 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of locked cyclohexene and cyclohexane nucleic acids (LCeNA and LCNA) with modified adenosine units†
Michal
Šála
*,
Milan
Dejmek
,
Eliška
Procházková
,
Hubert
Hřebabecký
,
Jiří
Rybáček
,
Martin
Dračínský
,
Pavel
Novák
,
Šárka
Rosenbergová
,
Jiří
Fukal
,
Vladimír
Sychrovský
,
Ivan
Rosenberg
and
Radim
Nencka
*
Gilead Sciences & IOCB Research Centre, Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic, v.v.i. Flemingovo n. 2, 16610, Prague, Czech Republic. E-mail: sala@uochb.cas.cz; nencka@uochb.cas.cz
First published on 2nd January 2015
Abstract
We describe here the preparation of conformationally locked cyclohexane nucleic acids designed as hybrids between locked nucleic acids (LNAs) and cyclohexene nucleic acids (CeNAs), both of which excel in hybridization with complementary RNAs. We have accomplished the synthesis of these adenine derivatives starting from a simple ketoester and installed all four chiral centres by means of total synthesis. The acquired monomers were incorporated into nonamer oligonucleotides.
Introduction
Oligonucleotides with modified sugar moieties have found many applications in modern technologies such as antisense oligonucleotides, RNA interference (RNAi), ribozymes, DNAzymes and aptamers. The stabilization of the duplexes with complementary mRNA of interest is an essential concept for oligonucleotide-mediated regulation of the gene expression. Extensive hybridization with the target sequence and selectivity towards mRNA in comparison with affinity to complementary DNA are important features of the desired technologies.1Although various sugar modifications have led to the enhancement of the hybridization properties of antisense oligonucleotides, probably the most famous modifications are based on monomers with a bridge between the 2′ and 4′ positions of the ribose ring. This results in the stabilization of the 3′-endo conformation and the formation of bridged nucleic acids (BNAs).2 Imanishi's3 and Wengel's4 groups have independently synthesized monomers for 2′,4′-bridged nucleic acids/locked nucleic acids (LNA, 1) and reported their hybridization properties after incorporation into oligonucleotides (Fig. 1). LNAs have also been successfully used for both RNAi5 and selection of aptamers.6 Since then, a number of compounds with alternative bridges (e.g.2–4) have been prepared, especially in order to increase the nuclease resistance of the resulting oligonucleosides.7 Carba-LNAs (e.g.2)8 have also been prepared. They seem to possess a significantly increased nuclease resistance in comparison with traditional LNAs without a dramatic effect on the RNAse H mediated cleavage of the target RNA.9 Recently LNAs modified on the nucleobase have been reported as well.10
 | ||
| Fig. 1 The structures of selected nucleic acids with sugar modification including LNA analogues (1–4) and six-membered carbohydrate mimics (5–7). | ||
In contrast to LNA-based oligonucleotides, which usually form stable duplexes with both RNA and DNA, cyclohexene nucleic acids (CeNA, 5), developed by Herdewijn et al., exert significant selectivity in hybridization with RNA over DNA.11 The same research team has also suggested that the cyclohexene moiety can serve as an appropriate bioisostere of the natural furanose ring11b and proved that this pseudosugar exerts significant flexibility while being incorporated into the structure of oligonucleotides. The crystal structures of duplexes with complementary DNA and RNA oligomers have clearly demonstrated that the cyclohexene moiety can interconvert between two distinct conformations 2H3 (similar to C2′-endo) and 3H2 (similar to C3′-endo).12,13
Recently, Seth et al. have shown that 2′-fluoro hexitol nucleic acids (FHNA, 6) exhibit higher duplex stability compared to 2′-fluoro CeNA (F-CeNA, 7) due to higher rigidity and superior stabilization in C3′-endo-like conformation.14,25
The major objective of our presented study was the preparation of hybrid derivatives merging LNAs and CeNAs in order to stabilize the cyclohexene moiety in 3H2 conformation resembling the C3′-endo, which is preferred by CeNA while forming a duplex with complementary RNA strands.13 In addition, the synthesis of locked cyclohexene nucleic acid (LCeNA) monomers made it easy to obtain saturated monomers bearing a cyclohexane ring instead of the original cyclohexene one (LCNA).
Although the obvious way to reach these compounds in an asymmetric fashion led through extending the synthesis of CeNA by methods for the preparation of LNA from sugar precursors,15 we decided to explore a synthetic approach, which would result in this type of compound starting from simple precursors avoiding the use of a chiral pool or enzymatic resolution of synthetically complicated nucleosides. In order to be able to determine the enantiomeric purity of our compounds, we initially performed racemic synthesis of the desired monomers (see the ESI†).
Results and discussion
The retrosynthetic analysis is outlined in Fig. 2. The crucial step of the synthesis is the construction of the first stereogenic centre by Michael conjugated addition of the acrolein to starting material 8 catalysed by the quinine based organocatalyst (Q-PHN-OH) immediately followed by cyclization to build a bicyclic ring system (bicyclo[3.2.1]octane). Further transformations of the functional groups lead to the desired final nucleoside 25.The asymmetric synthesis of the desired monomers started from the ester 816 (Scheme 1), which was treated with acrolein together with a quinine organocatalyst (Q-PHN-OH) by following the published synthetic protocol.17 The crude aldehyde intermediate 8a was cyclized with cesium carbonate18 in toluene to afford a mixture of bicyclic compounds 9a and 9b (87% yield, 2 steps, ratio ∼3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, GC-MS analysis). Alcohols 9a and 9b were used as a mixture (Scheme 1), their keto group protected as a ketal and the ester group of 10 was reduced by lithium aluminum hydride to an inseparable diastereomeric mixture of alcohols 11. The primary hydroxy group was protected by benzoylation at low temperature and the obtained mixture of the monobenzoylated compounds 13 (13a, 13b, separable) and the dibenzoylated compound 12 (only the compound with an equatorial hydroxyl group was dibenzoylated) was separated. The benzoylation procedure employing BzCN was also attempted but without any improvement in the yields of the monobenzoylated products 13. Compound 12 can be easily methanolyzed in high yield to the starting diol 11, which can be re-used in the benzoylation reaction. In one step, the hydroxy group of 13 was oxidized to a keto group and a double bond was introduced to the scaffold by IBX oxidation according to the procedure described by Nicolaou.19 This procedure progressed smoothly with an excellent yield (83%). Allylketone 14 was then subjected to the Luche reduction20 and the obtained alcohols 15 and 16 were easily separated by column chromatography. The undesired alcohol 15 can be oxidized back to ketone 14 by manganese dioxide and thus recycled.
2, GC-MS analysis). Alcohols 9a and 9b were used as a mixture (Scheme 1), their keto group protected as a ketal and the ester group of 10 was reduced by lithium aluminum hydride to an inseparable diastereomeric mixture of alcohols 11. The primary hydroxy group was protected by benzoylation at low temperature and the obtained mixture of the monobenzoylated compounds 13 (13a, 13b, separable) and the dibenzoylated compound 12 (only the compound with an equatorial hydroxyl group was dibenzoylated) was separated. The benzoylation procedure employing BzCN was also attempted but without any improvement in the yields of the monobenzoylated products 13. Compound 12 can be easily methanolyzed in high yield to the starting diol 11, which can be re-used in the benzoylation reaction. In one step, the hydroxy group of 13 was oxidized to a keto group and a double bond was introduced to the scaffold by IBX oxidation according to the procedure described by Nicolaou.19 This procedure progressed smoothly with an excellent yield (83%). Allylketone 14 was then subjected to the Luche reduction20 and the obtained alcohols 15 and 16 were easily separated by column chromatography. The undesired alcohol 15 can be oxidized back to ketone 14 by manganese dioxide and thus recycled.
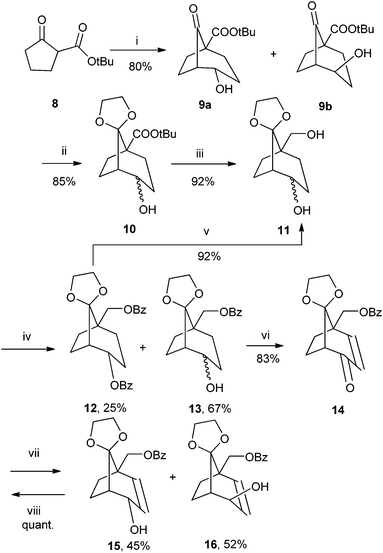 | ||
| Scheme 1 Synthesis of compound 16. Reagents and conditions: (i) (a) Q-PHN-OH,17 acrolein, CH2Cl2, −25 °C; (b) Cs2CO3, toluene, r.t.. (ii) ethylene glycol, PPTS, benzene, 100 °C; (iii) LiAlH4, Et2O; (iv) BzCl, pyridine, CH2Cl2, −40 °C; (v) MeONa, MeOH; (vi) IBX, DMSO, TsOH, 90 °C; (vii), NaBH4, CeCl3, MeOH, 0 °C; (viii) MnO2, CH2Cl2. | ||
The ketone-protecting group of 16 was easily removed by the reaction with p-toluenesulfonic acid in a refluxing acetone–water mixture (Scheme 2). The keto group of the derivative 17 was then reduced to a hydroxy group by sodium triacetoxyborohydride. The hydroxy group in the position C-4 participates in this reaction and allows to prepare exclusively the product with the desired orientation of the C-8 hydroxy group in diol 18.21 Although we tried numerous methodologies for the direct introduction of the purine nucleobase (including Tsuji–Trost reaction, Mitsunobu reaction and various direct alkylation methods) using diversely protected derivatives of compound 18 and its congeners with opposite configuration of the allylic hydroxyl, they all failed to give an appropriate product either due to low reactivity or undesired allylic rearrangements resulting in complex mixtures of products. Both the hydroxy groups were sequentially protected afterwards, the allylic hydroxyl was selectively protected by the TBDMS group and the C-8 hydroxyl by benzoylation. The TBDMS group was then cleaved by TBAF/acetic acid (the reaction mixture is less basic) at an elevated temperature (the reaction at r.t. is relatively slow) and the free allylic hydroxy group was converted to chloro derivative 21, followed by the introduction of the azido group by NaN3. At this stage, we were able to separate isomers 22 and 22a (a product of the allylic rearrangement) and we also discovered that the undesired isomer 22a can be easily converted to 22 by standing in acetonitrile solution or better by heating this solution overnight.22 The allylic rearrangement of 22a was monitored by 1H NMR spectroscopy (see the ESI†).
The key amine 23 was prepared by the Staudinger reaction, followed by the removal of the benzoyl protecting groups under basic conditions (Scheme 2). A purine nucleobase was then introduced in moderate yield (42%) by a recently described MW-assisted build-up protocol.23 Chloropurine derivative 24 was converted to adenine nucleoside 25 by ammonolysis with ethanolic ammonia under microwave conditions.23,24 The enantiomeric purity of this LCeNA monomer 25 was determined by chiral HPLC, which assessed the enantiomeric purity above 98%. The saturated analogue 26 was obtained after hydrogenation in high yield (89%). Both nucleosides (25 and 26) were used as building blocks for the synthesis of monomeric phosphoramidite units, which were subsequently used for the solid-state oligonucleotide synthesis.
