Open Access Article
Open Access ArticleAcid-promoted direct electrophilic trifluoromethylthiolation of phenols†
Marjan
Jereb
* and
Kaja
Gosak
Department of Organic Chemistry, Faculty of Chemistry and Chemical Technology, Večna pot 113, 1000 Ljubljana, Slovenia. E-mail: marjan.jereb@fkkt.uni-lj.si; Fax: (+386)1 241 9144; Tel: (+386)1 479 8577
First published on 13th January 2015
Abstract
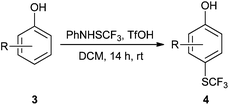
The electrophilic aromatic ring trifluoromethylthiolation of various substituted phenols was accomplished using PhNHSCF3 (N-trifluoromethylsulfanyl)aniline, (1) in the presence of BF3·Et2O (2) or triflic acid as the promoter. The functionalization was exclusively para-selective; phenols unsubstituted in both the ortho- and para positions solely gave the para-substituted SCF3-products in all cases, while para-substituted phenols gave the ortho-substituted SCF3-products. 3,4-Dialkyl substituted phenols yielded the corresponding products according to the Mills-Nixon effect, and estrone and estradiol furnished biologically interesting SCF3-analogues. The highly reactive catechol and pyrogallol substrates gave the expected products smoothly in the presence of BF3·Et2O, whereas less reactive phenols required triflic acid. 2-Allylphenol gave the expected p-SCF3 analogue, which underwent an addition/cyclization sequence and furnished a new di-trifluoromethylthio substituted 2,3-dihydrobenzofuran derivative. Some additional transformations of 4-(trifluoromethylthio)phenol with NBS, NIS, HNO3, HNO3/H2SO4 and 4-bromobenzyl bromide were performed giving bromo-, iodo-, nitro- and benzyl substituted products. The latter derivative underwent Suzuki–Miyaura coupling with phenylboronic acid.
Introduction
The introduction of a fluorine atom or a fluorine-containing substituent into an organic molecule often favorably modulates the compounds’ properties, thus making new functional and advanced materials.1–6 Fluorinated organic molecules frequently possess enhanced stability, binding affinity and biological activity in comparison with their non-fluorinated precursors.7,8 The trifluoromethyl group shares an exceedingly important part in biologically relevant molecules, and the number of newly introduced trifluoromethylated substances in the pharmaceutical and medicinal chemistry industries has been growing considerably.9–11 Consequently, there has been a strong interest in the direct introduction of CF3 groups into organic molecules regardless of the approach, via radical, nucleophilic or electrophilic modes, thus making trifluoromethylation a current topic of great interest.12–19An interesting variety of the CF3 group is the trifluoromethylthiol group SCF3, which considerably contributes to enhanced lipophilicity, and is an indispensable moiety in the agrochemistry and medicinal chemistry industries;20 however its introduction has received considerably less attention than the CF3 group. A 2′-SCF3 substituted uridine derivative was found to be a powerful label for probing the structure and function of RNA using 19F NMR spectroscopy.21
Recently, significant progress in the introduction of a SCF3 moiety has been achieved.22 The introduction of the SCF3 group could occur directly with CF3SCl23 or (CF3)2S,24 (extremely noxious and hazardous gases that are not suitable for non-specialized laboratories) or indirectly i.e. by interconversion of functional groups.25 Nucleophilic and radical sources of the SCF3 group are often copper-26 or silver-based27 metallic reagents, or [NH4][SCF3];28 furthermore, trifluoromethylthiolation can also be realized with a combination of two different sources for the sulfur functionality and CF3 group.29 In particular, the popularity of electrophilic trifluoromethylthiolation has grown remarkably in recent years; the new period of interest began with the work of Billard, Langlois and coworkers.30 They prepared PhNHSCF3 and its derivatives: easy-to-handle electrophilic SCF3-transfer reagents for use with alkenes and alkynes,31 indoles,32 tryptamines,33 organometallic species,34 amines,35 and allyl silanes.36 In addition, terminal alkynes were trifluoromethylthiolated in the presence of a catalytic amount of base.37 Internal alkynes reacted with PhNHSCF3, yielding the corresponding 3-(trifluoromethyl)thio derivatives of indoles,38 benzofurans, benzothiophenes39 and 1H-isochromen-1-ones.40 Similarly, 4-((trifluoromethyl)thio)-2H-benzo[e][1,2]thiazine 1,1-dioxides were efficiently prepared in the presence of BiCl3 in dichloroethane.41 An interesting trifluoromethanesulfonyl hypervalent iodonium ylide able to deliver the trifluoromethylthiol group was developed recently.42N-(Trifluoromethylthio)succinimide was utilized in the Pd-catalyzed trifluoromethylthiolation of arenes,43 while an in situ formed reagent from AgSCF3 and NCS was employed in the functionalization of alkynes.44 A new thioperoxide45,46 type of