (t, J = 7.0 Hz, 3 H, CH3); 13C NMR (100 MHz, CDCl3) δ = 201.3, 100.2, 94.6, 70.6, 38.28, 38.25, 31.8, 29.2, 29.05, 29.03, 28.8, 25.5, 22.6, 22.5, 22.49, 14.0; MS (EI) m/z (%): 236 (M+, 4.36), 99 (100); IR (neat): v = 3353, 2925, 2853, 1962, 1448, 1379, 1346, 1316, 1247, 1184, 1147, 1056 cm−1.
CH), 1.76 (s, 1 H, OH), 1.72–1.20 (m, 20 H, 10 CH2), 0.88 (t, J = 7.0 Hz, 3 H, CH3); 13C NMR (100 MHz, CDCl3) δ = 201.3, 100.2, 94.6, 70.6, 38.28, 38.25, 31.8, 29.2, 29.05, 29.03, 28.8, 25.5, 22.6, 22.5, 22.49, 14.0; MS (EI) m/z (%): 236 (M+, 4.36), 99 (100); IR (neat): v = 3353, 2925, 2853, 1962, 1448, 1379, 1346, 1316, 1247, 1184, 1147, 1056 cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (m.p.: 49–51 °C, we were not able to obtain the crystal from the solvent tested, the m.p. was determined by using the solid after evaporation of the eluent):12a 95% ee (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 95/5, 1.0 mL min−1, λ = 214 nm, tR(major) = 6.9 min, tR(minor) = 15.3 min); [α]28D = −346.3 (c = 1.01, CHCl3) (reported value: 93% ee; [α]20D = −341.4 (c = 1.00, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 7.33–7.25 (m, 4 H, Ar–H), 7.23–7.15 (m, 1 H, Ar–H), 6.30 (d, J = 6.0 Hz, 1 H, one proton from CH
1) as a white solid (m.p.: 49–51 °C, we were not able to obtain the crystal from the solvent tested, the m.p. was determined by using the solid after evaporation of the eluent):12a 95% ee (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 95/5, 1.0 mL min−1, λ = 214 nm, tR(major) = 6.9 min, tR(minor) = 15.3 min); [α]28D = −346.3 (c = 1.01, CHCl3) (reported value: 93% ee; [α]20D = −341.4 (c = 1.00, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 7.33–7.25 (m, 4 H, Ar–H), 7.23–7.15 (m, 1 H, Ar–H), 6.30 (d, J = 6.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.72 (d, J = 6.4 Hz, 1 H, one proton from CH
CH), 5.72 (d, J = 6.4 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 1.89 (s, 1 H, OH), 1.72–1.58 (m, 6 H, protons from Cy), 1.57–1.42 (m, 3 H, protons from Cy), 1.40–1.27 (m, 1 H, one proton from Cy); 13C NMR (100 MHz, CDCl3) δ = 202.8, 134.0, 128.6, 127.0, 126.6, 104.3, 97.6, 71.4, 38.33, 38.26, 25.4, 22.40, 22.38; MS (EI) m/z (%): 214 (M+, 2.81), 116 (100); IR (neat): v = 3327, 2929, 2859, 1948, 1599, 1492, 1444, 1407, 1346, 1318, 1291, 1244, 1185, 1145, 1113, 1057, 1033 cm−1.
CH), 1.89 (s, 1 H, OH), 1.72–1.58 (m, 6 H, protons from Cy), 1.57–1.42 (m, 3 H, protons from Cy), 1.40–1.27 (m, 1 H, one proton from Cy); 13C NMR (100 MHz, CDCl3) δ = 202.8, 134.0, 128.6, 127.0, 126.6, 104.3, 97.6, 71.4, 38.33, 38.26, 25.4, 22.40, 22.38; MS (EI) m/z (%): 214 (M+, 2.81), 116 (100); IR (neat): v = 3327, 2929, 2859, 1948, 1599, 1492, 1444, 1407, 1346, 1318, 1291, 1244, 1185, 1145, 1113, 1057, 1033 cm−1.
The following compounds (Sa)-4ba, (Sa)-4da, (Sa)-4de in Table 2 were also prepared according to the Typical Procedure I with the (R,Sa)-N-PINAP as the chiral ligand.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as an oil: 92% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 98/2, 1.0 mL min−1, λ = 214 nm, tR(minor) = 6.0 min, tR(major) = 7.0 min); [α]26D = +103.6 (c = 1.02, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 5.34 (t, J = 6.2 Hz, 1 H, one proton from CH
1) as an oil: 92% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 98/2, 1.0 mL min−1, λ = 214 nm, tR(minor) = 6.0 min, tR(major) = 7.0 min); [α]26D = +103.6 (c = 1.02, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 5.34 (t, J = 6.2 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.16 (dd, J1 = 6.2 Hz, J2 = 3.0 Hz, 1 H, one proton from CH
CH), 5.16 (dd, J1 = 6.2 Hz, J2 = 3.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.06–1.93 (m, 1 H, CH from Cy), 1.82–1.64 (m, 5 H, protons from Cy and C
CH), 2.06–1.93 (m, 1 H, CH from Cy), 1.82–1.64 (m, 5 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2CH3)2), 1.63–1.46 (m, 5 H, protons from Cy and C
CC(OH)(CH2CH3)2), 1.63–1.46 (m, 5 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2CH3)2), 1.35–1.03 (m, 5 H, protons from Cy and C
CC(OH)(CH2CH3)2), 1.35–1.03 (m, 5 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH2CH3)2COH), 0.89 (t, J = 7.2 Hz, 3 H, CH3), 0.87 (t, J = 5.4 Hz, 3 H, CH3); 13C NMR (100 MHz, CDCl3) δ = 199.6, 101.3, 99.6, 73.6, 37.3, 33.05, 32.98, 32.9, 32.8, 26.0, 25.94, 25.92, 8.0, 7.9; MS (EI) m/z (%): 208 (M+, 2.11), 87 (100); IR (neat): v = 3430, 2966, 2923, 2851, 1961, 1448, 1377, 1349, 1324, 1257, 1180, 1129 cm−1, HRMS calcd for C14H24O [M+]: 208.1827, found: 208.1824.
