Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Templating carbohydrate-functionalised polymer-scaffolded dynamic combinatorial libraries with lectins†
Clare S.
Mahon
ab,
Martin A.
Fascione
bc,
Chadamas
Sakonsinsiri
b,
Tom E.
McAllister
b,
W.
Bruce Turnbull
b and
David A.
Fulton
*a
aChemical Nanoscience Laboratory, School of Chemistry, Newcastle University, Newcastle-upon-Tyne, UK. E-mail: david.fulton@ncl.ac.uk; Fax: +44 (0)191 222 6929; Tel: +44 (0)191 208 7065
bSchool of Chemistry and Astbury Centre for Structural Molecular Biology, University of Leeds, Leeds, UK
cDepartment of Chemistry, University of York, Heslington, York YO10 5DD, UK
First published on 9th January 2015
Abstract
A conceptually new approach to the design of macromolecular receptors for lectins is outlined. Carbohydrate-functionalised Polymer-Scaffolded Dynamic Combinatorial Libraries (PS-DCLs) have been prepared in aqueous solution by the reversible conjugation of carbohydrates possessing acylhydrazide functionalities in their aglycone on to an aldehyde-functionalised polymer scaffold. PS-DCLs have been shown to undergo compositional change in response to the addition of lectin templates, with polymer scaffolds preferentially incorporating carbohydrate units which recognise the lectin added. This compositional change has been shown to generate polymers of significantly enhanced affinity for the lectin added, with enhancements in free energy of binding in the range of 5.2–8.8 kJ mol−1 observed. Experiments indicate that these enhancements are not only as a consequence of increased display of the preferred carbohydrate upon the polymer scaffold, but that templation also reorganises key residues into strategic positions in order to interact more strongly with the target.
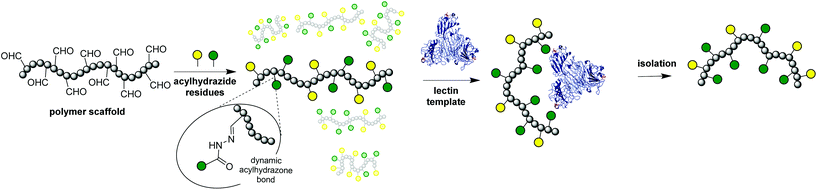
Polymer-Scaffolded Dynamic Combinatorial Libraries (PS-DCLs)1–4 (Fig. 1) have been demonstrated to present a viable route towards the generation of macromolecular receptors for synthetic polymers and proteins. PS-DCLs are mixtures of interconverting polymers generated through the reversible functionalization of synthetic polymer scaffolds with various side-chain residues, which undergo compositional exchange upon exposure to macromolecular templates to produce polymers of enhanced affinities towards the template. The macromolecular nature of PS-DCLs makes them ideally suited to the development of receptors for biologically-relevant macromolecules such as proteins, which typically interact with one another over large surface areas,5 and present the opportunity to harness multivalency6 in order to increase the influence of many relatively ‘weak’ supramolecular interactions. Indeed, PS-DCLs have been used to deliver polymers of measurably enhanced affinities4 towards synthetic polymers and proteins, by exploiting multivalent electrostatic interactions between charged polymeric receptors and templates.
Synthetic multivalent receptors such as those produced by the PS-DCL approach would be particularly useful in the recognition of carbohydrate-binding proteins (lectins) which are crucial to cellular recognition7 and often implicated in bacterial and viral infection.8 Some pathogenic bacteria, notably Vibrio cholerae, the causative agent of cholera, and Escherichia coli (E. coli), cause disease through the production of toxic lectins which bind to carbohydrates on cellular surfaces, facilitating entry to cells and initiating a biochemical cascade resulting in diarrhoea which may be life-threatening.9 Compounds which may inhibit these initial recognition processes are understandably of interest to chemists and clinicians.10
Lectins often display multiple identical recognition sites which may interact with multiple carbohydrates through low-affinity supramolecular interactions which reinforce one another to facilitate high-affinity binding, with greatly enhanced activities compared to monovalent inhibitors. The attachment of multiple carbohydrates to a molecular scaffold to facilitate their simultaneous binding at multiple sites is therefore a popular approach to inhibitor design, and is perhaps most successfully demonstrated by the success of glyco-dendrimers11 in inhibiting carbohydrate–protein interactions. Often, such inhibitors constitute elegant yet synthetically-challenging molecular architectures, with their production often time- and labour-intensive. An alternative strategy has seen polymers comprised of carbohydrate monomers connected through dynamic covalent linkages (‘dynamers’12) produced. These dynamers have been shown to recognise lectins, however, templation has not been used to enhance binding affinities of the polymers towards a target lectin.
