Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A multifunctional role of trialkylbenzenes for the preparation of aqueous colloidal mesostructured/mesoporous silica nanoparticles with controlled pore size, particle diameter, and morphology†
Hironori
Yamada‡
a,
Hiroto
Ujiie‡
a,
Chihiro
Urata
a,
Eisuke
Yamamoto
a,
Yusuke
Yamauchi
b and
Kazuyuki
Kuroda
*ac
aDepartment of Applied Chemistry, Faculty of Science and Engineering, Waseda University, Ohkubo 3-4-1, Shinjuku-ku, Tokyo, 169-8555, Japan. E-mail: kuroda@waseda.jp; Fax: +81-3-5286-3199; Tel: +81-3-5286-3199
bWorld Premier International (WPI) Research Center, International Center for Materials Nanoarchitectonics (MANA), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, 305-0044, Japan
cKagami Memorial Research Institute for Material Science and Technology, Waseda University, Nishiwaseda 2-8-26, Shinjuku-ku, Tokyo, 169-0051, Japan
First published on 6th October 2015
Abstract
Both the pore size and particle diameter of aqueous colloidal mesostructured/mesoporous silica nanoparticles (CMSS/CMPS) derived from tetrapropoxysilane were effectively and easily controlled by the addition of trialkylbenzenes (TAB). Aqueous highly dispersed CMPS with large pores were successfully obtained through removal of surfactants and TAB by a dialysis process. The pore size (from 4 nm to 8 nm) and particle diameter (from 50 nm to 380 nm) were more effectively enlarged by the addition of 1,3,5-triisopropylbenzene (TIPB) than 1,3,5-trimethylbenzene (TMB), and the enlargement did not cause the variation of the mesostructure and particle morphology. The larger molecular size and higher hydrophobicity of TIPB than TMB induce the incorporation of TIPB into micelles without the structural change. When TMB was used as TAB, the pore size of CMSS was also enlarged while the mesostructure and particle morphology were varied. Interestingly, when tetramethoxysilane and TIPB were used, CMSS with a very small particle diameter (20 nm) with concave surfaces and large mesopores were obtained, which may strongly be related to the initial nucleation of CMSS. A judicious choice of TAB and Si sources is quite important to control the mesostructure, size of mesopores, particle diameter, and morphology.
1. Introduction
Mesoporous silica nanoparticles (MSN) are promising for various potential applications through effective use of designed mesopores by nanoscale downsizing of mesoporous silica having various characteristics, such as high surface area, high pore volume, tunable mesopores, easiness of surface modification, and mechanical and chemical stabilities.1–18 MSN are expected to be applied in a wide variety of fields (adsorption, separation, drug delivery, and catalysis) because MSN also have colloidal characteristics, such as dispersity, transparency, and fluidity.19–37 In addition, their properties and potentialities geared for applications have stimulated the development of the control in the composition, structure, and morphology of MSN.38–46In particular, the control of pore size of MSN is quite important because it affects both the accessibility of in-coming guest molecules and the confinement effect of pores.47–54 The control of particle diameter of MSN also has a great significance in terms of the uptake, cytotoxicity, and dispersity of MSN.55–64 Thus, both the control of pore size and particle diameter of MSN is largely meaningful for the precise preparation of different types of MSN appropriate to various applications.
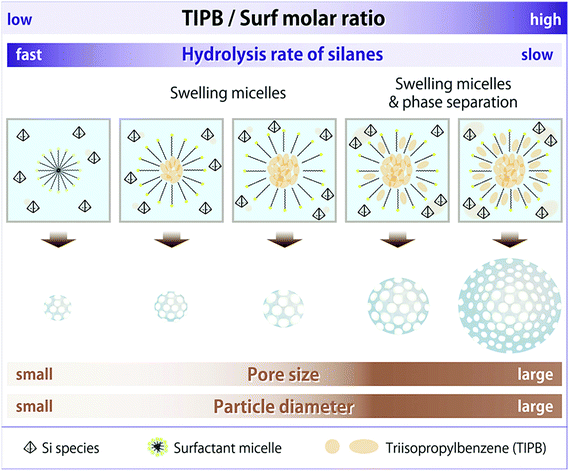
It has already been known that the pore size of mesoporous silica (including MSN) can be enlarged by adding substances, like alkanes, alcohols, and trialkylbenzenes (TAB), into amphiphilic molecules or varying experimental conditions including the concentration of surfactants and the kinds of catalysts.46–51,63,65–75 One of the most frequently used expanders is TAB such as 1,3,5-triisopropylbenzene (TIPB) or 1,3,5-trimethylbenzene (TMB).76–78 TAB can enlarge the pore size of MSN easily under basic conditions where it is easy to form MSN.47,50,75,79 Also, other particulate characteristics such as pore arrangement and particle diameter are varied by using TAB.47,50,75 In particular, it is quite reasonable to expect that the particle diameter of MSN can be enlarged by using the hydrophobicity of TAB. This is because the appropriate introduction of hydrophobicity into the reaction media can affect the hydrolysis rates of alkoxysilanes, followed by the variation of dominance between nucleation and particle growth.80 However, how the differences in the amount and kind of TAB affect the pore size, pore arrangement, and particle diameter has not yet been clarified.