C(CH2CH3)2COH), 0.89 (t, J = 7.2 Hz, 3 H, CH3), 0.87 (t, J = 5.4 Hz, 3 H, CH3); 13C NMR (100 MHz, CDCl3) δ = 199.6, 101.3, 99.6, 73.6, 37.3, 33.05, 32.98, 32.9, 32.8, 26.0, 25.94, 25.92, 8.0, 7.9; MS (EI) m/z (%): 208 (M+, 2.11), 87 (100); IR (neat): v = 3430, 2966, 2923, 2851, 1961, 1448, 1377, 1349, 1324, 1257, 1180, 1129 cm−1, HRMS calcd for C14H24O [M+]: 208.1827, found: 208.1824.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid (m.p.: 45–47 °C, we were not able to obtain the crystal from the solvent tested, the m.p. was determined by using the solid after evaporation of the eluent):12a 90% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR (minor) = 11.2 min, tR(major) = 12.0 min); [α]27D = +90.3 (c = 1.00, CHCl3) (reported value: 93% ee; [α]20D = +102.3 (c = 1.00, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.34–5.26 (m, 2 H, CH
1) as a white solid (m.p.: 45–47 °C, we were not able to obtain the crystal from the solvent tested, the m.p. was determined by using the solid after evaporation of the eluent):12a 90% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR (minor) = 11.2 min, tR(major) = 12.0 min); [α]27D = +90.3 (c = 1.00, CHCl3) (reported value: 93% ee; [α]20D = +102.3 (c = 1.00, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.34–5.26 (m, 2 H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.05–1.95 (m, 1 H, CH from Cy), 1.80–1.40 (m, 15 H, protons from Cy and C
CH), 2.05–1.95 (m, 1 H, CH from Cy), 1.80–1.40 (m, 15 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5), 1.37–1.02 (m, 6 H, protons from Cy and C
CC(OH)(CH2)5), 1.37–1.02 (m, 6 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5); 13C NMR (100 MHz, CDCl3) δ = 199.9, 101.3, 100.7, 70.4, 38.3, 38.2, 37.2, 33.03, 32.97, 25.97, 25.94, 25.44, 22.41, 22.39; MS (EI) m/z (%): 220 (M+, 1.27), 99 (100); IR (neat): v = 3310, 2921, 2848, 1961, 1444, 1398, 1351, 1267, 1249, 1185, 1140, 1098, 1060, 1034 cm−1; HRMS calcd for C15H24O [M+]: 220.1827, found: 220.1830.
CC(OH)(CH2)5); 13C NMR (100 MHz, CDCl3) δ = 199.9, 101.3, 100.7, 70.4, 38.3, 38.2, 37.2, 33.03, 32.97, 25.97, 25.94, 25.44, 22.41, 22.39; MS (EI) m/z (%): 220 (M+, 1.27), 99 (100); IR (neat): v = 3310, 2921, 2848, 1961, 1444, 1398, 1351, 1267, 1249, 1185, 1140, 1098, 1060, 1034 cm−1; HRMS calcd for C15H24O [M+]: 220.1827, found: 220.1830.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a white solid: 93% ee (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 90/10, 0.9 mL min−1, λ = 214 nm, tR(minor) = 6.0 min, tR(major) = 9.2 min); [α]26D = +322.3 (c = 1.02, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.33–7.25 (m, 4 H, Ar–H), 7.24–7.16 (m, 1 H, Ar–H), 6.31 (d, J = 6.4 Hz, 1 H, one proton from CH
1) as a white solid: 93% ee (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 90/10, 0.9 mL min−1, λ = 214 nm, tR(minor) = 6.0 min, tR(major) = 9.2 min); [α]26D = +322.3 (c = 1.02, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.33–7.25 (m, 4 H, Ar–H), 7.24–7.16 (m, 1 H, Ar–H), 6.31 (d, J = 6.4 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.73 (d, J = 6.4 Hz, 1 H, one proton from CH
CH), 5.73 (d, J = 6.4 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 1.79 (s, 1 H, OH), 1.72–1.59 (m, 6 H, protons from Cy), 1.57–1.41 (m, 3 H, protons from Cy), 1.44–1.29 (m, 1 H, one proton from Cy); 13C NMR (100 MHz, CDCl3) δ = 202.8, 134.0, 128.6, 127.0, 126.6, 104.3, 97.6, 71.5, 38.4, 38.3, 25.4, 22.42, 22.40; MS (EI) m/z (%): 214 (M+, 4.45), 116 (100); IR (neat): v = 3327, 3069, 3031, 2928, 2855, 1947, 1598, 1492, 1447, 1407, 1349, 1245, 1185, 1144, 1057 cm−1; HRMS calcd for C15H18O [M+]: 214.1358, found: 214.1362.
CH), 1.79 (s, 1 H, OH), 1.72–1.59 (m, 6 H, protons from Cy), 1.57–1.41 (m, 3 H, protons from Cy), 1.44–1.29 (m, 1 H, one proton from Cy); 13C NMR (100 MHz, CDCl3) δ = 202.8, 134.0, 128.6, 127.0, 126.6, 104.3, 97.6, 71.5, 38.4, 38.3, 25.4, 22.42, 22.40; MS (EI) m/z (%): 214 (M+, 4.45), 116 (100); IR (neat): v = 3327, 3069, 3031, 2928, 2855, 1947, 1598, 1492, 1447, 1407, 1349, 1245, 1185, 1144, 1057 cm−1; HRMS calcd for C15H18O [M+]: 214.1358, found: 214.1362.