The use of a system where pre-formed polymer scaffolds could be decorated with the required carbohydrates would enable convenient access to the large molecular architectures offered by dendrimers, with well-established living radical polymerisation methods such as RAFT13 allowing the necessary control over the molecular weight distribution of polymers. Most importantly, the dynamic nature of PS-DCLs would allow carbohydrates the opportunity to exchange and reshuffle their positions along polymer scaffolds in order to occupy optimum positions for interaction with a lectin template. This adaptive behaviour presents a conceptually new approach to the generation of polymeric receptors for carbohydrate-binding proteins, in addition to expanding the scope of the PS-DCL concept as a platform to new macromolecular receptors. Promising initial results investigating the potential of this concept are reported here.
Results and discussion
PS-DCL preparation and characterisation
The PS-DCLs in this work have been designed to explore specific molecular recognition between two very different lectins and their complementary carbohydrates. Concanavalin A (Con A) (Fig. 2) is a lectin isolated from Canavalia ensiformis (Jack bean), which exists at neutral pH as a tetramer of four identical 26 kDa subunits,14 each bearing a single mannose recognition site incorporating a penta-coordinated Ca2+ ion and a hexa-coordinated Mn2+ ion. These mannose-binding sites are located at the points of a tetrahedron, approximately 72 Å apart.14 In solutions of pH < 5.6, Con A tetramers dissociate to yield dimers which may also recognise mannose through complexation at two sites. Con A serves as a useful ‘model lectin’ as its recognition behaviour has been well-studied.15E. coli heat labile toxin (LTB)16 (Fig. 2) belongs to the AB5 family of toxins,17 and exhibits recognition behaviour identical to that of cholera toxin.18 The single A subunit is responsible for the toxicity of the protein, with the B-pentamer facilitating entry of the toxin into cells by binding to a galactose-terminated ganglioside displayed on cellular surfaces. For the purposes of this investigation, a modified B5 variant of the toxin has been used.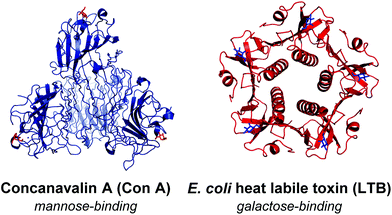 | ||
| Fig. 2 Lectin templates used within PS-DCLs. (a) Con A shown as a tetramer with four associated mannose residues shown in blue (5CNA.pdb). (b) LTB with five associated galactose residues shown in blue (1LTA.pdb). | ||
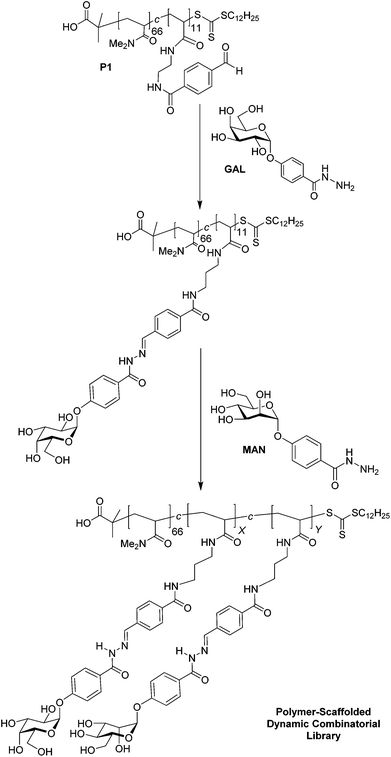
PS-DCLs incorporating GAL and MAN have been constructed on the aldehyde-functionalised poly(acrylamide) scaffold P1 (Scheme 1). Acylhydrazide residues GAL and MAN display galactose and mannose units and may be expected to interact favourably with LTB and Con A, respectively. Conjugation of GAL onto P1 generates a polymer displaying multiple galactose units, with complete reaction of all aldehyde units of the polymer confirmed by 1H NMR spectroscopy. Addition of a second acylhydrazide MAN initiates component exchange through a transimination-type process yielding a PS-DCL of polymers decorated with carbohydrate residues GAL and MAN. The composition of the carbohydrates appended to the polymer scaffold cannot be monitored directly using 1H NMR spectroscopy as a consequence of overlap of diagnostic signals corresponding to the residues attached to the polymer scaffold. Instead, the residual composition of carbohydrate-functionalised PS-DCLs may be determined indirectly using 1H NMR spectroscopy. Integral analysis of resonances corresponding to the anomeric protons of GAL and MAN was used to determine the relative concentration of these unconjugated residues in solution, allowing the relative proportion of each carbohydrate on the polymer scaffold to be determined. Equilibrium was attained overnight, with 1H NMR spectroscopic analysis revealing GAL and MAN to be present in solution in a 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 ratio, implying that the residual composition of the polymer scaffolds was also 1.0
1.0 ratio, implying that the residual composition of the polymer scaffolds was also 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0. No aldehyde signal was observed, indicating that polymers are fully functionalised with carbohydrate residues. The composition of the PS-DCL was monitored over a period of 48 h, without further deviation from this composition. This observation suggests that, in the absence of any template, the polymer scaffold displays no preference for the incorporation of either acylhydrazide GAL or MAN.