In this study, it has been clearly confirmed how trialkylbenzenes play a multifunctional role in the pore size, particle diameter, and morphology of colloidal mesostructured and mesoporous silica nanoparticles (CMSS and CMPS, respectively) under basic conditions (please see Scheme 1). As TAB, 1,3,5-triisopropylbenzene and 1,3,5-trimethylbenzene were used, and as Si sources, tetrapropoxysilane (TPOS) and tetramethoxysilane (TMOS) were used. When TIPB was used as TAB and TPOS as a Si source, both the pore size (from 4 nm to 8 nm) and particle diameter (from 50 nm to 380 nm) were enlarged, depending on the amount of TIPB. When an excess amount of TMB was used as TAB, the pore size of CMPS was enlarged above 10 nm, but the mesostructure and particle morphology were varied. When TMOS and TIPB were used, CMPS with a small particle diameter (20 nm), concave surfaces, and a large pore size (5–8 nm) were obtained, and they should be equivalent to particles at the initial nucleation. Thus, TAB played an important role in the enlargement of the pore size and diameter of particles as well as in the variation of the morphology. The differences in the mesostructure and particle diameter of CMSS by TAB should be ascribed to the larger molecular size and higher hydrophobicity of TIPB than those of TMB, which indicates the different interactions between surfactant micelles and TAB. Moreover, removal of surfactants and TAB by a dialysis process was successfully achieved by modifying the dialysis process reported previously by us. These findings can provide a method to control both the pore size and particle diameter of aqueous porous particles. This leads to the development of siliceous materials which can contain more and larger guest molecules and can reduce the nanorisks of toxicity toward applications for drug delivery, bioimaging, and catalysis.
2. Experimental section
2.1 Materials
Cetyltrimethylammonium bromide (CTAB) and triethanolamine (TEA) were purchased from Wako Pure Chem. Ind., Ltd. Tetramethoxysilane (TMOS: Si(OCH3)4), tetrapropoxysilane (TPOS: Si(OC3H7)4), 1,3,5-trimethylbenzene (TMB) and 1,3,5-triisopropylbenzene (TIPB) were purchased from Tokyo Kasei Co., Ltd. Acetic acid (AcOH), ethanol (EtOH), and 2-propanol (2-PrOH) were purchased from Kanto Chem. Co., Inc. A sheet of high density polyethylene (HDPE) was purchased from PlaPort Co. Ltd. All substances were used without any further purification.2.2 Characterization
TEM images were recorded on a Jeol JEM-2010 microscope operating at 200 kV. SEM images were obtained on a Hitachi S-5500 electron microscope operated at an acceleration voltage of 5–30 kV. Samples for TEM and SEM measurements were dropped and dried on a carbon-coated micro-grid (Okenshoji Co.). X-ray diffraction (XRD) patterns of dried powder samples were obtained on a Rigaku Ultima IV with Fe Kα radiation (40 kV, 30 mA). Nitrogen gas adsorption–desorption measurements were performed with an Autosorb-2 instrument (Quantachrome Instruments) at −196 °C. Samples were preheated at 120 °C for 24 h under 1 × 10−2 Torr. Brunauer–Emmett–Teller (BET) surface areas were calculated from adsorption data in the relative pressure range from 0.05 to 0.20. Pore volumes were calculated at P/P0 = 0.95. Pore size distributions were evaluated using the adsorption branch with non-local density functional theory (NLDFT). Thermogravimetry-differential thermal analysis (TG-DTA) was carried out with a Rigaku Thermo Plus 2 instrument under a dry air flow at a heating rate of 10 °C min−1 up to 900 °C. IR spectra were obtained by using a Jasco FT/IR 6100 spectrometer by a KBr disk method. Dynamic light scattering (DLS) measurements were conducted with a Horiba Nano Partica SZ-100-S at room temperature. The ζ-potential measurements were conducted with an Otsuka Electronics ELSZ-1 at 20 °C.2.3 Preparation of colloidal mesostructured silica nanoparticles (CMSS) by adding trialkylbenzenes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.50CTAB
0.50CTAB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25TEA
0.25TEA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1200H2O. The samples are denoted as P-as and M-as, corresponding to TPOS and TMOS, respectively. The phrase “as” means “as-synthesized” or “before removal of surfactants”.