The following compounds (Ra,R)-4ee, (Ra,S)-4ee, (Sa,R)-4ee, (Sa,R)-4ee in Scheme 3 and 4 were also prepared according to the Typical Procedure I with (R,Ra)-N-PINAP or (R,Sa)-N-PINAP as the chiral ligand.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow oil: 86% ee for (Ra,R)-4ee and 94% ee for (Ra,S)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 36
1) as a yellow oil: 86% ee for (Ra,R)-4ee and 94% ee for (Ra,S)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (Ra,S)-4ee/(Sa,S)-4ee = 13
1; (Ra,S)-4ee/(Sa,S)-4ee = 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 18.4 min, tR(major) = 19.9 min, tR(major) = 25.1 min, tR(minor) = 33.6 min); [α]28D = −184.7 (c = 1.00, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.41–7.11 (m, 10 H, Ar–H), 6.29 (dd, J1 = 6.4 Hz, J2 = 2.0 Hz, 1 H, one proton from CH
1; (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 18.4 min, tR(major) = 19.9 min, tR(major) = 25.1 min, tR(minor) = 33.6 min); [α]28D = −184.7 (c = 1.00, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.41–7.11 (m, 10 H, Ar–H), 6.29 (dd, J1 = 6.4 Hz, J2 = 2.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.79 (dd, J1 = 11.6 Hz, J2 = 6.4 Hz, 1 H, one proton from CH
CH), 5.79 (dd, J1 = 11.6 Hz, J2 = 6.4 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.27 (dd, J1 = 14.8 Hz, J2 = 6.4 Hz, 1 H, CH), 2.73 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) (Ra*,R*)-4ee: δ = 203.5, 142.7, 133.58, 128.6, 128.4, 127.71, 127.20, 126.80, 126.0, 99.9, 97.8, 72.1; (Ra*,S*)-4ee: δ = 203.8, 142.8, 133.57, 128.5, 128.4, 127.69, 127.16, 126.75, 125.9, 99.8, 97.5, 72.3; MS (EI) m/z (%): 222 (M+, 13.03), 116 (100); IR (neat): v = 3317, 3083, 3061, 3029, 1951, 1883, 1809, 1694, 1599, 1584, 1493, 1453, 1390, 1193, 1157, 1099, 1072, 1029 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1047 and 222.1042.
CH), 5.27 (dd, J1 = 14.8 Hz, J2 = 6.4 Hz, 1 H, CH), 2.73 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) (Ra*,R*)-4ee: δ = 203.5, 142.7, 133.58, 128.6, 128.4, 127.71, 127.20, 126.80, 126.0, 99.9, 97.8, 72.1; (Ra*,S*)-4ee: δ = 203.8, 142.8, 133.57, 128.5, 128.4, 127.69, 127.16, 126.75, 125.9, 99.8, 97.5, 72.3; MS (EI) m/z (%): 222 (M+, 13.03), 116 (100); IR (neat): v = 3317, 3083, 3061, 3029, 1951, 1883, 1809, 1694, 1599, 1584, 1493, 1453, 1390, 1193, 1157, 1099, 1072, 1029 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1047 and 222.1042.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow oil: 99% ee for (Ra,R)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 31
1) as a yellow oil: 99% ee for (Ra,R)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 19.9 min, tR(major) = 21.7 min, tR(minor) = 29.1 min, tR(minor) = 40.0 min); [α]28D = −205.3 (c = 1.025, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.42–7.15 (m, 10 H, Ar–H), 6.32 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, one proton from CH
1 (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 19.9 min, tR(major) = 21.7 min, tR(minor) = 29.1 min, tR(minor) = 40.0 min); [α]28D = −205.3 (c = 1.025, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.42–7.15 (m, 10 H, Ar–H), 6.32 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.82 (t, J = 6.2 Hz, 1 H, one proton from CH
CH), 5.82 (t, J = 6.2 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.28 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, CH), 2.54 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 133.6, 128.61, 128.5, 127.78, 127.3, 126.83, 126.0, 100.0, 97.9, 72.1; MS (EI) m/z (%): 222 (M+, 9.90), 116 (100); IR (neat): v = 3306, 3083, 3061, 3029, 2891, 1951, 1881, 1809, 1693, 1599, 1494, 1454, 1194, 1001 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1042.
CH), 5.28 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, CH), 2.54 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 133.6, 128.61, 128.5, 127.78, 127.3, 126.83, 126.0, 100.0, 97.9, 72.1; MS (EI) m/z (%): 222 (M+, 9.90), 116 (100); IR (neat): v = 3306, 3083, 3061, 3029, 2891, 1951, 1881, 1809, 1693, 1599, 1494, 1454, 1194, 1001 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1042.
The following signals are discernible for (Sa*,R*)-4ee: 1H NMR (400 MHz, CDCl3) δ = 5.31 (d, J = 6.8 Hz 1 H, CH); 13C NMR (100 MHz, CDCl3) δ = 128.58, 127.75, 127.2, 126.79, 125.9, 99.8, 97.6, 72.3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow solid: 99% ee for (Ra,S)-4ee; (Ra,S)-4ee/(Sa,S)-4ee = 12
1) as a yellow solid: 99% ee for (Ra,S)-4ee; (Ra,S)-4ee/(Sa,S)-4ee = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 22.9 min, tR(major) = 31.0 min, tR(minor) = 42.5 min); [α]28D = −187.9 (c = 1.03, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.40–7.10 (m, 10 H, Ar–H), 6.28 (dd, J1 = 6.0 Hz, J2 = 2.0 Hz, 1 H, one proton from CH
1 (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 22.9 min, tR(major) = 31.0 min, tR(minor) = 42.5 min); [α]28D = −187.9 (c = 1.03, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.40–7.10 (m, 10 H, Ar–H), 6.28 (dd, J1 = 6.0 Hz, J2 = 2.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.78 (t, J = 6.6 Hz, 1 H, one proton from CH
CH), 5.78 (t, J = 6.6 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.27 (dd, J1 = 6.6 Hz, J2 = 1.8 Hz, 1 H, CH), 2.92 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.8, 142.8, 133.6, 128.5, 128.4, 127.6, 127.1, 126.7, 125.87, 99.7, 97.4, 72.3; MS (EI) m/z (%): 222 (M+, 13.38), 116 (100); IR (neat): v = 3273, 3084, 3027, 2985, 1952, 1882, 1811, 1758, 1688, 1599, 1550, 1495, 1452, 1400, 1332, 1250, 1192, 1118, 1000 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1044.
