A domino reaction of tetrahalo-7,7-dimethoxybicyclo[2.2.1]heptenyl alcohols leading to indenones and a de novo synthesis of ninhydrin derivatives†
Received
17th September 2014
, Accepted 22nd October 2014
First published on 22nd October 2014
Abstract
An efficient acid induced rearrangement of a tetrahalo-7,7-dimethoxybicyclo[2.2.1]heptenyl system leading to substituted indenones is reported. This domino reaction involves dehydration, olefin isomerization, ketal hydrolysis, [3,3]-sigmatropic rearrangement and dehydrohalogenation. The resultant vicinal dihalo olefin moiety in the efficiently generated indenone derivatives was utilized to transform into ninhydrin derivatives by employing Ru(III)-catalyzed oxidation.
Introduction
In the recent past, the development of new synthetic routes involving one pot transformation has been an attractive area of research in organic synthesis. Among these, domino1 reactions play an important role, because of their ability to build new scaffolds in a single step. The indenone core is an important structural motif in several natural products of biological importance.2 For example, a structural analogue of indenone 1 is used as a fluorescent binding agent to estrogen receptors,3 neo-lignin42 was isolated from Virolasebifera plant fruits, and compound 3 is a structural analogue of the selective COX-2 inhibitor nimesulide.5 Indenones were used as intermediates in the synthesis of fluorenones6 (4), which are important structural motifs in various natural products, and alcyopterosin N7 contains an indanone core (Fig. 1).
 |
| | Fig. 1 Compounds containing indenone cores. | |
Additionally, indenone derivatives are used as fermentation activators,8 estrogen binding receptors,9 and precursors for C-nor-D-homo-steroids,10 gibberellins,11 liquid crystals such as indenes,12 indanones,13 2,4- and 3,4-disubstituted 1-naphthols14 and photochromic indenone oxides.15 On account of their importance, various methods have been developed to synthesize indenone derivatives, for example conventional organic synthetic methods such as Friedel–Crafts,16 Grignard17 and transition metal-mediated (rhodium,18a–c cobalt18d and ruthenium18e) reactions of alkynes and palladium catalysed coupling methods.18f–h It is worth mentioning that phenyl derivatives invariably serve as the precursors for indenones in most of the literature reports.
Results and discussion
Tetrahalobicyclo[2.2.1]heptenyl derivatives19 are readily available substrates through the Diels–Alder reaction, and are extensively used as convenient templates in organic syntheses, particularly natural products. As represented in Table 1, during the last decade we have reported the Grob-type fragmentation reaction of 5 leading to 2,3,4-trihalophenol derivatives.20 We thought that this methodology could be extended to prepare alkylated benzene derivatives from the corresponding exocyclic olefins through the same mechanistic pathway. The parent olefin 7, which was not attainable through a simple Wittig reaction, perhaps due to the enolizable nature of the ketone,20 was prepared via a methyl Grignard addition, followed by elimination. As expected, subjecting 7 to p-toluenesulfonic acid (PTSA) gave 8 in good yield. Based on the premise that tertiary alcohol 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 under PTSA conditions would also yield the corresponding alkylated benzene derivative through exocyclic olefin 10a, an experiment was carried out and the results are represented in Table 1. To our surprise, the compound 9 underwent a domino reaction via Cope rearrangement22 to deliver indenone 11 in excellent yield. It worth mentioning that 4,5,6,7-tetrachloro-3a,4,7,7a-tetrahydro-4,7-methanoinden-8-one was reported to undergo smooth Cope rearrangement rather than the anticipated CO extrusion reaction for these systems.23 Here, we wish to report this serendipitously observed, efficient acid-induced domino reaction of tetrahalo-7,7-dimethoxybicyclo[2.2.1]heptenyl alcohols leading to indenones. Furthermore, conversion of these indenones to ninhydrins, by employing Ru(III)-catalyzed oxidation, is also discussed. This, to the best our knowledge, represents a de novo synthesis.
21 under PTSA conditions would also yield the corresponding alkylated benzene derivative through exocyclic olefin 10a, an experiment was carried out and the results are represented in Table 1. To our surprise, the compound 9 underwent a domino reaction via Cope rearrangement22 to deliver indenone 11 in excellent yield. It worth mentioning that 4,5,6,7-tetrachloro-3a,4,7,7a-tetrahydro-4,7-methanoinden-8-one was reported to undergo smooth Cope rearrangement rather than the anticipated CO extrusion reaction for these systems.23 Here, we wish to report this serendipitously observed, efficient acid-induced domino reaction of tetrahalo-7,7-dimethoxybicyclo[2.2.1]heptenyl alcohols leading to indenones. Furthermore, conversion of these indenones to ninhydrins, by employing Ru(III)-catalyzed oxidation, is also discussed. This, to the best our knowledge, represents a de novo synthesis.
Table 1 Initial approach for synthesis of alkylated 2,3,4-trihalobenzene derivative 8via Grob-type Fragmentation (path-a) and unexpected formation of indenone 11 (path-b)a
|
Reagents and conditions: (a) MeMgI, Et2O, rt, 1 h, 91%, (b) SOCl2, pyridine, 0° C–rt, 5 h, 60%, (c) p-TsOH, toluene, reflux, 17 h, 84%, (d) p-TsOH, toluene, reflux, 23 h, 94%.
|

|
Initially, the Diels–Alder adducts of methyl acrylate and vinyl ketones 12 and 1324 obtained from 1,2,3,4-tetrachloro-5,5-dimethoxycyclopentadiene have been utilized to prepare alcohols 14a–d21 (yield 91–99%) and 15a–l (yield 92–99%), respectively, by treatment with Grignard reagents, as represented in Table 2. After preparing various alcohols, we turned our attention to executing the domino reaction as observed for 9. In the beginning, tertiary alcohols with two similar alkyl substituents (9, 14a–c) were treated with PTSA in refluxing toluene (23–60 h). As depicted in Table 3, indenones (11, 16a–c) were obtained in good yields (71–9 4%). Notably, in addition to the alkyl substituted alcohols, the cyclopentanol 14d delivered tricyclic cyclopentanulated indenone 16d in good yield.
Table 2 Synthesis of tertiary alcohols (14a–d and 15a–l) from 12 and 13
|

|
| Entry |
Product |
R, R1 |
Time (h) |
Yielda (%) |
|
Yields refers to isolated yields of chromatographically pure products. For compound 14a see ref. 21.
|
| 1 |
14a
|
R, R1 = –CH3 |
3.5 |
89 |
| 2 |
14b
|
R, R1 = –CH2–CH3 |
4 |
89 |
| 3 |
14c
|
R, R1 = –(CH2)2CH3 |
5 |
99 |
| 4 |
14d
|
R, R1 = –(CH2)4– |
6 |
91 |
| 5 |
15a
|
R = –CH3, R1 = –CH2–CH3 |
5 |
99 |
| 6 |
15b
|
R = –CH3, R1 = –(CH2)2CH3 |
9 |
98 |
| 7 |
15c
|
R = –CH3, R1 = –(CH2)3CH3 |
9 |
94 |
| 8 |
15d
|
R = –CH3, R1 = –(CH2)4CH3 |
13 |
99 |
| 9 |
15e
|
R = –CH3, R1 = –(CH2)5CH3 |
13 |
97 |
| 10 |
15f
|
R = –CH3, R1 = –(CH2)7CH3 |
16 |
99 |
| 11 |
15g
|
R = –CH3, R1 = –(CH2)11CH3 |
6 |
98 |
| 12 |
15h
|
R = –CH3, R1 = –CH2–C6H5 |
10 |
98 |
| 13 |
15i
|
R = –CH3, R1 = –C6H5 |
8 |
99 |
| 14 |
15j
|
R = –CH3, R1 = p-CH3–C6H4– |
15 |
98 |
| 15 |
15k
|
R = –CH3, R1 = o-OMe–C6H4– |
14 |
92 |
| 16 |
15l
|
R = –CH3, R1 = m-CH3–C6H4– |
13 |
99 |
Table 3 Acid mediated one pot synthesis of 2,3-dihaloindenones via [3,3]-sigmatropic rearrangementa
|
All reactions were carried out using 5 equiv. p-TsOH. Yields referreds to isolated yields of chromatographically pure products.
|

|
In order to determine the regioselectivity of this transformation, we became interested in probing the reaction of alcohols 15a–l, and the results are summarized in Table 4. However, the presence of two different groups (R and -CH2R1) in this case is expected to give two regioisomers, surprisingly in all cases isolated indenones (17a–l) as single regioisomers. The high regioselectivity leading toa single indenone may be attributed to the preferential formation of a more substituted alkene upon dehydration and isomerization of the initially formed exocyclic alkene. A plausible mechanism for formation of indenones (11, 16a) from alcohols (9, 14a) is depicted in Scheme 1. Under acidic conditions, the tertiary alcohols are expected to undergo dehydration to give a mixture of two interconvertible isomeric olefins (10a,b and 18a,b). This was confirmed by intercepting the reaction after 15 minutes and characterizing the inseparable mixture of isomeric olefins (10a,b and 18a,b) using 1H, 13C NMR (see ESI†). At this stage, refluxing the mixture of olefins (10a,b or 18a,b), thus intercepted, in xylene in the absence of PTSA did not afford the indenone. Evidently, PTSA plays a further role after dehydration to transform the ketal moiety into the carbonyl group. The resulting ketone would then furnish the fused bicycle via rearrangement,23 as detailed in Scheme 1. Finally, dehydrohalogenation of fused bicyclic intermediates leads to indenones (11, 16a).
 |
| | Scheme 1 A plausible mechanism for the formation of indenone. | |
Table 4 Regioselective transformation of alcohols 15a–lvia [3,3]-sigmatropic rearrangementa
|
All reactions were carried out by using 5 equiv. p-TsOH. Yields refers to isolated yields of chromatographically pure products.
|
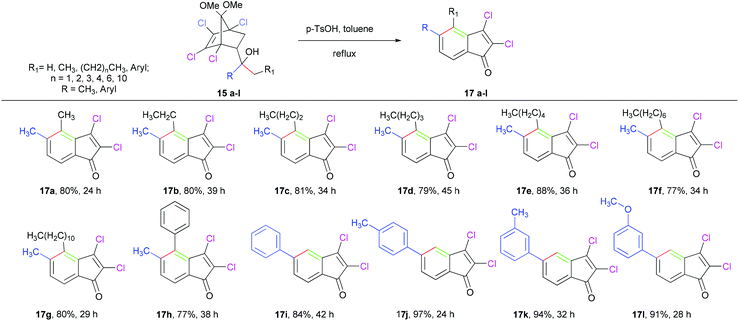
|
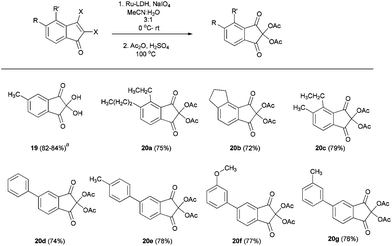
Having various substituted indenone derivatives with vicinal dihalo olefin functionality, we wanted to prepare ninhydrin derivatives by Ru(III) catalyzed oxidation.25 The use of ninhydrins received significant attention from many research groups globally, due to their wide ranging applications, which includes analysis and detection of amino acids, proteins, agricultural, analytical and forensic sciences, and especially for the detection of latent fingerprints.26 We anticipated that the synthesized vicinal halo indenones are the best precursors for the synthesis of ninhydrin derivatives due to the existence of a facile method for the conversion of vicinal dihalo alkene to the α-diketone moiety.25 The details of preparation of various ninhydrin derivatives (19, 20a–g) from indenones (11, 16a–b,d and 17b,i–l) are tabulated in Table 5. Apart from compound 1927 all the ninhydrin derivatives obtained after oxidation of substrates (16b,d, 17b and 17i–l) were characterized by converting them into the corresponding diacetate derivatives (20a–g).