All the compounds were appropriately characterized by 1H and 13C NMR and also by 2D NMR techniques (COSY, HSQC, HMBC). The configuration of the chiral centres at C8 and C4 of compound 25 was confirmed by 2D NMR techniques (COSY, ROESY). In the COSY spectrum, 2- and 3-bond spin–spin interactions are visible as cross-peaks. When the hydrogen atoms are in a W-like arrangement, it is possible to see 4-bond long-range couplings. Due to this fact, it was possible to confirm stereochemistry at C-8, where we found W-like long-range couplings between H8 and H7 (Fig. 3 top). The configuration was also confirmed by the ROESY spectrum, where the cross-peaks correspond to the through-space interactions. The H8–H8′ cross-peak clearly determined not only the configuration at the C8 atom, but also the C4 atom; the nucleobase must be above the cycle. For nucleoside 25 we also calculated spin–spin coupling constants by the DFT method (B3LYP/6-31+G(d,p)), which were in agreement with the experimental data (see Table S3 in ESI†).
Synthesis of the phosphoramidites 29 and 31 which were used in the solid phase oligonucleotide synthesis is depicted in Scheme 3. We used a traditional approach and obtained the desired compounds in good yields. The obtained phosphoramidites 29 and 31 were then used in the classical trityl-off phosphoramidite method for solid-supported oligonucleotide synthesis.
The hybridization properties of the modified oligonucleotides with their natural DNA and RNA counterparts were evaluated by UV thermal denaturation experiments and the obtained Tm values were compared with those of the corresponding unmodified duplexes (Table 1).
| Oligonucleotide | ssRNA Tm (ΔTm)b | ssDNA Tm (ΔTm)b |
|---|---|---|
| a 4 μM duplex in 50 mM NaH2PO4–Na2HPO4 pH 7.2 with 100 mM NaCl. b per modification. | ||
5′-d(GC![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 25T 25T![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 25TC 25TC![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 25C) 25C) |
22.0 (−4.3 °C) | No comp. form. |
5′-r(GC![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 25U 25U![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 25UC 25UC![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 25C) 25C) |
23.0 (−7.7 °C) | 22.0 (-4.3 °C) |
5′-d(GC![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 26T 26T![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 26TC 26TC![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 26C) 26C) |
No comp. form. | No comp. form. |
5′-r(GC![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 26U 26U![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 26UC 26UC![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) 26C) 26C) |
No comp. form. | No comp. form. |
To our surprise, a striking destabilization effect was observed for both LCeNA and LCNA. Although some destabilization was observed by Migawa et al.25 on structural related cANA derivatives, the drop in affinity is significantly larger in this case and cannot be clarified by the explanation suggested in their work, because the repulsion of the hydrogen in the bridge and the six-membered pseudosugar ring in cANA and LCeNA should lead to opposite effects (Fig. 4). Unfortunately, similar destabilization effects were observed also for homooligomers prepared from both LCeNA and LCNA subunits while hybridized with complementary oligothymidylates (see the ESI†).
To shed some light on the significant destabilization of the rLCeNA-RNA duplex, we performed the molecular dynamics simulations of RNA duplexes that included normal and locked units. (For a detailed analysis of the calculated results, structural models, and calculation method see ESI†). The values of backbone torsion angles α, γ, and δ calculated for the units of RNA oligonucleotides (Fig. S8–S13†) were analysed and statistical distributions of the torsion angles (Fig. S14†) were compared. The calculations unveiled a significant structural disorder of modified duplexes as compared to the A-RNA structure that was calculated for the duplex that included normal units. The deviations of modified oligonucleotides from canonical A-RNA occurred particularly owing to the irregular behaviour of the normal units neighbouring with modified units. The modified units were structurally more rigid though their behaviour was abnormal. In particular, the sugar of modified nucleosides was locked (δ torsion was ca. 60°) while the sugar of the normal units was flexible (δ torsion ranged from ca. 80° to ca. 160°). The overall values of α backbone torsions in the neighbourhood of modified residues were smaller by ca. 30° as compared to the typical value known for canonical A-RNA. The α-distribution calculated for locked units broadened, which indicated larger amplitudes of motion near phosphate. The α and γ torsions of phosphate groups bridging the locked unit with neighbouring normal units frequently flipped between the values characteristic to A-RNA, α/γ ≈ 290°/70°, and the values calculated owing to locked units, α/γ ≈ 180°/180°. The α-distributions of a normal A-RNA duplex were always single-modal and centered at 290° in contrast to bi- or even tri-modal α-distributions calculated in the neighbourhood of locked units (Fig. S14†). The calculations indicated instabilities and structural disorders of the normal residues in the neighbourhood of modified units. Moreover, the occurrence of α/γ flips depended on the positioning of phosphate groups with respect to 3′-end and 5′-end of locked units. The conformationally locked residues of RNA duplexes thus induced particularly irregular behaviour of backbone phosphates in the vicinity of the modified units.
Conclusions
In conclusion, we have prepared novel modified oligonucleotides containing monomers based on bicyclo[3.2.1]octene and octane skeletons as hybrids of CeNA/CNA and LNA. The appropriate monomers were synthesized from a simple achiral precursor – ketoester 8. As far as we know, this is the first synthesis of the LNA analogues performed by a total synthetic approach. Our molecular dynamics calculations suggest that a surprisingly low affinity of the modified oligonucleotides towards the complementary DNA and RNA results from the irregular behaviour of the nucleotides neighbouring with the locked units. The overall structure of the duplex containing locked units was significantly disordered and more conformationally labile in comparison with an A-RNA form of a normal duplex.Experimental section
General
Melting points were determined on a Büchi B-540 apparatus. NMR spectra (δ, ppm; J, Hz) were measured on a Bruker Avance II-600 and/or Bruker Avance II-500 instruments (600.1 or 500.0 MHz for 1H and 150.9 or 125.7 MHz for 13C) in hexadeuterated dimethyl sulfoxide and referenced to the solvent signal (δ 2.50 and 39.70, respectively). Mass spectra were measured on a LTQ Orbitrap XL (Thermo Fisher Scientific) by electrospray ionization (ESI). Column chromatography was performed on Silica gel 60 (Fluka) and thin-layer chromatography (TLC) on Silica gel 60 F254 foils (Merck). Solvents were evaporated at 2 kPa and bath temperature 30–60 °C; the compounds were dried at 13 Pa and 50 °C. The elemental analyses were obtained using a Perkin–Elmer CHN Analyzer 2400, Series II Sys (Perkin–Elmer). The elemental compositions for all compounds agreed to within ±0.4% of the calculated values. For all the tested compounds satisfactory elemental analysis was obtained supporting >95% purity. Optical rotation was measured on a polarimeter Autopol IV (Rudolph Research Analytical) at 589 nm wavelength in chloroform or methanol. Microwave syntheses were carried out using a CEM Discover instrument with a single-mode cavity and focused microwave heating (microwave power supply 0–300 W, 1 W increments, sealed vessel mode, pressure range 0–20 bar). GC-MS analyses were recorded by using a 5975B quadrupole mass spectrometer coupled to a 6890N gas chromatograph (Agilent, Santa Clara, CA, USA) equipped with a Phenomenex ZB-5 HT capillary column (30 m × 0.25 mm, film thickness 0.25 μm); temperature: 60 °C (2 min), then 10 °C min−1 to 320 °C (10 min).Preparation of compounds 9a and 9b
To a mixture of starting material 8 (9.05 g, 49.1 mmol) and a catalyst Q-PHN-OH17 (2.40 g, 4.93 mmol, 0.1 eq.) in dry CH2Cl2 (100 mL) at −25 °C under an argon atmosphere, a solution of acrolein (8.23 mL, 123 mmol, 2.5 eq.) in CH2Cl2 (30 mL) was added dropwise. The reaction mixture was occasionally stirred (>95% of time without stirring) and kept at −25 °C for 24 h. Then the reaction mixture was poured into a silica gel column (200 g, Et2O) and the crude product was eluted with Et2O. Fractions containing the product were collected and evaporated to afford a crude intermediate (12.53 g) which was used immediately in the next step. The crude catalyst was then eluted from the column using methanol and recycled (chromatography on a silica gel column in CH2Cl2–ethanol 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The crude intermediate was dissolved in toluene (350 mL), cesium carbonate (8.48 g, 26 mmol) was then added and the reaction mixture was stirred at r.t. overnight. Solids were removed by filtration through Celite and the filtrate was evaporated. The product was purified on a silica gel column (250 g, toluene–ethyl acetate 3
1). The crude intermediate was dissolved in toluene (350 mL), cesium carbonate (8.48 g, 26 mmol) was then added and the reaction mixture was stirred at r.t. overnight. Solids were removed by filtration through Celite and the filtrate was evaporated. The product was purified on a silica gel column (250 g, toluene–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 9.416 g (80%) of the mixture 9a and 9b (GC chromatogram, Fig. S1†). Analytical samples of both isomers were obtained by column chromatography of the sample (300 mg of the mixture, 100 g of silica gel, toluene–ethyl acetate 3
1) to afford 9.416 g (80%) of the mixture 9a and 9b (GC chromatogram, Fig. S1†). Analytical samples of both isomers were obtained by column chromatography of the sample (300 mg of the mixture, 100 g of silica gel, toluene–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). For the determination of the optical purity of this step see the ESI.†
1). For the determination of the optical purity of this step see the ESI.†
Preparation of compounds 10a and 10b
A mixture of alcohols 9a and 9b (9.1 g, 37.9 mmol) was dissolved in benzene (320 mL) and pyridinium p-toluenesulfonate (1.97 g, 7.8 mmol) and ethylene glycol (9.2 mL) were then added. The reaction mixture was heated to reflux with a Dean–Stark trap for 24 hours and then cooled to r.t., diluted with ethyl acetate (450 mL) and washed with water (300 mL) and saturated aq. sodium bicarbonate (2 × 300 mL). The organic phase was dried over sodium sulfate and evaporated. The residue was purified on a silica gel column (350 g, toluene–ethyl acetate 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to obtain 9.15 g (85%) of the mixture of 10a and 10b. Analytical samples of both isomers were obtained after chromatography of the sample (300 mg of the mixture, toluene–ethyl acetate 3
1) to obtain 9.15 g (85%) of the mixture of 10a and 10b. Analytical samples of both isomers were obtained after chromatography of the sample (300 mg of the mixture, toluene–ethyl acetate 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Preparation of compounds 13a and 13b
A mixture of alcohols 10a and 10b (9.79 g, 34.43 mmol) was dissolved in anhydrous ether (1000 mL) and cooled down with ice bath (argon atmosphere). A solution of LiAlH4 in THF (60.5 mL, 1 M solution, 1.75 eq.) was added dropwise in 30 minutes. The reaction was allowed to slowly reach room temperature and was stirred overnight, then cooled again to 0 °C and quenched with ice. Solids were removed by filtration through a Celite pad and thoroughly washed with ethanol. The filtrate was concentrated and the residue was chromatographed on a silica gel column (300 g, ethyl acetate–ethanol 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford an inseparable mixture of the diols (11, 6.86 g, 92%).
