Asymmetric synthesis of axially chiral anilides by Pd-catalyzed allylic substitutions with P/olefin ligands†
Received
27th May 2014
, Accepted 3rd October 2014
First published on 3rd October 2014
Abstract
As an attractive class of non-biaryl atropisomeric compounds, C–N axially chiral anilides have received considerable attention, and several methods have been successfully developed for their synthesis. Pd-catalyzed asymmetric allylic amination was proved to be an effective approach for the chiral anilide synthesis, although only moderate enantioselectivity and relatively narrow substrate scope have been achieved in the previous work. Searching for highly efficient methods for the synthesis of axially chiral anilides is therefore of great interest in synthetic and pharmaceutical chemistry. In this paper, a palladium-catalyzed asymmetric allylic substitution of ortho-substituted anilides using phosphorus amidite–olefin ligands was successfully achieved to afford a variety of axially chiral anilides in high yields with up to 84% ee. The absolute configurations of chiral anilides were also determined from X-ray and CD spectra.
Introduction
C–N axially chiral anilides as an attractive class of non-biaryl atropisomeric compounds have received considerable attention.1 One of the most famous examples is the herbicide metolachlor,2 although its axial chirality is found to have little effect on its biological activity. In 1994, Curran and coworkers reported their seminal work on the development of novel chiral auxiliaries based on axially chiral amides and imides.3 These axially chiral anilides gave high levels of asymmetric introduction in many types of important reactions, such as cycloaddition, alkylation, and aldol reaction.3,4 The axially chiral anilides can be also incorporated in chiral organocatalysts for asymmetric Michael addition and Friedel–Crafts amination.5
Due to their highly potential applications, a variety of strategies for accessing enantioenriched axially chiral anilides have been successfully developed. For example, Uemura and Simpkins described a desymmetrization of prochiral anilides using chiral lithium reagents, respectively.6 Taguchi, Shimizu, and Curran reported the chiral resolution of racemic compounds.7 Kamikawa and Uemura reported the stereoselective synthesis of chiral anilides by nucleophilic aromatic substitution of planar chiral arene chromium complexes.8 In contrast to the aforementioned work, using a catalytic amount of the chiral catalyst seems to be more desirable. In 2005, Taguchi and coworkers described a Pd-catalyzed asymmetric N-arylation reaction for the chiral anilide synthesis.9 In 2006, Jørgensen reported an organocatalytic amination of 2-naphthols.5 Tanaka and Hsung developed a Rh-catalyzed asymmetric [2 + 2 + 2] cycloaddition, respectively.10 Recently, Maruoka and coworkers employed chiral phase-transfer catalysis for the synthesis of axially chiral o-iodoanilides.11 As an extremely useful tool for the construction of C–N bonds, Pd-catalyzed asymmetric allylic aminations have also been employed for the chiral anilide synthesis to give a moderate enantioselectivity (30–56% ee) with a relatively narrow substrate scope.12,13 Despite these advances, searching for highly efficient methods for the synthesis of axially chiral anilides is still of great interest in synthetic and pharmaceutical chemistry.14,15
The lately emerging field of chiral olefin ligands has witnessed a rapid growth in recent years.16 As a novel type of ligand, chiral P/olefin hybrid ligands have been successfully developed for various transition-metal-catalyzed asymmetric reactions.17 Recently, our group has also described the application of chiral P/olefin ligands in Pd-catalyzed asymmetric allylic alkylations of indoles, pyrroles, and oximes.18 However, to the best of our knowledge, the construction of axial chirality using this type of ligand has not been reported.19 Herein, we wish to report our efforts on the asymmetric synthesis of axially chiral anilides through Pd-catalyzed allylic substitutions with phosphorus amidite–olefin ligands.
Results and discussion
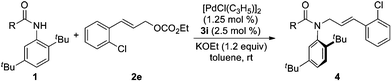
At the outset, we examined the ability of phosphorus amidite–olefin ligands for the Pd-catalyzed asymmetric allylic alkylation of ortho-tert-butylanilide (1a) with cinnamyl ethyl carbonate (2a). It was found that the reaction using ligand 3a proceeded smoothly at room temperature to give the desired product 4aa in 86% yield with 20% ee (Scheme 1). In a control experiment, ligand 3b without the vinyl group gave product 4aa in a reverse absolute configuration with a very low ee (Scheme 1).
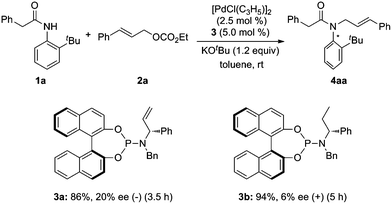 |
| | Scheme 1 Initial studies on Pd-catalyzed asymmetric allylic alkylation of ortho-tert-butylanilide 1a. | |
A variety of chiral P/olefin hybrid ligands 3c–i was next evaluated for this reaction (Fig. 1). Ligands 3c–d bearing methyl or diphenylmethyl on the nitrogen atom can only improve the enantioselectivity slightly. Ligands 3e–f containing different substituents on the carbon chiral center gave a lower ee. Using ligand 3g incorporated with an internal olefin led to an inverse configuration. Ligand 3h derived from H8-BINOL gave 12% ee. When a chirality matched ligand 3i was subjected to this reaction, the enantioselectivity can be improved from 20% to 46%.
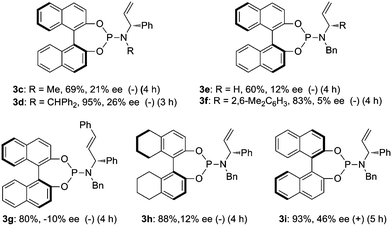 |
| | Fig. 1 Selective chiral P/olefin ligands for Pd-catalyzed asymmetric allylic alkylation of ortho-tert-butylanilide 1a. | |
The reaction conditions including bases and solvents were investigated to further improve the enantioselectivity. As shown in Table 1, bases were found to have a great impact on the enantioselectivity (entries 1–6). Significantly, the reaction with KOEt as a base can be accomplished in 0.5 h with 60% ee (Table 1, entry 6). Solvents can also influence the enantioselectivity, and toluene seems to be a better solvent (Table 1, entries 6–10). Decreasing the catalyst loading from 5 mol% to 2.5 mol% no loss of reactivity and enantioselectivity was observed (Table 1, entries 6 vs. 11). When anilide 1b containing one more tert-butyl group was used as a substrate, up to 79% ee was obtained (Table 1, entry 12).
Table 1 Optimization of reaction conditionsa
|
|
| Entry |
Base |
Solvent |
Time (h) |
Yieldb (%) |
eec (%) |
|
All reactions were carried out with 1a (0.20 mmol), 2a (0.24 mmol), Pd/3i = 1/1 (5.0 mol% Pd), base (0.24 mmol), and solvent (3.0 mL) unless otherwise stated.
Isolated yield based on 1.
The ee was determined by chiral HPLC.
The reaction was carried out with 1 (0.40 mmol), 2a (0.48 mmol), Pd/3i = 1/1 (2.5 mol% Pd), KOEt (0.48 mmol), and toluene (3.0 mL).
|
| 1 |
LiOtBu |
Toluene |
8 |
86 |
17 |
| 2 |
NaOtBu |
Toluene |
5 |
91 |
12 |
| 3 |
KOtBu |
Toluene |
5 |
93 |
46 |
| 4 |
KHMDS |
Toluene |
4 |
94 |
56 |
| 5 |
KH |
Toluene |
3.5 |
95 |
18 |
| 6 |
KOEt |
Toluene |
0.5 |
93 |
60 |
| 7 |
KOEt |
THF |
2 |
89 |
28 |
| 8 |
KOEt |
DCM |
2 |
88 |
56 |
| 9 |
KOEt |
MeCN |
4 |
93 |
26 |
| 10 |
KOEt |
Benzene |
3 |
95 |
61 |
| 11d |
KOEt |
Toluene |
1.5 |
92 |
61 |
| 12d |
KOEt |
Toluene |
3 |
91 |
79 |
Under the optimal reaction conditions, the allyl carbonate scope was examined using anilide 1b as a substrate. As shown in Table 2, a wide range of allyl carbonates were well tolerated to give the corresponding axially chiral anilide products in 74–92% yields with 68–84% ees (Table 2, entries 1–9). When a simple allyl carbonate 2k was used as a substrate, the desired product 4bk can be obtained in 51% yield with 46% ee (Table 2, entry 10).