Table 5 Ru(III) catalyzed oxidation of compounds 11, 16a–b,d and 17b,i–l
|
Compound 19 was isolated after Ru-LDH oxidation, from 11 yield 84% and from 16a yield 82%. Yields refers to the isolated two-step, overall yield, apart from compound 19.
|
|
Conclusions
In conclusion, we have demonstrated an unprecedented domino reaction for the formation of indenone derivatives, and an efficient synthesis of indenones starting from unconventional (non-aromatic) precursors is described. The methodology allows facile incorporation of two different types of substituents (alkyl and aryl) at the 4 and 5 positions (indenone numbering) of indenones. The two halogens that are present on the same side as the endo-substituent (shown in pink) are retained in the final structure and could serve as convenient handles for further elaboration. A plausible mechanistic pathway has been discussed. The indenones with vicinal dihalo alkene moiety were converted to ninhydrin derivatives in good yields using cat. Ru-LDH and NaIO4 as the co-oxidant.
Experimental section
General
All reactions were performed in oven dried apparatus. Commercial grade solvents were distilled before use. Melting points were obtained in open capillary tubes and are uncorrected. Infrared spectra were recorded as neat. The samples for NMR were made by dissolving in CDCl3 and TMS is used as an internal standard; the δ values for the peaks in 1H NMR were reported in terms of ppm with reference to TMS (0 ppm) peak and the coupling constants were reported in Hz. The multiplicities are reported as follows br = broad, s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, quin = quintet, sxt = sextet, m = multiplet. The chemical shift in 13C NMR is assigned by fixing the middle peak of CDCl3 at 77.00 ppm. HRMS was recorded using electron spray ionization (ESI) or atmospheric chemical ionization (APCI) mode. The reactions were monitored by thin layer chromatography (tlc) on microscopic slides coated with silica gel and visualization of the spots was accomplished by exposure to iodine vapor or spraying with 4% ethanolic H2SO4 solution or by UV radiation. The silica gel (100 × 200) column chromatography was carried for purification of compounds with various combinations of hexane and EtOAc solvent system as the eluent. Diethyl ether and tetrahydrofuran were distilled immediately prior to use from sodium/benzophenoneketyl under argon. Distilled water was used for the reactions. LDH refers to Layered Double Hydroxide.
General procedure for compounds 6, 14b–d, 15a–l (synthesis of tertiary alcohols)
A diethyl ether or tetrahydrofuran solution of alkyl or aryl magnesium halide was prepared from Mg turnings (4 mmol), a catalytic amount of iodine ≈5 mg, and alkyl or aryl halide (5 mmol) in 5 mL dry diethyl ether or THF as per standard procedures. After the metal was completely consumed, the substrate (1 mmol) in 3 mL ether or THF was added slowly under an argon atmosphere. The reaction mixture was stirred for a specified time at room temperature (completion of the starting material as per tlc), cooled, and quenched with saturated NH4Cl solution (1 mL). The reaction mixture was extracted thrice with ethyl acetate (3 × 10 mL). The combined organic layer was washed once with saturated brine solution and dried over anhydrous Na2SO4. The solvent was evaporated, and the crude reaction mixture was purified by chromatography using silica gel.
General procedure for the preparation of indenone derivatives (11, 16a–d, 17a–l) and compound 8 (synthesis of indenones)
p-Toluenesulfonic acid monohydrate (2 mmol), was added to a solution of the bicyclic alcohols (0.388 mmol) in toluene (6 mL) and the reaction mixture was refluxed for specified time. After the reaction was complete (tlc monitoring), the reaction mixture was diluted with water (2 mL) and the aqueous layer was extracted with EtOAc (3 × 10 mL). The combined organic layers were washed with saturated brine solution and dried over anhydrous Na2SO4. The solvent was concentrated under reduced pressure to give a residue, which was purified by silica gel column chromatography.
General procedure for compounds 10a, 10b, 18a and 18b
p-Toluenesulfonic acid monohydrate (0.151 mmol), was added to a solution of the bicyclic alcohol (0.076 mmol) in toluene (1 mL) and the reaction mixture was refluxed for 15 min. After the reaction was complete (tlc monitoring), the reaction mixture was diluted with water (2 mL) and the aqueous layer was extracted with EtOAc (3 × 5 mL). The combined organic layers were washed with brine (2 mL) and dried over anhydrous Na2SO4. The solvent was concentrated under reduced pressure to furnish a residue, which was purified by silica gel column chromatography.
General procedure for ninhydrin derivatives 20a–g (synthesis of ninhydrins)
To a stirred solution of indenones (0.418 mmol) in acetonitrile (3 mL) at 0 °C was added a solution of Ru-LDH (20, mg 0.83 mol% RuCl3 loaded on LDH) and NaIO4 (1.0455 mmol) in water (1 mL). The mixture was stirred for 5 h at −10 to 0 °C, after completion of the reaction (monitored by TLC). The resulting suspension was filtered through a thin pad of silica gel, which was then washed with ethyl acetate (10 mL). Concentration of the filtrate, and followed by silica gel column chromatography of the obtained residue, afforded a viscous liquid. Then the liquid was treated with acetic anhydride (1 mL) and a catalytic amount of concentrated (98%) sulfuric acid was added. The reaction mixture was stirred for 15 min at room temperature and was heated at 100 °C. Then the reaction mixture was cooled in an ice bath and diluted with water (0.5 mL). The solution was extracted with ethyl acetate (3 × 5 mL), washed with saturated NaHCO3 solution (2 mL) and dried over anhydrous Na2SO4. The solvent was then concentrated under reduced pressure to yield a residue, which was purified by silica gel column chromatography.
1,4,5,6-Tetrabromo-7,7-dimethoxy-2-methylbicyclo[2.2.1]hept-5-en-2-ol (6).
Yield 91%, Rf = 0.4 (15% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.89 (s, 1H), 3.69 (s, 3H), 3.60 (s, 3H), 2.33 (d, 1H, J = 12.8 Hz), 2.16 (d, 1H, J = 12.5 Hz), 1.12 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 128.8, 125.0, 113.1, 80.2, 68.0, 53.4, 51.9, 49.4, 23.5; IR νmax (neat) 3521, 2986, 2948, 2843, 1573, 1445, 1393, 1361, 1264, 1214, 1184, 1101, 978, 928, 852, 827, 718, 663 cm−1; HRMS (ESI) m/z calcd for C10H12Br4NaO3 [M + Na]+ 522.7376; found 522.7368.
Preparation of exocyclic double bond containing compound (7).
To a solution of compound 6, 217 mg (0.434 mmol) in 1.5 mL anhydrous pyridine cooled to −10 °C was treated for 1 min with 1 mL of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of thionyl chloride and anhydrous pyridine. The reaction was stirred at −10 °C to rt for 5 h and was poured into 50 mL cold pentane. The pentane solution was treated cautiously with ice chips and the resulting layers separated. Several pentane extracts were combined and washed with two portions of 3% aqueous hydrochloric acid, followed by brine until neutral. The pentane extract was dried with anhydrous Na2SO4. The solvent was concentrated under reduced pressure to yield a residue, which was purified by silica gel column chromatography to afford colorless solid 7, yield 60% (125 mg, 0.259 mmol), Rf = 0.64 (5% EtOAc in hexane, silica gel tlc), mp 69–71 °C; 1H NMR (400 MHz, CDCl3) δ 5.40 (s, 1H) 5.08 (s, 1H), 3.63 (s, 3H), 3.58 (s, 3H), 2.99 (d, 1H, J = 14.5 Hz), 2.6 (d, 1H, J = 18.5 Hz); 13C NMR (400 MHz, CDCl3) δ 143.7, 127.1, 125.2, 111.6, 109.5, 67.7, 53.0, 51.9, 42.7; IR νmax (neat) 2986, 2947, 2842, 1667, 1569, 1452, 1432, 1251, 1203, 1170, 1107, 1010, 949, 756, 694, 616 cm−1; HRMS (ESI) calcd for C10H14Br4O2N [M + NH4]+ 499.7717; found 499.7708.
1 mixture of thionyl chloride and anhydrous pyridine. The reaction was stirred at −10 °C to rt for 5 h and was poured into 50 mL cold pentane. The pentane solution was treated cautiously with ice chips and the resulting layers separated. Several pentane extracts were combined and washed with two portions of 3% aqueous hydrochloric acid, followed by brine until neutral. The pentane extract was dried with anhydrous Na2SO4. The solvent was concentrated under reduced pressure to yield a residue, which was purified by silica gel column chromatography to afford colorless solid 7, yield 60% (125 mg, 0.259 mmol), Rf = 0.64 (5% EtOAc in hexane, silica gel tlc), mp 69–71 °C; 1H NMR (400 MHz, CDCl3) δ 5.40 (s, 1H) 5.08 (s, 1H), 3.63 (s, 3H), 3.58 (s, 3H), 2.99 (d, 1H, J = 14.5 Hz), 2.6 (d, 1H, J = 18.5 Hz); 13C NMR (400 MHz, CDCl3) δ 143.7, 127.1, 125.2, 111.6, 109.5, 67.7, 53.0, 51.9, 42.7; IR νmax (neat) 2986, 2947, 2842, 1667, 1569, 1452, 1432, 1251, 1203, 1170, 1107, 1010, 949, 756, 694, 616 cm−1; HRMS (ESI) calcd for C10H14Br4O2N [M + NH4]+ 499.7717; found 499.7708.
Methyl 2,3,4-tribromo-5-methylbenzoate (8).
Colorless solid 8, yield 84%, Rf = 0.54 (15% EtOAc in hexane, silica gel tlc), mp 71–73 °C; 1H NMR (400 MHz, CDCl3) δ 7.47 (s, 1H) 3.93 (s, 3H), 2.47 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 166.5, 139.6, 134.0, 131.5, 130.5, 129.9, 121.3, 52.9, 25.0; IR νmax (neat) 2951, 2923, 2851, 1731, 1572, 1428, 1381, 1260, 1144, 1051, 989, 917, 891, 770, 641 cm−1; HRMS (APCI) calcd for C9H8Br3O2 [M + H]+ 386.8054; found 386.8052.