1) to afford an inseparable mixture of the diols (11, 6.86 g, 92%).
Benzoylation of the diol 11
Compound 11 (7.267 g, 33.9 mmol) was dissolved in CH2Cl2 (300 mL) and pyridine was added (5.5 mL, 68 mmol). The reaction mixture was cooled to −40 °C and a solution of benzoyl chloride (5.9 mL, 50.8 mmol) in CH2Cl2 (30 mL) was added dropwise for 1 h and the reaction mixture was stirred at −40 °C for 13 hours. The reaction was quenched with methanol and all volatiles were evaporated. The residue was dissolved in ethyl acetate (700 mL) and washed with water (300 mL) and satd sodium bicarbonate (300 mL), dried with sodium sulfate and evaporated. The residue was chromatographed on silica gel (400 g, toluene–ethyl acetate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 3.580 g of 12 (25%) and 7.261 g of 13a + 13b (67%, mixture). The mixture of monobenzoylated compounds 13a + 13b was separated on a small scale by column chromatography (250 mg of the mixture, 100 g, toluene–ethyl acetate 2
1) to afford 3.580 g of 12 (25%) and 7.261 g of 13a + 13b (67%, mixture). The mixture of monobenzoylated compounds 13a + 13b was separated on a small scale by column chromatography (250 mg of the mixture, 100 g, toluene–ethyl acetate 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Recyclation of diol 11 from 12
A freshly prepared sodium methoxide in methanol (prepared from 70 mg of sodium and 27 mL of absolute methanol) was added to a solution of the diol 12 (3.4 g, 8.1 mmol) in absolute methanol (55 mL). The reaction mixture was heated to 60 °C for 12 h and evaporated. The residue was chromatographed on a silica gel column (200 g, ethyl acetate) and 1.54 g (89%) of the recycled diol 11 was obtained. This diol was used again for the monobenzoylation reaction.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Viscous oil. [α]20D = +139.4 (c 0.307, CHCl3). Found: C, 68.54; H, 5.87. Calc. for C18H18O5: C, 68.78; H, 5.77%. 1H NMR (500 MHz, d6-DMSO): δ 1.32–1.39 (m, 1H, H-6en), 1.68–1.74 (m, 1H, H-7en), 1.97–2.03 (m, H-7ex), 2.10–2.17 (m, 1H, H-6ex), 2.77 (ddm, 1H, J5–3 = 1.7, J5–6ex = 7.7, 1H, H-5), 3.73–3.98 (m, 4H, −OCH2CH2O−), 4.44 and 4.51 (2 × d, 2H, Jgem = 11.1, BzOCH2−), 6.09 (dd, J3–5 = 1.7, J3–2 = 9.9, 1H, H-3), 7.38 (d, J2–3 = 9.9, 1H, H-2), 7.53–7.56 (m, 2H, Ph-m), 7.65–7.69 (m, 1H, Ph-p), 7.96–7.98 (m, 2H, Ph-o). 13C NMR (125.7 MHz, d6-DMSO): δ 20.81 (C-6), 30.76 (C-7), 50.87 (C-1), 57.47 (C-5), 63.67 (BzOCH2−), 65.15 and 65.40 (−OCH2CH2O−), 117.61 (C-8), 128.36 (C-3), 128.97 (C-m), 129.35 (C-o), 129.75 (C-i), 133.60 (C-p), 154.23 (C-2), 165.86 (COO), 200.87 (C-4). ESI MS, m/z (rel%): 337 (100) [M + Na]. HRMS: calcd for [M + Na]: 337.10464, found: 337.10469.
1). Viscous oil. [α]20D = +139.4 (c 0.307, CHCl3). Found: C, 68.54; H, 5.87. Calc. for C18H18O5: C, 68.78; H, 5.77%. 1H NMR (500 MHz, d6-DMSO): δ 1.32–1.39 (m, 1H, H-6en), 1.68–1.74 (m, 1H, H-7en), 1.97–2.03 (m, H-7ex), 2.10–2.17 (m, 1H, H-6ex), 2.77 (ddm, 1H, J5–3 = 1.7, J5–6ex = 7.7, 1H, H-5), 3.73–3.98 (m, 4H, −OCH2CH2O−), 4.44 and 4.51 (2 × d, 2H, Jgem = 11.1, BzOCH2−), 6.09 (dd, J3–5 = 1.7, J3–2 = 9.9, 1H, H-3), 7.38 (d, J2–3 = 9.9, 1H, H-2), 7.53–7.56 (m, 2H, Ph-m), 7.65–7.69 (m, 1H, Ph-p), 7.96–7.98 (m, 2H, Ph-o). 13C NMR (125.7 MHz, d6-DMSO): δ 20.81 (C-6), 30.76 (C-7), 50.87 (C-1), 57.47 (C-5), 63.67 (BzOCH2−), 65.15 and 65.40 (−OCH2CH2O−), 117.61 (C-8), 128.36 (C-3), 128.97 (C-m), 129.35 (C-o), 129.75 (C-i), 133.60 (C-p), 154.23 (C-2), 165.86 (COO), 200.87 (C-4). ESI MS, m/z (rel%): 337 (100) [M + Na]. HRMS: calcd for [M + Na]: 337.10464, found: 337.10469.
Preparation of compounds 15 and 16
To a solution of the starting material 14 (6.293 g, 20.02 mmol) in methanol (330 mL) at 0 °C, cerium(III) chloride heptahydrate (14.65 g, 35.3 mmol) was added and the reaction mixture was stirred at 0 °C for 1 h. Sodium borohydride (1.05 g, 27.8 mmol) was then added in three portions for 30 minutes, the reaction mixture was stirred at 0 °C for an additional hour, quenched with ice and evaporated. The residue was dissolved in ethyl acetate (600 mL) and washed with water (300 mL). The water phase was extracted with ethyl acetate (600 mL), the combined organic phases were dried with sodium sulfate and evaporated. The residue was chromatographed on silica gel (400 g, toluene–ethyl acetate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 1
1 → 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 3.293 g of 16 (52%) and 2.827 g of 15 (45%) (both colourless oils).
1) to afford 3.293 g of 16 (52%) and 2.827 g of 15 (45%) (both colourless oils).
Recycling of alcohol 15 to ketone 14
To a solution of allyl alcohol 15 (2.088 g, 6.6 mmol) in CH2Cl2 (100 mL) manganese(IV) oxide (6.4 g, 10 eq., activated, ∼ 90%) was added in one portion and the reaction mixture was stirred overnight. Solids were removed by filtration on a Celite pad and thoroughly washed with ethyl acetate. The filtrate was evaporated and the crude 15 (quant. yield) was re-used in the Luche reduction. The analytical sample was obtained by silica gel chromatography (toluene–ethyl acetate 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). NMR spectra match those for 14.
1). NMR spectra match those for 14.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 2
1 → 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 2.456 g (87%) of 17 as viscous oil. [α]20D = +15.3 (c 0.326, CHCl3). Found: C, 70.63; H, 6.27. Calc. for C16H16O4: C, 70.57; H, 5.92%. 1H NMR (500 MHz, d6-DMSO): δ 1.50–1.57 (m, 1H, H-6en), 1.86–1.95 (m, 2H, H-7en, H-7ex), 2.03–2.11 (m, 1H, H-6ex), 2.36 (dm, J5–6ex = 8.4, 1H, H-5), 4.32 and 4.35 (2 × d, 2H, Jgem = 11.3, BzOCH2−), 4.55 (bs, 1H, H-4), 5.39 (bs, 1H, 4-OH), 5.75 (ddd, J3–5 = 1.2, J3–4 = 3.8, J3–2 = 9.1, 1H, H-3), 5.96 (d, J2–3 = 9.1, 1H, H-2), 7.51–7.55 (m, 2H, Ph-m), 7.65–7.68 (m, 1H, Ph-p), 7.92–7.95 (m, 2H, Ph-o). 13C NMR (125.7 MHz, d6-DMSO): δ 19.35 (C-6), 29.16 (C-7), 49.88 (C-1), 50.10 (C-5), 63.75 (BzOCH2−), 79.92 (C-4), 129.01 (C-m), 129.39 (C-o), 129.56 (C-3), 129.74 (C-i), 133.55 and 133.66 (C-p and C-2), 165.86 (COO), 212.74 (C-8). ESI MS, m/z (rel%): 295 (100) [M + Na]. HRMS: calcd for [M + Na]: 295.09408, found: 295.09416.
1) to afford 2.456 g (87%) of 17 as viscous oil. [α]20D = +15.3 (c 0.326, CHCl3). Found: C, 70.63; H, 6.27. Calc. for C16H16O4: C, 70.57; H, 5.92%. 1H NMR (500 MHz, d6-DMSO): δ 1.50–1.57 (m, 1H, H-6en), 1.86–1.95 (m, 2H, H-7en, H-7ex), 2.03–2.11 (m, 1H, H-6ex), 2.36 (dm, J5–6ex = 8.4, 1H, H-5), 4.32 and 4.35 (2 × d, 2H, Jgem = 11.3, BzOCH2−), 4.55 (bs, 1H, H-4), 5.39 (bs, 1H, 4-OH), 5.75 (ddd, J3–5 = 1.2, J3–4 = 3.8, J3–2 = 9.1, 1H, H-3), 5.96 (d, J2–3 = 9.1, 1H, H-2), 7.51–7.55 (m, 2H, Ph-m), 7.65–7.68 (m, 1H, Ph-p), 7.92–7.95 (m, 2H, Ph-o). 13C NMR (125.7 MHz, d6-DMSO): δ 19.35 (C-6), 29.16 (C-7), 49.88 (C-1), 50.10 (C-5), 63.75 (BzOCH2−), 79.92 (C-4), 129.01 (C-m), 129.39 (C-o), 129.56 (C-3), 129.74 (C-i), 133.55 and 133.66 (C-p and C-2), 165.86 (COO), 212.74 (C-8). ESI MS, m/z (rel%): 295 (100) [M + Na]. HRMS: calcd for [M + Na]: 295.09408, found: 295.09416.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) affording 3.691 g (85% over two steps) of 19 as an oil. [α]20D = +54.4 (c 0.375, CHCl3). Found: C, 70.99; H, 7.36. Calc. for C29H36O5Si: C, 70.70; H, 7.37%. 1H NMR (500 MHz, d6-DMSO): δ 0.07 and 0.08 (2 × s, 2 × 3H, 2 × CH3), 0.88 (s, 9H, tBu), 1.33–1.39 (m, 1H, H-6en), 1.67–1.73 (m, 1H, H-7en), 1.95–2.01 (m, 1H, H-7ex), 2.04–2.12 (m, 1H, H-6ex), 2.38 (dm, J5–6ex = 7.9, 1H, H-5), 4.17–4.19 (m, 1H, H-4), 4.34 and 4.41 (2 × d, 2H, Jgem = 11.1, BzOCH2−), 5.46 (bs, 1H, H-8), 5.64 (ddd, J3–5 = 1.5, J3–4 = 4.1, J3–2 = 9.4, 1H, H-3), 5.96 (d, J2–3 = 9.4, 1H, H-2), 7.45–7.52 (m, 4H, Ph-m1, Ph-m2), 7.61–7.66 (m, 2H, Ph-p1, Ph-p2), 7.87–7.89 (m, 2H, Ph-o2), 7.95–7.97 (m, 2H, Ph-o1). 13C NMR (125.7 MHz, d6-DMSO): δ −4.30 and −4.55 (2 × CH3), 17.99 (C(CH3)3), 22.92 (C-6), 25.91 (tBu), 31.41 (C-7), 47.00 (C-5), 49.19 (C-1), 65.46 (BzOCH2−), 73.87 (C-4), 77.11 (C-8), 127.98 (C-3), 128.87 and 128.96 (C-m1, C-m2), 129.27 (C-o2), 129.44 (C-o1), 129.81 and 130.02 (C-i1 and C-i2), 133.54 (C-p1, C-p2), 135.02 (C-2), 164.93 (COO-1), 165.70 (COO-2). ESI MS, m/z (rel%): 515 (100) [M + Na]. HRMS: calcd for [M + Na]: 515.22242, found: 515.22244.