1200H2O. The samples are denoted as P-as and M-as, corresponding to TPOS and TMOS, respectively. The phrase “as” means “as-synthesized” or “before removal of surfactants”.
2.4 Removal of surfactants and TAB by a dialysis process
In order to remove surfactants and TAB, a dialysis process was conducted in the same way as shown in our previous papers.80–82,84–87 A colloidal suspension (50 mL) of one of the following ones (P-as, M-as, P_TIPBx-as, P_TMBx-as, or M_TIPB-as) was transferred into a dialysis membrane tube composed of cellulose (molecular weight cut off = 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and dialyzed for 12 h against 250 mL of a mixture containing 2 M acetic acid and 2-propanol (1
000) and dialyzed for 12 h against 250 mL of a mixture containing 2 M acetic acid and 2-propanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and this process was repeated five times. Then the tube, which contained CMPS, was immersed in water to remove AcOH/2-PrOH from the tube, and the process was repeated twice. Finally the pH value of the resulting solution was 3.4. The samples are denoted as P-dia, M-dia, P_TIPBx-dia, P_TMBx-dia, or M_TIPB-dia, respectively, where “dia” means “after dialysis” or “after removal of surfactants”. The removal of both organics has been proved by the absence of IR peaks due to those organic compounds and by no weight losses due to them, as shown in the TG curves (ESI, Fig. S1 and S2†). In addition, dried samples were obtained by heating a portion of CMPS at 120 °C for 12 h.
1, v/v) and this process was repeated five times. Then the tube, which contained CMPS, was immersed in water to remove AcOH/2-PrOH from the tube, and the process was repeated twice. Finally the pH value of the resulting solution was 3.4. The samples are denoted as P-dia, M-dia, P_TIPBx-dia, P_TMBx-dia, or M_TIPB-dia, respectively, where “dia” means “after dialysis” or “after removal of surfactants”. The removal of both organics has been proved by the absence of IR peaks due to those organic compounds and by no weight losses due to them, as shown in the TG curves (ESI, Fig. S1 and S2†). In addition, dried samples were obtained by heating a portion of CMPS at 120 °C for 12 h.
2.5 Measurement of swelling ratios of HDPE for TMB and TIPB
In order to estimate the extent of affinity of TAB to alkyl chains of surfactants, the swelling ratios of some fragments of HDPE sheets for TMB and TIPB were measured. These ratios were obtained by comparing the initial volume with the swollen volume after an immersion of HDPE fragments in TAB. Each piece was immersed in 1 mL of TAB and shaken at 100 rpm for one week. After the procedure, the pieces were dry-wiped to remove any residual liquid and weighed. Measurements were performed at least 3 times for each sample. The details on the calculation of the swelling ratios are shown in the ESI.†3. Results and discussion
3.1 Preparation of CMSS and CMPS with enlarged pore size and particle diameter by adding TIPB
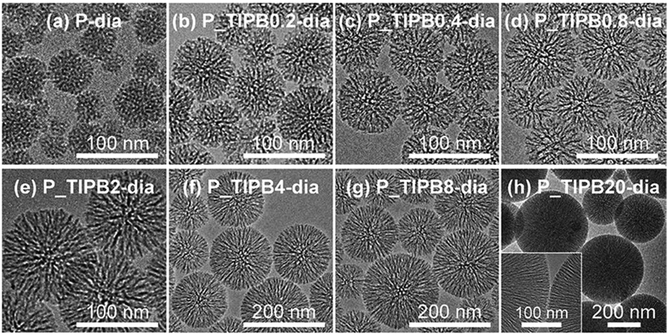 | ||
| Fig. 1 TEM images of P_TIPBx-dia: x = (a) 0, (b) 0.2, (c) 0.4, (d) 0.8, (e) 2, (f) 4, (g) 8, and (h) 20. | ||
 | ||
| Fig. 2 SEM images of P_TIPBx-dia: x = (a) 0, (b) 0.2, (c) 0.4, (d) 0.8, (e) 2, (f) 4, (g) 8, and (h) 20. | ||