electrophilic trifluoromethylthiolating reagent was able to react with β-ketoesters, boronic acids,47 alkynes and aliphatic carboxylic acids,48 giving the corresponding SCF3-substituted products. This thioperoxide reagent in combination with TMSOTf was also found to be a powerful activating agent of different thioglycosides.49 Additionally, the thioperoxide reagent was used in enantioselective catalytic trifluoromethylthiolations,50 as well as N-trifluoromethylthiophthalimide51 and an AgSCF3/trichloroisocyanuric acid system.52 The latter system was also utilized in the synthesis of 3-((trifluoromethyl)thio)-4H-chromen-4-ones.53 Electrophilic trifluoromethylthiolation of various carbonyl compounds54 and aromatics55 was accomplished using a new N-((trifluoromethyl)thio)benzenesulfonamide type of reagent. N-Trifluoromethylthiosaccharin was developed recently and utilized in the trifluoromethylation of alcohols, amines, thiols, electron-rich aromatics, aldehydes, ketones, acyclic β-ketoesters and alkynes.56
PhNHSCF3 (N-trifluoromethylsulfanyl)aniline, (1) is a simple and easy-to-handle electrophilic reagent for the direct introduction of a SCF3 group into organic molecules. Its electrophilic power usually requires activation with an appropriate promoter of the Lewis or Brønsted type. Its reactivity is mostly unexplored, and we decided to test it on phenols, since there are many biologically relevant phenols i.e. steroids of estrone type, epinephrine, thymol, and others. We report on a direct and remarkably highly regioselective trifluoromethylthiolation of phenols using PhNHSCF3 in combination with a boron trifluoride etherate complex or triflic acid.
Results and discussion
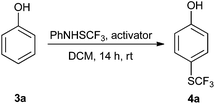
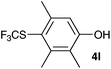
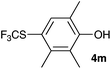
Initially, the reaction conditions were examined using phenol (3a) as a model substrate; the results are summarized in Table 1. Initially 3a was subjected to 1 without an activator, and remained unreacted (Table 1, entry 1). BF3·Et2O and p-TsOH·H2O were found to be rather unpromising promoters for this reaction (entries 2–5).We decided to examine the effect of considerably stronger activators, i.e. CH3SO3H (MSA) and triflic acid (TfOH). MSA was found to be a somewhat better activator (entries 6 and 7), while TfOH was the promoter of choice (entries 8 and 9). In all cases, 4-trifluoromethylthiophenol (4a) was obtained, and no ortho substitution was noted. Results from the functionalization of different phenols are presented in Table 2. 2-Methylphenol (3b) and 3-methylphenol (3c) both yielded the 4-SCF3-substituted products 4b and 4c exclusively (Table 2, entries 1 and 2). 4-Methylphenol (3d) and 4-i-propylphenol (3e) yielded the corresponding 2-SCF3-substituted products 4d and 4e as the sole products. In the cases of 2-t-butylphenol (3f) and 4-t-butylphenol (3g), ipso substitution could have been observed; however, the 4-SCF3-substituted 4f and 2-SCF3-substituted product 4g were formed as the sole products (entries 5 and 6). Similarly, 2-benzylphenol (3h) yielded the 4-SCF3-substituted product 4h, while no ipso substitution was observed. 4-Phenylphenol (3i) was regioselectively transformed into its 2-SCF3-substituted product 4i. The reactions of 2,5-dimethylphenol (3j) and 2,6-dimethylphenol (3k) cleanly furnished their 4-SCF3-substituted derivatives 4j and 4k (entries 9 and 10). 2,3,5-Trimethylphenol (3l) and 2,3,6-trimethylphenol (3m) gave their 4-SCF3-substituted derivatives 4l and 4m as the sole products (entries 11 and 12).
| Entry | Reactant | Product | Yieldb (%) |
|---|---|---|---|
| a Conditions: 3 (1 mmol), PhNHSCF3 (1, 1.2–1.3 mmol), TfOH (1.2–5 mmol), DCM (10 mL), 14 h, rt. b Isolated yield. | |||
| 1 | o-Cresol 3b |
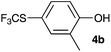
|
81 |
| 2 | m-Cresol 3c |
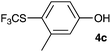
|
79 |
| 3 | p-Cresol 3d |
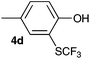
|
80 |
| 4 | 4-i-Propylphenol 3e |
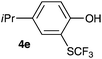
|
82 |
| 5 | 2-t-Butylphenol 3f |
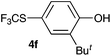
|
64 |
| 6 | 4-t-Butylphenol 3g |
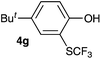
|
87 |
| 7 | 2-Benzylphenol 3h |
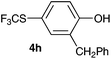
|
86 |
| 8 | 4-Phenylphenol 3i |
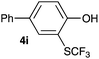
|
48 |
| 9 | 2,5-Dimethylphenol 3j |
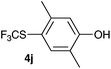
|
90 |
| 10 | 2,6-Dimethylphenol 3k |
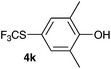
|
91 |
| 11 | 2,3,5-Trimethylphenol 3l |
|
84 |
| 12 | 2,3,6-Trimethylphenol 3m |
|
93 |
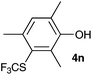
| 13 | 2,4,6-Trimethylphenol 3n |
|
83 |
2,4,6-Trimethylphenol (3n) was an interesting substrate, because all potentially reactive positions were substituted. None of the possible ipso adducts were observed, but 2,4,6-trimethyl-3-trifluoromethylthiophenol (4n) was isolated as the sole product (entry 13).