1) affording 3.691 g (85% over two steps) of 19 as an oil. [α]20D = +54.4 (c 0.375, CHCl3). Found: C, 70.99; H, 7.36. Calc. for C29H36O5Si: C, 70.70; H, 7.37%. 1H NMR (500 MHz, d6-DMSO): δ 0.07 and 0.08 (2 × s, 2 × 3H, 2 × CH3), 0.88 (s, 9H, tBu), 1.33–1.39 (m, 1H, H-6en), 1.67–1.73 (m, 1H, H-7en), 1.95–2.01 (m, 1H, H-7ex), 2.04–2.12 (m, 1H, H-6ex), 2.38 (dm, J5–6ex = 7.9, 1H, H-5), 4.17–4.19 (m, 1H, H-4), 4.34 and 4.41 (2 × d, 2H, Jgem = 11.1, BzOCH2−), 5.46 (bs, 1H, H-8), 5.64 (ddd, J3–5 = 1.5, J3–4 = 4.1, J3–2 = 9.4, 1H, H-3), 5.96 (d, J2–3 = 9.4, 1H, H-2), 7.45–7.52 (m, 4H, Ph-m1, Ph-m2), 7.61–7.66 (m, 2H, Ph-p1, Ph-p2), 7.87–7.89 (m, 2H, Ph-o2), 7.95–7.97 (m, 2H, Ph-o1). 13C NMR (125.7 MHz, d6-DMSO): δ −4.30 and −4.55 (2 × CH3), 17.99 (C(CH3)3), 22.92 (C-6), 25.91 (tBu), 31.41 (C-7), 47.00 (C-5), 49.19 (C-1), 65.46 (BzOCH2−), 73.87 (C-4), 77.11 (C-8), 127.98 (C-3), 128.87 and 128.96 (C-m1, C-m2), 129.27 (C-o2), 129.44 (C-o1), 129.81 and 130.02 (C-i1 and C-i2), 133.54 (C-p1, C-p2), 135.02 (C-2), 164.93 (COO-1), 165.70 (COO-2). ESI MS, m/z (rel%): 515 (100) [M + Na]. HRMS: calcd for [M + Na]: 515.22242, found: 515.22244.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of the residue afforded 2.05 g (92%) of 20 as a viscous oil. [α]20D = +120.60 (c 0.329, CHCl3). Found: C, 72.72; H, 6.10. Calc. for C23H22O5: C, 73.00; H; 5.86%. 1H NMR (500 MHz, d6-DMSO): δ 1.31–1.38 (m, 1H, H-6en), 1.69–1.74 (m, 1H, H-7en), 1.93–1.99 (m, 1H, H-7ex), 2.04–2.12 (m, 1H, H-6ex), 2.39 (d, J5–6ex = 8.2, 1H, H-5), 4.00–4.03 (m, 1H, H-4), 4.32 and 4.38 (2 × d, 2H, Jgem = 11.1, BzOCH2−), 5.24 (d, 1H, 4-OH), 5.48 (bs, 1H, H-8), 5.67 (ddd, J3–5 = 1.5, J3–4 = 3.9, J3–2 = 9.5, 1H, H-3), 5.96 (d, J2–3 = 9.5, 1H, H-2), 7.48–7.53 (m, 4H, Ph-m1, Ph-m2), 7.62–7.67 (m, 2H, Ph-p1, Ph-p2), 7.89–7.91 (m, 2H, Ph-o2), 7.95–7.97 (m, 2H, Ph-o1). 13C NMR (125.7 MHz, d6-DMSO): δ 23.46 (C-6), 31.79 (C-7), 46.88 (C-5), 49.18 (C-1), 65.51 (BzOCH2−), 72.86 (C-4), 77.22 (C-8), 128.63 (C-3), 128.89 and 128.97 (C-m1, C-m2), 129.31 (C-o2), 129.38 (C-o1), 129.76 and 130.04 (C-i1 and C-i2), 133.52 (C-p1, C-p2), 134.67 (C-2), 165.05 (COO-1), 165.72 (COO-2). ESI MS, m/z (rel%): 401 (100) [M + Na]. HRMS: calcd for [M + Na]: 401.13594, found: 401.13608.
1) of the residue afforded 2.05 g (92%) of 20 as a viscous oil. [α]20D = +120.60 (c 0.329, CHCl3). Found: C, 72.72; H, 6.10. Calc. for C23H22O5: C, 73.00; H; 5.86%. 1H NMR (500 MHz, d6-DMSO): δ 1.31–1.38 (m, 1H, H-6en), 1.69–1.74 (m, 1H, H-7en), 1.93–1.99 (m, 1H, H-7ex), 2.04–2.12 (m, 1H, H-6ex), 2.39 (d, J5–6ex = 8.2, 1H, H-5), 4.00–4.03 (m, 1H, H-4), 4.32 and 4.38 (2 × d, 2H, Jgem = 11.1, BzOCH2−), 5.24 (d, 1H, 4-OH), 5.48 (bs, 1H, H-8), 5.67 (ddd, J3–5 = 1.5, J3–4 = 3.9, J3–2 = 9.5, 1H, H-3), 5.96 (d, J2–3 = 9.5, 1H, H-2), 7.48–7.53 (m, 4H, Ph-m1, Ph-m2), 7.62–7.67 (m, 2H, Ph-p1, Ph-p2), 7.89–7.91 (m, 2H, Ph-o2), 7.95–7.97 (m, 2H, Ph-o1). 13C NMR (125.7 MHz, d6-DMSO): δ 23.46 (C-6), 31.79 (C-7), 46.88 (C-5), 49.18 (C-1), 65.51 (BzOCH2−), 72.86 (C-4), 77.22 (C-8), 128.63 (C-3), 128.89 and 128.97 (C-m1, C-m2), 129.31 (C-o2), 129.38 (C-o1), 129.76 and 130.04 (C-i1 and C-i2), 133.52 (C-p1, C-p2), 134.67 (C-2), 165.05 (COO-1), 165.72 (COO-2). ESI MS, m/z (rel%): 401 (100) [M + Na]. HRMS: calcd for [M + Na]: 401.13594, found: 401.13608.
Preparation of compounds 22a and 22
A solution of PPh3 (2.99 g, 11.4 mmol) and N-chlorosuccinimide (1.53 g, 11.45 mmol) in CH2Cl2 (32 mL) was stirred at 0 °C for 30 minutes. A solution of hydroxy derivative 20 (2.155 g, 5.65 mmol) in CH2Cl2 (32 mL + 5 mL for rinsing the flask) was then added dropwise for 30 minutes. The reaction mixture was stirred at 0 °C for 2 h and then treated with methanol (5 mL) and evaporated. The residue was chromatographed on a silica gel column (250 g, hexanes–ethyl acetate 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the isolated intermediate was immediately used in the following step. Chloro derivative 21 was dissolved in DMF (44 mL) and treated with sodium azide (1.836 g, 28.3 mmol) at 65 °C for 12 h. Volatiles were evaporated, the residue was dissolved in ethyl acetate (350 mL) and washed with water (200 mL). The organic phase was dried with sodium sulfate, evaporated and the crude product was chromatographed on a silica gel column (250 g, hexanes–ethyl acetate 20
1) and the isolated intermediate was immediately used in the following step. Chloro derivative 21 was dissolved in DMF (44 mL) and treated with sodium azide (1.836 g, 28.3 mmol) at 65 °C for 12 h. Volatiles were evaporated, the residue was dissolved in ethyl acetate (350 mL) and washed with water (200 mL). The organic phase was dried with sodium sulfate, evaporated and the crude product was chromatographed on a silica gel column (250 g, hexanes–ethyl acetate 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → 10
1 → 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 22a (505 mg, 22% over 2 steps) and 22 (1.64 g, 72% over 2 steps, both were oils).
1) to afford 22a (505 mg, 22% over 2 steps) and 22 (1.64 g, 72% over 2 steps, both were oils).
Allylic rearrangement of 22a to 22
A solution of compound 22a (505 mg, 1.25 mmol) in acetonitrile (35 mL) was heated to 95 °C for 24 h. The reaction mixture was evaporated and the residue was chromatographed (100 g, hexanes–ethyl acetate 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 241 mg (48%) of 22.