1.0. No aldehyde signal was observed, indicating that polymers are fully functionalised with carbohydrate residues. The composition of the PS-DCL was monitored over a period of 48 h, without further deviation from this composition. This observation suggests that, in the absence of any template, the polymer scaffold displays no preference for the incorporation of either acylhydrazide GAL or MAN.
PS-DCLs undergo compositional change in response to addition of lectin templates
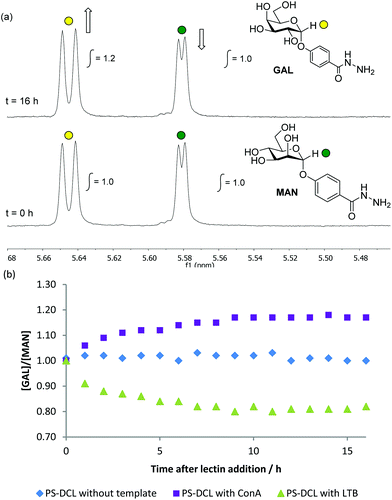
Initial templating experiments were performed using Con A as a template. Upon addition of Con A, changes in the composition of the PS-DCL as a function of time were monitored using 1H NMR spectroscopy, which revealed an increase in the relative concentration of GAL compared to MAN of 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 (Fig. 3(a) and (b)). This observation suggests that polymer scaffolds have responded to the addition of Con A by preferentially incorporating the mannose-functionalised MAN at the expense of the galactose-functionalised GAL. It is proposed that this templating effect proceeds as a consequence of additional stability gained by library members through their favourable interactions with Con A dimers, which preferentially bind to mannose in preference to galactose residues. The templating effect of LTB upon the PS-DCL was also investigated. Upon addition of LTB, 1H NMR spectroscopy revealed a decrease in the relative concentration of GAL compared to MAN of 0.8
1.0 (Fig. 3(a) and (b)). This observation suggests that polymer scaffolds have responded to the addition of Con A by preferentially incorporating the mannose-functionalised MAN at the expense of the galactose-functionalised GAL. It is proposed that this templating effect proceeds as a consequence of additional stability gained by library members through their favourable interactions with Con A dimers, which preferentially bind to mannose in preference to galactose residues. The templating effect of LTB upon the PS-DCL was also investigated. Upon addition of LTB, 1H NMR spectroscopy revealed a decrease in the relative concentration of GAL compared to MAN of 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 from an initial ratio of 1.0
1.0 from an initial ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 (Fig. 3(b)). This observation suggests that polymer scaffolds have preferentially incorporated the galactose-functionalised GAL, rejecting the mannose-functionalised MAN. This templating effect is likely to be a consequence of favourable interactions between LTB and polymers functionalised primarily with the preferred residue GAL. These observations demonstrate that carbohydrate-functionalised PS-DCLs possess that same capacity for template-induced re-equilibration exhibited by other PS-DCLs,2–4 and suggest that favourable multivalent interactions between template and library members provide enough of a thermodynamic driving force to cause compositional exchange upon template addition.