Next, we examined the reactivity of 3,4-dimethylphenol (5a), 5-indanol (5b) and 5,6,7,8-tetrahydro-2-naphthol (5c) (Scheme 1). Such compounds possess unequally reactive ortho positions; this phenomenon is known as the Mills–Nixon effect.575a furnished the expected 6a as the major product, while no 6aa was detected. Instead, double functionalization took place, yielding 6a′ as a minor product. The relative distribution ratio of 6a/6a′ was 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. 5b yielded only one expected product 6b; whereas no 6bb was detected. 5c reacted in accordance with the anticipated reactivity giving 6c and 6cc in a relative ratio of 56
6. 5b yielded only one expected product 6b; whereas no 6bb was detected. 5c reacted in accordance with the anticipated reactivity giving 6c and 6cc in a relative ratio of 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44. The regioselectivity of trifluoromethylthiolation for 3,4-dimethylphenol and 5-indanol is similar to that of their bromination reactions,58 while bromination of 5,6,7,8-tetrahydro-2-naphthol was more selective (78
44. The regioselectivity of trifluoromethylthiolation for 3,4-dimethylphenol and 5-indanol is similar to that of their bromination reactions,58 while bromination of 5,6,7,8-tetrahydro-2-naphthol was more selective (78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22) than its trifluoromethylthiolation. Nitration of 3,4-dimethylphenol (57
22) than its trifluoromethylthiolation. Nitration of 3,4-dimethylphenol (57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 43) and 5-indanol (58
43) and 5-indanol (58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42) with NaNO2/H2O2/H2SO4 was less regioselective than trifluoromethylthiolation, while nitration was more selective in the case of 5,6,7,8-tetrahydro-2-naphthol (63
42) with NaNO2/H2O2/H2SO4 was less regioselective than trifluoromethylthiolation, while nitration was more selective in the case of 5,6,7,8-tetrahydro-2-naphthol (63![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37).59
37).59
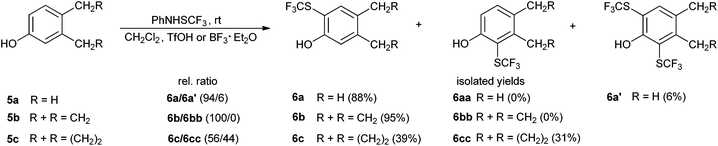 | ||
| Scheme 1 The effect of the structure of 3,4-dialkyl substituted phenols on trifluoromethylthiolation. | ||
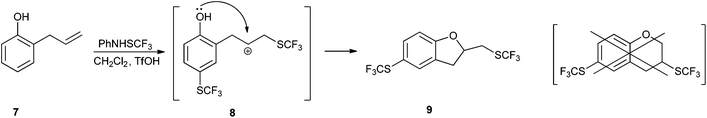
It is known that PhNHSCF3 reacts with alkenes,31 and so we examined the reactivity of 2-allylphenol (7) due to the two diverse potential reaction sites (Scheme 2). We established that 7 reacted with 1 in the presence of TfOH as both phenol and alkene, thus proposed a secondary carbocation intermediate 8. The reaction proceeded in dichloromethane in the absence of a good nucleophile, and the phenolic oxygen atom took part in an intramolecular cyclization thus producing five-membered product 9 as a novel type of trifluoromethylthiolated product. It appears that the stability of 8 was of crucial importance for the reaction selectivity. The other possible product, a six-membered isomeric product originating from a reversed addition of PhNHSCF3 to the double bond that would have generated a primary carbocation, was not observed. The structure of 9 was confirmed using NMR spectroscopy, and the key experiment was DEPT 135. The carbon atom attached to the SCF3 group appeared as a quartet in the same phase with the second aliphatic signal, whereas the third aliphatic signal was in the opposite phase. This was a clear indication that the SCF3 group was attached to the CH2, and not to a CH group.