1,2,3,4-Tetrabromo-7,7-dimethoxy-5-(propan-2-ylidene)bicyclo[2.2.1]hept-2-ene (10a).
10a,b, yield 98%, ratio of isomers 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47, colorless liquid, Rf = 0.62 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.61 (s, 3H), 3.59 (s, 3H), 2.92 (d, 1H, J = 13.6 Hz), 2.41 (d, 1H, J = 13.6 Hz), 2.04 (s, 3H), 1.63 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.8, 129.5, 126.0, 125.3, 111.1, 72.7, 67.5, 52.3, 51.7, 42.6, 23.8; IR νmax (neat) 3080, 2980, 2946, 2841, 1641, 1568, 1451, 1373, 1263, 1180, 1111, 971, 893, 842, 722, 647, 571 cm−1; HRMS (APCI) calcd for C12H15Br4O2 [M + H]+ 510.7764; found 510.7762.
47, colorless liquid, Rf = 0.62 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.61 (s, 3H), 3.59 (s, 3H), 2.92 (d, 1H, J = 13.6 Hz), 2.41 (d, 1H, J = 13.6 Hz), 2.04 (s, 3H), 1.63 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.8, 129.5, 126.0, 125.3, 111.1, 72.7, 67.5, 52.3, 51.7, 42.6, 23.8; IR νmax (neat) 3080, 2980, 2946, 2841, 1641, 1568, 1451, 1373, 1263, 1180, 1111, 971, 893, 842, 722, 647, 571 cm−1; HRMS (APCI) calcd for C12H15Br4O2 [M + H]+ 510.7764; found 510.7762.
1,2,3,4-Tetrabromo-7,7-dimethoxy-5-(prop-1-en-2yl)bicyclo[2.2.1]hept-2-ene (10b).
1H NMR (400 MHz, CDCl3) δ 5.00 (s, 1H), 4.73 (s, 1H), 3.64 (s, 3H), 3.63 (s, 3H), 3.28 (dd, 1H, J = 9.3, 4.5 Hz), 2.54 (dd, 1H, J = 12, 9.3 Hz), 1.99 (dd, 1H, J = 12, 4.5 Hz), 1.82 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.8, 126.5, 125.5, 116.5, 112.3, 73.5, 68.3, 53.2, 52.3, 43.8, 19.6; IR (neat) 2916, 1717, 1607, 1546, 1455, 1343, 1228, 1187, 1092, 833, 763, 693, 638, 601 cm−1; HRMS (APCI) calcd for C12H15Br4O2 [M + H]+ 510.7764; found 510.7762.
2,3-Dibromo-5-methyl-1H-inden-1-one (11).
Yellow solid 11, yield 94%, Rf = 0.6 (5% EtOAc in hexane, silica gel tlc), mp 91–93 °C; 1H NMR (400 MHz, CDCl3) δ 7.35 (d, 1H, J = 7.3, Hz), 7.08 (d, 1H, J = 7.3, Hz), 7.00 (s, 1H), 2.41 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 186.2, 145.8, 142.2, 129.7, 126.6, 123.1, 122.7, 122.3, 22.1; IR νmax (neat) 3080, 2980, 2946, 2841, 1641, 1568, 1451, 1373, 1263, 1180, 1111, 971, 893, 842, 722, 647, 571 cm−1; HRMS (ESI) calcd for C10H7Br2O [M + H]+ 302.8843; found 302.8837.
3-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)pentan-3-ol (14b).
Colorless liquid 14b, yield 98%, Rf = 0.53 (10% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.6 (s, 3H), 3.54 (s, 3H), 2.76 (dd,1H, J = 9.3, 4.9 Hz), 2.34 (dd, 1H, J = 11.2, 9.3 Hz), 2.11 (dd, 1H, J = 11.2, 4.9 Hz), 1.94–1.87 (m, 1H), 1.74–1.69 (m, 1H), 1.40–1.28 (m, 2H), 1.07 (s, 1H), 0.9 (t, 3H, J = 7.3), 0.81 (t, 3H, J = 7.6 Hz); 13C NMR (400 MHz, CDCl3) δ 128.6, 128.3, 112.3, 77.9, 75.7, 74.0, 52.8, 51.6, 51.0, 38.0, 29.2, 8.7, 7.1; IR νmax (neat) 3605, 3493, 2970, 2883, 2845, 1607, 1457, 1278, 1193, 1117 cm−1; HRMS (APCI) m/z calcd for C14H19Cl4O2 [(M + H)+(–H2O)]+ 361.0062; found 361.0096.
4-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)heptan-4-ol (14c).
Colorless solid 14c, yield 99%, Rf = 0.5 (10% EtOAc in hexane, silica gel tlc), mp 66–68 °C; 1H NMR (400 MHz, CDCl3) δ 3.58 (s, 3H), 3.53 (s, 3H), 2.74 (dd, 1H, J = 9.3, 4.9 Hz), 2.34 (t, 1H, J = 10.0 Hz), 2.12 (dd, 1H, J = 11.2, 4.9 Hz), 1.88–1.81 (m, 1H), 1.59 (td, 1H J = 13.0, 4.4 Hz), 1.43–1.34 (m, 1H), 1.26–1.21 (m, 5H), 1.08 (s, 1H), 0.93–0.88 (m, 6H); 13C NMR (400 MHz, CDCl3) δ 129.0, 128.3, 112.3, 77.9, 75.2, 73.9, 52.7, 51.6, 40.0, 39.6, 38.0, 17.8, 15.8, 14.4, 14.2; IR νmax (neat) 3563, 2960, 2871, 1609, 1259, 1189, 1089, 1012, 794 cm−1; HRMS (APCI) m/z calcd for C16H23Cl4O2 [(M + H)+(–H2O)]+ 389.0423; found 389.0412.
1-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)cyclopentanol (14d).
Colorless liquid 14d, yield 91%, Rf = 0.5 (10% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.60 (s, 3H), 3.55 (s, 3H), 2.69 (dd, 1H, J = 9.3, 4.9 Hz), 2.44 (dd, 1H, J = 11.2, 9.3 Hz), 2.05 (dd, 1H, J = 11.7, 4.9 Hz), 1.88–1.80 (m, 3H), 1.79–1.70 (m, 2H), 1.65–1.57 (m, 2H), 1.46–1.40 (m, 1H), 1.08 (s, 1H); 13C NMR (400 MHz, CDCl3) δ 128.9, 128.3, 112.5, 82.1, 78.2, 74.2, 55.0, 53.0, 51.6, 39.8, 39.6, 38.5, 23.8, 22.0; IR (neat) 3600, 2951, 2874, 2846, 1607, 1445, 1275, 1189, 1119, 1040, 985, 897, 798, 781 cm−1; HRMS (APCI) m/z calcd for C14H17Cl4O2 [(M + H)(–H2O)]+ 358.9953; found 358.9945.
2-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)butan-2-ol (15a).
Colorless liquid 15a, yield 99%, Rf = 0.56 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.60 (s, 3H), 3.55 (s, 3H), 2.67 (dd,1H, J = 9.3, 4.9 Hz), 2.36 (dd, 1H, J = 11.2, 9.3 Hz), 2.1 (dd, 1H, J = 11.2, 4.9 Hz), 1.42–1.36 (m, 5H), 1.14 (s, 1H), 0.88 (t, 3H, J = 7.6 Hz); 13C NMR (400 MHz, CDCl3) δ 128.9, 128.2, 112.4, 78.0, 74.1, 73.3, 53.3, 52.8, 51.6, 37.9, 34.8, 25.5, 7.8; IR νmax (neat) 3603, 2950, 2847, 1606, 1458, 1381, 1275, 1186, 1114, 1035, 986, 905, 796, 724, 610 cm−1; HRMS (ESI) m/z calcd for C13H19Cl4O3 [M + H]+ 365.0059; found 365.0046.
2-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)pentan-2-ol (15b).
Colorless liquid 15b, yield 98%, Rf = 0.51 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.60 (s, 3H), 3.54 (s, 3H), 2.67 (dd, 1H, J = 9.3, 4.9 Hz), 2.37 (dd, 1H, J = 11.2, 9.3 Hz), 2.11 (dd, 1H, J = 11.7, 4.9 Hz), 1.41 (s, 3H), 1.36–1.15 (m, 4H), 1.15 (br, 1H), 0.9 (br, 3H); 13C NMR (400 MHz, CDCl3) δ 128.8, 128.2, 112.4, 78.0, 74.1, 73.2, 53.6, 52.8, 51.6, 45.0, 37.9, 26.1, 16.7, 14.5; IR νmax (neat) 3602, 2955, 2846, 1606, 1459, 1380, 1329, 1275, 1187, 1115, 1033, 905, 873, 802, 745, 616, 581 cm−1; HRMS (ESI) m/z calcd for C14H19Cl4O2 [(M + H)+(–H2O)]+ 361.0109; found 361.0106.
2-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)hexan-2-ol (15c).
Colorless liquid 15c, yield 94%, Rf = 0.51 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.60 (s, 3H), 3.54 (s, 3H), 2.67 (dd,1H, J = 9.3, 4.9 Hz), 2.39–2.33 (m, 1H), 2.11 (dd, 1H, J = 11.7, 4.9 Hz), 1.41 (s, 3H), 1.34–1.26 (m, 6H), 1.15 (br, 1H), 0.9 (t, 3H, J = 6.8 Hz); 13C NMR (400 MHz, CDCl3) δ 128.8, 128.2, 112.4, 77.9, 74.1, 73.2, 53.6, 52.8, 51.6, 42.2, 38.0, 26.2, 25.7, 23.1, 14.0; IR νmax (neat) 3602, 2949, 2856, 1608, 1451, 1379, 1271, 1180, 1113, 1032, 985, 884, 801, 731, 615 cm−1; HRMS (ESI) m/z calcd for C15H26Cl4NO3 [M + NH4]+ 410.0637; found 410.0635.
2-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)heptan-2-ol (15d).
Colorless liquid 15d, yield 96%, Rf = 0.53 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.60 (s, 3H), 3.55 (s, 3H), 2.67 (dd, 1H, J = 9.3, 4.9 Hz), 2.36 (dd, 1H, J = 11.2, 9.3 Hz), 2.11 (dd, 1H, J = 11.2, 4.9 Hz), 1.41 (s, 3H), 1.41–1.14 (m, 8H), 1.14 (br, 1H) 0.89 (t, 3H, J = 6.8 Hz); 13C NMR (400 MHz, CDCl3) δ 128.9, 128.2, 112.4, 78.0, 74.1, 73.2, 53.6, 52.8, 51.6, 42.5, 37.9, 32.2, 26.2, 23.2, 22.5, 14.0; IR νmax (neat) 3604, 2947, 2855, 1606, 1459, 1380, 1275, 1190, 1115, 1034, 989, 889, 802, 736, 616 cm−1; HRMS (ESI) m/z calcd for C16H23Cl4O2 [(M + H)+(–H2O)]+ 389.0422; found 389.0413.
2-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)octan-2-ol (15e).