1.0 (Fig. 3(b)). This observation suggests that polymer scaffolds have preferentially incorporated the galactose-functionalised GAL, rejecting the mannose-functionalised MAN. This templating effect is likely to be a consequence of favourable interactions between LTB and polymers functionalised primarily with the preferred residue GAL. These observations demonstrate that carbohydrate-functionalised PS-DCLs possess that same capacity for template-induced re-equilibration exhibited by other PS-DCLs,2–4 and suggest that favourable multivalent interactions between template and library members provide enough of a thermodynamic driving force to cause compositional exchange upon template addition.
The best-binding polymers within PS-DCLs may be isolated
A key validation of the hypothesis that templating PS-DCLs presents a viable route to polymeric receptors for lectins is the isolation from a PS-DCL of the best-binding fraction of the library, and demonstration that these polymers exhibit significantly enhanced affinity for the lectin template. We have previously4 isolated best-binding polymers from PS-DCLs using solid-supported templates and envisioned a similar strategy could be employed in this case.‘Templation vessels’ were developed by functionalising commercially-available streptavidin-functionalised 96-well plates with biotinylated Con A or LTB, allowing for convenient exposure of PS-DCLs to surface-immobilised lectin templates. It was proposed that those library members which interacted most favourably with the template would become attached to the surfaces of the wells, presenting a straightforward route to their isolation from the rest of the system, which may simply be pipetted away.
Templating experiments were performed using PS-DCLs constructed with acylhydrazides GAL and MAN upon polymer scaffold P1. PS-DCLs were shown to contain equal concentrations of unconjugated GAL and MAN using 1H NMR spectroscopy prior to templation, indicating that both carbohydrates were incorporated onto polymer scaffolds in equal proportions. After 18 h incubation at 5 °C in lectin-functionalised wells, 1H NMR spectroscopic analysis revealed compositional change within both systems – a decrease in the concentration of MAN compared to GAL of 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 from an initial ratio of 1.0
1.0 from an initial ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 in Con A-functionalised wells, and a decrease in the relative concentration of GAL compared to MAN of 0.9
1.0 in Con A-functionalised wells, and a decrease in the relative concentration of GAL compared to MAN of 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 from an initial ratio of 1.0
1.0 from an initial ratio of 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 in LTB-functionalised wells. In both cases, polymer scaffolds have preferentially incorporated the carbohydrate known to interact favourably with the displayed lectin. There was no significant change in the composition of PS-DCLs incubated in 96-well plates which had not been treated with biotinyl-LTB or biotinyl-Con A, eliminating the possibility that streptavidin could induce unwanted compositional change within these PS-DCLs. Polymer members of the library which do not bind well to the lectin-functionalized surface were then removed from the wells, and the surfaces of the wells were washed with a denaturant solution (50 mM EDTA in D2O in the case of ConA-functionalised wells, or 5.0 M guanidinium chloride, 0.5 M NaCl in D2O in the case of LTB-functionalised wells) to disrupt interactions between templates and those polymers bound to the surfaces of wells. These washings were shown using 1H NMR spectroscopy to contain polymeric species which were reduced using NaCNBH3 to prevent unwanted compositional change during binding studies and then purified by dialysis. The concentration of carbohydrate-functionalised polymers in the material isolated may be determined by absorbance measurements (ε = 0.264 μM cm−1) (ESI†). In each case around 10% by mass of polymers within the PS-DCL was isolated. These polymers (T-Con A, T-LTB) were then titrated against Con A or LTB to determine association constants. For comparison, polymer scaffolds with identical residual composition to an untemplated PS-DCL (UT), a PS-DCL templated with the relevant lectin (TC-Con A and TC-LTB) or functionalised solely with GAL or MAN (P-GAL and P-MAN) were prepared and their affinities towards the lectins determined in the same way (Table 1). Binding affinities were established at pH 4.5, under conditions identical to those used during templating, and under neutral conditions, to establish if interactions between polymers and lectins differed at increased pH. It should be noted that while TC-Con A and TC-LTB display GAL and MAN in the same relative concentrations as polymers within a templated PS-DCL, they have not been exposed to either lectin and therefore lack possible sequence-specific information which may be gained during the templating process.