On the basis of the TEM images (Fig. 1), it was also confirmed that the mesostructure was not varied after dialysis. The mean pore size in each particle, roughly estimated by using the SEM images (Fig. 2), varied from ca. 4 nm to ca. 10 nm with the amount of TIPB, in the range of x ≤ 0.8. The N2 adsorption–desorption isotherms (Fig. S8†) show type IV isotherms for all types of dried samples of CMPS, meaning that they have mesopores. The relative pressure of capillary condensation for P-dia is P/P0 = 0.4, while in the cases of x = 0.2, 0.4, 0.8, and 2, the values are P/P0 = 0.45, 0.5, 0.6, and 0.65, respectively. The mean pore size of P-dia is 4.3 nm, while in the cases of x = 0.2, 0.4, 0.8, and 2, the pore sizes are enlarged to 4.9 nm, 6.6 nm, 7.0 nm, and 8.1 nm, respectively (Fig. S8†). In this range (x ≤ 2), TIPB should be incorporated into micelles of surfactants and the micelles are sufficiently swollen. On the other hand, in the range of 2 ≤ x < 8, the relative pressure is P/P0 = 0.65 and the mean pore size is 8.1 nm regardless of the amount of TIPB. This means that the extent of swelling of micelles is limited. Moreover, in the case of x = 20, the mean pore size is 6.8 nm, smaller than those in the cases of 2 ≤ x < 8. The isotherm in this case (x = 20) shows that the adsorbed amount of nitrogen is less than those of the cases of other x values and that the shape of isotherm is different from others. An excessive amount of TIPB should lead to the variation of the curvature and the shape of micelles,88 resulting in the transformation of the structure.
The XRD patterns (Fig. S9†) show that the mesostructure is enlarged to some extent with the amount of TIPB. In the region of x ≤ 2, the periodicity of structure is varied (d = from 5.6 nm to 9.0 nm). In the region of 2 ≤ x < 8, the variation of the periodicity of structure was not observed. However, in the case of x = 20, the periodicity slightly decreased to 7.9 nm. The tendency will be discussed in the next section.
 | ||
| Fig. 3 Variation of particle diameter and pore size of P_TIPBx-dia (x = 0, 0.2, 0.4, 0.8, 2, 4, 8, and 20); the particle diameter was obtained from Fig. S4 and S7,† and the pore size was obtained from Fig. S8. | ||
The general view about the enlargement of both the pore size and particle diameter is explained as follows. In terms of pore size, TIPB is incorporated into micelles of surfactants, as reported previously.76–78,89–91 In terms of particle diameter, the decrease in the hydrolysis rate of alkoxysilanes leads to particle growth more dominant than nucleation.80,82 In the present case, the interaction of TPOS with TIPB due to hydrophobic interactions should inhibit the contact of TPOS with water. It results in the decrease in the hydrolysis rate of TPOS and then particle growth. After separate descriptions on the following two ranges (sections (3.1.3.i) and (3.1.3.ii)), the present case is compared with the previously reported particle growth by the addition of alcohols (section (3.1.3.iii)).80
3.1.3.i 0 ≤ x ≤ 8. Firstly, the enlargement of the pore size with the increase in the molar ratio of TIPB to CTAB (that is, the value ‘x’) is explained. As shown in Scheme 1, when x is low (x ≤ 2), TIPB molecules are probably located at the center of micelles of surfactants. This means that the swelling of micelles is due to the incorporation of TIPB. On the other hand, when x is larger (2 < x ≤ 8), the pore size is not enlarged. In this region, the oil phase of TIPB in the resulting solution was obviously separated from water. This means that, in the case of an excess amount of TIPB, TIPB should be more stable as a separated phase due to the aggregation of TIPB itself than as a solubilized phase in micelles.