Colorless liquid 15e, yield 97%, Rf = 0.54 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.60 (s, 3H), 3.55 (s, 3H), 2.67 (dd, 1H, J = 9.3, 4.9 Hz), 2.36 (dd, 1H, J = 11.2, 9.3 Hz), 2.11 (dd, 1H, J = 11.2, 4.9 Hz), 1.41 (s, 3H), 1.33–1.26 (m, 10H), 1.15 (br, 1H) 0.88 (t, 3H, J = 6.6 Hz); 13C NMR (400 MHz, CDCl3) δ 128.8, 128.2, 112.4, 78.0, 74.1, 73.2, 53.6, 52.8, 51.6, 42.5, 37.9, 31.7, 29.7, 26.9, 23.5, 22.6, 14.0; IR νmax (neat) 3606, 2932, 2853, 1606, 1459, 1379, 1332, 1275, 1191, 1114, 1034, 987, 905, 868, 804, 731, 616, 583 cm−1; HRMS (ESI) m/z calcd for C17H25Cl4O2, [(M + H)+(–H2O)]+ 403.0579; found 403.0575.
2-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)decan-2-ol (15f).
Colorless liquid 15f, yield 99%, Rf = 0.56 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.58 (s, 3H), 3.53 (s, 3H), 2.65 (dd, 1H, J = 9.3, 4.9 Hz), 2.32 (dd, 1H, J = 11.2, 9.3 Hz), 2.09 (dd, 1H, J = 11.2, 4.9 Hz), 1.39 (s, 3H), 1.32–1.25 (m, 14H), 1.18 (br, 1H), 0.86 (t, 3H, J = 6.8 Hz); 13C NMR (400 MHz, CDCl3) δ 128.7, 128.2, 112.4, 77.9, 74.0, 73.1, 53.5, 52.7, 51.5, 42.5, 37.9, 31.8, 30.0, 29.4, 29.2, 26.1, 23.5, 22.5, 14.0; IR νmax (neat) 3606, 2926, 2853, 1714, 1606, 1459, 1379, 1275, 1190, 1115, 1304, 989, 879, 804, 744, 616 cm−1; HRMS (ESI) m/z calcd for C19H29Cl4O2 [(M + H)+(–H2O)]+ 431.0892; found 431.0898.
2-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)tetradecan-2-ol (15g).
Colorless liquid 15g, yield 98%, Rf = 0.58 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.59 (s, 3H), 3.53 (s, 3H), 2.66 (dd, 1H, J = 9, 4.6 Hz), 2.35 (dd, 1H, J = 11.2, 9.3 Hz), 2.1 (dd, 1H, J = 11.5, 4.6 Hz), 1.39 (s, 3H), 1.39–1.25 (m, 22H), 1.18 (br, 1H) 0.87 (t, 3H, J = 6.6 Hz); 13C NMR (400 MHz, CDCl3) δ 128.8, 128.2, 112.4, 77.9, 74.1, 73.1, 53.6, 52.7, 51.6, 42.5, 37.9, 31.9, 30.0, 29.6, 29.6, 29.5, 29.5, 29.3, 26.1, 23.5, 22.6, 14.1; IR νmax (neat) 3608, 2852, 1606, 1460, 1379, 1275, 1191, 1115, 1035, 989, 873, 804, 723, 617 cm−1; HRMS (ESI) m/z calcd for C23H37Cl4O2 [(M + H)+(–H2O)]+ 487.1518; found 487.1517.
1-Phenyl-2-(1,4,5,6-tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)propan-2-ol (15h).
White solid 15h, yield 98%, Rf = 0.52 (10% EtOAc in hexane, silica gel tlc), mp 98–100 °C; 1H NMR (400 MHz, CDCl3) δ 7.30–7.14 (m, 5H), 3.58 (s, 3H), 3.54 (s, 3H), 2.73–2.67 (m, 2H), 2.56–2.45 (m, 2H), 2.33 (dd, 1H, J = 11.2, 4.9 Hz), 1.31 (s, 3H), 1.13 (s, 1H); 13C NMR (400 MHz, CDCl3) δ 135.9, 130.8, 128.7, 128.4, 128.2, 126.8, 112.5, 78.0, 74.1, 72.9, 54.1, 52.8, 51.6, 48.1, 38.3, 26.1; IR νmax (neat) 3585, 2979, 2949, 2844, 1605, 1494, 1453, 1381, 1279, 1190, 1116, 1096, 1032, 987, 910, 754, 727, 701, 607 cm−1; HRMS (ESI) m/z calcd for C18H19Cl4O2 [(M + H)+(–H2O)]+ 409.0109; found 409.0110.
1-Phenyl-1-(1,4,5,6-tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)ethanol (15i).
White solid 15i, yield 99%, Rf = 0.61 (10% EtOAc in hexane, silica gel tlc), mp 89–91 °C; 1H NMR (400 MHz, CDCl3) δ 7.32–7.30 (m, 2H), 7.24–7.22 (2H, m), 7.16–7.13 (1H, m), 3.49 (s, 3H), 3.46 (s, 3H), 2.97–2.94 (m,1H), 1.81–1.79 (m, 2H), 1.72 (s, 3H), 1.5 (s, 1H); 13C NMR (400 MHz, CDCl3) δ 147.4, 128.9, 128.2, 126.8, 124.5, 112.4, 78.0, 74.5, 74.0, 55.4, 52.7, 51.6, 37.9, 30.4; IR (neat) νmax 3585, 2954, 2924, 2848, 1603, 1442, 1326, 1261, 1180, 1160, 1098, 1066, 1029, 981, 900, 868, 782, 696, 581 cm−1; HRMS (ESI) m/z calcd for C17H17Cl4O2 [(M + H)+(–H2O)]+ 394.9953; found 394.9949.
1-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)-1-p-tolylethanol (15j).
White solid 15j, yield 98%, Rf = 0.53 (5% EtOAc in hexane, silica gel tlc), mp 107–109 °C; 1H NMR (400 MHz, CDCl3) δ 7.29 (d, 2H, J = 8.3 Hz), 7.15 (d, 2H, J = 8.3 Hz), 3.58 (s, 3H), 3.56 (s, 3H), 3.03 (t, 1H, J = 7.3 Hz), 2.34 (s, 3H), 1.9 (d, 2H, J = 7.3 Hz), 1.79 (s, 3H), 1.53 (s, 1H); 13C NMR (400 MHz, CDCl3) δ 144.6, 136.4, 128.9, 128.2, 124.5, 124.4, 78.0, 74.5, 74.0, 55.4, 52.8, 51.6, 38.0, 30.5, 20.9; IR νmax (neat) 3548, 2984, 2945, 2847, 1606, 1507, 1447, 1359, 1280, 1194, 1109, 1079, 1033, 987, 904, 874, 814, 762, 721, 584 cm−1; HRMS (ESI) m/z calcd for C18H19Cl4O2 [(M + H)+(–H2O)]+ 409.0109; found 409.0108.
1-(3-Methoxyphenyl)-1-(1,4,5,6-tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)ethanol (15k).
White solid 15k, yield 92%, Rf = 0.45 (5% EtOAc in hexane, silica gel tlc), mp 119–121 °C; 1H NMR (400 MHz, CDCl3) δ 7.24 (t, 1H, J = 8.1 Hz), 6.96–6.93 (m, 2H), 6.76 (d, 1H, J = 7.8 Hz), 3.80 (s, 3H), 3.57 (s, 3H), 3.54 (s, 3H), 3.04–3.01 (m, 1H), 1.90–1.88 (m, 1H), 1.78 (s, 3H), 1.56 (s, 1H); 13C NMR (400 MHz, CDCl3) δ 159.6, 149.4, 129.3, 128.9, 128.3, 117.0, 112.4, 111.6, 111.0, 78.1, 27.5, 74.0, 55.2, 52.8, 51.6, 38.0, 30.4; IR νmax (neat) 3483, 2948, 2844, 1605, 1581, 1484, 1435, 1317, 1280, 1182, 1152, 1035, 990, 913, 879, 789, 757, 706, 621 cm−1; HRMS (ESI) m/z calcd for C18H19Cl4O3 [(M + H)+(–H2O)]+ 425.0058; found 425.0056.
1-(1,4,5,6-Tetrachloro-7,7-dimethoxybicyclo[2.2.1]hept-5-en-2-yl)-1-m-tolylethanol (15l).
White solid 15l, yield 99%, Rf = 0.49 (5% EtOAc in hexane, silica gel tlc), mp 79–81 °C; 1H NMR (400 MHz, CDCl3) δ 7.21 (quin, 3H, J = 7.7 Hz), 3.60 (s, 3H), 3.56 (s, 3H), 3.04 (t, 1H, J = 7.1 Hz), 2.37 (s, 3H), 1.91 (d, 2H, J = 7.3 Hz), 1.80 (s, 3H), 1.55 (s, 1H); 13C NMR (400 MHz, CDCl3) δ 147.5, 137.9, 128.9, 128.2, 128.1, 127.5, 125.2, 121.6, 112.4, 78.1, 74.5, 74.0, 55.4, 52.8, 51.7, 37.9, 30.5, 21.6; IR νmax (neat) 3574, 2988, 2949, 2846, 1604, 1448, 1379, 1267, 1184, 1115, 1028, 984, 908, 880, 823, 786, 707, 616, 570, 616 cm−1; HRMS (ESI) m/z calcd for C18H19Cl4O2 [(M + H)+(–H2O)]+ 409.0109; found 409.0105.
2,3-Dichloro-5-methyl-1H-inden-1-one (16a).
Yellow solid 16a, yield 83%, Rf = 0.44 (hexane, silica gel tlc), mp 85–87 °C; 1H NMR (400 MHz, CDCl3) δ 7.43 (s, 1H), 7.25 (d, 1H, J = 7.3, Hz), 7.15 (d, 1H, J = 7.8, Hz), 2.44 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 192.1, 149.5, 148.8, 132.5, 132.0, 127.6, 123.8, 78.4, 73.0, 22.4; IR νmax (neat) 2949, 2846, 1720, 1599, 1565, 1374, 1192, 1113, 829, 764, 697 cm−1; HRMS (APCI) m/z calcd for C10H7Cl2O [M + H]+ 212.9874; found 212.9862.
2,3-Dichloro-5-ethyl-4-methyl-1H-inden-1-one (16b).
Yellow solid 16b, yield 79%, Rf = 0.46 (hexane, silica gel, tlc), mp 75–77 °C; 1H NMR (400 MHz, CDCl3) δ 7.27 (d, 1H), 7.07 (d, 1H), 2.65 (q, 2H, J = 7.5, Hz), 2.54 (s, 3H), 1.2 (t, 3H, J = 7.3, Hz); 13C NMR (400 MHz, CDCl3) δ 185.9, 152.1, 151.7, 136.0, 131.8, 129.1, 128.1, 127.4, 121.0, 27.0, 14.3, 13.6; IR νmax (neat) 2968, 2879, 1722, 1593, 1452, 1213, 1132, 846, 798, 718 cm−1; HRMS (APCI) m/z calcd for C12H11Cl2O [M + H]+ 241.0187; found 241.0176.