The reactivity of highly reactive bicyclic-, alkoxy- and hydroxyphenols with PhNHSCF3 is summarized in Table 3.
| Entry | Reactant | Product | Yieldc (%) |
|---|---|---|---|
| a Conditions: 10 (1 mmol), PhNHSCF3 (1, 1.2–1.3 mmol), TfOH (1.3–4 mmol) or BF3·Et2O (2, 2–3 mmol), DCM (10 mL), 14 h, rt. b TfOH was used in entries 1, 2, 5, 6 and 7. BF3·Et2O was used in rest of the entries. c Isolated yield. | |||
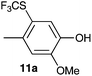
| 1 | 2-Methoxy-4-methylphenol 10a |
|
97 |
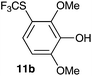
| 2 | 2,6-Dimethoxyphenol 10b |
|
84 |
| 3 | 5,6,7,8-Tetrahydronaphth-1-ol 10c |
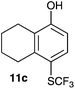
|
93 |
| 4 | 1-Naphthol 10d |
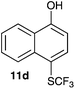
|
95 |
| 5 | 2-Naphthol 10e |
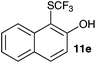
|
82 |
| 6 | 2,7-Dihidroxynaphthalene 10f |
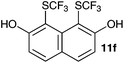
|
86 |
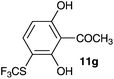
| 7 | 2′,6′-Dihydroxyacetophenone 10g |
|
79 |
| 8 | Catechol 10h |
|
70 |
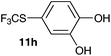
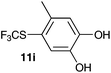
| 9 | 4-Methylcatechol 10i |
|
88 |
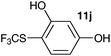
| 10 | Resorcinol 10j |
|
80 |
| 11 | Pyrogallol 10k |
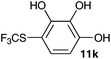
|
60 |
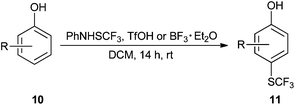
2-Methoxy-4-methylphenol (10a) produced 6-SCF3 analogue 11a as the sole product, while 2,6-dimethoxyphenol (10b) selectively yielded its 3-SCF3 derivative 11b (Table 3, entries 1 and 2). 5,6,7,8-Tetrahydro-1-naphthol (10c) was regioselectively transformed into its 4-SCF3 analogue 11c in good yield. The transformations of 1-naphthol (10d) and 2-naphthol (10e) were completely selective in both cases. The former led to its 4-SCF3 derivative 11d, whereas the latter led to its 1-SCF3 analogue 11e (entries 4 and 5). Additionally, 2,7-dihydroxynaphthalene (10f) was tested due to the possibility of double functionalization. Indeed, 1,8-diSCF3 derivative 11f was isolated as a single product in good yield. 2′,6′-dihydroxyacetophenone (10g) was smoothly converted into its 3-SCF3 derivative 11g (entry 7). In continuation, some naturally occurring phenols were successfully transformed into their trifluoromethylthio analogues. Catechol (10h) selectively produced its 4-SCF3 derivative 11h, and 4-methylcatechol (10i) yielded its 5-SCF3 derivative 11i exclusively (entries 8 and 9). Functionalization of resorcinol (10j) smoothly afforded its 4-SCF3 analogue 11j in a good yield. The highly oxidation-prone pyrogallol (10k) was also efficiently transformed into its 3-SCF3 derivative 11k (entry 11). It could be concluded that the reaction system of PhNHSCF3/activator is compatible with highly electron-rich phenols and that introduction of the SCF3 group took place efficiently without a noticeable amount of oxidation.
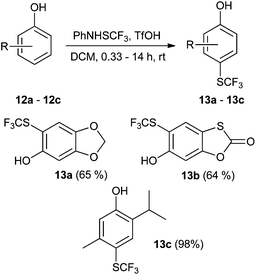
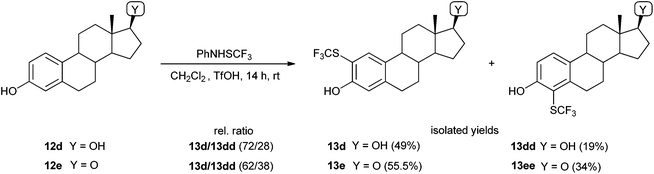
In addition, we tested some biologically relevant molecules possessing a phenolic functionality (Scheme 3). 3,4-(Methylenedioxy)phenol (12a) as a highly reactive substance afforded the corresponding 6-SCF3 derivative 13a as the sole product. The reaction was complete within 20 minutes, and the product, 13a, was obtained in good yield. 6-Hydroxy-1,3-benzoxathiol-2-one (12b) was successfully converted into its 5-SCF3 derivative 13b in spite of the acid-sensitive oxathiolone functional group. This is a good demonstration that the acidic reaction system is also compatible with sensitive functionalities. Thymol (12c) was effectively transformed into its 4-SCF3 derivative 13c in high yield. Estrone (12d) and estradiol (12e) are important steroid hormones bearing a phenolic functionality. Both were regioselectively transformed into the corresponding o-SCF3 analogues (13d, 13dd, and 13e, 13ee; Scheme 4). The regioselectivity of functionalization of estrone was similar to that of the nitration reaction;59 however, the selectivity of trifluoromethylthiolation was higher in the case of estradiol.