CH), 5.27 (dd, J1 = 6.6 Hz, J2 = 1.8 Hz, 1 H, CH), 2.92 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.8, 142.8, 133.6, 128.5, 128.4, 127.6, 127.1, 126.7, 125.87, 99.7, 97.4, 72.3; MS (EI) m/z (%): 222 (M+, 13.38), 116 (100); IR (neat): v = 3273, 3084, 3027, 2985, 1952, 1882, 1811, 1758, 1688, 1599, 1550, 1495, 1452, 1400, 1332, 1250, 1192, 1118, 1000 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1044.
The following signals are discernible for (Sa*,S*)-4ee: 1H NMR (400 MHz, CDCl3) δ = 5.23 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, CH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 127.2, 126.8, 125.9, 99.8, 97.7, 72.0.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow oil: 88% ee for (Sa,R)-4ee and 77% ee (Sa,S)-4ee; (Sa,R)-4ee/(Ra,R)-4ee = 7
1) as a yellow oil: 88% ee for (Sa,R)-4ee and 77% ee (Sa,S)-4ee; (Sa,R)-4ee/(Ra,R)-4ee = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (Sa,S)-4ee/(Ra,S)-4ee = 17
1; (Sa,S)-4ee/(Ra,S)-4ee = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(major) = 19.3 min, tR(minor) = 21.2 min, tR(minor) = 27.9 min, tR(major) = 37.7 min); [α]28D = +187.5 (c = 1.00, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.42–7.13 (m, 10 H, Ar–H), 6.31 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, one proton from CH
1; (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(major) = 19.3 min, tR(minor) = 21.2 min, tR(minor) = 27.9 min, tR(major) = 37.7 min); [α]28D = +187.5 (c = 1.00, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.42–7.13 (m, 10 H, Ar–H), 6.31 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.81 (dd, J1 = 11.4 Hz, J2 = 6.2 Hz, 1 H, one proton from CH
CH), 5.81 (dd, J1 = 11.4 Hz, J2 = 6.2 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), [5.31 (dd, J1 = 6.6 Hz, J2 = 1.8 Hz), 5.27 (dd, J1 = 7.2 Hz, J2 = 2.4 Hz), 1 H, CH], 2.65 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) (Sa*,R*)-4ee: δ = 203.8, 142.8, 128.57, 128.46, 127.72, 127.18, 126.77, 125.9, 99.8, 97.6, 72.3; (Sa*,S*)-4ee: δ = 203.5, 142.7, 128.58, 128.46, 127.75, 127.23, 126.81, 126.0, 99.9, 97.9, 72.1; MS (EI) m/z (%): 222 (M+, 12.57), 116 (100); IR (neat): v = 3332, 3083, 3061, 3029, 1950, 1882, 1809, 1699, 1598, 1494, 1456, 1193, 1157, 1100, 1072, 1027 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1041 and 222.1048.
CH), [5.31 (dd, J1 = 6.6 Hz, J2 = 1.8 Hz), 5.27 (dd, J1 = 7.2 Hz, J2 = 2.4 Hz), 1 H, CH], 2.65 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) (Sa*,R*)-4ee: δ = 203.8, 142.8, 128.57, 128.46, 127.72, 127.18, 126.77, 125.9, 99.8, 97.6, 72.3; (Sa*,S*)-4ee: δ = 203.5, 142.7, 128.58, 128.46, 127.75, 127.23, 126.81, 126.0, 99.9, 97.9, 72.1; MS (EI) m/z (%): 222 (M+, 12.57), 116 (100); IR (neat): v = 3332, 3083, 3061, 3029, 1950, 1882, 1809, 1699, 1598, 1494, 1456, 1193, 1157, 1100, 1072, 1027 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1041 and 222.1048.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow solid: 99% ee for (Sa,R)-4ee; (Sa,R)-4ee/(Ra,R)-4ee = 8
1) as a yellow solid: 99% ee for (Sa,R)-4ee; (Sa,R)-4ee/(Ra,R)-4ee = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(major) = 19.1 min, tR(minor) = 21.0 min, tR(minor) = 27.4 min, tR(minor) = 36.7 min); [α]27D = +168.6 (c = 1.00, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.43–7.12 (m, 10 H, Ar–H), 6.31 (dd, J1 = 6.4 Hz, J2 = 2.0 Hz, 1 H, one proton from CH
1 (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(major) = 19.1 min, tR(minor) = 21.0 min, tR(minor) = 27.4 min, tR(minor) = 36.7 min); [α]27D = +168.6 (c = 1.00, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.43–7.12 (m, 10 H, Ar–H), 6.31 (dd, J1 = 6.4 Hz, J2 = 2.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.81 (t, 1 H, J = 6.4 Hz, one proton from CH
CH), 5.81 (t, 1 H, J = 6.4 Hz, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.31 (dd, J1 = 6.8 Hz, J2 = 1.6 Hz, 1 H, CH), 2.68 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.8, 142.8, 133.57, 128.57, 128.5, 127.7, 127.19, 126.77, 125.9, 99.8, 97.6, 72.3; MS (EI) m/z (%): 222 (M+, 12.90), 116 (100); IR (neat): v = 3458, 3085, 3061, 3027, 1953, 1882, 1812, 1758, 1691, 1599, 1585, 1550, 1496, 1455, 1387, 1332, 1251, 1192, 1161, 1120, 1072, 1001 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1050.