1) to afford 241 mg (48%) of 22.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Potassium hydroxide (1.2 g, 21.4 mmol) was added and the reaction mixture was heated to reflux for 5 h, neutralized with aq. HCl and purified on a DOWEX 50 (100 mL, H+ cycle). The column was washed with water (400 mL), methanol (400 mL) and the product was then eluted with aq. NH3–MeOH (1
1). Potassium hydroxide (1.2 g, 21.4 mmol) was added and the reaction mixture was heated to reflux for 5 h, neutralized with aq. HCl and purified on a DOWEX 50 (100 mL, H+ cycle). The column was washed with water (400 mL), methanol (400 mL) and the product was then eluted with aq. NH3–MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, v/v). Fractions containing the product were evaporated and the oily residue was converted to hydrochloride salt with hydrogen chloride in dioxane (2 M) (659 mg, 79%, slightly hygroscopic yellowish solid). [α]20D = −51.4 (c 0.292, CH3OH). Found: C, 51.21; H, 7.70; N, 6.23. Calc. for C9H16ClNO2·1/3H2O: C, 51.06; H, 7.94; N, 6.62%. 1H NMR (500 MHz, d6-DMSO): δ 1.26 (ddd, J6en–7ex = 5.9, J6en–7en = 9.1, Jgem = 13.7, 1H, H-6en), 1.37–1.43 (m, 1H, H-7en), 1.52–1.59 (m, 1H, H-7ex), 1.98–2.06 (m, 1H, H-6ex), 2.24 (d, J5–6ex = 8.0, 1H, H-5), 3.41 (d, Jgem = 10.7, 1H, CHbOH), 3.52 (m, 2H, H-4, CHaOH), 4.00 (bs, 1H, H-8), 4.62 and 4.89 (2 × bs, 2H, 8-OH, CH2OH), 5.48 (dd, J3–5 = 1.7, J3–4 = 3.6, J3–2 = 9.5, 1H, H-3), 6.13 (dd, J2–4 = 0.8, J2–3 = 9.5, 1H, H-2), 8.20 (bs, 3H, NH3+). 13C NMR (125.7 MHz, d6-DMSO): δ 25.78 (C-6), 31.86 (C-7), 43.97 (C-5), 51.88 (C-1), 55.09 (C-4), 62.24 (CH2OH), 71.21 (C-8), 120.68 (C-3), 142.49 (C-2). CI MS, m/z (rel%): 152 (100) [M + H-H2O]. HRMS: calcd for [M + H]: 171.1181, found: 170.1176.
4, v/v). Fractions containing the product were evaporated and the oily residue was converted to hydrochloride salt with hydrogen chloride in dioxane (2 M) (659 mg, 79%, slightly hygroscopic yellowish solid). [α]20D = −51.4 (c 0.292, CH3OH). Found: C, 51.21; H, 7.70; N, 6.23. Calc. for C9H16ClNO2·1/3H2O: C, 51.06; H, 7.94; N, 6.62%. 1H NMR (500 MHz, d6-DMSO): δ 1.26 (ddd, J6en–7ex = 5.9, J6en–7en = 9.1, Jgem = 13.7, 1H, H-6en), 1.37–1.43 (m, 1H, H-7en), 1.52–1.59 (m, 1H, H-7ex), 1.98–2.06 (m, 1H, H-6ex), 2.24 (d, J5–6ex = 8.0, 1H, H-5), 3.41 (d, Jgem = 10.7, 1H, CHbOH), 3.52 (m, 2H, H-4, CHaOH), 4.00 (bs, 1H, H-8), 4.62 and 4.89 (2 × bs, 2H, 8-OH, CH2OH), 5.48 (dd, J3–5 = 1.7, J3–4 = 3.6, J3–2 = 9.5, 1H, H-3), 6.13 (dd, J2–4 = 0.8, J2–3 = 9.5, 1H, H-2), 8.20 (bs, 3H, NH3+). 13C NMR (125.7 MHz, d6-DMSO): δ 25.78 (C-6), 31.86 (C-7), 43.97 (C-5), 51.88 (C-1), 55.09 (C-4), 62.24 (CH2OH), 71.21 (C-8), 120.68 (C-3), 142.49 (C-2). CI MS, m/z (rel%): 152 (100) [M + H-H2O]. HRMS: calcd for [M + H]: 171.1181, found: 170.1176.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 24 (631 mg, 42%). The analytical sample was crystalized from ethanol (white solid). M.p. 176.5–177 °C (decomposition, EtOH). [α]20D = −18.6 (c 0.297, CH3OH). Found: C, 54.50; H, 4.91; Cl, 11.30; N, 17.87. Calc. for C14H15ClN4O2: C, 54.82; H, 4.93; Cl, 11.56; N, 18.26%. 1H NMR (500 MHz, d6-DMSO): δ 1.46–1.53 (m, 1H, H-6en), 1.54–1.60 (m, 1H, H-7en), 1.65–1.72 (m, 1H, H-7ex), 2.06–2.13 (m, 1H, H-6ex), 2.42 (dm, J5–6ex = 7.8, 1H, H-5), 3.51 (dd, JCH–OH = 5.3, Jgem = 10.5, 1H, CHbOH), 3.63 (dd, JCH–OH = 5.3, Jgem = 10.5, 1H, CHaOH), 3.74 (d, J8-OH = 3.6, 1H, H-8), 4.53 (t, JOH–CH2 = 5.3, 1H, CH2OH), 4.63 (d, JOH-8 = 3.6, 1H, 8-OH), 5.10–5.12 (m, 1H, H-4), 5.76 (ddd, J3–5 = 1.7, J3–4 = 3.9, J3–2 = 9.4, 1H, H-3), 6.40 (dd, J2–4 = 1.2, J2–3 = 9.4, 1H, H-2), 8.49 (s, 1H, H-8′), 8.83 (s, 1H, H-2′). 13C NMR (125.7 MHz, d6-DMSO): δ 25.10 (C-6), 32.31 (C-7), 46.26 (C-5), 51.70 (C-1), 59.74 (C-4), 62.18 (CH2OH), 71.81 (C-8), 119.57 (C-3), 131.47 (C-5′), 143.33 (C-2), 145.84 (C-8′), 149.32 (C-6′), 151.48 (C-4′), 151.67 (C-2′). ESI MS, m/z (rel%): 329/331 (100/33) [M + Na]. HRMS: calcd for [M + Na]: 329.07757, found: 329.07771.
1) to afford 24 (631 mg, 42%). The analytical sample was crystalized from ethanol (white solid). M.p. 176.5–177 °C (decomposition, EtOH). [α]20D = −18.6 (c 0.297, CH3OH). Found: C, 54.50; H, 4.91; Cl, 11.30; N, 17.87. Calc. for C14H15ClN4O2: C, 54.82; H, 4.93; Cl, 11.56; N, 18.26%. 1H NMR (500 MHz, d6-DMSO): δ 1.46–1.53 (m, 1H, H-6en), 1.54–1.60 (m, 1H, H-7en), 1.65–1.72 (m, 1H, H-7ex), 2.06–2.13 (m, 1H, H-6ex), 2.42 (dm, J5–6ex = 7.8, 1H, H-5), 3.51 (dd, JCH–OH = 5.3, Jgem = 10.5, 1H, CHbOH), 3.63 (dd, JCH–OH = 5.3, Jgem = 10.5, 1H, CHaOH), 3.74 (d, J8-OH = 3.6, 1H, H-8), 4.53 (t, JOH–CH2 = 5.3, 1H, CH2OH), 4.63 (d, JOH-8 = 3.6, 1H, 8-OH), 5.10–5.12 (m, 1H, H-4), 5.76 (ddd, J3–5 = 1.7, J3–4 = 3.9, J3–2 = 9.4, 1H, H-3), 6.40 (dd, J2–4 = 1.2, J2–3 = 9.4, 1H, H-2), 8.49 (s, 1H, H-8′), 8.83 (s, 1H, H-2′). 13C NMR (125.7 MHz, d6-DMSO): δ 25.10 (C-6), 32.31 (C-7), 46.26 (C-5), 51.70 (C-1), 59.74 (C-4), 62.18 (CH2OH), 71.81 (C-8), 119.57 (C-3), 131.47 (C-5′), 143.33 (C-2), 145.84 (C-8′), 149.32 (C-6′), 151.48 (C-4′), 151.67 (C-2′). ESI MS, m/z (rel%): 329/331 (100/33) [M + Na]. HRMS: calcd for [M + Na]: 329.07757, found: 329.07771.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) to afford 433 mg (86%) of the product 25. The analytical sample was crystallized from ethanol (white solid).
1.2) to afford 433 mg (86%) of the product 25. The analytical sample was crystallized from ethanol (white solid).
Enantiomeric purity was determined by chiral HPLC on a Chirapak IA (Daicel) column with heptane–ethanol 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 0.1% Et2NH as an eluent (Fig. S4 and S5†).
1 + 0.1% Et2NH as an eluent (Fig. S4 and S5†).
M.p. 158.5–159.5 °C (EtOH). [α]20D = −50.1 (c 0.154, CH3OH). Found: C, 53.48; H, 6.24; N, 22.14. Calc. for C14H17N5O2·1.5H2O: C, 53.49; H, 6.41; N, 22.28%. 1H NMR (500 MHz, d6-DMSO): δ 1.41–1.48 (m, 1H, H-6en), 1.51–1.57 (m, 1H, H-7en), 1.63–1.70 (m, 1H, H-7ex), 2.02–2.10 (m, 1H, H-6ex), 2.37 (dm, J5–6ex = 7.9, 1H, H-5), 3.49 (dd, JCH–OH = 5.5, Jgem = 10.5, 1H, CHbOH), 3.62 (dd, JCH–OH = 5.5, Jgem = 10.5, 1H, CHaOH), 3.73 (d, J8-OH = 3.5, 1H, H-8), 4.53 (t, JOH–CH2 = 5.3, 1H, CH2OH), 4.63 (d, JOH-8 = 3.5, 1H, 8-OH), 4.91–4.93 (m, 1H, H-4), 5.71 (ddd, J3–5 = 1.6, J3–4 = 3.9, J3–2 = 9.5, 1H, H-3), 6.40 (dd, J2–4 = 1.1, J2–3 = 9.5, 1H, H-2), 7.27 (bs, 2H, NH2), 7.90 (s, 1H, H-8′), 8.17 (s, 1H, H-2′). 13C NMR (125.7 MHz, d6-DMSO): δ 25.20 (C-6), 32.41 (C-7), 46.46 (C-5), 51.77 (C-1), 58.82 (C-4), 62.28 (CH2OH), 71.69 (C-8), 119.39 (C-5′), 120.42 (C-3), 139.06 (C-8′), 142.64 (C-2), 149.18 (C-4′), 151.68 (C-2′), 156.30 (C-6′). ESI MS, m/z (rel%): 310 (100) [M + Na]. HRMS: calcd for [M + H]: 288.14550, found: 288.14557; calcd for [M + Na]: 310.12745, found: 310.12747.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.8
1.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2)) to afford 450 mg (89%) of the product 26. The analytical sample was crystalized from ethanol (white solid). M.p. 241.5–242.5 °C (EtOH). [α]20D = +54.5 (c 0.301, CH3OH). Found: C, 56.33; H, 6.46; N; 23.23. Calc. for C14H19N5O2·0.5H2O: C, 56.36; H, 6.76; N, 23.47%. 1H NMR (600 MHz, d6-DMSO): δ 1.40–1.45 (m, 1H, H-7en), 1.52–1.58 (m, 3H, H-2eq, H-6en, H-7ex), 1.92–2.02 (m, 2H, H-2ax, H-6ex), 2.07–2.17 (m, 2H, H-3), 2.65 (dd, J5–4 = 3.9, J5–6ex = 7.0, 1H, H-5), 3.32 (dd, JCH–OH = 5.0, Jgem = 10.5, 1H, CHbOH), 3.38 (d, J8-OH = 3.5, 1H, H-8), 3.53 (dd, JCH–OH = 5.0, Jgem = 10.5, 1H, CHaOH), 4.32 (t, JOH–CH2 = 5.0, 1H, CH2OH), 4.42 (d, JOH-8 = 3.5, 1H, 8-OH), 4.52–4.54 (m, 1H, H-4), 7.21 (bs, 2H, NH2), 8.14 (s, 1H, H-2′), 8.15 (s, 1H, H-8′). 13C NMR (150.92 MHz, d6-DMSO): δ 21.51 (C-3), 25.63 (C-6), 28.26 (C-7), 31.75 (C-2), 47.10 (C-5), 49.54 (C-1), 56.99 (C-4), 64.92 (CH2OH), 74.66 (C-8), 119.12 (C-5′), 138.98 (C-8′), 149.81 (C-4′), 152.44 (C-2′), 156.21 (C-6′). ESI MS, m/z (rel%): 312 (100) [M + Na]. HRMS: calcd for [M + H]: 290.16115, found: 290.16124; calcd for [M + Na]: 312.14310, found: 312.14315.