Table 2 Pd-catalyzed asymmetric allylic alkylation of ortho-tert-butylanilide 1ba
|
|
| Entry |
Product (4) |
Time (h) |
Yieldb (%) |
eec (%) |
|
All reactions were carried out with 1b (0.40 mmol), 2 (0.48 mmol), Pd/3i = 1/1 (2.5 mol% Pd), KOEt (0.48 mmol), and toluene (3.0 mL) unless otherwise stated.
Isolated yield based on 1b.
The ee was determined by chiral HPLC.
|
| 1 |
4bb: R = 4-CF3C6H4 |
3.5 |
92 |
68 |
| 2 |
4bc: R = 3-ClC6H4 |
2 |
80 |
75 |
| 3 |
4bd: R = 3-MeOC6H4 |
2.5 |
74 |
76 |
| 4 |
4be: R = 2-ClC6H4 |
1.5 |
80 |
80 |
| 5 |
4bf: R = 2-MeOC6H4 |
1.5 |
87 |
84 |
| 6 |
4bg: R = 2-BnOC6H4 |
2.5 |
76 |
80 |
| 7 |
4bh: R = 2-iPrOC6H4 |
2 |
81 |
79 |
| 8 |
4bi: R = 2-MeC6H4 |
1.5 |
81 |
75 |
| 9 |
4bj: R = 1-naphthyl |
2 |
76 |
83 |
| 10 |
4bk: R = H |
4 |
51 |
46 |
Subsequently, the asymmetric reaction of various N-acylanilides and allyl carbonate 2e was investigated. It was found that all these reactions went smoothly to furnish chiral anilides 4 in high yields with 43–83% ees (Table 3, entries 1–8). The substrate scope can be further extended to other anilides. The reaction of ortho-haloanilides 1k–m and allyl carbonate 2e can proceed well to give the desired products in high yields with relatively lower ees (Scheme 2), which can be used as chiral building blocks for further transformation.20 Anilide 1n bearing an ortho-phenyl substituent was also a suitable substrate to give product 4ne in 78% yield with 33% ee (Scheme 2).
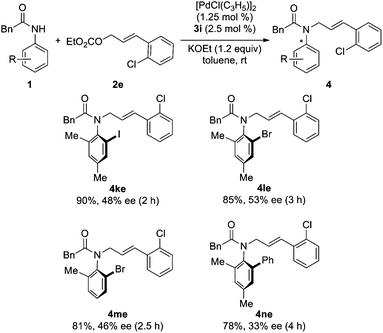 |
| | Scheme 2 Pd-catalyzed asymmetric synthesis of other types of axially chiral anilides. | |
Table 3 Pd-catalyzed asymmetric allylic alkylation of N-acylanilidea
|
|
| Entry |
Product (4) |
Time (h) |
Yieldb (%) |
eec (%) |
|
All reactions were carried out with 1 (0.40 mmol), 2e (0.48 mmol), Pd/3i = 1/1 (2.5 mol% Pd), KOEt (0.48 mmol), and toluene (3.0 mL) unless otherwise stated.
Isolated yield based on 1.
The ee was determined by chiral HPLC.
|
| 1 |
4ce: R = PhCH2CH2 |
2.5 |
83 |
47 |
| 2 |
4de: R = trans-PhCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
4.5 |
79 |
44 |
| 3 |
4ee: R = Ph |
4 |
71 |
46 |
| 4 |
4fe: R = 2-furyl |
3.5 |
82 |
43 |
| 5 |
4ge: R = Cy |
2 |
84 |
74 |
| 6 |
4he: R = 4-Cl-C6H4CH2 |
4 |
86 |
66 |
| 7 |
4ie: R = 2-Cl-C6H4CH2 |
4 |
90 |
75 |
| 8 |
4je: R = 2,5-Me2C6H4CH2 |
4 |
85 |
83 |
The absolute configuration of product 4 was tentatively assigned as S by analogy with compounds (S)-4de and (S)-4ge, which was determined by their X-ray structures on the basis of the anomalous dispersion of the heavy chlorine atom (Fig. 2).21 To determine the absolute configurations of the other products, the CD spectra were measured in CH3CN, and some typical ones are summarized in Fig. 3. As shown in Fig. 3, all of the compounds exhibited a similar (−)-Cotton effect in the CD spectra with compounds (S)-4de and (S)-4ge, which indicated that these compounds possess the same S configuration as (S)-4de and (S)-4ge.
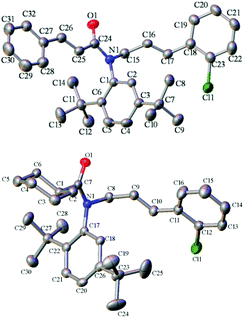 |
| | Fig. 2 The X-ray structure of (S)-4de (above) and (S)-4ge (below). | |
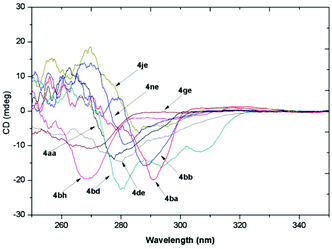 |
| | Fig. 3 CD spectra of selected typical compounds 4 (c = 5 × 10−4 M in CH3CN). | |
Experimental section
Representative procedure for 3i/Pd-catalyzed asymmetric allylic alkylation of ortho-tert-butylanilide (1a) (Table 1, entry 11)
To a dried Schlenk flask charged with [PdCl(C3H5)]2 (0.0018 g, 0.005 mmol, 2.5 mol% Pd) and ligand 3i (0.0054 g, 0.010 mmol, 2.5 mol%) was added toluene (0.5 mL) under argon. After the mixture was stirred for 30 min at room temperature, cinnamyl ethyl carbonate 2a (0.0990 g, 0.48 mmol) and toluene (1.3 mL) were added sequentially, and the solution was stirred for 10 min. Meanwhile a suspension of ortho-tert-butylanilide (1a) (0.1070 g, 0.40 mmol) and KOEt (0.0404 g, 0.48 mmol) in toluene (1.2 mL) was stirred for 3 min at room temperature. Then the solution was added to the suspension with a syringe. After the resulting mixture was stirred at room temperature for 1.5 h, it was filtered by crude flash column chromatography with ethyl acetate, and evaporated under vacuum. The crude residue was purified by flash chromatography on silica gel (PE–EA = 10/1, v/v) to afford the desired product 4aa as a light yellow oil (0.1411 g, 92% yield, 61% ee).
Spectral data
(11bS)-N-Benzhydryl-N-((R)-1-phenylallyl)dinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-amine (3d).
White solid; mp 103–105 °C; [α]D20 = −70.3 (c 1.00, CH2Cl2); IR (film): 1468, 1232, 1050, 938 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 8.8 Hz, 1H), 7.89 (d, J = 8.0 Hz, 1H), 7.79 (d, J = 8.0 Hz, 1H), 7.54 (t, J = 8.8 Hz, 2H), 7.43 (d, J = 7.2 Hz, 1H), 7.40–7.28 (m, 10H), 7.25–7.15 (m, 7H), 7.14–7.03 (m, 3H), 5.94 (ddd, J = 17.2, 10.0, 7.2 Hz, 1H), 5.72 (d, J = 11.2 Hz, 1H), 5.06 (dd, J = 12.0, 7.2 Hz, 1H), 4.87 (d, J = 10.0 Hz, 1H), 4.51(d, J = 17.2 Hz, 1H); 31P NMR (121 MHz, CDCl3) δ 148.8; HRMS (ESI): calcd for C42H33NO2P (M + H)+: 614.2243; Found: 614.2246.
(11bS)-N-Benzyl-N-((R)-1-phenylallyl)-8,9,10,11,12,13,14,15-octahydrodinaphtho[2,1-d:1′,2′-f][1,3,2]dioxaphosphepin-4-amine (3h).