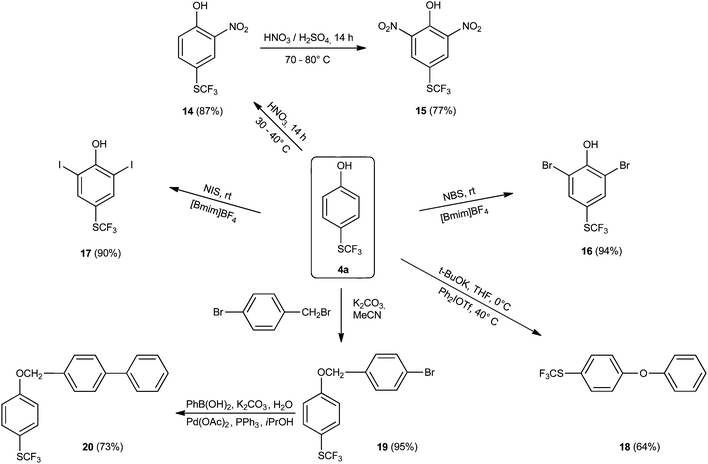
In addition, some further functionalizations of 4-(trifluoromethylthio)phenol (4a) were studied (Scheme 5). The strong electron-withdrawing nature of the trifluoromethylthio group significantly influences the reactivity of such compounds. 4a was nitrated with 65% HNO3 under solvent-free reaction conditions for 14 hours at 30–40 °C, selectively yielding mono-nitrated product 14, and no over-nitration took place. The reason for the perfect selectivity is that dinitration required considerably more rigorous reaction conditions to occur: 70–80° C and the presence of concentrated sulfuric acid. The dinitrophenol derivative 15 was isolated in a 78% yield. The introduction of halogens is highly significant because of the possibility for additional reactions, particularly various cross-couplings. Bromination of 4a with NBS in 1-methyl-3-butylimidazolium tetrafluoroborate ([Bmim]BF4) and in the presence of a small amount of water60 afforded dibromo analogue 16 in 94% yield. Iodination was performed using the same reaction medium, producing diiodo derivative 17. Preparation of diphenyl ether derivative 18 was accomplished using t-BuOK and diphenyliodonium triflate61 in THF. Finally, 4a was converted into its 4-bromobenzyl ether 19, which reacted with phenyl boronic acid in the presence of Pd(OAc)2 and PPh3 to yield a cross-coupled derivative 20 in good yield.
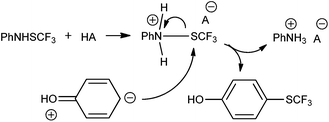
The detailed reaction mechanism is not known; however, the most likely reaction pathway is an electrophilic one. The reaction selectivity for para- and ortho- positions is one of the strong arguments in favor of an electrophilic pathway. The reagent PhNHSCF3 (1) is principally an amine and a relatively weak electrophilic reagent. The strong Brønsted acid TfOH seemingly protonates 1, thus forming a corresponding salt with considerably more pronounced polarization between the nitrogen and sulfur atoms. The strong electron deficiency of the nitrogen atom presumably tends to attract electron density; thus resulting in a weaker C–S bond and a stronger sulfur electrophile able to react with phenols (Scheme 6).
Trifluoromethylthiolation of m-cresol in the presence of the free radical TEMPO62,63 took place in the same manner as without TEMPO. This is another indication that a radical pathway is not very likely to be the chief reaction course.
Conclusions
In summary, we have developed a new highly regioselelective and efficient method for the trifluoromethylthiolation of phenols. The reaction pathway is most likely to be an electrophilic substitution pathway. The method is suitable for non-specialized laboratories because the transformation was accomplished with PhNHSCF3 and without the extremely noxious CF3SCl or (CF3)2S. An additional advantage was operational simplicity; specifically, the reactions were performed with non-dried dichloromethane in an air atmosphere at room temperature. The reaction selectivity was remarkably high; when both the para- and ortho- sites were unsubstituted, only para- functionalization took place. When the para- position was already substituted, functionalization of the ortho- site took place, and no ipso-substitution was noted. The reaction conditions were also demonstrated to be compatible with acid sensitive groups i.e. the thioxolone moiety.Experimental section
General information
All transformations were carried out in untreated dichloromethane under an air atmosphere with stirring at room temperature. The starting phenols and other chemicals were obtained from commercial sources; PhNHSCF31 was prepared using the literature procedure.30 The crude products were purified by column chromatography on silica gel (63–200 μm, 70–230 mesh ASTM; Fluka). TLC was performed on Merck-60-F254 plates using mixtures of hexane and diethyl ether. The melting points were determined in open-capillaries on Büchi 535 apparatus and are uncorrected. All products were characterized using 1H, 13C and 19F NMR spectra, IR, HRMS and/or elemental analysis, and also using the melting points when solid. The 1H and 13C NMR spectra were recorded on Bruker Avance 300 DPX and Bruker Avance III 500 instruments, while the 19F NMR spectra were only recorded on the latter instrument. Chemical shifts are reported in δ (ppm), values are relative to δ = 7.26 ppm in CDCl3 or δ = 2.05 ppm in acetone-d6 for 1H NMR, and to the central peak of CDCl3 (δ = 77.00 ppm) or the central peak of acetone-d6 (δ = 30.83 ppm) for 13C NMR. 19F NMR spectra are referenced to CFCl3 (δ = 0.00 ppm).Representative procedure for the acid-promoted trifluoromethylthiolaton of phenols
To a solution of phenol (1 mmol, or 0.5 mmol in the case of the steroids) in dichloromethane (10 mL), PhNHSCF3 (1.2–1.3 equiv.) was added along with a corresponding amount of triflic acid (1.2–5 equiv.) or BF3·Et2O (2–3 equiv.). The resulting mixture was stirred at room temperature for up to 16 h. In most cases, the starting phenol was fully consumed, as determined by TLC. The reaction mixture was diluted with 10 ml of dichloromethane, washed with a 10% solution of NaHCO3, then water and then dried over anhydrous Na2SO4. The crude reaction mixture was subjected to column chromatography after removal of the solvent. In numerous cases, this was only a ‘filtration’ over silica gel, because of the excellent reaction selectivity.Reactivity of 4a with different reagents
Spectroscopic and analytical data
Acknowledgements
Mrs T. Stipanovič and Prof. B. Stanovnik for elemental combustion analyses, Dr D. Urankar and Prof. J. Košmrlj for HRMS, and the Ministry of Higher Education, Science and Technology (P1-0134) for financial support are gratefully acknowledged.References
- W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed.