1.2)) to afford 450 mg (89%) of the product 26. The analytical sample was crystalized from ethanol (white solid). M.p. 241.5–242.5 °C (EtOH). [α]20D = +54.5 (c 0.301, CH3OH). Found: C, 56.33; H, 6.46; N; 23.23. Calc. for C14H19N5O2·0.5H2O: C, 56.36; H, 6.76; N, 23.47%. 1H NMR (600 MHz, d6-DMSO): δ 1.40–1.45 (m, 1H, H-7en), 1.52–1.58 (m, 3H, H-2eq, H-6en, H-7ex), 1.92–2.02 (m, 2H, H-2ax, H-6ex), 2.07–2.17 (m, 2H, H-3), 2.65 (dd, J5–4 = 3.9, J5–6ex = 7.0, 1H, H-5), 3.32 (dd, JCH–OH = 5.0, Jgem = 10.5, 1H, CHbOH), 3.38 (d, J8-OH = 3.5, 1H, H-8), 3.53 (dd, JCH–OH = 5.0, Jgem = 10.5, 1H, CHaOH), 4.32 (t, JOH–CH2 = 5.0, 1H, CH2OH), 4.42 (d, JOH-8 = 3.5, 1H, 8-OH), 4.52–4.54 (m, 1H, H-4), 7.21 (bs, 2H, NH2), 8.14 (s, 1H, H-2′), 8.15 (s, 1H, H-8′). 13C NMR (150.92 MHz, d6-DMSO): δ 21.51 (C-3), 25.63 (C-6), 28.26 (C-7), 31.75 (C-2), 47.10 (C-5), 49.54 (C-1), 56.99 (C-4), 64.92 (CH2OH), 74.66 (C-8), 119.12 (C-5′), 138.98 (C-8′), 149.81 (C-4′), 152.44 (C-2′), 156.21 (C-6′). ESI MS, m/z (rel%): 312 (100) [M + Na]. HRMS: calcd for [M + H]: 290.16115, found: 290.16124; calcd for [M + Na]: 312.14310, found: 312.14315.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4) to afford 27 (464 mg, 62%) as a yellowish foam. [α]20D = −86.1 (c 0.296, CH3OH). Found: C, 63.40; H, 5.46; N, 17.38. Calc. for C21H21N5O3·1/3H2O: C, 63.46; H, 5.50; N, 17.62%. 1H NMR (600 MHz, d6-DMSO): δ 1.48–1.54 (m, 1H, H-6en), 1.55–1.60 (m, 1H, H-7en), 1.66–1.73 (m, 1H, H-7ex), 2.07–2.15 (m, 1H, H-6ex), 2.44 (dm, J5–6ex = 8.0, 1H, H-5), 3.51 (dd, JCH–OH = 5.1, Jgem = 10.5, 1H, CHbOH), 3.64 (dd, JCH–OH = 5.1, Jgem = 10.5, 1H, CHaOH), 3.77 (d, J8-OH = 3.5, 1H, H-8), 4.54 (t, JOH–CH2 = 5.1, 1H, CH2OH), 4.69 (d, JOH-8 = 3.5, 1H, 8-OH), 5.09–5.11 (m, 1H, H-4), 5.77 (ddd, J3–5 = 1.6, J3–4 = 3.9, J3–2 = 9.5, 1H, H-3), 6.39 (dd, J2–4 = 1.1, J2–3 = 9.5, 1H, H-2), 7.53–7.57 (m, 2H, Ph-m), 7.63–7.66 (m, 1H, Ph-p), 8.04–8.06 (m, 2H, Ph-o), 8.24 (s, 1H, H-8′), 8.77 (s, 1H, H-2′), 11.21 (bs, 1H, NH). 13C NMR (150.92 MHz, d6-DMSO): δ 25.16 (C-6), 32.37 (C-7), 46.45 (C-5), 51.72 (C-1), 59.18 (C-4), 62.23 (CH2OH), 71.82 (C-8), 119.96 (C-3), 126.09 (C-5′), 128.61 (C-m,o), 132.55 (C-p), 133.62 (C-i), 142.87 (C-8′), 143.04 (C-2), 150.48 (C-6′), 151.53 (C-2′), 151.98 (C-4′), 165.87 (COO). ESI MS, m/z (rel%): 414 (100) [M + Na]. HRMS: calcd for [M + Na]: 414.15366, found: 414.15359.
0.4) to afford 27 (464 mg, 62%) as a yellowish foam. [α]20D = −86.1 (c 0.296, CH3OH). Found: C, 63.40; H, 5.46; N, 17.38. Calc. for C21H21N5O3·1/3H2O: C, 63.46; H, 5.50; N, 17.62%. 1H NMR (600 MHz, d6-DMSO): δ 1.48–1.54 (m, 1H, H-6en), 1.55–1.60 (m, 1H, H-7en), 1.66–1.73 (m, 1H, H-7ex), 2.07–2.15 (m, 1H, H-6ex), 2.44 (dm, J5–6ex = 8.0, 1H, H-5), 3.51 (dd, JCH–OH = 5.1, Jgem = 10.5, 1H, CHbOH), 3.64 (dd, JCH–OH = 5.1, Jgem = 10.5, 1H, CHaOH), 3.77 (d, J8-OH = 3.5, 1H, H-8), 4.54 (t, JOH–CH2 = 5.1, 1H, CH2OH), 4.69 (d, JOH-8 = 3.5, 1H, 8-OH), 5.09–5.11 (m, 1H, H-4), 5.77 (ddd, J3–5 = 1.6, J3–4 = 3.9, J3–2 = 9.5, 1H, H-3), 6.39 (dd, J2–4 = 1.1, J2–3 = 9.5, 1H, H-2), 7.53–7.57 (m, 2H, Ph-m), 7.63–7.66 (m, 1H, Ph-p), 8.04–8.06 (m, 2H, Ph-o), 8.24 (s, 1H, H-8′), 8.77 (s, 1H, H-2′), 11.21 (bs, 1H, NH). 13C NMR (150.92 MHz, d6-DMSO): δ 25.16 (C-6), 32.37 (C-7), 46.45 (C-5), 51.72 (C-1), 59.18 (C-4), 62.23 (CH2OH), 71.82 (C-8), 119.96 (C-3), 126.09 (C-5′), 128.61 (C-m,o), 132.55 (C-p), 133.62 (C-i), 142.87 (C-8′), 143.04 (C-2), 150.48 (C-6′), 151.53 (C-2′), 151.98 (C-4′), 165.87 (COO). ESI MS, m/z (rel%): 414 (100) [M + Na]. HRMS: calcd for [M + Na]: 414.15366, found: 414.15359.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) to afford 358 mg (65%) of the product 28 as a foam (contains some inseparable impurities according 13C NMR, the compound was pure enough for the next step). [α]20D = −8.0 (c 0.313, CH3OH). Found: C, 72.43; H, 5.83; N, 9.71. Calc. for C42H39N5O5: C, 72.71; H, 5.67; N, 10.09%. 1H NMR (500 MHz, d6-DMSO): δ 1.50–1.56 (m, 1H, H-6en), 1.70 (m, 2H, H-7en, H-7ex), 2.08–2.15 (m, 1H, H-6ex), 2.48 (bs, 1H, H-5), 3.06 and 3.29 (2 × d, Jgem = 8.4, 2H, OCH2), 3.74 (2 × s, 2 × 3H, 2 × OCH3), 3.88 (bs, 1H, H-8), 4.76 (d, J8-OH = 3.4, 1H, H-8), 5.13–5.15 (m, 1H, H-4), 5.82 (ddd, J3–5 = 1.6, J3–4 = 3.9, J3–2 = 9.4, 1H, H-3), 6.31 (dd, J2–4 = 1.1, J2–3 = 9.4, 1H, H-2), 6.89–6.92 (m, 4H, H-3′′), 7.21–7.24 (m, 1H, 4′′′), 7.28–7.30 (m, 4H, H-2′′), 7.31–7.34 (m, 2H, H-3′′′), 7.42–7.44 (m, 2H, H-2′′′), 7.54–7.57 (m, 2H, Bz-m), 7.63–7.66 (m, 1H, Bz-p), 8.04–8.07 (m, 2H, Bz-o), 8.28 (s, 1H, H-8′), 8.79 (s, 1H, H-2′), 11.25 (bs, 1H, NH). 13C NMR (125.7 MHz, d6-DMSO): δ 24.99 (C-6), 32.99 (C-7), 46.55 (C-5), 50.48 (C-1), 55.19 and 55.20 (2 × OCH3), 59.20 (C-4), 64.87 (OCH2), 72.14 (C-8), 85.10 (Ph3CO−), 113.30 and 113.31 (2 × C-3′′), 120.00 (C-3), 126.17 (C-5′), 126.75 (C-4′′′), 127.92 and 127.96 (C-2′′′, C-3′′′), 128.64 and 128.66 (Bz-o, Bz-m), 129.95 and 129.99 (2 × C-2′′), 132.60 (Bz-p), 133.61 (Bz-i), 135.99 and 136.19 (2 × C-1′′), 142.68 and 142.75 (C-2 and C-8′), 145.51 (C-1′′′), 150.55 (C-6′), 151.61 (C-2′), 152.04 (C-4′), 158.16 and 158.17 (C-4′′), 165.93 (COO). ESI MS, m/z (rel%): 716 (100) [M + Na]. HRMS: calcd for [M + Na]: 716.28434, found: 716.28429.