White solid; mp 70–72 °C; [α]D20 = +108.6 (c 0.50, CH2Cl2); IR (film): 1468, 1232, 1050, 938 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.33–7.20 (m, 10H), 7.06–7.01 (m, 2H), 6.92 (d, J = 8.0 Hz, 1H), 6.83 (d, J = 8.0 Hz, 1H), 6.18 (ddd, J = 16.8, 10.0, 4.0 Hz, 1H), 5.25 (d, J = 10.0 Hz, 1H), 5.09 (d, J = 16.8 Hz, 1H), 4.62 (dd, J = 16.8, 8.0 Hz, 1H), 3.98 (dd, J = 14.8, 2.4 Hz, 1H), 3.45 (dd, J = 15.2, 3.2 Hz, 1H), 2.81–2.71 (m, 4H), 2.66–2.56 (m, 2H), 2.27–2.20 (m, 2H), 1.79–1.71 (m, 6H), 1.55–1.45 (m, 2H); 31P NMR (121 MHz, CDCl3) δ 140.5; HRMS (ESI): calcd for C36H37NO2P (M + H)+: 546.2556; Found: 546.2559.
(S)-N-(2-(tert-Butyl)phenyl)-N-cinnamyl-2-phenylacetamide (4aa).
Light yellow oil; [α]20D = +18.9 (c 2.99, CH2Cl2) (61% ee); IR (film): 2959, 1650, 1490, 1392, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.61 (d, J = 8.0 Hz, 1H), 7.35–7.17 (m, 9H), 7.10 (t, J = 7.2 Hz, 3H), 6.81 (d, J = 8.0 Hz, 1H), 6.42–6.31 (m, 2H), 5.14 (dd, J = 13.6, 4.4 Hz, 1H), 3.49 (dd, J = 13.6, 7.6 Hz, 1H), 3.36 (dd, J = 27.2, 15.2 Hz, 2H), 1.45 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.4, 146.5, 139.8, 136.9, 135.2, 134.0, 132.7, 130.1, 129.6, 128.9, 128.7, 128.4, 127.8, 127.0, 126.8, 126.7, 124.0, 53.9, 42.0, 36.4, 32.5; HRMS (TOF-EI): calcd for C27H29NO (M): 383.2249; Found: 383.2253.
(S)-N-Cinnamyl-N-(2,5-di-tert-butylphenyl)-2-phenylacetamide (4ba).
Light yellow oil; [α]20D = −18.6 (c 1.72, CH2Cl2) (79% ee); IR (film): 2954, 1655, 1490, 1406, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 8.4 Hz, 1H), 7.32 (dd, J = 8.4, 2.0 Hz, 1H), 7.29–7.16 (m, 8H), 7.12 (d, J = 7.2 Hz, 2H), 6.72 (d, J = 2.4 Hz, 1H), 6.38 (ddd, J = 12.8, 8.0, 4.8 Hz, 1H), 6.30 (t, J = 16.0 Hz, 1H), 5.16 (dd, J = 14.0, 4.8 Hz, 1H), 3.45 (dd, J = 13.6, 8.0 Hz, 1H), 3.39 (dd, J = 27.2, 14.6 Hz, 2H), 1.44 (s, 9H), 1.07 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.4, 149.7, 143.0, 139.2, 136.9, 135.4, 134.4, 130.0, 129.6, 129.4, 128.6, 128.5, 127.7, 126.7, 126.5, 125.8, 124.0, 77.4, 53.6, 42.1, 35.9, 34.1, 32.5, 31.0; HRMS (TOF-EI): calcd for C31H37NO (M): 439.2875; Found: 439.2881.
(S)-N-(2,5-Di-tert-butylphenyl)-2-phenyl-N-(3-(4-(trifluoromethyl)phenyl)allyl)acetamide (4bb).
Light yellow oil; [α]20D = +9.0 (c 4.18, CH2Cl2) (68% ee); IR (film): 2959, 1655, 1495, 1410, 1330, 1121, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 8.4 Hz, 3H), 7.37–7.32 (m, 3H), 7.26–7.19 (m, 3H), 7.11 (d, J = 6.4 Hz, 2H), 6.69 (d, J = 2.0 Hz, 1H), 6.47 (ddd, J = 16.0, 8.4, 4.8 Hz, 1H), 6.34 (d, J = 16.0 Hz, 1H), 5.17 (dd, J = 14.0, 4.8 Hz, 1H), 3.48 (dd, J = 14.0, 8.4 Hz, 1H), 3.40 (dd, J = 28.0, 14.4 Hz, 2H), 1.44 (s, 9H), 1.08 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.5, 149.9, 143.1, 140.4, 139.2, 135.3, 132.9, 129.8, 129.4, 129.3 (q, JC–F = 29.0 Hz), 128.6, 126.9, 126.7, 126.0, 125.8, 125.7, 125.6, 124.4 (q, JC–F = 270.0 Hz), 53.5, 42.1, 36.0, 34.1, 32.5, 31.1; HRMS (TOF-EI): calcd for C32H37F3NO (M + H)+: 508.2822; Found: 508.2818.
(S)-N-(2,5-Di-tert-butylphenyl)-2-phenyl-N-(3-(3-chlorophenyl)allyl)acetamide (4bc).
Light yellow oil; [α]20D = +2.6 (c 5.07, CH2Cl2) (75% ee); IR (film): 2963, 1655, 1495, 1401, 1246, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 8.4 Hz, 1H), 7.33 (dd, J = 8.8, 2.4 Hz, 1H), 7.26–7.18 (m, 7H), 7.11 (d, J = 6.4 Hz, 2H), 6.68 (d, J = 2.4 Hz, 1H), 6.35 (ddd, J = 16.0, 8.8, 4.8 Hz, 1H), 6.25 (d, J = 16.0 Hz, 1H), 5.15 (dd, J = 14.0, 4.8 Hz, 1H), 3.43 (dd, J = 14.0, 8.8 Hz, 1H), 3.39 (dd, J = 28.0, 14.4 Hz, 2H), 1.44 (s, 9H), 1.08 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.5, 149.8, 143.0, 139.2, 135.4, 135.3, 133.4, 133.0, 129.9, 129.7, 129.4, 128.9, 128.5, 127.7, 126.8, 125.9, 124.8, 53.5, 42.1, 36.0, 34.1, 32.5, 31.1; HRMS (TOF-EI): calcd for C31H36NOCl (M): 473.2485; Found: 473.2491.
(S)-N-(2,5-Di-tert-butylphenyl)-2-phenyl-N-(3-(3-methyloxyphenyl)allyl)acetamide (4bd).
Light yellow oil; [α]20D = +10.8 (c 4.58, CH2Cl2) (76% ee); IR (film): 2959, 1659, 1490, 1406, 1259, 965 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 8.4 Hz, 1H), 7.32 (dd, J = 8.4, 2.4 Hz, 1H), 7.26–7.16 (m, 4H), 7.11 (d, J = 6.8 Hz, 2H), 6.86 (d, J = 7.6 Hz, 1H), 6.82 (s, 1H), 6.77 (dd, J = 8.0, 2.0 Hz, 1H), 6.72 (d, J = 2.0 Hz, 1H), 6.37 (ddd, J = 16.0, 8.8, 4.8 Hz, 1H), 6.28 (d, J = 16.0 Hz, 1H), 5.15 (dd, J = 14.0, 4.8 Hz, 1H), 3.78 (s, 3H), 3.44 (dd, J = 13.6, 8.4 Hz, 1H), 3.40 (dd, J = 27.6, 14.8 Hz, 2H), 1.44 (s, 9H), 1.09 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.4, 159.9, 149.8, 143.0, 139.2, 138.4, 135.4, 134.3, 130.0, 129.6, 129.4, 128.5, 126.8, 125.8, 124.3, 119.4, 113.8, 111.4, 55.4, 53.6, 42.1, 36.0, 34.1, 32.5, 31.1; HRMS (TOF-EI): calcd for C32H39NO2 (M): 469.2981; Found: 469.2986.