1.0 in LTB-functionalised wells. In both cases, polymer scaffolds have preferentially incorporated the carbohydrate known to interact favourably with the displayed lectin. There was no significant change in the composition of PS-DCLs incubated in 96-well plates which had not been treated with biotinyl-LTB or biotinyl-Con A, eliminating the possibility that streptavidin could induce unwanted compositional change within these PS-DCLs. Polymer members of the library which do not bind well to the lectin-functionalized surface were then removed from the wells, and the surfaces of the wells were washed with a denaturant solution (50 mM EDTA in D2O in the case of ConA-functionalised wells, or 5.0 M guanidinium chloride, 0.5 M NaCl in D2O in the case of LTB-functionalised wells) to disrupt interactions between templates and those polymers bound to the surfaces of wells. These washings were shown using 1H NMR spectroscopy to contain polymeric species which were reduced using NaCNBH3 to prevent unwanted compositional change during binding studies and then purified by dialysis. The concentration of carbohydrate-functionalised polymers in the material isolated may be determined by absorbance measurements (ε = 0.264 μM cm−1) (ESI†). In each case around 10% by mass of polymers within the PS-DCL was isolated. These polymers (T-Con A, T-LTB) were then titrated against Con A or LTB to determine association constants. For comparison, polymer scaffolds with identical residual composition to an untemplated PS-DCL (UT), a PS-DCL templated with the relevant lectin (TC-Con A and TC-LTB) or functionalised solely with GAL or MAN (P-GAL and P-MAN) were prepared and their affinities towards the lectins determined in the same way (Table 1). Binding affinities were established at pH 4.5, under conditions identical to those used during templating, and under neutral conditions, to establish if interactions between polymers and lectins differed at increased pH. It should be noted that while TC-Con A and TC-LTB display GAL and MAN in the same relative concentrations as polymers within a templated PS-DCL, they have not been exposed to either lectin and therefore lack possible sequence-specific information which may be gained during the templating process.
| Library | Template | K a/M−1 | ΔG/kJ mol−1 | ΔΔG/kJ mol−1 | |
|---|---|---|---|---|---|
| a Errors associated with these measurements are very large, as a consequence of very limited association between these polymers and lectins. | |||||
| pH 4.5 | T-ConA | Con A | 3.76 × 106 ± 2.26 × 105 | −36.9 | −5.2 |
| UT | Con A | 4.45 × 105 ± 2.64 × 104 | −31.7 | 0 | |
| TC-Con A | Con A | 5.32 × 105 ± 3.20 × 104 | −32.1 | −0.4 | |
| P-MAN | Con A | 5.26 × 105 ± 4.77 × 104 | −32.1 | −0.4 | |
| P-GALa | Con A | 4.66 ± 1.12 × 104 | −9.36 | +22.3 | |
| T-LTB | LTB | 1.74 × 106 ± 6.97 × 104 | −35.0 | −5.7 | |
| UT | LTB | 1.68 × 105 ± 1.69 × 104 | −29.3 | 0 | |
| TC-LTB | LTB | 6.27 × 105 ± 5.05 × 104 | −32.5 | −3.2 | |
| P-MANa | LTB | 1.06 ± 985.8 | −0.1 | +29.2 | |
| P-GAL | LTB | 2.42 × 105 ± 4.83 × 103 | −30.2 | −0.9 | |
| pH 7.1 | T-Con A | Con A | 7.69 × 106 ± 4.63 × 105 | −38.6 | −7.1 |
| UT | Con A | 4.12 × 105 ± 6.32 × 104 | −31.5 | 0 | |
| TC-Con A | Con A | 4.77 × 105 ± 1.43 × 104 | −31.9 | −0.4 | |
| P-MAN | Con A | 6.62 × 105 ± 4.65 × 104 | −32.7 | −1.2 | |
| P-GALa | Con A | 0.181 ± 5.53 × 103 | 4.17 | +35.7 | |
| T-LTB | LTB | 6.11 × 106 ± 9.38 × 105 | −38.1 | −8.8 | |
| UT | LTB | 1.64 × 105 ± 1.32 × 104 | −29.3 | 0 | |
| TC-LTB | LTB | 1.76 × 105 ± 1.24 × 104 | −29.4 | −0.1 | |
| P-MANa | LTB | 0.625 ± 4.27 × 104 | 1.10 | +30.4 | |
| P-GAL | LTB | 2.45 × 105 ± 9.83 × 103 | −30.2 | −0.9 | |
Polymers isolated from the surfaces of wells display significantly enhanced affinities for the relevant lectin, with an order of magnitude enhancement in association constants observed compared to the untemplated library UT (Table 1). Enhancements in free energy of binding of 5.2–8.8 kJ mol−1 compared to UT were observed for T-Con A and T-LTB, which also display significantly improved recognition characteristics compared to polymers functionalised only with the preferred carbohydrate (P-GAL and P-MAN) and polymer scaffolds with residual compositions matching those of templated PS-DCLs (TC-Con A and TC-LTB). Polymers functionalised only with one carbohydrate (P-GAL and P-MAN) do not display affinities towards the lectin which does not recognise the carbohydrate, as may be expected. These observations suggest that templating leads to the positioning of key residues along polymer scaffolds in specific positions in order to more strongly interact with the lectin