CH), 5.31 (dd, J1 = 6.8 Hz, J2 = 1.6 Hz, 1 H, CH), 2.68 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.8, 142.8, 133.57, 128.57, 128.5, 127.7, 127.19, 126.77, 125.9, 99.8, 97.6, 72.3; MS (EI) m/z (%): 222 (M+, 12.90), 116 (100); IR (neat): v = 3458, 3085, 3061, 3027, 1953, 1882, 1812, 1758, 1691, 1599, 1585, 1550, 1496, 1455, 1387, 1332, 1251, 1192, 1161, 1120, 1072, 1001 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1050.
The following signals are discernible for (Ra*,R*)-4ee: 1H NMR (400 MHz, CDCl3), 5.27 (dd, J1 = 6.2 Hz, J2 = 2.2 Hz, 1 H, CH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 133.58, 128.60, 127.8, 127.23, 126.81, 126.0, 99.9, 97.9, 72.1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow oil: 99% ee for (Sa,S)-4ee; (Sa,S)-4ee/(Ra,S)-4ee = 19 (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 19.1 min, tR(minor) = 20.8 min, tR(minor) = 26.9 min, tR(minor) = 35.7 min); [α]27D = +201.3 (c = 1.04, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.45–7.16 (m, 10 H, Ar–H), 6.33 (dd, J1 = 6.4 Hz, J2 = 2.8 Hz, 1 H, one proton from CH
1) as a yellow oil: 99% ee for (Sa,S)-4ee; (Sa,S)-4ee/(Ra,S)-4ee = 19 (determined by HPLC) (HPLC conditions: Chiralcel OD column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 19.1 min, tR(minor) = 20.8 min, tR(minor) = 26.9 min, tR(minor) = 35.7 min); [α]27D = +201.3 (c = 1.04, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.45–7.16 (m, 10 H, Ar–H), 6.33 (dd, J1 = 6.4 Hz, J2 = 2.8 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.83 (t, J = 6.0 Hz, 1 H, one proton from CH
CH), 5.83 (t, J = 6.0 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.29 (dd, J1 = 6.0 Hz, J2 = 2.4 Hz, 1 H, CH), 2.52 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 133.6, 128.6, 128.5, 127.8, 127.3, 126.83, 126.0, 100.0, 98.0, 72.1; MS (EI) m/z (%): 222 (M+, 11.03), 116 (100); IR (neat): v = 3373, 3083, 3061, 3029, 1951, 1883, 1809, 1700, 1599, 1494, 1455, 1389, 1194, 1157, 1111, 1073, 1001 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1043.
CH), 5.29 (dd, J1 = 6.0 Hz, J2 = 2.4 Hz, 1 H, CH), 2.52 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 133.6, 128.6, 128.5, 127.8, 127.3, 126.83, 126.0, 100.0, 98.0, 72.1; MS (EI) m/z (%): 222 (M+, 11.03), 116 (100); IR (neat): v = 3373, 3083, 3061, 3029, 1951, 1883, 1809, 1700, 1599, 1494, 1455, 1389, 1194, 1157, 1111, 1073, 1001 cm−1; HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1043.
The following signals are discernible for (Ra*,S*)-4ee: 13C NMR (100 MHz, CDCl3) δ = 128.59, 127.78, 127.23, 126.8, 125.9, 99.9, 72.3.
Typical procedure II
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford a mixture of (Ra,R)-4ee and (Ra,S)-4ee (171.1 mg, 77%) as a yellow oil: 83% ee for (Ra,R)-4ee and 93% ee for (Ra,S)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 26
1) to afford a mixture of (Ra,R)-4ee and (Ra,S)-4ee (171.1 mg, 77%) as a yellow oil: 83% ee for (Ra,R)-4ee and 93% ee for (Ra,S)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (Ra,S)-4ee/(Sa,S)-4ee = 11
1; (Ra,S)-4ee/(Sa,S)-4ee = 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (determined by HPLC) (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 17.9 min, tR(major) = 20.0 min, tR(major) = 25.6 min, tR(minor) = 35.6 min); [α]23D = −167.5 (c = 1.03, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.50–7.10 (m, 10 H, Ar–H), 6.33–6.28 (m, 1 H, one proton from CH
1; (determined by HPLC) (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 17.9 min, tR(major) = 20.0 min, tR(major) = 25.6 min, tR(minor) = 35.6 min); [α]23D = −167.5 (c = 1.03, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.50–7.10 (m, 10 H, Ar–H), 6.33–6.28 (m, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.80 (dd, J1 = 11.8 Hz, J2 = 5.8 Hz, 1 H, one proton from CH
CH), 5.80 (dd, J1 = 11.8 Hz, J2 = 5.8 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.28 (dd, J1 = 14.0 Hz, J2 = 6.0 Hz, 1 H, CH), 2.69 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) (Ra*,R*)-4ee: δ = 203.5, 142.7, 133.57, 128.58, 128.444, 127.74, 127.22, 126.80, 125.98, 99.9, 97.8, 72.1; (Ra*,S*)-4ee: δ = 203.7, 142.8, 133.56, 128.55, 128.436, 127.71, 127.18, 126.76, 125.90, 99.8, 97.5, 72.3; MS (EI) m/z (%): 222 (M+, 27.67), 204 (100); IR (neat): v = 3450, 3062, 3029, 2924, 2852, 1950, 1598, 1492, 1450, 1256, 1189, 1025 cm−1.