(S)-N-(2,5-Di-tert-Butylphenyl)-2-phenyl-N-(3-(2-chlorophenyl)allyl)acetamide (4be).
Light yellow oil; [α]20D = +35.4 (c 2.21, CH2Cl2) (80% ee); IR (film): 2963, 1655, 1495, 1406, 1263, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 8.8 Hz, 1H), 7.48 (dd, J = 7.6, 1.6 Hz, 1H), 7.34–7.28 (m, 2H), 7.26–7.16 (m, 4H), 7.15–7.11 (m, 3H), 6.71 (s, 1H), 6.69 (d, J = 13.6 Hz, 1H), 6.40 (ddd, J = 13.6, 8.8, 4.8 Hz, 1H), 5.15 (dd, J = 14.0, 4.8 Hz, 1H), 3.52 (dd, J = 14.0, 8.8 Hz, 1H), 3.40 (dd, J = 26.8, 14.4 Hz, 2H), 1.45 (s, 9H), 1.10 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.5, 149.9, 143.0, 139.3, 135.4, 135.0, 133.1, 130.1, 129.8, 129.7, 129.6, 129.4, 128.8, 128.5, 127.2, 127.1, 127.0, 126.8, 126.0, 53.8, 42.1, 36.0, 34.1, 32.5, 31.1; HRMS (TOF-EI): calcd for C31H36NOCl (M): 473.2485; Found: 473.2491.
(S)-N-(2,5-Di-tert-butylphenyl)-2-phenyl-N-(3-(2-methyloxyphenyl)allyl)acetamide (4bf).
Light yellow oil; [α]20D = +18.3 (c 2.63, CH2Cl2) (84% ee); IR (film): 2965, 1651, 1486, 1242, 748 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.4 Hz, 1H), 7.36 (d, J = 6.8 Hz, 1H), 7.31 (dd, J = 8.4, 2.0 Hz, 1H), 7.25–7.16 (m, 4H), 7.11 (d, J = 6.8 Hz, 2H), 6.88 (t, J = 7.6 Hz, 1H), 6.81 (d, J = 8.0 Hz, 1H), 6.75 (d, J = 2.0 Hz, 1H), 6.62 (d, J = 16.0 Hz, 1H), 6.38 (ddd, J = 16.0, 8.8, 4.8 Hz, 1H), 5.15 (dd, J = 14.0, 4.4 Hz, 1H), 3.74 (s, 3H), 3.48 (dd, J = 13.6, 8.8 Hz, 1H), 3.39 (dd, J = 25.6, 14.8 Hz, 1H), 1.44 (s, 9H), 1.08 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.3, 156.8, 149.8, 143.0, 139.5, 135.6, 130.0, 129.5, 129.4, 129.1, 128.8, 128.5, 127.3, 126.7, 126.1, 125.7, 124.8, 120.8, 110.8, 55.4, 54.2, 42.2, 36.0, 34.1, 32.5, 31.0; HRMS (TOF-EI): calcd for C32H39NO2 (M): 469.2981; Found: 469.2986.
(S)-N-(2,5-Di-tert-butylphenyl)-2-phenyl-N-(3-(2-benzyloxyphenyl)allyl)acetamide (4bg).
Light yellow oil; [α]20D = +26.3 (c 2.54, CH2Cl2) (80% ee); IR (film): 2963, 1650, 1495, 1401, 1241, 752 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.4 Hz, 1H), 7.40 (dd, J = 7.6, 1.2 Hz, 1H), 7.34–7.27 (m, 6H), 7.24–7.11 (m, 6H), 6.89 (t, J = 7.6 Hz, 1H), 6.83 (d, J = 8.4 Hz, 1H), 6.72 (s, 1H), 6.72 (d, J = 15.6 Hz, 1H), 6.38 (ddd, J = 15.6, 8.8, 4.8 Hz, 1H), 5.16 (ddd, J = 13.2, 9.2, 8.0 Hz, 1H), 5.05–4.99 (m, 2H), 3.51 (dd, J = 13.6, 4.8 Hz, 1H), 3.39 (dd, J = 26.4, 14.4 Hz, 2H), 1.43 (s, 9H), 1.06 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.4, 155.9, 149.7, 143.1, 139.2, 137.3, 135.5, 130.0, 129.5, 129.4, 128.9, 128.8, 128.7, 128.5, 127.9, 127.2, 127.0, 126.7, 126.5, 125.8, 124.7, 121.1, 112.6, 70.3, 54.0, 42.2, 36.0, 34.1, 32.5, 31.1; HRMS (TOF-EI): calcd for C38H43NO2 (M): 545.3294; Found: 545.3299.
(S)-N-(2,5-Di-tert-butylphenyl)-2-phenyl-N-(3-(2-isopropoxyphenyl)allyl)acetamide (4bh).
Light yellow solid; mp 77–79 °C; [α]20D = +21.7 (c 4.11, CH2Cl2) (79% ee); IR (film): 2963, 1646, 1486, 1410, 1237, 952 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.51 (d, J = 8.8 Hz, 1H), 7.35 (d, J = 6.4 Hz, 1H), 7.30 (dd, J = 8.8, 2.0 Hz, 1H), 7.25–7.11 (m, 6H), 6.87–6.80 (m, 2H), 6.74 (d, J = 2.4 Hz, 1H), 6.61 (d, J = 16.0 Hz, 1H), 6.38 (ddd, J = 16.0, 8.8, 5.2 Hz, 1H), 5.15 (dd, J = 14.0, 5.2 Hz, 1H), 4.50–4.43 (m, 1H), 3.51 (dd, J = 13.6, 8.8 Hz, 1H), 3.39 (dd, J = 26.4, 14.4 Hz, 1H), 1.44 (s, 9H), 1.25 (t, J = 5.6 Hz, 6H), 1.07 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.3, 155.2, 149.7, 143.0, 139.4, 135.5, 129.9, 129.4, 129.4, 129.3, 128.6, 128.5, 127.3, 127.0, 126.7, 125.7, 124.4, 120.6, 114.0, 70.7, 54.1, 42.2, 35.9, 34.1, 32.5, 31.1, 22.3, 22.3; HRMS (TOF-EI): calcd for C34H43NO2 (M): 497.3294; Found: 497.3299.
(S)-N-(2,5-Di-tert-butylphenyl)-2-phenyl-N-(3-(2-methylphenyl)allyl)acetamide (4bi).
Light yellow oil; [α]20D = +25.2 (c 2.91, CH2Cl2) (75% ee); IR (film): 2958, 1654, 1494, 1401, 1249, 969 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.52 (d, J = 8.4 Hz, 1H), 7.37 (dd, J = 5.2, 4.0 Hz, 1H), 7.32 (dd, J = 8.4, 2.0 Hz, 1H), 7.24–7.16 (m, 3H), 7.14–7.07 (m, 5H), 6.72 (d, J = 2.0 Hz, 1H), 6.54 (d, J = 15.6 Hz, 1H), 6.25 (ddd, J = 15.6, 8.8, 5.2 Hz, 1H), 5.16 (dd, J = 14.0, 5.2 Hz, 1H), 3.52 (dd, J = 14.0, 8.8 Hz, 1H), 3.39 (dd, J = 28.8, 14.4 Hz, 2H), 2.19 (s, 3H), 1.45 (s, 9H), 1.09 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.3, 149.8, 143.0, 139.3, 136.0, 135.4, 135.4, 132.0, 130.3, 129.7, 129.6, 129.3, 128.5, 127.6, 126.8, 126.2, 126.1, 125.9, 125.5, 53.9, 42.1, 36.0, 34.1, 32.5, 31.1, 19.9; HRMS (TOF-EI): calcd for C32H39NO (M): 453.3032; Found: 453.3038.
(S)-N-(2,5-Di-tert-butylphenyl)-2-phenyl-N-(3-(1-naphthyl)allyl)acetamide (4bj).