2,3-Dichloro-4-ethyl-5propyl-1H-inden-1-one (16c).
Yellow solid 16c, yield 75%, Rf = 0.41 (hexane, silica gel tlc), mp 62–64 °C; 1H NMR (400 MHz, CDCl3) δ 7.28–7.25 (m, 1H), 7.3 (d, 1H J = 7.3, Hz), 3.01 (q, 2H, J = 7.3, Hz), 2.62–2.58 (m, 2H), 1.62 (sxt, 2H, J = 7.5, Hz), 1.23 (t,3H, J = 7.6, Hz), 1.01 (t, 3H, J = 7.3, Hz); 13C NMR (400 MHz, CDCl3) δ 185.8, 151.7, 149.7, 138.3, 135.5, 130.4, 128.2, 127.6, 120.9, 34.9, 24.2, 20.0, 16.2, 14.1; IR νmax (neat) 2961, 2872, 1722, 1593, 1555, 1450, 1203, 1131, 798, 741 cm−1; HRMS (APCI) m/z calcd for C14H15Cl2O [M + H]+ 269.0500; found 269.0496.
1,2-Dichloro-7,8-dihydroas-indacen-3(6H)-one(16d).
Yellow solid 16d, yield 71%, Rf = 0.45 (hexane, silica gel tlc), mp 150–152 °C; 1H NMR (400 MHz, CDCl3) δ 7.3 (d, 1H J = 6.8, Hz), 7.12 (d, 1H J = 7.3, Hz), 3.15 (t, 2H, J = 7.3 × 2, Hz), 2.88 (t, 2H, J = 7.3 × 2, Hz), 2.18–2.10 (2H, m); 13C NMR (400 MHz, CDCl3) δ 186.1, 154.4, 150.8, 138.6, 134.6, 127.2, 127.0, 124.8, 121.8, 32.5, 30.2, 25.3; IR νmax (neat) 2964, 2987, 2897, 1276, 1587, 1553, 1453, 1413, 1344, 1290, 1203, 1176, 1112, 787, 780 cm−1; HRMS (APCI) m/z calcd for C12H9Cl2O [M + H]+ 239.0030; found 239.0022.
2,3-Dichloro-4,5-methyl-1H-inden-1-one (17a).
Yellow solid 17a, yield 80%, Rf = 0.42 (hexane, silica gel tlc), mp 139–141 °C; 1H NMR (400 MHz, CDCl3) δ 7.19 (d, 1H, J = 7.3, Hz), 7.04 (d, 1H, J = 7.3, Hz), 2.48 (s, 3H), 2.28 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 185.7, 151.9, 146.1, 135.6, 132.4, 130.5, 127.4, 120.7, 21.0, 14.0; IR νmax (neat) 2948, 2836, 1712, 1587, 1547, 1446, 1379, 1338, 1256, 1211, 1128, 965, 921, 848, 791, 760, 712 cm−1; HRMS (ESI) m/z calcd for C11H9Cl2O [M + H]+ 227.0030; found 227.0026.
2,3-Dichloro-4-ethyl-5-1H-inden-1-one (17b).
Yellow solid 17b, yield 80%, Rf = 0.49 (hexane, silica gel tlc), mp 56–58 °C; 1H NMR (400 MHz, CDCl3) δ 7.23 (d, 1H, J = 7.3, Hz), 7.06 (d, 1H, J = 6.8, Hz), 3.00 (q, 2H, J = 7.7, Hz), 2.34 (s, 3H), 1.2 (t, 3H, J = 7.6, Hz); 13C NMR (400 MHz, CDCl3) δ 185.8, 151.6, 145.3, 138.6, 135.2, 131.1, 128.2, 127.7, 120.9, 20.6, 20.0, 15.0; IR νmax (neat) 2966, 2930, 2872, 1715, 1585, 1549, 1448, 1340, 1244, 1207, 1125, 1053, 973, 906, 860, 828, 770, 727 cm−1; HRMS (ESI) m/z calcd for C12H11Cl2O [M + H]+ 241.0187; found 241.0182.
2,3-Dichloro-5-methyl-4-propyl-1H-inden-1-one (17c).
Yellow solid 17c, yield 81%, Rf = 0.46 (hexane, silica gel tlc), mp 78–80 °C; 1H NMR (400 MHz, CDCl3) δ 7.21 (d, 1H, J = 7.3, Hz), 7.05 (d, 1H, J = 7.3, Hz), 2.92–2.88 (m, 2H), 2.32 (s, 3H), 1.60–1.51 (m, 2H), 1.04 (t, 3H, J = 7.1, Hz); 13C NMR (400 MHz, CDCl3) δ 185.7, 151.6, 145.5, 137.3, 135.4, 131.0, 128.2, 120.9, 29.3, 24.4, 20.2, 14.0 νmax.33; IR (neat) 2960, 2929, 2871, 1720, 1588, 1553, 1456, 1346, 1232, 1212, 1131, 948, 844, 806, 772, 735 cm−1; HRMS (ESI) m/z calcd for C13H13Cl2O [M + H]+ 255.0343; found 255.0328.
4-Butyl-2,3-dichloro-5-methyl-1H-inden-1-one (17d).
Yellow solid 17d, yield 79%, Rf = 0.43 (hexane, silica gel tlc), mp 49–51 °C; 1H NMR (400 MHz, CDCl3) δ 7.23 (d, 1H, J = 7.3, Hz), 7.06 (d, 1H, J = 7.3, Hz), 2.96–2.92 (m, 2H), 2.33 (s, 3H), 1.55–1.42 (m, 4H), 0.98 (t, 3H, J = 7.6, Hz); 13C NMR (400 MHz, CDCl3) δ 185.8, 151.7, 145.4, 137.5, 135.4, 131.0, 128.3, 127.7, 120.9, 33.2, 27.2, 23.1, 20.2, 13.9; IR νmax (neat) 2957, 2925, 2863, 1720, 1588, 1552, 1457, 1343, 1219, 1129, 1030, 855, 806, 773, 731 cm−1; HRMS (ESI) m/z calcd for C14H15Cl2O [M + H]+ 269.0500; found 269.0498.
2,3-Dichloro-5-methyl-4-pentyl-1H-inden-1-one (17e).
Yellow solid, 17e yield 88%, Rf = 0.43 (hexane, silica gel tlc), mp 79–81 °C; 1H NMR (400 MHz, CDCl3) δ 7.23 (d, 1H, J = 7.3, Hz), 7.06 (d, 1H, J = 7.3, Hz), 2.93–2.91 (m, 2H), 2.33 (s, 3H), 1.55–1.49 (m, 2H), 1.45–1.33 (m, 4H), 0.93 (t, 3H, J = 7.1, Hz); 13C NMR (400 MHz, CDCl3) δ 185.8, 151.7, 145.4, 137.6, 135.4, 131.0, 128.3, 127.7, 120.9, 32.2, 30.8, 27.5, 22.4, 20.2, 14.0; IR νmax (neat) 2955, 2927, 2858, 1723, 1585, 1554, 1461, 1345, 1223, 1132, 1081, 818, 804, 773, 735 cm−1; HRMS (ESI) m/z calcd for C15H17Cl2O [M + H]+ 283.0656; found 283.0650.
2,3-Dichloro-4-heptyl-5-methyl-1H-inden-1-one (17f).
Yellow solid, 17f yield 77%, Rf = 0.49 (hexane, silica gel tlc), mp 62–64 °C; 1H NMR (400 MHz, CDCl3) δ 7.23 (d, 1H, J = 7.3, Hz), 7.06 (d, 1H, J = 7.3, Hz), 2.95–2.91 (m, 2H), 2.33 (s, 3H), 1.56–1.49 (m, 2H), 1.47–1.40 (m, 2H), 1.37–1.25 (m, 6H), 0.89 (t, 3H, J = 6.8, Hz); 13C NMR (400 MHz, CDCl3) δ 185.8, 151.7, 145.4, 137.6, 135.4, 131.0, 128.3, 127.7, 120.9, 31.8, 31.2, 29.0, 27.5, 22.6, 20.2, 14.1; IR νmax (neat) 2923, 2854, 1724, 1587, 1555, 1459, 1374, 1227, 1129, 1086, 977, 812, 767, 728 cm−1; HRMS (ESI) m/z calcd for C17H21Cl2O [M + H]+ 311.0969; found 311.0963.
2,3-Dichloro-5-methyl-4-undecyl-1H-inden-1-one (17g).
Yellow solid 17g, yield 80%, Rf = 0.52 (hexane, silica gel tlc), mp 63–65 °C; 1H NMR (400 MHz, CDCl3) δ 7.22 (d, 1H, J = 6.8, Hz), 7.05 (d, 1H, J = 7.3, Hz), 2.94–2.90 (m, 2H), 2.33 (s, 3H), 1.56–1.48 (m, 2H), 1.47–1.40 (m, 2H), 1.35–1.27 (m, 16H), 0.88 (t, 3H, J = 6.8, Hz); 13C NMR (400 MHz, CDCl3) δ 185.8, 151.6, 145.4, 137.6, 135.3, 131.0, 128.3, 127.7, 120.9, 31.9, 31.1, 30.0, 29.3, 27.5, 22.7, 20.2, 14.1; IR (neat) 2920, 2851, 1718, 1587, 1552, 1458, 1216, 1130, 850, 809, 771, 730 cm−1; HRMS (ESI) m/z calcd for C21H29Cl2O [M + H]+ 367.1595; found 367.1595.
2,3-Dichloro-5-methyl-4-phenyl-1H-inden-1-one (17h).
Yellow solid 17h, yield 77%, Rf = 0.48 (hexane, silica gel tlc), mp 149–151 °C; 1H NMR (400 MHz, CDCl3) δ 7.41 (m, 4H), 7.18 (dd, 3H, J = 6.4, Hz), 2.03 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 185.8, 151.6, 145.4, 136.9, 135.9, 130.4, 129.3, 128.4, 128.2, 128.0, 126.9, 122.0, 21.1; IR νmax (neat) 3047, 2922, 2859, 1718, 1588, 1553, 1444, 1372, 1336, 1219, 1225, 1125, 1083, 892, 832, 765, 726, 696, 648 cm−1; HRMS (ESI) m/z calcd for C16H11Cl2O [M + H]+ 289.0187; found 289.0181.
2,3-Dichloro-5-phenyl-1H-inden-1-one (17i).
Yellow solid 17i, yield 84%, Rf = 0.42 (hexane, silica gel tlc), mp 98–100 °C; 1H NMR (400 MHz, CDCl3) δ 7.59 (d, 2H, J = 6.8, Hz), 7.52–7.42 (m, 6H); 13C NMR (400 MHz, CDCl3) δ 185.5, 149.7, 147.7, 140.9, 139.2, 129.0, 128.8, 128.1, 127.3, 127.0, 126.9, 123.4, 118.7; IR νmax (neat) 3040, 2919, 2861, 1726, 1603, 1565, 1452, 1418, 1340, 1303, 1213, 1109, 1008, 959, 856, 842, 750, 690 cm−1; HRMS (ESI) m/z calcd for C15H9Cl2O [M + H]+ 275.0030; found 275.0034.