they have been exposed to. It is perhaps unimportant which residues are present on those sections of polymer scaffold which bridge the binding sites of the lectin template, but it is conceivable that clusters of preferred residues may compete for interaction with binding sites, introducing steric crowding which may prove detrimental to recognition. Similar behaviour has been observed by Whitesides et al.19 in their investigation of polyvalent sialic acid inhibitors of haemagglutinin, with some polymeric receptors demonstrated to display poorer inhibitory activity than monomer units on a per monomer basis, observations which were attributed to heteroglycocluster effects.20 Heteroglycoconjugates displaying mannose and galactose units have been demonstrated21 to display higher relative potencies in their interactions with Con A than the corresponding mannose-homoglycoconjugates. Additionally, Haddleton et al.22 have reported that increasing mannose content beyond 75% in a library of mannose and galactose containing copolymers yields no improvement in agglutination activity of polymers towards Con A, implying a higher efficiency for heteroglycopolymers on a per mannose basis.
These observations demonstrate that templating PS-DCLs may deliver polymers of markedly improved affinity to lectins, with affinities surpassing those of expected ‘ideal’ binders, and validates the concept as a route to the generation of macromolecular receptors for these species.
Conclusions
Carbohydrate-functionalised PS-DCLs have been shown to adapt their composition in response to the addition of lectin templates, with polymer scaffolds preferentially incorporating the carbohydrate known to interact favourably with the lectin added. Experiments suggest that recognition processes between lectins and carbohydrate-functionalised polymers are highly specific, with polymers adapting their compositions so as to display the required carbohydrate residues at strategic positions along the polymer chain to facilitate stronger recognition at binding sites on the lectin.Thermodynamically-driven templating has been shown to afford compositional change within PS-DCLs which delivers polymers of enhanced affinities for lectin templates. The immobilisation of lectins on 96-well plates to produce ‘templation vessels’ has enabled the isolation of the best-binding fraction of the PS-DCL, separating those polymers of highest affinities towards the template from the rest of the system. These polymers have been shown to display significantly enhanced affinities for the lectin added, with enhancements in free energy of binding of 5.2–8.8 kJ mol−1 observed. The development of a method to immobilise protein templates onto solid supports using commercially-available materials allows for the rapid expansion of the PS-DCL concept to provide polymeric receptors for a wide range of bacterial toxins and other proteins of interest. The use of 96-well plates for the immobilisation of templates may also facilitate the application of high-throughput techniques for the discovery of macromolecular receptors using PS-DCLs. The best-binding fractions of PS-DCLs could also be conveniently separated from the bulk of the library to undergo a second exposure to template in the presence of additional acylhydrazides, with the possibility of further enhancing the affinities of these polymers to the template. Recognition properties could also be improved by harnessing favourable secondary interactions between polymers and lectins.23 Basic information about a lectin, such as its isoelectric point (pI) and amino acid composition, could be used to aid in selection from a palette of ‘amino-acid like’ residues which may interact favourably with the surfaces of lectins, potentially facilitating a synergistic effect in combination with carbohydrate recognition processes.
The PS-DCL approach could also be used to develop polymeric receptors for lectins whose favoured carbohydrates are not known, by allowing polymer scaffolds to ‘self-select’ favoured residues from a pool of different carbohydrates. Additionally, we anticipate that the incorporation of more complex carbohydrate-recognition motifs, e.g. ganglioside-derivatives may lead to polymeric receptors of even greater affinities towards AB5 toxins and other lectins.
Acknowledgements
The authors wish to thank COST action CM1102 for funding. The authors also thank the Engineering and Physical Sciences Research Council for generous support (EP/G066507/1 – C.S.M. and D.A.F); (EP/G043302/1 – W.B.T., M.A.F. and T.E.M.).Notes and references
- D. A. Fulton, Org. Lett., 2008, 10, 3291–3294 CrossRef CAS PubMed.