Light yellow solid; mp 96–98 °C; [α]20D = +46.9 (c 3.69, CH2Cl2) (83% ee); IR (film): 2963, 1637, 1490, 1406, 1174, 974 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.4 Hz, 1H), 7.82 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.55 (d, J = 8.8 Hz, 1H), 7.52 (d, J = 7.2 Hz, 1H), 7.47–7.38 (m, 3H), 7.35 (dd, J = 8.4, 2.0 Hz, 1H), 7.25–7.18 (m, 3H), 7.17–7.07 (m, 3H), 6.77 (d, J = 2.0 Hz, 1H), 6.41 (ddd, J = 15.6, 8.8, 5.2 Hz, 1H), 5.24 (dd, J = 14.0, 5.2 Hz, 1H), 3.63 (dd, J = 14.0, 8.8 Hz, 1H), 3.42 (dd, J = 30.0, 14.4 Hz, 2H), 1.47 (s, 9H), 1.05 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.4, 149.9, 143.0, 139.4, 135.4, 134.7, 133.7, 131.4, 131.2, 129.7, 129.4, 128.59, 128.57, 128.1, 127.4, 126.8, 126.1, 126.0, 125.9, 125.8, 124.3, 124.1, 54.0, 42.1, 36.0, 34.1, 32.5, 31.1; HRMS (TOF-EI): calcd for C35H39NO (M): 489.3032; Found: 489.3038.
(S)-N-Allyl-N-(2,5-di-tert-butylphenyl)-2-phenylacetamide (4bk).
Light yellow oil; [α]20D = +49.6 (c 1.69, CH2Cl2) (46% ee); IR (film): 2963, 1655, 1490, 1401, 1259, 925 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.4 Hz, 1H), 7.32 (dd, J = 8.4, 2.0 Hz, 1H), 7.26–7.17 (m, 3H), 7.10 (d, J = 7.2 Hz, 2H), 6.72 (d, J = 2.0 Hz, 1H), 5.99 (dddd, J = 16.8, 10.0, 6.4, 4.8 Hz, 1H), 5.12 (d, J = 10.0 Hz, 1H), 5.01 (d, J = 16.8 Hz, 1H), 4.96 (d, J = 4.8 Hz, 1H), 3.37 (dd, J = 25.6, 14.4 Hz, 2H), 3.31 (dd, J = 13.6, 8.4 Hz, 1H), 1.41 (s, 9H), 1.17 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.3, 149.7, 142.9, 139.5, 135.4, 133.1, 129.8, 129.6, 129.4, 128.5, 126.7, 125.8, 118.8, 54.3, 42.1, 35.9, 34.2, 32.4, 31.1; HRMS (TOF-EI): calcd for C25H33NO (M): 363.2562; Found: 363.2566.
(S)-N-(3-(2-Chlorophenyl)allyl)-N-(2,5-di-tert-butylphenyl)-3-phenylpropanamide (4ce).
Light yellow oil; [α]20D = −8.0 (c 2.89, CH2Cl2) (47% ee); IR (film): 2963, 1650, 1472, 1401, 1272, 965 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.55 (dd, J = 7.6, 1.2 Hz, 1H), 7.45 (d, J = 8.4 Hz, 1H), 7.32–7.28 (m, 2H), 7.25–7.11 (m, 5H), 7.07 (d, J = 6.8 Hz, 2H), 6.80 (d, J = 2.0 Hz, 1H), 6.72 (d, J = 15.6 Hz, 1H), 6.44 (ddd, J = 15.6, 9.2, 5.2 Hz, 1H), 5.15 (dd, J = 14.0, 5.2 Hz, 1H), 3.48 (dd, J = 14.0, 9.2 Hz, 1H), 3.01–2.87 (m, 2H), 2.37–2.25 (m, 2H), 1.35 (s, 9H), 1.16 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 172.9, 150.2, 143.1, 141.6, 139.4, 135.0, 133.1, 130.0, 129.8, 129.6, 129.1, 128.8, 128.7, 128.6, 127.2, 127.2, 127.0, 126.2, 125.7, 53.8, 37.6, 35.8, 34.2, 32.3, 31.8, 31.2; HRMS (TOF-EI): calcd for C32H38NOCl (M): 487.2642; Found: 487.2647.
(S)-N-(3-(2-Chlorophenyl)allyl)-N-(2,5-di-tert-butylphenyl)-cinnamamide (4de).
Light yellow solid; mp 95–97 °C; [α]20D = −12.8 (c 2.99, CH2Cl2) (44% ee); IR (film): 2963, 1655, 1468, 1379, 1232, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 15.6 Hz, 1H), 7.59 (d, J = 7.6 Hz, 1H), 7.54 (d, J = 8.4 Hz, 1H), 7.38 (d, J = 8.4 Hz, 1H), 7.32–7.27 (m, 6H), 7.23–7.14 (m, 2H), 6.95 (s, 1H), 6.77 (d, J = 16.0 Hz, 1H), 6.56 (ddd, J = 16.0, 9.2, 5.2 Hz, 1H), 6.21 (d, J = 15.6 Hz, 1H), 5.25 (dd, J = 14.0, 5.2 Hz, 1H), 3.62 (dd, J = 14.0, 9.2 Hz, 1H), 1.40 (s, 9H), 1.19 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 166.6, 150.4, 143.9, 141.9, 139.2, 135.5, 135.0, 133.1, 130.1, 129.8, 129.7, 129.4, 128.9, 128.8, 128.0, 127.2, 127.0, 125.8, 119.5, 54.2, 35.9, 34.3, 32.4, 31.2; HRMS (TOF-EI): calcd for C32H36NOCl (M): 485.2485; Found: 485.2491.
(S)-N-(3-(2-Chlorophenyl)allyl)-N-(2,5-di-tert-butylphenyl)-benzamide (4ee).
Light yellow oil; [α]20D = −16.7 (c 3.16, CH2Cl2) (46% ee); IR (film): 2963, 1633, 1468, 1410, 1281, 965 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 6.0 Hz, 1H), 7.36–7.29 (m, 4H), 7.26–7.15 (m, 4H), 7.10 (t, J = 7.2 Hz, 2H), 7.04 (d, J = 2.0 Hz, 1H), 6.86 (d, J = 16.0 Hz, 1H), 6.60 (ddd, J = 16.0, 8.8, 4.8 Hz, 1H), 5.33 (dd, J = 14.0, 4.8 Hz, 1H), 3.72 (dd, J = 14.0, 8.8 Hz, 1H), 1.25 (s, 9H), 1.16 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 169.3, 149.5, 142.9, 139.4, 136.3, 135.0, 133.1, 130.3, 130.2, 130.1, 129.8, 129.7, 129.4, 128.9, 127.6, 127.2, 127.2, 127.0, 125.3, 55.5, 35.9, 34.1, 32.5, 31.1; HRMS (TOF-EI): calcd for C30H34NOCl (M): 459.2329; Found: 459.2335.
(S)-N-(3-(2-Chlorophenyl)allyl)-N-(2,5-di-tert-butylphenyl)-2-furylamide (4fe).
Light yellow oil; [α]20D = −22.4 (c 3.53, CH2Cl2) (43% ee); IR (film): 2954, 1642, 1472, 1410, 1188, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.58 (d, J = 7.6 Hz, 1H), 7.52 (d, J = 8.8 Hz, 1H), 7.40 (d, J = 8.8 Hz, 2H), 7.31 (d, J = 8.0 Hz, 1H), 7.23–7.14 (m, 2H), 7.00 (d, J = 2.0 Hz, 1H), 6.80 (d, J = 15.6 Hz, 1H), 6.59 (ddd, J = 15.6, 9.2, 4.8 Hz, 1H), 6.14 (d, J = 2.0 Hz, 1H), 5.32 (dd, J = 13.6, 4.8 Hz, 1H), 5.26 (d, J = 3.2 Hz, 1H), 3.64 (dd, J = 13.6, 9.2 Hz, 1H), 1.31 (s, 9H), 1.17 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 159.4, 150.4, 147.4, 144.7, 143.7, 139.1, 134.9, 133.1, 130.4, 129.9, 129.79, 129.77, 128.9, 127.2, 127.0, 126.8, 126.0, 116.6, 111.2, 54.9, 35.9, 34.2, 32.3, 31.1; HRMS (TOF-EI): calcd for C28H32NO2Cl (M): 449.2122; Found: 449.2126.