- K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed.
- P. Kirsch, Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, Wiley-VCH, Weinheim, 2006 Search PubMed.
- Modern Fluoropolymers: High Performance Polymers for Diverse Applications, ed. J. Scheirs, John Wiley & Sons, Chichester, 2000 Search PubMed.
- W. Grot, Fluorinated Ionomers, Elsevier, Oxford, 2011 Search PubMed.
- J. A. Gladysz, D. P. Curran and I. T. Horváth, Handbook of Fluorous Chemistry, Wiley-VCH, Weinheim, 2004 Search PubMed.
- A. M. Thayer, Chem. Eng. News, 2006, 84, 15–25 Search PubMed.
- K. L. Kirk, J. Fluorine Chem., 2006, 127, 1013–1029 CrossRef CAS PubMed.
- D. O'Hagan, J. Fluorine Chem., 2010, 131, 1071–1081 CrossRef PubMed.
- J.-P. Bégué and D. Bonnet-Delpon, J. Fluorine Chem., 2006, 127, 992–1012 CrossRef PubMed.
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC.
- (a) Z. Jin, G. B. Hammond and B. Xu, Aldrichimica Acta, 2012, 45, 67–83 CAS; (b) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294–8308 CrossRef CAS PubMed.
- J.-A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1–PR43 CrossRef CAS.
- N. Shibata, A. Matsnev and D. Cahard, Beilstein J. Org. Chem., 2010, 6, 65 Search PubMed.
- O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475–4521 CrossRef CAS PubMed.
- A. Studer, Angew. Chem., Int. Ed., 2012, 51, 8950–8958 CrossRef CAS PubMed.
- (a) P. Chen and G. Liu, Synthesis, 2013, 2919–2939 CAS; (b) G. Landelle, A. Panossian, S. Pazenok, J.-P. Vors and F. R. Leroux, Beilstein J. Org. Chem., 2013, 9, 2476–2536 CrossRef PubMed.
- X.-F. Wu, H. Neumann and M. Beller, Chem. – Asian J., 2012, 7, 1744–1754 CrossRef CAS PubMed.
- L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513–1522 CrossRef CAS PubMed.
- G. Landelle, A. Panossian and F. R. Leroux, Curr. Top. Med. Chem., 2014, 14, 941–951 CrossRef CAS.
- K. Fauster, C. Kreutz and R. Micura, Angew. Chem., Int. Ed., 2012, 51, 13080–13084 CrossRef CAS PubMed.
- (a) F. Toulgoat, S. Alazet and T. Billard, Eur. J. Org. Chem., 2014, 2415–2428 CrossRef CAS; (b) A. Tlili and T. Billard, Angew. Chem., Int. Ed., 2013, 52, 6818–6819 CrossRef CAS PubMed.
- S. Munavalli, D. K. Rohrbaugh, D. I. Rossman and H. D. Durst, J. Fluorine Chem., 1999, 98, 3–9 CrossRef CAS.
- L. Dieu, I. Popov and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 18237–18240 CrossRef PubMed.
- V. N. Boiko, Beilstein J. Org. Chem., 2010, 6, 880–921 CrossRef PubMed.