2,3-Dichloro-5-p-tolyl-1H-inden-1-one (17j).
Yellow solid 17j, yield 97%, Rf = 0.49 (hexane, silica gel tlc), mp 138–140 °C; 1H NMR (400 MHz, CDCl3) δ 7.48–7.43 (m, 4H), 7.38 (s, 1H), 7.8 (d, 2H, J = 7.8, Hz), 2.39, (s, 3H); 13C NMR (400 MHz, CDCl3) δ 185.5, 149.6, 147.6, 140.9, 138.9, 136.9, 129.7, 127.7, 127.3, 126.6, 123.4, 118.5, 21.2; IR νmax (neat) 3010, 2911, 2831, 1719, 1601, 1663, 1455, 1424, 1337, 1301, 1216, 1169, 1116, 856, 810, 765, 696, 648 cm−1; HRMS (ESI) m/z calcd for C16H11Cl2O [M + H]+ 289.0187; found 289.0182.
2,3-Dichloro-5-m-tolyl-1H-inden-1-one (17k).
Yellow solid 17k, yield 94%, Rf = 0.48 (hexane, silica gel tlc), mp 111–113 °C; 1H NMR (400 MHz, CDCl3) δ 7.54–7.48 (m, 2H), 7.42–7.35 (m, 4H), 7.25 (d, 2H, J = 6.8, Hz), 2.45, (s, 3H); 13C NMR (400 MHz, CDCl3) δ 185.6, 149.7, 147.9, 140.8, 139.2, 138.7, 129.5, 128.9, 128.1, 127.7, 127.3, 126.8, 124.2, 123.4, 118.8, 21.5; IR νmax (neat) 3014, 2918, 2848, 1714, 1604, 1565, 1455, 1379, 1338, 1219, 1112, 1036, 994, 880, 846, 787, 763, 696, 641 cm−1; HRMS (ESI) m/z calcd for C16H11Cl2O [M + H]+ 289.0187; found 289.0178.
2,3-Dichloro-5-(3-methoxyphenyl)-1H-inden-1-one (17l).
Yellow solid 17l, yield 91%, Rf = 0.48 (hexane, silica gel tlc), mp 138–140 °C; 1H NMR (400 MHz, CDCl3) δ 7.53–7.48 (m, 2H), 7.41–7.37 (m, 2H), 7.17 (d, 1H, J = 7.3, Hz), 7.10 (s, 1H), 6.96 (dd, 1H, J = 8.1, 2.2 Hz), 3.88 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 185.5, 160.0, 149.7, 147.5, 140.9, 140.7, 130.1, 128.2, 127.3, 127.1, 123.3, 119.5, 118.8, 113.9, 112.9, 55.4; IR νmax (neat) 3013, 2917, 2849, 1714, 1606, 1566, 1462, 1339, 1281, 1199, 1168, 1115, 1048, 1019, 962, 848, 804, 769, 692, 660, 612 cm−1; HRMS (ESI) m/z calcd for C16H11Cl2O2 [M + H]+ 305.0136; found 305.0135.
1,2,3,4-Tetrachloro-7,7-dimethoxy-5-(propan-2-ylidene)bicyclo[2.2.1]hept-2-ene (18a).
18a,b, yield 98%, ratio of isomers 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81, colorless liquid, Rf = 0.69 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.58 (s, 3H), 3.57 (s, 3H), 3.41 (d, 1H J = 2.9 Hz), 2.37 (d, 1H J = 13.7 Hz), 1.98 (s, 3H), 1.64 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.7, 129.3, 129.0, 125.8, 111.2, 79.2, 73.6, 52.1, 42.2, 23.6, 19.0; IR νmax (neat) 2982, 2950, 2844, 1644, 1603, 1454, 1271, 1190, 1156, 1117, 1040, 985, 902, 756, 591 cm−1; HRMS (APCI) calcd for C12H13Cl4O [(M + H)+(–H2O)]+ 312.9720; found 312.9790.
81, colorless liquid, Rf = 0.69 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.58 (s, 3H), 3.57 (s, 3H), 3.41 (d, 1H J = 2.9 Hz), 2.37 (d, 1H J = 13.7 Hz), 1.98 (s, 3H), 1.64 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.7, 129.3, 129.0, 125.8, 111.2, 79.2, 73.6, 52.1, 42.2, 23.6, 19.0; IR νmax (neat) 2982, 2950, 2844, 1644, 1603, 1454, 1271, 1190, 1156, 1117, 1040, 985, 902, 756, 591 cm−1; HRMS (APCI) calcd for C12H13Cl4O [(M + H)+(–H2O)]+ 312.9720; found 312.9790.
1,2,3,4-Tetrachloro-7,7-dimethoxy-5-(prop-1-en-2yl)bicycle[2.2.1]hept-2-ene (18b).
1H NMR (400 MHz, CDCl3) δ 4.98 (s, 1H), 4.70 (s, 1H), 3.62 (s, 3H), 3.55 (s, 3H), 3.23 (dd, 1H, J = 9.3, 4.4 Hz), 2.50 (dd, 1H, J = 12.2, 9.3 Hz), 1.93 (dd, 1H, J = 11.7, 4.4 Hz), 1.80 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.7, 129.1, 115.6, 112.2, 79.4, 74.5, 52.6, 51.8, 51.6, 40.6, 23.5; IR (neat) 2982, 2950, 2844, 1644, 1603, 1454, 1271, 1190, 1156, 1117, 1040, 985, 902, 756, 591 cm−1; HRMS (APCI) calcd for C12H13Cl4O [(M + H)+(–H2O)]+ 312.9720; found 312.9790.
2,2-Dihydroxy-5-methyl-1H-indene-1,3(2H)-dione (19).
To a stirred solution of 50 mg 11 (0.234 mmol) in acetonitrile (3 mL) at 0 °C was added Ru-LDH (20 mol%) and NaIO4 (125 mg, 0.586 mmol) in water (1 mL). The mixture was stirred for 5 h at 0 °C–rt; after the reaction was complete (tlc monitoring), the resulting suspension was filtered through a thin pad of silica gel, which was then washed with ethyl acetate (15 mL). Concentration of the filtrate followed by silica gel column chromatography afford white solid 19, yield 84% (38 mg, 0.197 mmol), Rf = 0.23 (50% EtOAc in hexane, silica gel tlc), mp 170–172 °C; 1H NMR (400 MHz, CDCl3) δ 7.83 (d, 1H J = 7.8, Hz), 7.62 (s, 1H), 7.42 (d, 1H, J = 7.8, Hz), 7.70 (br, 2H), 2.47 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 174.0, 173.0, 143.2, 132.1, 132.0, 129.9, 129.6, 127.5, 21.4; IR νmax (neat) 3403, 2925, 2855, 1752, 1720, 1600, 1428, 1381, 1257, 1224, 1192, 1093, 981, 907, 730 cm−1; HRMS (APCI) m/z calcd for C10H9O4 [M + H]+ 193.0501; found 193.0496.
4-Ethyl-1,3-dioxo-5-propyl-2,3-dihydro-1H-indene-2,2-diyldiacetate (20a).
White solid 20a, yield 75%, Rf = 0.54 (20% EtOAc in hexane, silica gel tlc), mp 71–73 °C; 1H NMR (400 MHz, CDCl3) δ 7.76 (d, 1H J = 7.8, Hz), 7.64 (d, 1H J = 7.8, Hz), 3.17 (q, 2H, J = 7.3, Hz), 2.77–2.73 (m, 2H), 2.13 (s, 6H), 1.67 (sxt, 2H, J = 7.5, Hz), 1.19 (t, 3H, J = 7.6, Hz), 1.04 (t, 3H, J = 7.3, Hz); 13C NMR (400 MHz, CDCl3) δ 191.2, 190.2, 167.8, 150.9, 143.2, 138.2, 137.1, 136.8, 121.0, 34.7, 24.4, 21.2, 20.1, 14.6, 14.2; IR νmax (neat) 2960, 2924, 2853, 1749, 1723, 1583, 1460, 1371, 1259, 1217, 1059, 961, 799, 755 cm−1; HRMS (APCI) m/z calcd for C18H24NO6 [M + NH4]+ 350.1604; found 350.1599.
1,3-Dioxo-1,2,3,6,7,8-hexahydroas-indacene-2,2-diyldiacetate (20b).
White solid 20b, yield 72%, Rf = 0.52 (20% EtOAc in hexane, silica gel tlc), mp 158–160 °C; 1H NMR (400 MHz, CDCl3) δ 7.79 (d, 1H J = 7.3, Hz), 7.69 (d, 1H J = 7.8, Hz), 3.31 (t, 2H, J = 7.6, Hz), 3.04 (t, 2H, J = 7.6, Hz), 2.24 (quin, 2H, J = 7.6, Hz), 2.13 (s, 6H); 13C NMR (400 MHz, CDCl3) δ 191.3, 190.1, 167.9, 155.5, 143.3, 138.0, 134.7, 131.8, 121.9, 32.9, 32.9, 31.1, 25.2, 20.2; IR νmax (neat) 2924, 2853, 1749, 1747, 1585, 1464, 1431, 1370, 1219, 1062, 976 cm−1; HRMS (APCI) m/z calcd for C16H14NaO6 [M + Na]+ 325.0688; found 325.0677.
4-Ethyl-5-methyl-1,3-dioxo-2,3-dihydro-1H-indene-2,2-diyldiacetate (20c).
White solid 20c, yield 79%, Rf = 0.56 (20% EtOAc in hexane, silica gel tlc), mp 145–147 °C; 1H NMR (400 MHz, CDCl3) δ 7.73 (d, 1H J = 7.8, Hz), 7.62 (d, 1H J = 7.8, Hz), 3.17 (q, 2H, J = 7.5, Hz), 2.49 (s, 3H), 2.13 (s, 6H), 1.18 (t, 3H, J = 7.6, Hz); 13C NMR (400 MHz, CDCl3) δ 191.3, 190.3, 167.9, 146.6, 143.6, 138.2, 137.8, 136.4, 120.9, 21.6, 20.1, 19.4, 13.5; IR νmax (neat) 2965, 2935, 1747, 1723, 1583, 1472, 1370, 1215, 1192, 1058, 1014, 956, 801, 753, 666 cm−1; HRMS (ESI) m/z calcd for C16H16O6Na [M + Na]+ 327.0844; found 327.0837.
1,3-Dioxo-5-phenyl-2,3-dihydro-1H-indene-2,2-diyldiacetate (20d).