CH), 5.28 (dd, J1 = 14.0 Hz, J2 = 6.0 Hz, 1 H, CH), 2.69 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) (Ra*,R*)-4ee: δ = 203.5, 142.7, 133.57, 128.58, 128.444, 127.74, 127.22, 126.80, 125.98, 99.9, 97.8, 72.1; (Ra*,S*)-4ee: δ = 203.7, 142.8, 133.56, 128.55, 128.436, 127.71, 127.18, 126.76, 125.90, 99.8, 97.5, 72.3; MS (EI) m/z (%): 222 (M+, 27.67), 204 (100); IR (neat): v = 3450, 3062, 3029, 2924, 2852, 1950, 1598, 1492, 1450, 1256, 1189, 1025 cm−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow oil: 81% ee for (Ra,R)-4ee and 91% ee for (Ra,S)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 23
1) as a yellow oil: 81% ee for (Ra,R)-4ee and 91% ee for (Ra,S)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (Ra,S)-4ee/(Sa,S)-4ee = 9
1; (Ra,S)-4ee/(Sa,S)-4ee = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; (determined by HPLC) (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 18.6 min, tR(major) = 20.1 min, tR(major) = 25.9 min, tR(minor) = 34.1 min); [α]28D = −188.7 (c = 1.02, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.43–7.11 (m, 10 H, Ar–H), 6.32 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, one proton from CH
1; (determined by HPLC) (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 18.6 min, tR(major) = 20.1 min, tR(major) = 25.9 min, tR(minor) = 34.1 min); [α]28D = −188.7 (c = 1.02, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.43–7.11 (m, 10 H, Ar–H), 6.32 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.84–5.79 (m, 1 H, one proton from CH
CH), 5.84–5.79 (m, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), [5.32 (dd, J1 = 6.4 Hz, J2 = 2.0 Hz), 5.28 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz), 1 H, CH], 2.56 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) (Ra*,R*)-4ee: δ = 203.5, 142.7, 133.60, 128.61, 128.48, 127.78, 127.3, 126.83, 126.0, 99.9, 97.9, 72.1; (Ra*,S*)-4ee: δ = 203.8, 142.8, 133.59, 128.58, 128.47, 127.75, 127.2, 126.78, 125.9, 99.8, 97.6, 72.3.
CH), [5.32 (dd, J1 = 6.4 Hz, J2 = 2.0 Hz), 5.28 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz), 1 H, CH], 2.56 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) (Ra*,R*)-4ee: δ = 203.5, 142.7, 133.60, 128.61, 128.48, 127.78, 127.3, 126.83, 126.0, 99.9, 97.9, 72.1; (Ra*,S*)-4ee: δ = 203.8, 142.8, 133.59, 128.58, 128.47, 127.75, 127.2, 126.78, 125.9, 99.8, 97.6, 72.3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow oil: 99% ee for (Ra,R)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 25
1) as a yellow oil: 99% ee for (Ra,R)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (based on HPLC) (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 17.7 min, tR(major) = 19.7 min, tR(minor) = 25.0 min, tR(minor) = 35.6 min); [α]24D = −198.0 (c = 0.995, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.42–7.10 (m, 10 H, Ar–H), 6.30–6.25 (m, 1 H, one proton from CH
1 (based on HPLC) (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 17.7 min, tR(major) = 19.7 min, tR(minor) = 25.0 min, tR(minor) = 35.6 min); [α]24D = −198.0 (c = 0.995, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.42–7.10 (m, 10 H, Ar–H), 6.30–6.25 (m, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.78 (t, J = 6.2 Hz, 1 H, one proton from CH
CH), 5.78 (t, J = 6.2 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.23 (d, J = 6.0 Hz, 1 H, CH), 2.83 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 133.6, 128.54, 128.4, 127.7, 127.2, 126.8, 126.0, 99.8, 97.7, 72.0; MS (EI) m/z (%): 222 (M+, 22.71), 131 (100); HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1046.
CH), 5.23 (d, J = 6.0 Hz, 1 H, CH), 2.83 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 133.6, 128.54, 128.4, 127.7, 127.2, 126.8, 126.0, 99.8, 97.7, 72.0; MS (EI) m/z (%): 222 (M+, 22.71), 131 (100); HRMS calcd for C16H14O [M+]: 222.1045, found: 222.1046.
The following signals are discernible for (Ra*,S*)-4ee: 13C NMR (100 MHz, CDCl3) δ = 203.8, 142.8, 128.51, 127.6, 127.1, 126.7, 125.9, 99.7, 97.4, 72.3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as a yellow oil: 99% ee for (Ra,R)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 20
1) as a yellow oil: 99% ee for (Ra,R)-4ee; (Ra,R)-4ee/(Sa,R)-4ee = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (based on HPLC) (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 19.6 min, tR(major) = 21.2 min, tR(minor) = 27.7 min, tR(minor) = 36.5 min; [α]24D = −181.0 (c = 1.02, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.54–7.00 (m, 10 H, Ar–H), 6.31 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, one proton from CH
1 (based on HPLC) (HPLC conditions: Chiralcel OD-H column, hexane/i-PrOH = 96/4, 1.0 mL min−1, λ = 214 nm, tR(minor) = 19.6 min, tR(major) = 21.2 min, tR(minor) = 27.7 min, tR(minor) = 36.5 min; [α]24D = −181.0 (c = 1.02, CHCl3); 1H NMR (400 MHz, CDCl3) δ = 7.54–7.00 (m, 10 H, Ar–H), 6.31 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.81 (t, J = 6.4 Hz, 1 H, one proton from CH
CH), 5.81 (t, J = 6.4 Hz, 1 H, one proton from CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 5.27 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, CH), 2.26 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 133.6, 128.59, 128.5, 127.75, 127.23, 126.82, 125.99, 99.9, 97.9, 72.1.
CH), 5.27 (dd, J1 = 6.4 Hz, J2 = 2.4 Hz, 1 H, CH), 2.26 (s, 1 H, OH); 13C NMR (100 MHz, CDCl3) δ = 203.5, 142.7, 133.6, 128.59, 128.5, 127.75, 127.23, 126.82, 125.99, 99.9, 97.9, 72.1.