3) to afford 358 mg (65%) of the product 28 as a foam (contains some inseparable impurities according 13C NMR, the compound was pure enough for the next step). [α]20D = −8.0 (c 0.313, CH3OH). Found: C, 72.43; H, 5.83; N, 9.71. Calc. for C42H39N5O5: C, 72.71; H, 5.67; N, 10.09%. 1H NMR (500 MHz, d6-DMSO): δ 1.50–1.56 (m, 1H, H-6en), 1.70 (m, 2H, H-7en, H-7ex), 2.08–2.15 (m, 1H, H-6ex), 2.48 (bs, 1H, H-5), 3.06 and 3.29 (2 × d, Jgem = 8.4, 2H, OCH2), 3.74 (2 × s, 2 × 3H, 2 × OCH3), 3.88 (bs, 1H, H-8), 4.76 (d, J8-OH = 3.4, 1H, H-8), 5.13–5.15 (m, 1H, H-4), 5.82 (ddd, J3–5 = 1.6, J3–4 = 3.9, J3–2 = 9.4, 1H, H-3), 6.31 (dd, J2–4 = 1.1, J2–3 = 9.4, 1H, H-2), 6.89–6.92 (m, 4H, H-3′′), 7.21–7.24 (m, 1H, 4′′′), 7.28–7.30 (m, 4H, H-2′′), 7.31–7.34 (m, 2H, H-3′′′), 7.42–7.44 (m, 2H, H-2′′′), 7.54–7.57 (m, 2H, Bz-m), 7.63–7.66 (m, 1H, Bz-p), 8.04–8.07 (m, 2H, Bz-o), 8.28 (s, 1H, H-8′), 8.79 (s, 1H, H-2′), 11.25 (bs, 1H, NH). 13C NMR (125.7 MHz, d6-DMSO): δ 24.99 (C-6), 32.99 (C-7), 46.55 (C-5), 50.48 (C-1), 55.19 and 55.20 (2 × OCH3), 59.20 (C-4), 64.87 (OCH2), 72.14 (C-8), 85.10 (Ph3CO−), 113.30 and 113.31 (2 × C-3′′), 120.00 (C-3), 126.17 (C-5′), 126.75 (C-4′′′), 127.92 and 127.96 (C-2′′′, C-3′′′), 128.64 and 128.66 (Bz-o, Bz-m), 129.95 and 129.99 (2 × C-2′′), 132.60 (Bz-p), 133.61 (Bz-i), 135.99 and 136.19 (2 × C-1′′), 142.68 and 142.75 (C-2 and C-8′), 145.51 (C-1′′′), 150.55 (C-6′), 151.61 (C-2′), 152.04 (C-4′), 158.16 and 158.17 (C-4′′), 165.93 (COO). ESI MS, m/z (rel%): 716 (100) [M + Na]. HRMS: calcd for [M + Na]: 716.28434, found: 716.28429.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to afford 297 mg (72%) of the product 29 as a white foam. 31P{1H} NMR (202 MHz, d6-DMSO): 145.73, 145.21. ESI MS, m/z (rel%): 916 (100) [M + Na]. HRMS: calcd for [M + H]: 894.41025, found: 894.41126; calcd for [M + Na]: 916.39219, found: 916.39245.
1 to afford 297 mg (72%) of the product 29 as a white foam. 31P{1H} NMR (202 MHz, d6-DMSO): 145.73, 145.21. ESI MS, m/z (rel%): 916 (100) [M + Na]. HRMS: calcd for [M + H]: 894.41025, found: 894.41126; calcd for [M + Na]: 916.39219, found: 916.39245.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) afforded a foam, which was directly used for the tritylation. The foam was co-evaporated with pyridine (2 × 10 mL), then dissolved in pyridine (10 mL) and cooled to 0 °C. DMTrCl (636 mg, 1.88 mmol) was added in one portion, the reaction mixture was slowly warmed to r.t. and then stirred for 48 h. Volatiles were evaporated and the residue was dissolved in ethyl acetate (300 mL), washed with a satd solution of sodium bicarbonate (2 × 150 mL) and brine (150 mL), dried with sodium sulfate, evaporated and chromatographed on a silica gel column (200 g – deactivated with triethylamine, ethyl acetate–acetone 10
1) afforded a foam, which was directly used for the tritylation. The foam was co-evaporated with pyridine (2 × 10 mL), then dissolved in pyridine (10 mL) and cooled to 0 °C. DMTrCl (636 mg, 1.88 mmol) was added in one portion, the reaction mixture was slowly warmed to r.t. and then stirred for 48 h. Volatiles were evaporated and the residue was dissolved in ethyl acetate (300 mL), washed with a satd solution of sodium bicarbonate (2 × 150 mL) and brine (150 mL), dried with sodium sulfate, evaporated and chromatographed on a silica gel column (200 g – deactivated with triethylamine, ethyl acetate–acetone 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 727 mg (83%) of 30 as a white foam. [α]20D = −18.5 (c 0.313, CH3OH). Found: C, 72.01; H, 7.40; N, 11.17. Calc. for C44H54N6O4: C, 72.30; H, 7.45; N, 11.50%. 1H NMR (500 MHz, d6-DMSO): δ 0.93 (q, J4–3 = 7.5, 6H, 2 × CH3), 1.28–1.37 (m, 4H, H-4), 1.46–1.51 (m, 1H, H-7′′ex), 1.55–1.65 (m, 6H, H-2, H-6′′en, H-7′′en), 1.74–1.78 (m, 1H, H-2′′a), 1.94–2.00 (m, 1H, H-6′′en), 2.04–2.11 (m, 1H, H-2′′b), 2.15–2.20 (m, 2H, H-3′′ax, H-3′′eq), 2.71 (dd, J5′′–6′′ex = 7.3, J5′′–4′′ = 3.8, H-5′′), 2.90 and 3.16 (2 × d, Jgem = 8.4, 2H, OCH2), 3.40 (d, J8′′OH = 3.9, 1H, H-8′′), 3.42–3.45 and 3.55–3.64 (2 × m, 2 × 2H, H-2), 3.73 (2 × s, 2 × 3H, 2 × OCH3), 4.59–4.62 (m, 1H, H-4′′), 6.86–6.89 (m, 4H, H-3′′′), 7.19–7.22 (m, 1H, H-p), 7.24–7.27 (m, 4H, H-2′′′), 7.28–7.31 (m, 2H, H-m), 7.39–7.41 (m, 2H, H-p), 8.33 (s, 1H, H-8′), 8.42 (s, 1H, H-2′), 8.96 (s, 1H, N
1) to afford 727 mg (83%) of 30 as a white foam. [α]20D = −18.5 (c 0.313, CH3OH). Found: C, 72.01; H, 7.40; N, 11.17. Calc. for C44H54N6O4: C, 72.30; H, 7.45; N, 11.50%. 1H NMR (500 MHz, d6-DMSO): δ 0.93 (q, J4–3 = 7.5, 6H, 2 × CH3), 1.28–1.37 (m, 4H, H-4), 1.46–1.51 (m, 1H, H-7′′ex), 1.55–1.65 (m, 6H, H-2, H-6′′en, H-7′′en), 1.74–1.78 (m, 1H, H-2′′a), 1.94–2.00 (m, 1H, H-6′′en), 2.04–2.11 (m, 1H, H-2′′b), 2.15–2.20 (m, 2H, H-3′′ax, H-3′′eq), 2.71 (dd, J5′′–6′′ex = 7.3, J5′′–4′′ = 3.8, H-5′′), 2.90 and 3.16 (2 × d, Jgem = 8.4, 2H, OCH2), 3.40 (d, J8′′OH = 3.9, 1H, H-8′′), 3.42–3.45 and 3.55–3.64 (2 × m, 2 × 2H, H-2), 3.73 (2 × s, 2 × 3H, 2 × OCH3), 4.59–4.62 (m, 1H, H-4′′), 6.86–6.89 (m, 4H, H-3′′′), 7.19–7.22 (m, 1H, H-p), 7.24–7.27 (m, 4H, H-2′′′), 7.28–7.31 (m, 2H, H-m), 7.39–7.41 (m, 2H, H-p), 8.33 (s, 1H, H-8′), 8.42 (s, 1H, H-2′), 8.96 (s, 1H, N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH−NBu2). 13C NMR (150.92 MHz, d6-DMSO): δ 13.77 and 13.95 (2 × CH3), 19.34 and 19.83 (2 × C-3), 21.37 (C-3′′), 25.45 (C-6′′), 28.88 and 30.69 (2 × C-3), 29.22 (C-7′′), 32.62 (C-2′′), 44.55 and 51.07 (2 × C-1), 47.14 (C-5′′), 48.68 (C-1′′), 55.16 (2 × OCH3), 57.12 (C-4′′), 66.45 (OCH2), 74.81 (C-8′′), 84.91 (Ph3CO−), 113.18 (C-3′′′), 125.64 (C-5′), 126.61 and 127.85 and 127.97 (Ph-o, m, p), 129.96 and 129.98 (C-2′′′), 136.19 and 136.38 (C-1′′′), 140.98 (C-8′), 145.67 (Ph-i), 151.84 Č-2′), 151.88 (C-4′), 158.07 (C-4′′′), 158.13 (N
CH−NBu2). 13C NMR (150.92 MHz, d6-DMSO): δ 13.77 and 13.95 (2 × CH3), 19.34 and 19.83 (2 × C-3), 21.37 (C-3′′), 25.45 (C-6′′), 28.88 and 30.69 (2 × C-3), 29.22 (C-7′′), 32.62 (C-2′′), 44.55 and 51.07 (2 × C-1), 47.14 (C-5′′), 48.68 (C-1′′), 55.16 (2 × OCH3), 57.12 (C-4′′), 66.45 (OCH2), 74.81 (C-8′′), 84.91 (Ph3CO−), 113.18 (C-3′′′), 125.64 (C-5′), 126.61 and 127.85 and 127.97 (Ph-o, m, p), 129.96 and 129.98 (C-2′′′), 136.19 and 136.38 (C-1′′′), 140.98 (C-8′), 145.67 (Ph-i), 151.84 Č-2′), 151.88 (C-4′), 158.07 (C-4′′′), 158.13 (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH−NBu2), 159.52 (C-6′). ESI MS, m/z (rel%): 731 (100) [M + H]. HRMS: calcd for [M + H]: 731.42793, found: 731.42774.
CH−NBu2), 159.52 (C-6′). ESI MS, m/z (rel%): 731 (100) [M + H]. HRMS: calcd for [M + H]: 731.42793, found: 731.42774.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) to afford 693 mg (80%) of the product 31 as a white foam. 31P{1H} NMR (202 MHz, C6D6): 147.67, 146.63. ESI MS, m/z (rel%): 931 (100) [M + H]. HRMS: calcd for [M + H]: 931.53578, found: 931.53558; calcd for [M + Na]: 953.51772, found: 953.51730.