White solid 20d, yield 74%, Rf = 0.51 (20% EtOAc in hexane, silica gel tlc), mp 145–147 °C; 1H NMR (400 MHz, CDCl3) δ 8.16 (s, 1H), 8.11–8.03 (m, 2H), 7.67 (d, 2H, J = 8.3, Hz), 7.53–7.46 (m, 3H), 2.15 (s, 6H); 13C NMR (400 MHz, CDCl3) δ 190.7, 190.2, 168.1, 149.6, 139.9, 138.7, 137.6, 135.1, 129.2, 127.5, 124.0, 121.6, 20.0; IR νmax (neat) 3020, 2926, 1749, 1719, 1599, 1423, 1260, 1214, 1017, 1056, 1012, 948, 801, 752, 697, 667 cm−1; HRMS (ESI) m/z calcd for C19H14O6Na [M + Na]+ 361.0688; found 361.30675.
1,3-Dioxo-5-p-tolyl-2,3-dihydro-1H-indene-2,2-diyldiacetate (20e).
White solid 20e, yield 78%, Rf = 0.54 (15% EtOAc in hexane, silica gel tlc), mp 150–152 °C; 1H NMR (400 MHz, CDCl3) δ 8.14 (s, 1H), 8.09–8.07 (m, 1H), 8.03–8.01 (m, 1H), 7.56 (d, 1H, J = 8.3, Hz), 7.31 (d, 1H, J = 8.3, Hz), 2.42 (s, 3H), 2.15 (s, 6H); 13C NMR (400 MHz, CDCl3) δ 190.9, 190.2, 168.2, 149.7, 140.0, 139.6, 137.6, 135.9, 134.9, 130.1, 127.5, 124.1, 121.3, 21.3, 20.1; IR νmax (neat) 3027, 2924, 1750, 1727, 1601, 1432, 1370, 1305, 1216, 1161, 1161, 1061, 1015, 945, 911, 816, 754, 553 cm−1; HRMS (ESI) m/z calcd for C20H20NO6 [M + NH4]+ 370.1290; found 370.1288.
5-(3-Methoxyphenyl)-1,3-dioxo-2,3-dihydro-1H-indene-2,2-diyldiacetate (20f).
White solid 20f, yield 77%, Rf = 0.51 (20% EtOAc in hexane, silica gel tlc), mp 163–165 °C; 1H NMR (400 MHz, CDCl3) δ 8.15 (s, 1H), 8.10–8.07 (m, 1H), 8.03–8.04 (m, 1H), 7.44–7.40 (m, 1H), 7.26–7.23 (m, 1H), 7.17 (d, 1H, J = 2.4, Hz), 7.00 (dd, 1H, J = 8.1, 2.2 Hz), 3.88 (s, 3H), 2.15 (s, 6H); 13C NMR (400 MHz, CDCl3) δ 190.7, 190.1, 168.1, 149.5, 139.8, 137.7, 135.1, 130.3, 124.0, 121.6, 119.9, 114.8, 133.0, 55.4, 20.0; IR νmax (neat) 3027, 2940, 2838, 1751, 1723, 1601, 1474, 1371, 1218, 1156, 1063, 1026, 957, 915, 844, 782, 732, 687 cm−1; HRMS (ESI) m/z calcd for C20H16NaO7 [M + Na]+ 391.0793; found 391.0787.
1,3-Dioxo-5-m-tolyl-2,3-dihydro-1H-indene-2,2-diyl diacetate (20g).
White solid 20g yield 76%, Rf = 0.53 (20% EtOAc in hexane, silica gel tlc), mp 159–161 °C, 1H NMR (400 MHz, CDCl3) δ 8.13 (s, 1H), 8.07–8.05 (m, 1H), 8.01–7.99 (m, 1H), 7.45–7.35(m, 3H), 7.24–7.23 (m, 2H), 2.42 (s, 3H), 2.13 (s, 6H); 13C NMR (400 MHz, CDCl3) δ 190.8, 190.2, 168.1, 149.8, 139.8, 139.0, 138.6, 137.5, 135.0, 130.0, 129.1, 128.3, 124.6, 124.0, 121.5, 21.5, 20.0; IRνmax (neat) 3025, 2923, 2852, 1748, 1720, 1601, 1463, 1415, 1305, 1216, 1156, 1089, 1053, 883, 794, 757 cm−1; HRMS (ESI) m/z calcd for C20H20NO6 [M + NH4]+ 370.1290; found 370.1289.
Acknowledgements
FAK gratefully acknowledges CSIR for financial support. KRB thanks UGC for the award of a fellowship.
Notes and references
-
(a) L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed. Engl., 1993, 32, 131 CrossRef;
(b) H. Pellissier, Chem. Rev., 2013, 113, 442 CrossRef CAS PubMed;
(c) Y. Liu and J.-P. Wan, Org. Biomol. Chem., 2011, 9, 6873 RSC;
(d) P. J. Parsons, C. S. Penkett and A. J. Shell, Chem. Rev., 1996, 96, 195 CrossRef CAS PubMed;
(e) F. Geng, J. Liu and L. A. Paquette, Org. Lett., 2002, 4, 71 CrossRef CAS PubMed;
(f) L. A. Paquette, Eur. J. Org. Chem., 1998, 1709 CrossRef CAS;
(g) W. Giersch and I. Farris, Helv. Chim. Acta, 2004, 87, 1601 CrossRef CAS;
(h) A. Srikrishna, S. Venkateswarlu, S. Nagaraju and K. Krishuan, Tetrahedron, 1994, 50, 8765 CrossRef CAS;
(i) M. Hiersemann, L. Abraham and A. Pollex, Synlett, 2003, 1088 CrossRef CAS;
(j)
L. F. Tietze, G. Brasche and K. M. Gericke, Domino Reactions in Organic Synthesis, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Die Deutsche Bibliothek, 2006 Search PubMed.
-
(a) P. Wessig and J. Teubner, Synlett, 2006, 1543 CrossRef CAS PubMed;
(b) D. C. Harrowven, N. A. Newman and C. A. Knight, Tetrahedron Lett., 1998, 39, 6757 CrossRef;
(c) W. M. Clark, A. M. Tickner-Eldridge, G. K. Huang, L. N. Pridgen, M. A. Olsen, R. J. Mills, I. Lantos and N. H. Baine, J. Am. Chem. Soc., 1998, 120, 4550 CrossRef CAS;
(d) M. A. Ernst-Russell, C. L. L. Chai, J. H. Wardlaw and J. A. Elix, J. Nat. Prod., 2000, 63, 129 CrossRef CAS PubMed.
-
(a) R. E. McDevitt, M. S. Malamas, E. S. Manas, R. J. Unwalla, Z. B. Xu, C. P. Miller and H. A. Harris, Bioorg. Med. Chem. Lett., 2005, 15, 3137 CrossRef CAS PubMed;
(b) M. Anstead, S. R. Wilson and J. A. Katzenellenbogen, J. Med. Chem., 1989, 32, 2163 CrossRef.
-
(a) L. M. X. Lopes, M. Yoshida and O. R. Gottlieb, Phytochemistry, 1984, 23, 2021 CrossRef CAS;
(b) D. C. Harrowven, N. A. Newman and C. A. Knight, Tetrahedron Lett., 1998, 39, 6757 CrossRef.
- C. H. Park, X. Siomboing, S. Yous, B. Gressier, M. Luyckxand and P. Chavatte, Eur. J. Med. Chem., 2002, 37, 461 CrossRef CAS.
- O. A. Akrawi, A. Khan, M. Hussain, H. Mohammed, A. Villinger and P. Langer, Tetrahedron Lett., 2013, 54, 3037 CrossRef CAS PubMed.
- J. A. Palermo, M. F. Rodriguez Brasco, C. Spagnuolo and A. M. Seldes, J. Org. Chem., 2000, 65, 4482 CrossRef CAS.
- R. L. Frank, H. Eklund, J. W. Richter, C. R. Vanneman and A. N. Wennerberg, J. Am. Chem. Soc., 1944, 66, 1 CrossRef CAS.
- G. M. Anstead, R. J. Altenbach, R. W. Scott and J. A. Katzenellenbogen, J. Med. Chem., 1988, 31, 1316 CrossRef CAS.
-
(a) G. Jammaer, H. Martens and G. J. Hoornaert, J. Org. Chem., 1974, 32, 2163 Search PubMed;
(b) A. Chatterjee and S. Banerjee, Tetrahedron, 1970, 26, 2599 CrossRef CAS.
- H. O. House and J. K. Larson, J. Org. Chem., 1968, 33, 448 CrossRef CAS.
-
(a) S. Basurto, S. Garcia, A. G. Neo, T. Torroba, C. F. Marcos, D. Miguel, J. Barbera, M. B. Ros and M. R. de la Fuente, Chem. – Eur. J., 2005, 11, 5362 CrossRef CAS PubMed;
(b) E. N. Alesso, D. G. Tombari, A. F. Ibañez, G. Y. Moltrasio Iglesias and J. M. Aguirre, Can. J. Chem., 1991, 69, 1166 CrossRef CAS.
- H. E. Zimmerman, J. Am. Chem. Soc., 1956, 78, 1168 CrossRef CAS.
- K. Buggle, U. N. Ghógáin and D. O'Sullivan, J. Chem. Soc., Perkin Trans. 1, 1983, 2075 RSC.
- E. F. Ulman and W. A. J. Henderson, J. Am. Chem. Soc., 1966, 88, 4942 CrossRef.
-
(a) J. F. Feeman and E. D. Amstutz, J. Am. Chem. Soc., 1950, 72, 1522 CrossRef CAS;
(b) M. B. Floyd and G. R. J. Allen, J. Org. Chem., 1970, 35, 2647 CrossRef CAS;
(c) G. Jammaer, H. Martens and G. Hoornaert, Tetrahedron, 1975, 31, 2293 CrossRef CAS;
(d) H. Martens and G. Hoornaert, Synth. Commun., 1972, 2, 147 CrossRef.
- C. Manning, M. R. McClory and J. J. McCullough, J. Org. Chem., 1981, 46, 919 CrossRef CAS.
-
(a) Y. Harada, J. Nakanishi, H. Fujihara, M. Tobisu, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2007, 129, 5766 CrossRef CAS PubMed;
(b) B. J. Li, H.-Y. Wang, Q.-L. Zhu and Z.-J. Shi, Angew. Chem., Int. Ed., 2012, 51, 3948 CrossRef CAS PubMed;
(c) T. Morimoto, K. Yamasaki, A. Hirano, K. Tsutsumi, N. Kagawa, K. Kakiuchi, Y. Harada, Y. Fukumoto, N. Chatani and T. Nishioka, Org. Lett., 2009, 11, 1777 CrossRef CAS PubMed;
(d) K. Gao and N. Yoshikai, Chem. Commun., 2012, 48, 4305 RSC;
(e) Y. Kuninobu, T. Matsuki and K. Takai, Org. Lett., 2010, 12, 2948 CrossRef CAS PubMed;
(f) N. B. Alexey, R. Beatrice, M. Lieven, S. Ian, A. Patrick, C. Andrei and C. Janine, Org. Biomol. Chem., 2014, 12, 728 RSC;
(g) H. Tsukamoto and Y. Kondo, Org. Lett., 2007, 9, 4227 CrossRef CAS PubMed;
(h) R. C. Larock and M. J. Doty, J. Org. Chem., 1993, 58, 4579 CrossRef CAS.