(S)-N-(3-(2-Chlorophenyl)allyl)-N-(2,5-di-tert-butylphenyl)-2-cyclohexanylamide (4ge).
Light yellow oil; [α]20D = +9.3 (c 3.22, CH2Cl2) (74% ee); IR (film): 2937, 1650, 1468, 1424, 1268, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 7.6 Hz, 1H), 7.49 (d, J = 8.4 Hz, 1H), 7.33–7.30 (m, 2H), 7.23–7.14 (m, 2H), 6.95 (d, J = 2.0 Hz, 1H), 6.73 (d, J = 16.0 Hz, 1H), 6.45 (ddd, J = 16.0, 8.8, 4.8 Hz, 1H), 5.11 (dd, J = 14.0, 4.8 Hz, 1H), 3.47 (dd, J = 14.0, 8.8 Hz, 1H), 1.99–1.94 (m, 1H), 1.75–1.64 (m, 4H), 1.62–1.44 (m, 3H), 1.39 (s, 9H), 1.19 (s, 9H), 1.03–0.78 (m, 3H); 13C NMR (100 MHz, CDCl3) δ 176.9, 149.7, 143.1, 139.5, 135.1, 133.1, 129.8, 129.7, 129.4, 129.3, 128.7, 127.5, 127.2, 127.0, 125.3, 53.7, 42.9, 35.8, 34.2, 32.4, 31.1, 28.3, 25.9, 25.4; HRMS (TOF-EI): calcd for C30H40NOCl (M): 465.2798; Found: 465.2804.
(S)-N-(2,5-Di-tert-butylphenyl)-2-(4-chlorophenyl)-N-(3-(2-chlorophenyl)allyl)acetamide (4he).
Light yellow oil; [α]20D = +50.2 (c 2.19, CH2Cl2) (66% ee); IR (film): 2959, 1655, 1490, 1094, 1023, 965 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 8.8 Hz, 1H), 7.48 (dd, J = 8.0, 1.6 Hz, 1H), 7.34 (dd, J = 8.4, 2.0 Hz, 1H), 7.30 (dd, J = 8.0, 1.2 Hz, 1H), 7.22–7.13 (m, 4H), 7.06 (d, J = 8.0 Hz, 2H), 6.71 (d, J = 2.0 Hz, 1H), 6.70 (d, J = 15.2 Hz, 1H), 6.38 (ddd, J = 15.2, 8.8, 4.0 Hz, 1H), 5.13 (dd, J = 14.4, 4.0 Hz, 1H), 3.53 (dd, J = 14.4, 8.8 Hz, 1H), 3.40–3.31 (m, 2H), 1.44 (s, 9H), 1.12 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.0, 150.1, 143.0, 139.2, 134.9, 133.8, 133.1, 132.7, 130.8, 130.3, 129.81, 129.77, 129.5, 128.9, 128.6, 127.2, 127.0, 126.9, 126.1, 53.8, 41.3, 36.0, 34.2, 32.5, 31.1; HRMS (ESI): calcd for C31H36NOCl2 (M + H)+: 508.2169; Found: 508.2169.
(S)-N-(2,5-Di-tert-butylphenyl)-2-(2-chlorophenyl)-N-(3-(2-chlorophenyl)allyl)acetamide (4ie).
Light yellow oil; [α]20D = +21.0 (c 3.05, CH2Cl2) (75% ee); IR (film): 2965, 1657, 1470, 1407, 1249, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.54–7.50 (m, 2H), 7.36 (dd, J = 7.6, 1.2 Hz, 1H), 7.33–7.27 (m, 3H), 7.24–7.13 (m, 4H), 6.83 (d, J = 2.0 Hz, 1H), 6.74 (d, J = 15.6 Hz, 1H), 6.48 (ddd, J = 15.6, 8.8, 4.8 Hz, 1H), 5.17 (dd, J = 14.0, 4.8 Hz, 1H), 3.71 (d, J = 16.0 Hz, 1H), 3.55 (dd, J = 14.0, 8.8 Hz, 1H), 3.36 (d, J = 16.0 Hz, 1H), 1.47 (s, 9H), 1.10 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 170.7, 150.2, 143.2, 139.0, 135.1, 134.5, 133.9, 133.1, 131.3, 130.3, 129.9, 129.8, 129.6, 129.3, 128.8, 128.3, 127.2, 127.06, 127.05, 126.9, 126.0, 54.0, 39.9, 36.0, 34.1, 32.6, 31.1; HRMS (ESI): calcd for C31H36NOCl2 (M + H)+: 508.2169; Found: 508.2169.
(S)-N-(2,5-Di-tert-butylphenyl)-2-(2,5-dimethylphenyl)-N-(3-(2-chlorophenyl)allyl)acetamide (4je).
Light yellow oil; [α]20D = +29.9 (c 3.07, CH2Cl2) (83% ee); IR (film): 2963, 1655, 1472, 1406, 1254, 974 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.53 (dd, J = 7.6, 1.2 Hz, 1H), 7.49 (d, J = 8.4 Hz, 1H), 7.32–7.28 (m, 2H), 7.23–7.14 (m, 2H), 7.08 (s, 1H), 6.92–6.88 (m, 2H), 6.73 (d, J = 16.0 Hz, 1H), 6.66 (d, J = 2.4 Hz, 1H), 6.50 (ddd, J = 16.0, 8.8, 4.0 Hz, 1H), 5.18 (dd, J = 13.6, 4.0 Hz, 1H), 3.51 (dd, J = 13.6, 8.8 Hz, 1H), 3.44 (d, J = 15.2 Hz, 1H), 3.27 (d, J = 15.2 Hz, 1H), 2.25 (s, 3H), 1.83 (s, 3H), 1.45 (s, 9H), 1.02 (s, 9H); 13C NMR (100 MHz, CDCl3) δ 171.8, 149.9, 143.0, 139.3, 135.5, 135.1, 133.9, 133.3, 133.1, 130.3, 130.1, 129.8, 129.6, 129.3, 128.8, 127.5, 127.2, 127.2, 127.0, 125.9, 53.8, 39.5, 36.0, 34.0, 32.5, 30.9, 21.1, 19.3; HRMS (ESI): calcd for C33H41NOCl (M + H)+: 502.2871; Found: 502.2866.
(S)-N-(2,4-Di-methyl-6-iodophenyl)-2-phenyl-N-(3-(2-chloro-phenyl)allyl)acetamide (4ke).
Light yellow oil; [α]20D = +11.6 (c 2.71, CH2Cl2) (48% ee); IR (film): 2923, 1664, 1468, 1383, 1263, 965 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.63 (s, 1H), 7.43 (dd, J = 7.6, 1.6 Hz, 1H), 7.32–7.18 (m, 5H), 7.16–7.13 (m, 1H), 7.12–7.09 (m, 1H), 7.03 (s, 1H), 6.71 (d, J = 15.6 Hz, 1H), 6.39 (dt, J = 15.6, 7.6 Hz, 1H), 4.53 (dd, J = 14.0, 6.4 Hz, 1H), 4.23 (dd, J = 14.0, 8.0 Hz, 1H), 3.43 (d, J = 14.8 Hz, 1H), 3.24 (d, J = 14.8 Hz, 1H), 2.31 (s, 3H), 2.04 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.0, 140.5, 140.5, 138.7, 138.4, 135.0, 134.7, 133.1, 132.5, 129.84, 129.80, 129.7, 129.2, 128.8, 128.4, 127.5, 127.2, 127.0, 126.9, 102.2, 51.8, 41.6, 20.7, 19.6; HRMS (ESI): calcd for C25H24ONClI (M + H)+: 516.0586; Found: 516.0583.
(S)-N-(2,4-Di-methyl-6-bromophenyl)-2-phenyl-N-(3-(2-chloro-phenyl)allyl)acetamide (4le).