9) to afford 693 mg (80%) of the product 31 as a white foam. 31P{1H} NMR (202 MHz, C6D6): 147.67, 146.63. ESI MS, m/z (rel%): 931 (100) [M + H]. HRMS: calcd for [M + H]: 931.53578, found: 931.53558; calcd for [M + Na]: 953.51772, found: 953.51730.
Synthesis of oligonucleotides
The oligonucleotides were synthesised from the appropriate monomers on a ∼0.5 μmol scale by a standard trityl-off phosphoramidite method using the LCAA CPG with attached 2′-deoxy-5′-O-dimethoxytritylcytidine-3′-O-hemisuccinate as the first nucleoside. Deprotection and release of oligonucleotides from CPG was achieved with gaseous ammonia (0.7 MPa) at r.t. for 12 h. Oligonucleotides were purified at 55 °C on DNAPac PA100 10 × 250 mm Nucleic Acid Column (Dionex) at a flow rate of 3 mL min−1 using a linear gradient of sodium chloride (20 mM → 500 mM, 60 min) in 50 mM sodium acetate buffer pH 7.0 containing 20% (v) of acetonitrile. Desalting of pure oligonucleotides was performed on 10 μm Luna C18 (2) 10 × 100 mm column (Phenomenex) at a flow rate of 3 mL min−1 using a gradient of acetonitrile (0 → 25%, 30 min) in 0.1 M triethylammonium hydrogencarbonate. Desalted oligonucleotides were freeze-dried and characterized by MALDI TOF (Table 2).| Oligonucleotide | Calcd mass | Found mass |
|---|---|---|
5′-d(GC![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 25T 25T![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 25TC 25TC![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 25C) 25C) |
2790.82 | 2790.2 |
5′-r(GC![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 25U 25U![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 25UC 25UC![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 25C) 25C) |
2858.76 | 2858.4 |
5′-d(GC![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 26T 26T![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 26TC 26TC![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 26C) 26C) |
2796.82 | 2796.2 |
5′-r(GC![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 26U 26U![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 26UC 26UC![[A with combining low line]](https://www.rsc.org/images/entities/b_char_0041_0332.gif) 26C) 26C) |
2864.76 | 2864.0 |
Hybridization study
Thermal experiments with oligonucleotide complexes were performed at 260 nm using a CARY 100 Bio UV Spectrophotometer (Varian Inc.) equipped with a Peltier temperature controller and thermal analysis software. The aqueous solutions of modified and natural complementary strands (4 nmol of each) were mixed, freeze-dried and dissolved in 50 mM NaH2PO4 – Na2HPO4 pH 7.2 with 100 mM NaCl (1 mL) to give a 4 μM duplex solution. A heating–cooling cycle over a range of 15–60 °C with a gradient of 0.5 °C min−1 was applied. The Tm value of each complex was determined from the first derivative plots (dA260/dT versus temperature) as the temperature at a local maximum of dA260/dT.Acknowledgements
This study was supported by the Czech Science Foundation (grant no. P207/12/P625) and Gilead Sciences, Inc. (Foster City, CA, USA). Subvention for the development of a research organization (RVO: 61388963) is also acknowledged.Notes and references
- (a) C. J. Leumann, Bioorg. Med. Chem., 2002, 10, 841–854 CrossRef CAS; (b) Antisense Drug Technology: Principles, Strategies, and Applications, ed. S. T. Crooke, CRC Press, Boca Raton, 2nd edn, 2007 Search PubMed; (c) S. Kauppinen, B. Vester and J. Wengel, Drug Discovery Today Technol., 2005, 2, 287–290 CrossRef PubMed.
- T. Imanishi and S. Obika, Chem. Commun., 2002, 1653–1659 RSC.
- S. Obika, D. Nanbu, Y. Hari, K. Morio, Y. In, T. Ishida and T. Imanishi, Tetrahedron Lett., 1997, 38, 8735–8738 CrossRef CAS.
- S. K. Singh, P. Nielsen, A. A. Koshkin and J. Wengel, Chem. Commun., 1998, 455–456 RSC.
- (a) R. N. Veedu and J. Wengel, Chem. Biodiversity, 2010, 7, 536–542 CrossRef CAS PubMed; (b) T. P. Prakash, Chem. Biodiversity, 2011, 8, 1616–1641 CrossRef CAS PubMed.
- (a) M. A. Campbell, B. Vester and J. Wengel, Nucleic Acid Ther., 2011, 21, A4 Search PubMed; (b) K. K. Karlsen and J. Wengel, Nucleic Acid Ther., 2012, 22, 366–370 CAS; (c) A. S. Jorgensen, L. H. Hansen, B. Vester and J. Wengel, Bioorg. Med. Chem. Lett., 2014, 24, 2273–2277 CrossRef PubMed; (d) M. Hollenstein, Molecules, 2012, 17, 13569–13591 CrossRef CAS PubMed.
- H. Kaur, B. R. Babu and S. Maiti, Chem. Rev., 2007, 107, 4672–4697 CrossRef CAS PubMed.
- J. F. Xu, Y. Liu, C. Dupouy and J. Chattopadhyaya, J. Org. Chem., 2009, 74, 6534–6554 CrossRef CAS PubMed.
- (a) C. Zhou and J. Chattopadhyaya, Chem. Rev., 2012, 112, 3808–3832 CrossRef CAS PubMed; (b) P. P. Seth, C. R. Allerson, A. Berdeja, A. Siwkowski, P. S. Pallan, H. Gaus, T. P. Prakash, A. T. Watt, M. Egli and E. E. Swayze, J. Am. Chem. Soc., 2010, 132, 14942–14950 CrossRef CAS PubMed.
- (a) M. Kaura, D. C. Guenther and P. J. Hrdlicka, Org. Lett., 2014, 16, 3311 CrossRef PubMed; (b) M. Kaura, P. Kumar and P. J. Hrdlicka, J. Org. Chem., 2014, 79, 6256–6268 CrossRef CAS PubMed; (c) P. Kumar, M. E. Østergaard, B. Baral, B. A. Anderson, D. C. Guenther, M. Kaura, D. J. Raible, P. K. Sharma and P. J. Hrdlicka, J. Org. Chem., 2014, 79, 5047–5061 CrossRef CAS PubMed.
- (a) J. Wang, B. Verbeure, I. Luyten, E. Lescrinier, M. Froeyen, C. Hendrix, H. Rosemeyer, F. Seela, A. Van Aerschot and P. Herdewijn, J. Am. Chem. Soc., 2000, 122, 8595–8602 CrossRef CAS; (b) P. Herdewijn and E. De Clercq, Bioorg. Med. Chem. Lett., 2001, 11, 1591–1597 CrossRef CAS; (c) B. Verbeure, E. Lescrinier, J. Wang and P. Herdewijn, Nucleic Acids Res., 2001, 29, 4941–4947 CrossRef CAS PubMed; (d) J. Wang, B. Verbeure, I. Luyten, M. Froeyen, C. Hendrix, H. Rosemeyer, F. Seela, A. Van Aerschot and P. Herdewijn, Nucleosides, Nucleotides Nucleic Acids, 2001, 20, 785–788 CrossRef CAS PubMed.
- K. Robeyns, P. Herdewijn and L. Van Meervelt, Artif. DNA: PNA & XNA, 2010, 1, 2–8 Search PubMed.
- M. Ovaere, P. Herdewijn and L. Van Meervelt, Chem. – Eur. J., 2011, 17, 7823–7830 CrossRef CAS PubMed.
- (a) M. Egli, P. S. Pallan, C. R. Allerson, T. P. Prakash, A. Berdeja, J. H. Yu, S. Lee, A. Watt, H. Gaus, B. Bhat, E. E. Swayze and P. P. Seth, J. Am. Chem. Soc., 2011, 133, 16642–16649 CrossRef CAS PubMed; (b) P. P. Seth, J. H. Yu, A. Jazayeri, P. S. Pallan, C. R. Allerson, M. E. Østergaard, F. W. Liu, P. Herdewijn, M. Egli and E. E. Swayze, J. Org. Chem., 2012, 77, 5074–5085 CrossRef CAS PubMed; (c) M. E. Jung, T. A. Dwight, F. Vigant, M. E. Østergaard, E. E. Swayze and P. P. Seth, Angew. Chem., Int. Ed., 2014, 37, 9893–9897 CrossRef PubMed.
- A. A. Koshkin, J. Fensholdt, H. M. Pfundheller and C. Lomholt, J. Org. Chem., 2001, 66, 8504–8512 CrossRef CAS PubMed.
- E. Abraham, C. W. Bailey, T. D. W. Claridge, S. G. Davies, K. B. Ling, B. Odell, T. L. Rees, P. M. Roberts, A. J. Russell, A. D. Smith, L. J. Smith, H. R. Storr, M. J. Sweet, A. L. Thompson, J. E. Thomson, G. E. Tranter and D. J. Watkin, Tetrahedron: Asymmetry, 2010, 21, 1797–1815 CrossRef CAS PubMed.
- F. H. Wu, R. Hong, J. H. Khan, X. F. Liu and L. Deng, Angew. Chem., Int. Ed., 2006, 45, 4301–4305 CrossRef CAS PubMed.
- M.-H. Filippini, R. Faure and J. Rodriguez, J. Org. Chem., 1995, 60, 6872–6882 CrossRef CAS.
- K. C. Nicolau, Y.-L. Zhong and P. S. Baran, J. Am. Chem. Soc., 2000, 122, 7596–7597 CrossRef.
- J. L. Luche, J. Am. Chem. Soc., 1978, 100, 2226–2227 CrossRef CAS.
- P. S. Jones, P. W. Smith, G. W. Hardy, P. D. Howes, R. J. Upton and R. C. Bethell, Bioorg. Med. Chem. Lett., 1999, 9, 605–610 CrossRef CAS.
- C. Ding, Y. Zhang, H. Chen, C. Wild, T. Wang, M. A. White, Q. Shen and J. Zhou, Org. Lett., 2013, 15, 3718–3721 CrossRef CAS PubMed.
- M. Dejmek, S. Kovačková, E. Zborníková, H. Hřebabecký, M. Šála, M. Dračínský and R. Nencka, RSC Adv., 2012, 2, 6970–6980 RSC.
- M. Dejmek, M. Šála, P. Plačková, M. Hřebabecký, L. Mascarell Borredà, J. Neyts, M. Dračínský, E. Procházková, P. Jansa, P. Leyssen, H. Mertlíková-Kaiserová and R. Nencka, Arch. Pharm., 2014, 347, 478–485 CrossRef CAS PubMed.
- M. T. Migawa, T. P. Prakash, G. Vasquez, P. P. Seth and E. E. Swayze, Org. Lett., 2013, 15, 4316–4319 CrossRef CAS PubMed.
- M. R. Harnden, P. G. Wyatt, R. B. Boyd and D. Sutton, J. Med. Chem., 1990, 33, 187–196 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ob02193b |
| This journal is © The Royal Society of Chemistry 2015 |