- (a) D. J. Adams, A. Goddard, J. H. Clark and D. J. Macquarrie, Chem. Commun., 2000, 987–988 RSC; (b) M. Hu, J. Rong, W. Miao, C. Ni, Y. Han and J. Hu, Org. Lett., 2014, 16, 2030–2033 CrossRef CAS PubMed; (c) M. Rueping, N. Tolstoluzhsky and P. Nikolaienko, Chem. – Eur. J., 2013, 19, 14043–14046 CrossRef CAS PubMed; (d) G. Danoun, B. Bayarmagnai, M. F. Gruenberg and L. J. Goossen, Chem. Sci., 2014, 5, 1312–1316 RSC; (e) Q. Lefebvre, E. Fava, P. Nikolaienko and M. Rueping, Chem. Commun., 2014, 50, 6617–6619 RSC; (f) D. Kong, Z. Jiang, S. Xin, Z. Bai, Y. Yuan and Z. Weng, Tetrahedron, 2013, 69, 6046–6050 CrossRef CAS PubMed; (g) P. Zhu, X. He, X. Chen, Y. You, Y. Yuan and Z. Weng, Tetrahedron, 2014, 70, 672–677 CrossRef CAS PubMed; (h) Z. Weng, W. He, C. Chen, R. Lee, D. Tan, Z. Lai, D. Kong, Y. Yuan and K.-W. Huang, Angew. Chem., Int. Ed., 2013, 52, 1548–1552 CrossRef CAS PubMed; (i) Z. Wang, Q. Tu and Z. Weng, J. Organomet. Chem., 2014, 751, 830–834 CrossRef CAS PubMed; (j) B. Bayarmagnai, C. Matheis, E. Risto and L. J. Goossen, Adv. Synth. Catal., 2014, 356, 2343–2348 CrossRef CAS.
- (a) Q. Xiao, J. Sheng, Q. Ding and J. Wu, Eur. J. Org. Chem., 2014, 217–221 CrossRef CAS; (b) F. Yin and X.-S. Wang, Org. Lett., 2014, 16, 1128–1131 CrossRef CAS PubMed; (c) X. Wang, Y. Zhou, G. Ji, G. Wu, M. Li, Y. Zhang and J. Wang, Eur. J. Org. Chem., 2014, 3093–3096 CrossRef CAS; (d) G. Teverovskiy, D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 7312–7314 CrossRef CAS PubMed; (e) D. J. Adams and J. H. Clark, J. Org. Chem., 2000, 65, 1456–1460 CrossRef CAS PubMed; (f) C. Chen, X.-H. Xu, B. Yang and F.-L. Qing, Org. Lett., 2014, 16, 3372–3375 CrossRef CAS PubMed.
- (a) C.-P. Zhang and D. A. Vicic, J. Am. Chem. Soc., 2012, 134, 183–185 CrossRef CAS PubMed; (b) C.-P. Zhang and D. A. Vicic, Chem. – Asian J., 2012, 7, 1756–1758 CrossRef CAS PubMed.
- See, for example: (a) C. Pooput, M. Medebielle and W. R. Dolbier Jr., Org. Lett., 2004, 6, 301–303 CrossRef CAS PubMed; (b) I. Kieltsch, P. Eisenberger and A. Togni, Angew. Chem., Int. Ed., 2007, 46, 754–757 CrossRef CAS PubMed; (c) N. Santschi and A. Togni, J. Org. Chem., 2011, 76, 4189–4193 CrossRef CAS PubMed; (d) S. Capone, I. Kieltsch, O. Flögel, G. Lelais, A. Togni and D. Seebach, Helv. Chim. Acta, 2008, 91, 2035–2056 CrossRef CAS; (e) C. Chen, Y. Xie, L. Chu, R.-W. Wang, X. Zhang and F.-L. Qing, Angew. Chem., Int. Ed., 2012, 51, 2492–2495 CrossRef CAS PubMed; (f) C. Chen, L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 12454–12457 CrossRef CAS PubMed; (g) L. Zhai, Y. Li, J. Yin, K. Jin, R. Zhang, X. Fu and C. Duan, Tetrahedron, 2013, 69, 10262–10266 CrossRef CAS PubMed; (h) V. N. Movchun, A. A. Kolomeitsev and Y. L. Yagupolskii, J. Fluorine Chem., 1995, 70, 255–257 CrossRef CAS; (i) T. Billard, S. Large and B. R. Langlois, Tetrahedron Lett., 1997, 38, 65–68 CrossRef CAS; (j) Y. Huang, X. He, X. Lin, M. Rong and Z. Weng, Org. Lett., 2014, 16, 3284–3287 CrossRef CAS PubMed.
- A. Ferry, T. Billard, B. R. Langlois and E. Bacqué, J. Org. Chem., 2008, 73, 9362–9365 CrossRef CAS PubMed.
- A. Ferry, T. Billard, B. R. Langlois and E. Bacqué, Angew. Chem., Int. Ed., 2009, 48, 8551–8555 CrossRef CAS PubMed.
- A. Ferry, T. Billard, E. Bacqué and B. R. Langlois, J. Fluorine Chem., 2012, 134, 160–163 CrossRef CAS PubMed.
- Y. Yang, X. Jiang and F.-L. Qing, J. Org. Chem., 2012, 77, 7538–7547 CrossRef CAS PubMed.
- F. Baert, J. Colomb and T. Billard, Angew. Chem., Int. Ed., 2012, 51, 10382–10385 CrossRef CAS PubMed.
- S. Alazet, K. Ollivier and T. Billard, Beilstein J. Org. Chem., 2013, 9, 2354–2357 CrossRef PubMed.