Light yellow oil; [α]20D = +7.0 (c 4.52, CH2Cl2) (53% ee); IR (film): 2919, 1659, 1468, 1388, 1263, 974 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 7.2 Hz, 1H), 7.36 (s, 1H), 7.29–7.11 (m, 6H), 7.07 (d, J = 7.2 Hz, 2H), 7.00 (s, 1H), 6.70 (d, J = 15.6 Hz, 1H), 6.35 (dt, J = 15.6, 7.6 Hz, 1H), 4.43 (dd, J = 14.0, 6.8 Hz, 1H), 4.34 (dd, J = 14.0, 7.2 Hz, 1H), 3.44 (d, J = 14.8 Hz, 1H), 3.26 (d, J = 14.8 Hz, 1H), 2.33 (s, 3H), 2.02 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.2, 140.2, 138.9, 137.2, 135.1, 134.8, 133.0, 132.1, 131.5, 129.8, 129.7, 128.8, 128.4, 127.4, 127.2, 127.0, 126.9, 124.8, 51.3, 41.3, 21.0, 19.1; HRMS (ESI): calcd for C25H24ONBrCl (M + H)+: 468.0724; Found: 468.0723.
(S)-N-(6-Bromo-2-methyl-phenyl)-2-phenyl-N-(3-(2-chlorophenyl)allyl)acetamide (4me).
Light yellow oil; [α]20D = +5.9 (c 2.91, CH2Cl2) (46% ee); IR (film): 2927, 1664, 1464, 1388, 1263, 970 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.55 (dd, J = 7.6, 1.2 Hz, 1H), 7.42 (dd, J = 7.6, 1.6 Hz, 1H), 7.29 (dd, J = 7.6, 1.6 Hz, 1H), 7.25–7.12 (m, 7H), 7.05 (dd, J = 7.6, 1.6 Hz, 2H), 6.70 (d, J = 16.0 Hz, 1H), 6.35 (dt, J = 16.0, 7.6 Hz, 1H), 4.47–4.34 (m, 2H), 3.45 (d, J = 14.8 Hz, 1H), 3.26 (d, J = 14.8 Hz, 1H), 2.05 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.0, 139.8, 139.7, 135.1, 134.6, 133.1, 131.7, 130.8, 130.0, 129.9, 129.7, 129.7, 128.8, 128.5, 127.3, 127.3, 127.0, 127.0, 125.4, 51.3, 41.6, 19.1; HRMS (TOF-EI): calcd for C24H21NOClBr (M): 453.0495; Found: 453.0500.
(S)-N-(3-(2-Chlorophenyl)allyl)-N-(3,5-dimethyl-[1,1′-biphenyl]-2-yl)-2-phenylacetamide (4ne).
Light yellow oil; [α]20D = +4.3 (c 1.46, CH2Cl2) (33% ee); IR (film): 2954, 1655, 1468, 1392, 1263, 965 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.32–7.21 (m, 11H), 7.16–7.12 (m, 4H), 7.10 (d, J = 4.4 Hz, 1H), 6.59 (d, J = 16.0 Hz, 1H), 6.05 (dt, J = 16.0, 8. 4 Hz, 1H), 4.32 (dd, J = 14.4, 6.4 Hz, 1H), 3.74 (dd, J = 14.4, 8.0 Hz, 1H), 3.47 (dd, J = 25.6, 15.2 Hz, 2H), 2.40 (s, 3H), 2.12 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 171.5, 140.6, 139.6, 138.5, 136.8, 136.5, 135.1, 135.0, 133.0, 131.6, 130.4, 129.9, 129.7, 129.1, 129.0, 128.7, 128.6, 128.5, 127.67, 127.66, 127.2, 126.94, 126.89, 52.4, 41.1, 21.3, 19.0; HRMS (TOF-EI): calcd for C31H28NOCl (M): 465.1859; Found: 465.1864.
Conclusions
In summary, a Pd-catalyzed asymmetric allylic alkylation of anilides using chiral phosphorus amidite–olefin ligands is successfully developed, and a wide range of highly desirable optically active C–N axially chiral anilides were obtained in high yields with up to 84% ee. The absolute configurations of the corresponding chiral anilide products were determined from X-ray and CD spectra.
Acknowledgements
The authors are thankful for the financial support from the National Natural Science Foundation of China (21072194, 21172222, 21222207), and the 973 program (2010CB833300).
Notes and references
- O. Kitagawa and T. J. Taguchi, Synth. Org. Chem., Jpn., 2001, 59, 680 CrossRef CAS.
- H.-U. Blaser and F. Spindler, Top. Catal., 1997, 4, 275 CrossRef.
- D. P. Curran, H. Qi, S. J. Geib and N. C. DeMello, J. Am. Chem. Soc., 1994, 116, 3131 CrossRef CAS.
- A. D. Hughes, D. A. Price, O. Shishkin and N. S. Simpkins, Tetrahedron Lett., 1996, 37, 7607 CrossRef CAS.
-
(a) S. Brandes, M. Bella, A. Kjærsgaard and K. A. Jørgensen, Angew. Chem., Int. Ed., 2006, 45, 1147 CrossRef CAS PubMed;
(b) S. Brandes, B. Niess, M. Bella, A. Prieto, J. Overgaard and K. A. Jørgensen, Chem. – Eur. J., 2006, 12, 6039 CrossRef CAS PubMed.
-
(a) T. Hata, H. Koide, N. Taniguchi and M. Uemura, Org. Lett., 2000, 2, 1907 CrossRef CAS PubMed;
(b) D. J. Bennett, P. L. Pickering and N. S. Simpkins, Chem. Commun., 2004, 1392 RSC;
(c) H. Koide, T. Hata, K. Yoshihara, K. Kamikawa and M. Uemura, Tetrahedron, 2004, 60, 4527 CrossRef CAS PubMed.
-
(a) O. Kitagawa, H. Izawa, T. Taguchi and M. Shiro, Tetrahedron Lett., 1997, 38, 4447 CrossRef CAS;
(b) O. Kitagawa, H. Izawa, K. Sato, A. Dobashi and T. Taguchi, J. Org. Chem., 1998, 63, 2634 CrossRef CAS PubMed;
(c) K. D. Shimizu, H. O. Freyer and R. D. Adams, Tetrahedron Lett., 2000, 41, 5431 CrossRef CAS;
(d) A. Ates and D. P. Curran, J. Am. Chem. Soc., 2001, 123, 5130 CrossRef CAS.
-
(a) K. Kamikawa, S. Kinoshita, H. Matsuzaka and M. Uemura, Org. Lett., 2006, 8, 1097 CrossRef CAS PubMed;
(b) K. Kamikawa, S. Kinoshita, M. Furusyo, S. Takemoto, H. Matsuzaka and M. Uemura, J. Org. Chem., 2007, 72, 3394 CrossRef CAS PubMed.
-
(a) O. Kitagawa, M. Takahashi, M. Yoshikawa and T. Taguchi, J. Am. Chem. Soc., 2005, 127, 3676 CrossRef CAS PubMed;
(b) O. Kitagawa, M. Yoshikawa, H. Tanabe, T. Morita, M. Takahashi, Y. Dobashi and T. Taguchi, J. Am. Chem. Soc., 2006, 128, 12923 CrossRef CAS PubMed.
-
(a) K. Tanaka, K. Takeishi and K. Noguchi, J. Am. Chem. Soc., 2006, 128, 4586 CrossRef CAS PubMed;
(b) K. Tanaka and K. Takeishi, Synthesis, 2007, 2920 CrossRef CAS PubMed;
(c) J. Oppenheimer, R. P. Hsung, R. Figueroa and W. L. Johnson, Org. Lett., 2007, 9, 3969 CrossRef CAS PubMed;
(d) K. Tanaka, Y. Takahashi, T. Suda and M. Hirano, Synlett, 2008, 1724 CrossRef CAS PubMed;
(e) J. Oppenheimer, W. L. Johnson, R. Figueroa, R. Hayashi and R. P. Hsung, Tetrahedron, 2009, 65, 5001 CrossRef CAS PubMed.
-
(a) S. Shirakawa, K. Liu and K. Maruoka, J. Am. Chem. Soc., 2012, 134, 916 CrossRef CAS PubMed;
(b) K. Liu, X. Wu, S. B. J. Kan, S. Shirakawa and K. Maruoka, Chem. – Asian J., 2013, 8, 3214 CrossRef CAS PubMed.