Next, the gradual enlargement of the particle diameter is described as follows. In particular, when x is low (x ≤ 2), the enlargement was not clear, but when x is larger (2 < x ≤ 8), the enlargement is clearly shown. In the case of low x (x ≤ 2), the additional TIPB was mainly absorbed into micelles and it was unlikely for TIPB to be separated from water and to come into contact with TPOS. In the case of higher x (2 < x ≤ 8), the phase separation of TIPB as an oil leads to the interaction with TPOS and the lack of contact of TPOS with water. It causes the decrease in the hydrolysis rate of TPOS as shown in Scheme 1, followed by particle growth more dominant than nucleation. This phenomenon is similar to the previous study80 in which the addition of alcohols (typically butanol) leads to the decrease in the hydrolysis rates of alkoxysilanes and the increase in the particle growth. Moreover, the enlargement of the particle diameter (0 < x ≤ 2) compared to the particle diameter at x = 0 is due to a slight interaction of TIPB with TPOS, although most of TIPB is absorbed into micelles. This enlargement is also explained by the decrease in the hydrolysis rate of TPOS.
Consequently, when x is low (x ≤ 2), the pore size is evidently enlarged due to the incorporation of TIPB into micelles of surfactants, and the partial interaction of TIPB with TPOS causes the decrease in the hydrolysis rate of TPOS, leading to a slight increase in the particle diameter. When x becomes higher (2 < x ≤ 8), the pore size is not enlarged because there is little space to incorporate TIPB into micelles. The phase separation of TIPB from water and the increase in the interaction of TIPB with TPOS lead to an obvious increase in the particle diameter.
3.1.3.ii 8 < x ≤ 20. In the cases of 8 ≤ x ≤ 20, the pore size of P_TIPBx-as decreased and the particle diameter clearly increased. Nitrogen adsorption–desorption isotherms (Fig. S8†) show that the mesostructure changed. This variation should be due to the relatively large amount of oil (including TIPB) and also due to the formation of complicated emulsion consisting of water, oil, and surfactants.92 In the present case, the presence of an excess amount of TIPB varied the structures of micelles, siliceous species, and their composites, probably leading to the variation of the mesostructure. Thus, the pore size was not enlarged and decreased in the range of 8 < x ≤ 20.
In addition, as x increased, the volume of oil increased and the amount of TPOS incorporated into the oil phase also increased. As shown in Scheme 1, the particle was grown successively to 380 nm.
3.1.3.iii Comparison of the present case with the case of the addition of propanol. As reported previously,80,82 the decrease in the hydrolysis rates of alkoxysilanes leads to the enlargement of the particle diameter. In the case of alcohols, it is necessary to add 88 mmol of propanol (using TPOS) in order to enlarge the particle diameter from 40 nm to 80 nm and to add 88 mmol of butanol (using TBOS) to enlarge from 80 nm to 330 nm. On the other hand, in the case of TAB (in particular TIPB), 11 mmol of TIPB can enlarge the particle diameter from 40 nm to 380 nm. That is, the effect of TAB on the enlargement of the particle diameter is much stronger than that of alcohols. It should be ascribed to the lower relative electric permittivity (and thus higher hydrophobicity) of TAB than that of butanol (water: 80, TAB: 2–2.3, and butanol: 18). This means that TAB delays the hydrolysis of alkoxysilanes more effectively than alcohols, followed by particle growth more dominant than nucleation.
3.2 Preparation of CMSS and CMPS by adding TMB
The SEM image of P_TMB0.8-dia also shows that the pore size is ca. 4 nm and that this value was similar to that of P-dia which were made without the addition of trialkylbenzenes (Fig. S10a and S10d†). The N2 adsorption–desorption isotherms of the cases using TMB show that the values of P/P0 corresponding to capillary condensation are around 0.4 and that these are similar to that of P-dia (Fig. S12†). Besides, in the cases of P_TMBx-dia (0 ≤ x ≤ 0.8), the pore sizes were almost constant and independent of the values of x and the distinct enlargement of the pore size according to the increase in the value of x was not observed (Fig. S13†). On the other hand, in the cases of P_TIPBx-dia (0 ≤ x ≤ 0.8), the relative pressures (P/P0) corresponding to capillary condensation were high (around 0.7), showing the enlargement of the pore size with the increase in the value of x.