- C. S. Mahon, A. W. Jackson, B. S. Murray and D. A. Fulton, Chem. Commun., 2011, 47, 7209–7211 RSC.
- C. S. Mahon, A. W. Jackson, B. S. Murray and D. A. Fulton, Polym. Chem., 2013, 4, 368–377 RSC.
- C. S. Mahon and D. A. Fulton, Chem. Sci., 2013, 4, 3661–3666 RSC.
- W. E. Stites, Chem. Rev., 1997, 97, 1233–1250 CrossRef CAS PubMed.
- M. Mammen, S.-K. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2754–2794 CrossRef.
- A. Imberty and A. Varrot, Curr. Opin. Struct. Biol., 2008, 18, 567–576 CrossRef CAS PubMed.
- A. Imberty, Y. M. Chabre and R. Roy, Chem. – Eur. J., 2008, 14, 7490–7499 CrossRef CAS PubMed.
- C. J. O'Neal, M. G. Jobling, R. K. Holmes and W. G. J. Hol, Science, 2005, 309, 1093–1096 CrossRef PubMed.
- A. Bernardi, J. Jimenez-Barbero, A. Casnati, C. De Castro, T. Darbre, F. Fieschi, J. Finne, H. Funken, K.-E. Jaeger, M. Lahmann, T. K. Lindhorst, M. Marradi, P. Messner, A. Molinaro, P. V. Murphy, C. Nativi, S. Oscarson, S. Penades, F. Peri, R. J. Pieters, O. Renaudet, J.-L. Reymond, B. Richichi, J. Rojo, F. Sansone, C. Schaffer, W. B. Turnbull, T. Velasco-Torrijos, S. Vidal, S. Vincent, T. Wennekes, H. Zuilhof and A. Imberty, Chem. Soc. Rev., 2013, 42, 4709–4727 RSC.
- U. Boas and P. M. H. Heegaard, Chem. Soc. Rev., 2004, 33, 43–63 RSC.
- Y. Ruff, E. Buhler, S.-J. Candau, E. Kesselman, Y. Talmon and J.-M. Lehn, J. Am. Chem. Soc., 2010, 132, 2573–2584 CrossRef CAS PubMed.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2005, 58, 379–410 CrossRef CAS.
- K. D. Hardman and C. F. Ainsworth, Biochemistry, 1972, 11, 4910–4919 CrossRef CAS.
- V. Wittmann and R. J. Pieters, Chem. Soc. Rev., 2013, 42, 4492–4503 RSC.
- E. A. Merritt, T. K. Sixma, K. H. Kalk, B. A. M. van Zanten and W. G. J. Hol, Mol. Microbiol., 1994, 13, 745–753 CrossRef CAS PubMed.
- E. A. Merritt and W. G. J. Hol, Curr. Opin. Struct. Biol., 1995, 5, 165–171 CrossRef CAS.
- T. R. Branson and W. B. Turnbull, Chem. Soc. Rev., 2013, 42, 4613–4622 RSC.
- W. J. Lees, A. Spaltenstein, J. E. Kingery-Wood and G. M. Whitesides, J. Med. Chem., 1994, 37, 3419–3433 CrossRef CAS.
- J. L. Jimenez Blanco, C. Ortiz Mellet and J. M. Garcia Fernandez, Chem. Soc. Rev., 2013, 42, 4518–4531 RSC.
- M. Gomez-Garcia, J. M. Benito, R. Gutierrez-Gallego, A. Maestre, C. O. Mellet, J. M. G. Fernandez and J. L. J. Blanco, Org. Biomol. Chem., 2010, 8, 1849–1860 CAS.
- V. Ladmiral, G. Mantovani, G. J. Clarkson, S. Cauet, J. L. Irwin and D. M. Haddleton, J. Am. Chem. Soc., 2006, 128, 4823–4830 CrossRef CAS PubMed.
- M. W. Jones, L. Otten, S. J. Richards, R. Lowery, D. J. Phillips, D. M. Haddleton and M. I. Gibson, Chem. Sci., 2014, 5, 1611–1616 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and experimental procedures and spectroscopic data. See DOI: 10.1039/c4ob02587c |
| This journal is © The Royal Society of Chemistry 2015 |