- J. Liu, L. Chu and F.-L. Qing, Org. Lett., 2013, 15, 894–897 CrossRef CAS PubMed.
- S. Alazet, L. Zimmer and T. Billard, Angew. Chem., Int. Ed., 2013, 52, 10814–10817 CrossRef CAS PubMed.
- J. Sheng, S. Li and J. Wu, Chem. Commun., 2014, 50, 578–580 RSC.
- J. Sheng, C. Fan and J. Wu, Chem. Commun., 2014, 50, 5494–5496 RSC.
- Y. Li, G. Li and Q. Ding, Eur. J. Org. Chem., 2014, 5017–5022 CrossRef CAS.
- Q. Xiao, J. Sheng, Z. Chen and J. Wu, Chem. Commun., 2013, 49, 8647–8649 RSC.
- Y.-D. Yang, A. Azuma, E. Tokunaga, M. Yamasaki, M. Shiro and N. Shibata, J. Am. Chem. Soc., 2013, 135, 8782–8785 CrossRef CAS PubMed.
- C. Xu and Q. Shen, Org. Lett., 2014, 16, 2046–2049 CrossRef CAS PubMed.
- S.-Q. Zhu, X.-H. Xu and F.-L. Qing, Eur. J. Org. Chem., 2014, 4453–4456 CrossRef CAS.
- X. Shao, X. Wang, T. Yang, L. Lu and Q. Shen, Angew. Chem., Int. Ed., 2013, 52, 3457–3460 CrossRef CAS PubMed.
- E. V. Vinogradova, P. Müller and S. L. Buchwald, Angew. Chem., Int. Ed., 2014, 53, 3125–3128 CrossRef CAS PubMed.
- X. Shao, T. Liu, L. Lu and Q. Shen, Org. Lett., 2014, 16, 4738–4741 CrossRef CAS PubMed.
- F. Hu, X. Shao, D. Zhu, L. Lu and Q. Shen, Angew. Chem., Int. Ed., 2014, 53, 6105–6109 CrossRef CAS PubMed.
- H. He and X. Zhu, Org. Lett., 2014, 16, 3102–3105 CrossRef CAS PubMed.
- (a) X. Wang, T. Yang, X. Cheng and Q. Shen, Angew. Chem., Int. Ed., 2013, 52, 12860–12864 CrossRef CAS PubMed; (b) Q.-H. Deng, C. Rettenmeier, H. Wadepohl and L. H. Gade, Chem. – Eur. J., 2014, 20, 93–97 CrossRef CAS PubMed.
- (a) T. Bootwicha, X. Liu, R. Pluta, I. Atodiresei and M. Rueping, Angew. Chem., Int. Ed., 2013, 52, 12856–12859 CrossRef CAS PubMed; (b) M. Rueping, X. Liu, T. Bootwicha, R. Pluta and C. Merkens, Chem. Commun., 2014, 50, 2508–2511 RSC.
- X.-L. Zhu, J.-H. Xu, D.-J. Cheng, L.-J. Zhao, X.-Y. Liu and B. Tan, Org. Lett., 2014, 16, 2192–2195 CrossRef CAS PubMed.
- H. Xiang and C. Yang, Org. Lett., 2014, 16, 5686–5689 CrossRef CAS PubMed.
- S. Alazet, L. Zimmer and T. Billard, Chem. – Eur. J., 2014, 20, 8589–8593 CrossRef CAS PubMed.
- S. Alazet and T. Billard, Synlett, 2015, 76–78 CAS.
- C. Xu, B. Ma and Q. Shen, Angew. Chem., Int. Ed., 2014, 53, 9316–9320 CrossRef CAS PubMed.
- W. H. Mills and I. G. Nixon, J. Chem. Soc., 1930, 2510–2524 RSC.
- J. L. G. Nilsson, H. Selander, H. Sievertsson, I. Skånberg and K.-G. Svensson, Acta Chem. Scand., 1971, 25, 94–100 CrossRef CAS PubMed.
- M. Jereb, Curr. Org. Chem., 2013, 17, 1694–1700 CrossRef CAS.
- R.-Y. Tang, P. Zhong and Q.-L. Lin, J. Fluorine Chem., 2007, 128, 636–640 CrossRef CAS PubMed.
- N. Jalalian, T. B. Petersen and B. Olofsson, Chem. – Eur. J., 2012, 18, 14140–14149 CrossRef CAS PubMed.
- W. Kong, M. Casimiro, N. Fuentes, E. Merino and C. Nevado, Angew. Chem., Int. Ed., 2013, 52, 13086–13090 CrossRef CAS PubMed.
- E. Pair, N. Monteiro, D. Bouyssi and O. Baudoin, Angew. Chem., Int. Ed., 2013, 52, 5346–5349 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Copies of the 1H, 13C and 19F NMR spectra for all products. See DOI: 10.1039/c4ob02633k |
| This journal is © The Royal Society of Chemistry 2015 |