The following signals are discernible for (Ra*,S*)-4ee: 1H NMR (400 MHz, CDCl3) δ = 5.32–5.29 (m, 1 H, CH); 13C NMR (100 MHz, CDCl3) δ = 128.57, 127.72, 127.18, 126.77, 125.92, 99.8, 97.6, 72.3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford (Ra)-4da (153.9 mg, 69%) as a white solid12a: 95% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR(major) = 9.9 min, tR (minor) = 10.6 min; [α]25D = −107.1 (c = 1.02, CHCl3) (reported value: 96% ee; [α]20D = −108.6 (c = 0.98, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.35–5.25 (m, 2 H, CH
1) to afford (Ra)-4da (153.9 mg, 69%) as a white solid12a: 95% ee (HPLC conditions: Chiralcel AD-H column, hexane/i-PrOH = 95/5, 0.6 mL min−1, λ = 214 nm, tR(major) = 9.9 min, tR (minor) = 10.6 min; [α]25D = −107.1 (c = 1.02, CHCl3) (reported value: 96% ee; [α]20D = −108.6 (c = 0.98, CHCl3)); 1H NMR (400 MHz, CDCl3) δ = 5.35–5.25 (m, 2 H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.05–1.90 (m, 1 H, CH from Cy), 1.80–1.42 (m, 15 H, protons from Cy and C
CH), 2.05–1.90 (m, 1 H, CH from Cy), 1.80–1.42 (m, 15 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5), 1.39–1.02 (m, 6 H, protons from Cy and C
CC(OH)(CH2)5), 1.39–1.02 (m, 6 H, protons from Cy and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(OH)(CH2)5); 13C NMR (100 MHz, CDCl3) δ = 199.9, 101.3, 100.7, 70.4, 38.3, 38.2, 37.2, 33.1, 33.0, 25.98, 25.96, 25.5, 22.43, 22.41; IR (neat): v = 3310, 2921, 2848, 1961, 1444, 1398, 1351, 1265, 1140, 1061; MS (EI) m/z (%): 220 (M+, 20.5), 99 (100).
CC(OH)(CH2)5); 13C NMR (100 MHz, CDCl3) δ = 199.9, 101.3, 100.7, 70.4, 38.3, 38.2, 37.2, 33.1, 33.0, 25.98, 25.96, 25.5, 22.43, 22.41; IR (neat): v = 3310, 2921, 2848, 1961, 1444, 1398, 1351, 1265, 1140, 1061; MS (EI) m/z (%): 220 (M+, 20.5), 99 (100).
Acknowledgements
Financial support from National Basic Research Program (2015CB856600) and National Natural Science Foundation of China (21232006) is greatly appreciated. We thank Miss Jing Zhou of our research group for reproducing the results presented in entries 8, 11 in Table 2, and eqn (2) in Scheme 4.Notes and references
- For reviews on the synthesis of allenes, see:
(a) L. K. Sydnes, Chem. Rev., 2003, 103, 1133 CrossRef CAS PubMed
; (b) N. Krause and A. Hoffmann-Röder, Tetrahedron, 2004, 60, 11671 CrossRef CAS
; (c) K. M. Brummond and J. E. Deforrest, Synthesis, 2007, 795 CrossRef CAS
; (d) M. Ogasawara, Tetrahedron: Asymmetry, 2009, 20, 259 CrossRef CAS
; (e) S. Yu and S. Ma, Chem. Commun., 2011, 47, 5384 RSC
; (f) R. K. Neff and D. E. Frantz, ACS Catal., 2014, 4, 519 CrossRef CAS
.
- For the most recent reviews on the chemistry of allenes, see:
(a) S. Ma, Chem. Rev., 2005, 105, 2829 CrossRef PubMed
; (b) S. Ma, Aldrichimica Acta, 2007, 40, 91 CAS
; (c) M. Brasholz, H.-U. Reissig and R. Zimmer, Acc. Chem. Res., 2009, 42, 45 CrossRef CAS PubMed
; (d) S. Ma, Acc. Chem. Res., 2009, 42, 1679 CrossRef CAS PubMed
; (e) B. Alcaide, P. Almendros and T. M. d. Campo, Chem. – Eur. J., 2010, 16, 5836 CrossRef CAS PubMed
; (f) C. Aubert, L. Fensterbank, P. Garcia, M. Malacria and A. Simonneau, Chem. Rev., 2011, 111, 1954 CrossRef CAS PubMed
; (g) F. Inagaki, S. Kitagaki and C. Mukai, Synlett, 2011, 594 CAS
; (h) F. Ĺopez and J. L. Mascareňas, Chem. – Eur. J., 2011, 17, 418 CrossRef PubMed
; (i) J. Ye and S. Ma, Acc. Chem. Res., 2014, 47, 989 CrossRef CAS PubMed
; (j) S. Kitagaki, F. Inagaki and C. Mukai, Chem. Soc. Rev., 2014, 43, 2956 RSC
; (k) B. Alcaide and P. Almendros, Acc. Chem. Res., 2014, 47, 939 CrossRef CAS PubMed
.
-
(a)
The Chemistry of the Allenes, ed. S. R. Landor, Academic Press, London, 1982 Search PubMed
; (b) Modern Allene Chemistry, ed. N. Krause and A. S. K. Hashmi, Wiley-VCH, Weinheim, 2004 Search PubMed
; (c) A. Hoffmann-Röder and N. Krause, Angew. Chem., Int. Ed., 2004, 43, 1196 CrossRef PubMed
.
-
(a) S. Ma, Acc. Chem. Res., 2003, 36, 701 CrossRef CAS PubMed
; (b) N. Krause, V. Belting, C. Deutsch, J. Erdsack, H.-T. Fan, B. Gockel, A. Hoffmann-Röder, N. Morita and F. Volz, Pure Appl. Chem., 2008, 80, 1063 CrossRef CAS
; (c) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS PubMed
.