- For leading reviews, see:
(a) B. M. Trost and D. L. Van Vranken, Chem. Rev., 1996, 96, 395 CrossRef CAS PubMed;
(b) B. M. Trost and M. L. Crawley, Chem. Rev., 2003, 103, 2921 CrossRef CAS PubMed;
(c) Z. Lu and S. Ma, Angew. Chem., Int. Ed., 2008, 47, 258 CrossRef CAS PubMed.
-
(a) O. Kitagawa, M. Kohriyama and T. Taguchi, J. Org. Chem., 2002, 67, 8682 CrossRef CAS PubMed;
(b) J. Terauchi and D. P. Curran, Tetrahedron: Asymmetry, 2003, 14, 587 CrossRef CAS.
- M. Fujita, O. Kitagawa, H. Izawa, A. Dobashi, H. Fukaya and T. Taguchi, Tetrahedron Lett., 1999, 40, 1949 CrossRef CAS.
-
(a) E. W. Byrnes, P. D. McMaster, E. R. Smith, M. R. Blair, R. N. Boyes, B. R. Duce, H. S. Feldman, G. H. Kronberg, B. H. Takman and P. A. Tenthorey, J. Med. Chem., 1979, 22, 1171 CrossRef CAS;
(b) B. Paul, G. L. Butterfoss, M. G. Boswell, P. D. Renfrew, F. G. Yeung, N. H. Shah, C. Wolf, R. Bonneau and K. Kirshenbaum, J. Am. Chem. Soc., 2011, 133, 10910 CrossRef CAS PubMed;
(c) H.-U. Blaser, Adv. Synth. Catal., 2002, 344, 17 CrossRef CAS;
(d) H.-U. Blaser, B. Pugin, F. Spindler and M. Thommen, Acc. Chem. Res., 2007, 40, 1240 CrossRef CAS PubMed;
(e) A. Mannschreck, H. Koller, G. Stuhler, M. A. Davies and J. Traber, Eur. J. Med. Chem., 1984, 19, 381 CAS.
- For leading reviews on chiral olefin ligands, see:
(a) F. Glorius, Angew. Chem., Int. Ed., 2004, 43, 3364 CrossRef CAS PubMed;
(b) J. B. Johnson and T. Rovis, Angew. Chem., Int. Ed., 2008, 47, 840 CrossRef CAS PubMed;
(c) C. Defieber, H. Grützmacher and E. M. Carreira, Angew. Chem., Int. Ed., 2008, 47, 4482 CrossRef CAS PubMed;
(d) R. Shintani and T. Hayashi, Aldrichimica Acta, 2009, 42, 31 CAS;
(e) C.-G. Feng, M.-H. Xu and G.-Q. Lin, Synlett, 2011, 1345 CAS;
(f) P. Tian, H.-Q. Dong and G.-Q. Lin, ACS Catal., 2012, 2, 95 CrossRef CAS;
(g) X. Feng and H. Du, Asian J. Org. Chem., 2012, 1, 204 CrossRef CAS;
(h) Y. Li and M.-H. Xu, Chem. Commun., 2014, 50, 3771 RSC.
- Selected examples on chiral P/olefin ligands, see:
(a) P. Maire, S. Deblon, F. Breher, J. Geier, C. Böhler, H. Rüegger, H. Schönberg and H. Grützmacher, Chem. – Eur. J., 2004, 10, 4198 CrossRef CAS PubMed;
(b) R. Shintani, W.-L. Duan, T. Nagano, A. Okada and T. Hayashi, Angew. Chem., Int. Ed., 2005, 44, 4611 CrossRef CAS PubMed;
(c) P. Štěpnička and I. Císařová, Inorg. Chem., 2006, 45, 8785 CrossRef PubMed;
(d) C. Defieber, M. A. Ariger, P. Moriel and E. M. Carreira, Angew. Chem., Int. Ed., 2007, 46, 3139 CrossRef CAS PubMed;
(e) R. T. Stemmler and C. Bolm, Synlett, 2007, 1365 CAS;
(f) T. Minuth and M. M. K. Boysen, Org. Lett., 2009, 11, 4212 CrossRef CAS PubMed;
(g) T. J. Hoffman and E. M. Carreira, Angew. Chem., Int. Ed., 2011, 50, 10670 CrossRef CAS PubMed;
(h) R. Shintani, R. Narui, Y. Tsutsumi, S. Hayashi and T. Hayashi, Chem. Commun., 2011, 6123 RSC;
(i) M. A. Schafroth, D. Sarlah, S. Krautwald and E. M. Carreira, J. Am. Chem. Soc., 2012, 134, 20276 CrossRef CAS PubMed;
(j) J. Y. Hamilton, D. Sarlah and E. M. Carreira, J. Am. Chem. Soc., 2013, 135, 994 CrossRef CAS PubMed;
(k) J. Y. Hamilton, D. Sarlah and E. M. Carreira, Angew. Chem., Int. Ed., 2013, 52, 7532 CrossRef CAS PubMed;
(l) O. F. Jeker, A. G. Kravina and E. M. Carreira, Angew. Chem., Int. Ed., 2013, 52, 12166 CrossRef CAS PubMed;
(m) S. Krautwald, D. Sarlah, M. A. Schafroth and E. M. Carreira, Science, 2013, 340, 1065 CrossRef CAS PubMed;
(n) S. Krautwald, M. A. Schafroth, D. Sarlah and E. M. Carreira, J. Am. Chem. Soc., 2014, 136, 3020 CrossRef CAS PubMed.
-
(a) Z. Liu and H. Du, Org. Lett., 2010, 12, 3054 CrossRef CAS PubMed;
(b) Z. Cao, Y. Liu, Z. Liu, X. Feng, M. Zhuang and H. Du, Org. Lett., 2011, 13, 2164 CrossRef CAS PubMed;
(c) Z. Cao, Z. Liu, Y. Liu and H. Du, J. Org. Chem., 2011, 76, 6401 CrossRef CAS PubMed;
(d) Z. Liu, Z. Cao and H. Du, Org. Biomol. Chem., 2011, 9, 5369 RSC;
(e) Y. Liu, Z. Cao and H. Du, J. Org. Chem., 2012, 77, 4479 CrossRef CAS PubMed;
(f) Y. Liu and H. Du, Org. Lett., 2013, 15, 740 CrossRef CAS PubMed.
- For using chiral diene ligands, see: S.-S. Zhang, Z.-Q. Wang, M.-H. Xu and G.-Q. Lin, Org. Lett., 2010, 12, 5546 CrossRef CAS PubMed.
-
(a) D. P. Curran, C. H.-T. Chen, S. J. Geib and A. J. B. Lapierre, Tetrahedron, 2004, 60, 4413 CrossRef CAS PubMed;
(b) M. Petit, A. J. B. Lapierre and D. P. Curran, J. Am. Chem. Soc., 2005, 127, 14994 CrossRef CAS PubMed;
(c) A. J. B. Lapierre, S. J. Geib and D. P. Curran, J. Am. Chem. Soc., 2007, 129, 494 CrossRef CAS PubMed;
(d) A. Bruch, A. Ambrosius, R. Fröhlich, A. Studer, D. B. Guthrie, H. Zhang and D. P. Curran, J. Am. Chem. Soc., 2010, 132, 11452 CrossRef CAS PubMed;
(e) D. B. Guthrie, S. J. Geib and D. P. Curran, J. Am. Chem. Soc., 2011, 133, 115 CrossRef CAS PubMed.
- The ee of compound 4ge was improved from 74% to 90% after a recrystallization in the solvents of dichloromethane and hexane. Then a single crystal of 4ge was obtained by further recrystallization.
Footnote |
| † Electronic supplementary information (ESI) available: The procedure for palladium-catalyzed asymmetric alkylation of N-nonsubstituted anilides, characterization of ligands and products, X-ray structural data, and data for the determination of enantiomeric excesses. CCDC 1005254 and 1025346. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ob01087f |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 

![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH
CH