The reason for the difference in the enlargement of the pore size among various kinds of trialkylbenzenes was discussed in the previous report by Fukuoka et al.91 It is described that in the case of mesoporous silica made from CTAB micelles and trialkylbenzenes, the position of trialkylbenzenes in micelles is a key factor for pore size enlargement. On the basis of this report, the behavior of the formation of micelles is described as follows. Because the structure of TMB is planar, a number of TMB molecules can get into the space between surfactant molecules in micelles (Scheme 2b), thus micelles should not be enlarged clearly when the additional amount of TMB increases. On the other hand, because the bulkiness of TIPB is larger than that of TMB, lesser amounts of TIPB should exist in the spaces between surfactants in micelles. Thus, it should be easy for TIPB to get into the hydrophobic central space of micelles (Scheme 2c). For this reason, the additional amount of TIPB led to the increased accumulation in the hydrophobic center of micelles and the distinct enlargement of micelles.
 | ||
| Scheme 2 Proposed structures of micelles and formation of CMPS; (a) without TAB, (b) with TMB, and (c) with TIPB. | ||
When the amount of TMB was increased (x = 20), radial mesopores were observed from the TEM image and the size of mesopores was dozens of nanometers, as shown in the SEM image (Fig. 4). The XRD pattern of the dried sample (P_TMB20-dia) showed a very broad peak and a peak due to mesostructures was not confirmed clearly (Fig. S14†). The reason for the formation of particles with large radial mesopores will be discussed in the next section on the basis of several previous reports about particles with similar structures.
In order to obtain MSN with radial pores, typically shown in Fig. 4, the following two points are important, that is (1) suppression of the adsorption of soluble silica species on the micelles of surfactants and (2) the release of TAB encapsulated in the micelles to vary the micelle-directed mesostructure. On the basis of this consideration, it has been reported that TMB can have cation–π interactions with the head groups of surfactants as well as hydrophobic interactions with the alkyl chains of surfactants.89,93 It was also reported that the cation–π interaction becomes stronger in an aqueous system.94 Thus, in the present system, the phenomenon (1) must have occurred because the adsorption of soluble silica species on the head groups of surfactants is hindered due to the cation–π interaction between TMB and surfactants. In the case of x = 0.8, particles with a disordered morphology were obtained (Fig. S10†). This should be ascribed to the adsorption of TMB on micelles in competition with soluble silica species, therefore, the contact points of silica species with surfactants are heterogeneously limited. Moreover, in terms of case (2), TMB should exist in the state as shown in Scheme 2b and TMB near the outer surface of micelles can come into contact with solvents. In addition, TMB should be more soluble gradually in the aqueous phase because the hydrophobicity of solvents increases due to the presence of propanol generated from the hydrolysis of TPOS. For these reasons, TMB can leak and diffuse from the inner space of micelles to the outer (but still inside) space to make the shape of micelles larger along the axis of rod-like micelles.
On the other hand, as shown in Scheme 2c, TIPB should exist in the inner space of micelles and it is less likely for TIPB to make contact with solvents in the outer space of micelles. Thus, compared to the case of TMB, TIPB should be less affected by the hydrophobization of solvents due to the presence of propanol (derived from TPOS) and be less likely to leak and diffuse to the outside of micelles. It should lead to the suppression of the formation of large radial pores as found for the case of TMB. In addition, the steric hindrance of TIPB is larger than that of TMB, and thus the cation–π interaction between the head groups of surfactants and TIPB should be less likely. On the basis of this consideration, it can be explained that spherical particles were obtained in the case of x = 0.8 because the adsorption of soluble silica species on micelles is thought to occur in a relatively homogeneous way (Scheme 2b).
Moreover, it should be noted that the volume of TIPB is about twice larger than that of TMB at the same molar concentration. This means TIPB can have a stronger effect on expanding the size of micelles than TMB, regardless of how TAB interacts with surfactants. However, in the present case, the expansion by TIPB (ca. 4 nm to 8 nm, in the range of 0 ≤ x ≤ 0.8) occurred clearly, while the expansion by TMB was not observed (almost the same ca. 4 nm, in the range of 0 ≤ x ≤ 0.8). Thus, it is shown that relative locations of TAB and micelles have a greater influence on the expansion of pore size than the difference in volumes of TAB.