- For selected recent reports, see:
(a) W. Zhang, H. Xu, H. Xu and W. Tang, J. Am. Chem. Soc., 2009, 131, 3832 CrossRef CAS PubMed
; (b) H. Qian, X. Yu, J. Zhang and J. Sun, J. Am. Chem. Soc., 2013, 135, 18020 CrossRef CAS PubMed
; (c) I. T. Crouch, R. K. Neff and D. E. Frantz, J. Am. Soc. Chem., 2013, 135, 4970 CrossRef CAS PubMed
; (d) T. Hashimoto, K. Sakata, F. Tamakuni, M. J. Dutton and K. Maruoka, Nat. Chem., 2013, 5, 240 CrossRef CAS PubMed
; (e) Y. Wang, W. Zhang and S. Ma, J. Am. Chem. Soc., 2013, 135, 11517 CrossRef CAS PubMed
; (f) B. Wan and S. Ma, Angew. Chem., Int. Ed., 2013, 52, 441 CrossRef CAS PubMed
.
- For a seminal work on the reactions with paraformaldehyde, see: P. Crabbé, H. Fillion, D. André and J. Luche, J. Chem. Soc., Chem. Commun., 1979, 859 RSC
.
-
(a) S. Ma, H. Hou, S. Zhao and G. Wang, Synthesis, 2002, 1643 CrossRef CAS
; (b) U. Kazmaier, S. Lucas and M. Klein, J. Org. Chem., 2006, 71, 2429 CrossRef CAS PubMed
; (c) H. Nakamura, T. Sugiishi and Y. Tanaka, Tetrahedron Lett., 2008, 49, 7230 CrossRef CAS
; (d) J. Kuang and S. Ma, J. Org. Chem., 2009, 74, 1763 CrossRef CAS PubMed
; (e) H. Luo and S. Ma, Eur. J. Org. Chem., 2013, 3041 CrossRef CAS
; (f) J. Kuang, X. Xie and S. Ma, Synthesis, 2013, 592 CAS
; (g) X. Huang, C. Fu and S. Ma, Synthesis, 2014, 2917 Search PubMed
.
- For a seminal work on the reactions with aldehydes mediated with ZnX2, see: J. Kuang and S. Ma, J. Am. Chem. Soc., 2010, 132, 1786 CrossRef CAS PubMed
.
- S. Kitagaki, M. Komizu and C. Mukai, Synlett, 2011, 1129 CrossRef CAS
.
- J. Kuang, H. Luo and S. Ma, Adv. Synth. Catal., 2012, 354, 933 CrossRef CAS
.
- For a seminal paper on the reactions with ketones, see: X. Tang, C. Zhu, T. Cao, J. Kuang, W. Lin, S. Ni, J. Zhang and S. Ma, Nat. Commun., 2013, 4, 2450 Search PubMed
.
- For the reaction using chiral α,α-dephenylprolinol for allene synthesis, see:
(a) J. Ye, S. Li, B. Chen, W. Fan, J. Kuang, J. Liu, Y. Liu, B. Miao, B. Wan, Y. Wang, X. Xie, Q. Yu, W. Yuan and S. Ma, Org. Lett., 2012, 14, 1346 CrossRef CAS PubMed
; (b) M. Periasamy, N. Sanjeevakumar, M. Dalai, R. Gurubrahamam and P. O. Reddy, Org. Lett., 2012, 14, 2932 CrossRef CAS PubMed
; (c) J. Ye, R. Lü, W. Fan and S. Ma, Tetrahedron, 2013, 69, 8959 CrossRef CAS
; (d) R. Lü, J. Ye, T. Cao, B. Chen, W. Fan, W. Lin, J. Liu, H. Luo, B. Miao, S. Ni, X. Tang, N. Wang, Y. Wang, X. Xie, Q. Yu, W. Yuan, W. Zhang, C. Zhu and S. Ma, Org. Lett., 2013, 15, 2254 CrossRef PubMed
; (e) J. Ye, W. Fan and S. Ma, Chem. – Eur. J., 2013, 19, 716 CrossRef CAS PubMed
; (f) J. Ye and S. Ma, Org. Synth., 2014, 91, 233 CrossRef CAS
; (g) X. Zhang, Y. Qiu, C. Fu and S. Ma, Org. Chem. Front., 2014, 1, 247 RSC
.
-
(a) V. K. Lo, Y. Liu, M. Wong and C. Che, Org. Lett., 2006, 8, 1529 CrossRef CAS PubMed
; (b) V. K. Lo, M. Wong and C. Che, Org. Lett., 2008, 10, 517 CrossRef CAS PubMed
; (c) V. K. Lo, C. Zhou, M. Wong and C. Che, Chem. Commun., 2010, 46, 213 RSC
.
- R. Gurubrahamam and M. Periasamy, J. Org. Chem., 2013, 78, 1463 CrossRef CAS PubMed
.
-
(a) A. Claesson and L.-I. Olsson, J. Am. Chem. Soc., 1979, 101, 7302 CrossRef CAS
; (b) T. Miura, M. Shimada, S.-Y. Ku, T. Tamai and M. Murakami, Angew. Chem., Int. Ed., 2007, 46, 7101 CrossRef CAS PubMed
; (c) J. Li, W. Kong, C. Fu and S. Ma, J. Org. Chem., 2009, 74, 5104 CrossRef CAS PubMed
; (d) Z. Li and S. Z. Zard, Org. Lett., 2009, 11, 2868 CrossRef CAS PubMed
.
- After CuBr and (R,Ra)-N-PINAP were treated in 2 mL of toluene for half an hour at room temperature, a yellow clear solution was formed; however, a pale yellow solid was formed from CuBr and (R,Sa)-N-PINAP under the same conditions.
-
(a) G. Lowe, Chem. Commun., 1965, 411 RSC
; (b) J. H. Brewster, Top. Stereochem., 1967, 2, 1 CAS
.
-
(a) D. Xu, Z. Li and S. Ma, Tetrahedron Lett., 2003, 44, 6343 CrossRef CAS
; (b) C. Raminelli, N. C. da Silva, A. A. D. Santos, A. L. M. Porto, L. H. Andrade and J. V. Comasseto, Tetrahedron, 2005, 61, 409 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: The experimental procedures, characterization data, and copies of 1H and 13C NMR spectra for all products. See DOI: 10.1039/c4ob02673j |
| This journal is © The Royal Society of Chemistry 2015 |