-
(a) F. A. Khan and C. H. Sudheer, Org. Lett., 2008, 10, 3029 CrossRef CAS PubMed;
(b) F. A. Khan and B. Rout, Tetrahedron Lett., 2006, 47, 5251 CrossRef CAS PubMed.
-
(a) F. A. Khan, J. Dash, D. Jain and B. P. Das, J. Chem. Soc., Perkin Trans. 1, 2001, 3132 RSC;
(b) F. A. Khan and L. Soma, Tetrahedron Lett., 2007, 48, 85 CrossRef CAS PubMed;
(c) F. A. Khan and S. Choudhury, Eur. J. Org. Chem., 2006, 672 CrossRef CAS.
- F. A. Khan, J. Dash, N. Sahu and Ch. Sudheer, J. Org. Chem., 2002, 67, 3783 CrossRef CAS PubMed.
-
(a) R. P. Lutz, Chem. Rev., 1984, 84, 205 CrossRef CAS;
(b) L. A. Paquette, J. L. Romine, H.-S. Lin and J. Wright, J. Am. Chem. Soc., 1990, 112, 9284 CrossRef CAS . And references cited therein.
-
(a) R. B. Woodward, Tetrahedron, 1959, 5, 70 CrossRef CAS;
(b) P. Yates and P. Eaton, Tetrahedron Lett., 1960, 5 CrossRef CAS;
(c) P. Yates and P. Eaton, Tetrahedron, 1961, 12, 13 CrossRef CAS;
(d) R. Breslow and J. M. Hoffman Jr., J. Am. Chem. Soc., 1972, 94, 2111 CrossRef CAS.
- E. T. McBee, W. R. Diveley and J. E. Burch, J. Am. Chem. Soc., 1955, 77, 385 CrossRef CAS.
-
(a) F. A. Khan and N. Sahu, J. Catal., 2005, 231, 438 CrossRef CAS PubMed;
(b) F. A. Khan, B. Prabhudas, J. Dash and N. Sahu, J. Am. Chem. Soc., 2000, 122, 9558 CrossRef CAS.
-
(a) R. R. Hark, D. B. Hauze, O. Petrovskaia and M. M. Joullié, Can. J. Chem., 2001, 79, 1632 CrossRef CAS PubMed;
(b) R. J. Heffner and M. M. Joullié, Synth. Commun., 1991, 21, 2231 CrossRef CAS;
(c) D. J. McCaldin, Chem. Rev., 1960, 60, 39 CrossRef CAS;
(d) M. B. Rubin, Chem. Rev., 1975, 75, 177 CrossRef CAS;
(e) A. Schönberg and E. Singer, Tetrahedron, 1978, 34, 1283 CrossRef;
(f) M. M. Joullié, T. R. Thompson and N. H. Nemeroff, Tetrahedron, 1991, 47, 8791 CrossRef;
(g) M. B. Rubin and R. Gleiter, Chem. Rev., 2000, 100, 1121 CrossRef CAS PubMed;
(h) J. Almog, A. Hirshfeld and J. T. Klug, Forensic. Sci., 1982, 27, 912 CAS.
-
(a) K. Bowden and S. Rumpal, J. Chem. Soc., Perkin Trans. 2, 1997, 983 RSC;
(b) R. R. Hark, D. B. Hauze, O. Petrovskaia, M. M. Joullié, R. Jaouhari and P. M. McComiskey, Tetrahedron Lett., 1994, 35, 7719 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1015482. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ob01977f |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 under PTSA conditions would also yield the corresponding alkylated benzene derivative through exocyclic olefin 10a, an experiment was carried out and the results are represented in Table 1. To our surprise, the compound 9 underwent a domino reaction via Cope rearrangement22 to deliver indenone 11 in excellent yield. It worth mentioning that 4,5,6,7-tetrachloro-3a,4,7,7a-tetrahydro-4,7-methanoinden-8-one was reported to undergo smooth Cope rearrangement rather than the anticipated CO extrusion reaction for these systems.23 Here, we wish to report this serendipitously observed, efficient acid-induced domino reaction of tetrahalo-7,7-dimethoxybicyclo[2.2.1]heptenyl alcohols leading to indenones. Furthermore, conversion of these indenones to ninhydrins, by employing Ru(III)-catalyzed oxidation, is also discussed. This, to the best our knowledge, represents a de novo synthesis.
21 under PTSA conditions would also yield the corresponding alkylated benzene derivative through exocyclic olefin 10a, an experiment was carried out and the results are represented in Table 1. To our surprise, the compound 9 underwent a domino reaction via Cope rearrangement22 to deliver indenone 11 in excellent yield. It worth mentioning that 4,5,6,7-tetrachloro-3a,4,7,7a-tetrahydro-4,7-methanoinden-8-one was reported to undergo smooth Cope rearrangement rather than the anticipated CO extrusion reaction for these systems.23 Here, we wish to report this serendipitously observed, efficient acid-induced domino reaction of tetrahalo-7,7-dimethoxybicyclo[2.2.1]heptenyl alcohols leading to indenones. Furthermore, conversion of these indenones to ninhydrins, by employing Ru(III)-catalyzed oxidation, is also discussed. This, to the best our knowledge, represents a de novo synthesis.




![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of thionyl chloride and anhydrous pyridine. The reaction was stirred at −10 °C to rt for 5 h and was poured into 50 mL cold pentane. The pentane solution was treated cautiously with ice chips and the resulting layers separated. Several pentane extracts were combined and washed with two portions of 3% aqueous hydrochloric acid, followed by brine until neutral. The pentane extract was dried with anhydrous Na2SO4. The solvent was concentrated under reduced pressure to yield a residue, which was purified by silica gel column chromatography to afford colorless solid 7, yield 60% (125 mg, 0.259 mmol), Rf = 0.64 (5% EtOAc in hexane, silica gel tlc), mp 69–71 °C; 1H NMR (400 MHz, CDCl3) δ 5.40 (s, 1H) 5.08 (s, 1H), 3.63 (s, 3H), 3.58 (s, 3H), 2.99 (d, 1H, J = 14.5 Hz), 2.6 (d, 1H, J = 18.5 Hz); 13C NMR (400 MHz, CDCl3) δ 143.7, 127.1, 125.2, 111.6, 109.5, 67.7, 53.0, 51.9, 42.7; IR νmax (neat) 2986, 2947, 2842, 1667, 1569, 1452, 1432, 1251, 1203, 1170, 1107, 1010, 949, 756, 694, 616 cm−1; HRMS (ESI) calcd for C10H14Br4O2N [M + NH4]+ 499.7717; found 499.7708.
1 mixture of thionyl chloride and anhydrous pyridine. The reaction was stirred at −10 °C to rt for 5 h and was poured into 50 mL cold pentane. The pentane solution was treated cautiously with ice chips and the resulting layers separated. Several pentane extracts were combined and washed with two portions of 3% aqueous hydrochloric acid, followed by brine until neutral. The pentane extract was dried with anhydrous Na2SO4. The solvent was concentrated under reduced pressure to yield a residue, which was purified by silica gel column chromatography to afford colorless solid 7, yield 60% (125 mg, 0.259 mmol), Rf = 0.64 (5% EtOAc in hexane, silica gel tlc), mp 69–71 °C; 1H NMR (400 MHz, CDCl3) δ 5.40 (s, 1H) 5.08 (s, 1H), 3.63 (s, 3H), 3.58 (s, 3H), 2.99 (d, 1H, J = 14.5 Hz), 2.6 (d, 1H, J = 18.5 Hz); 13C NMR (400 MHz, CDCl3) δ 143.7, 127.1, 125.2, 111.6, 109.5, 67.7, 53.0, 51.9, 42.7; IR νmax (neat) 2986, 2947, 2842, 1667, 1569, 1452, 1432, 1251, 1203, 1170, 1107, 1010, 949, 756, 694, 616 cm−1; HRMS (ESI) calcd for C10H14Br4O2N [M + NH4]+ 499.7717; found 499.7708.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47, colorless liquid, Rf = 0.62 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.61 (s, 3H), 3.59 (s, 3H), 2.92 (d, 1H, J = 13.6 Hz), 2.41 (d, 1H, J = 13.6 Hz), 2.04 (s, 3H), 1.63 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.8, 129.5, 126.0, 125.3, 111.1, 72.7, 67.5, 52.3, 51.7, 42.6, 23.8; IR νmax (neat) 3080, 2980, 2946, 2841, 1641, 1568, 1451, 1373, 1263, 1180, 1111, 971, 893, 842, 722, 647, 571 cm−1; HRMS (APCI) calcd for C12H15Br4O2 [M + H]+ 510.7764; found 510.7762.
47, colorless liquid, Rf = 0.62 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.61 (s, 3H), 3.59 (s, 3H), 2.92 (d, 1H, J = 13.6 Hz), 2.41 (d, 1H, J = 13.6 Hz), 2.04 (s, 3H), 1.63 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.8, 129.5, 126.0, 125.3, 111.1, 72.7, 67.5, 52.3, 51.7, 42.6, 23.8; IR νmax (neat) 3080, 2980, 2946, 2841, 1641, 1568, 1451, 1373, 1263, 1180, 1111, 971, 893, 842, 722, 647, 571 cm−1; HRMS (APCI) calcd for C12H15Br4O2 [M + H]+ 510.7764; found 510.7762.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 81, colorless liquid, Rf = 0.69 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.58 (s, 3H), 3.57 (s, 3H), 3.41 (d, 1H J = 2.9 Hz), 2.37 (d, 1H J = 13.7 Hz), 1.98 (s, 3H), 1.64 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.7, 129.3, 129.0, 125.8, 111.2, 79.2, 73.6, 52.1, 42.2, 23.6, 19.0; IR νmax (neat) 2982, 2950, 2844, 1644, 1603, 1454, 1271, 1190, 1156, 1117, 1040, 985, 902, 756, 591 cm−1; HRMS (APCI) calcd for C12H13Cl4O [(M + H)+(–H2O)]+ 312.9720; found 312.9790.
81, colorless liquid, Rf = 0.69 (5% EtOAc in hexane, silica gel tlc); 1H NMR (400 MHz, CDCl3) δ 3.58 (s, 3H), 3.57 (s, 3H), 3.41 (d, 1H J = 2.9 Hz), 2.37 (d, 1H J = 13.7 Hz), 1.98 (s, 3H), 1.64 (s, 3H); 13C NMR (400 MHz, CDCl3) δ 140.7, 129.3, 129.0, 125.8, 111.2, 79.2, 73.6, 52.1, 42.2, 23.6, 19.0; IR νmax (neat) 2982, 2950, 2844, 1644, 1603, 1454, 1271, 1190, 1156, 1117, 1040, 985, 902, 756, 591 cm−1; HRMS (APCI) calcd for C12H13Cl4O [(M + H)+(–H2O)]+ 312.9720; found 312.9790.