Consequently, the effects of the kind of TAB on the preparation of CMSS and CMPS can be summarized in the following three points: (i) regardless of the kind of TAB, colloidal mesoporous silica nanoparticles dispersed in aqueous solution can be prepared, (ii) in the case of TIPB, the pore size of CMPS can be controlled easily with the amount of TIPB, and (iii) by varying the kind of TAB, the mesostructures of CMSS and CMPS can be changed. These phenomena are explained by the steric hindrance and hydrophobicity of TAB, that is, relative locations of TAB and micelles. Such relationship should affect the mesostructure as well as the particle morphology of CMSS.
3.3 Pore size enlargement of colloidal mesoporous silica nanoparticles with small particle diameters
By using TIPB, the enlargement of the pore size of small CMPS (20 nm) was investigated. In terms of particle diameter, it was observed by using SEM image (Fig. 5) that the particle diameters of both M-dia and M_TIPB-dia were about 20 nm regardless of the presence of TIPB. Aggregates were not observed apparently. However, as shown in particle size distributions measured by DLS (Fig. 5), the hydrodynamic diameter was about 40 nm and this means that the secondary particles were partly formed by the aggregation of the primary particles. | ||
| Fig. 5 Schematic images, SEM images, appearances, and the particle size distributions (DLS, hydrodynamic diameter) of (a) M-dia and (b) M_TIPB-dia. | ||
It was confirmed by the N2 adsorption–desorption isotherms (Fig. S15†) that the pore size of M-dia, which was prepared without the addition of TIPB, was about 4 nm. On the other hand, in the case of M_TIPB-dia, the pore size was about 5 nm. The degree of the enlargement for the cases of M-dia and M_TIPB-dia (from ca. 4 nm (M-dia) to ca. 5–8 nm (M_TIPB-dia)) is less than that of P-dia and P_TIPB0.8-dia (from ca. 4 nm to ca. 7 nm). The state and composition of the solution before the addition of Si sources are similar to those of P_TIPB0.8-as, and the micelle size of M_TIPB-as should be about 7 nm. In view of the fact that the particle diameter was about 20 nm, the size of micelles and pore size (ca. 5–8 nm) are too large to form particles. Thus, all surfaces of micelles did not contribute to the formation of the framework of particles but a part of surfaces contributed to form the framework, leading to the formation of a silica nanostructure with concave surfaces.
The characteristics of silica nanostructures with concave surfaces are explained as follows. In the step of the preparation of mesostructured silica nanoparticles, the structure which consists of several micelles of surfactants, corresponds to the structure in the initial step of nucleation. Taking into account the mechanism proposed by Hollamby,95 several species of silicate–surfactant composite micelles are formed in the initial step of nucleation of mesostructured silica nanoparticles. As shown in Scheme 3, the proposed model is applied to the present case judging from the size of micelles and the particle diameter of CMSS. This means that the silica nanostructure with concave surfaces in this case is comparable to particles in the initial step of nucleation. The present mesostructured and mesoporous nanoparticles with concave surfaces had much smaller particle diameter and the curvature of concaves was smaller than those reported previously on MSN.81,95 These particles should contribute to the development of siliceous materials which have both convex and concave surfaces for various applications of catalysis and drug delivery.
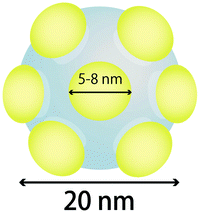 | ||
| Scheme 3 Proposed model of M_TIPB-as, which was prepared by using TIPB as TAB and using TMOS as a Si source. Gray: siloxane frameworks, yellow: micelles embedded in the siloxane frameworks. | ||
4. Conclusion
Aqueous colloidal mesostructured silica nanoparticles (CMSS) were prepared by varying the kind and amount of trialkylbenzenes (TAB) and by varying Si sources. When 1,3,5-triisopropylbenzene (TIPB) was used as TAB and tetrapropoxysilane (TPOS) was used as a Si source, both the pore size (from 4 nm to 8 nm) and particle diameter (from 50 nm to 380 nm) were enlarged with the amount of TIPB. In the case of TPOS and an excess amount of 1,3,5-trimethylbenzene (TMB), the pore size of CMSS was also enlarged above 10 nm, accompanied by the variation of the mesostructure and deformation of the spherical morphology. When tetramethoxysilane and TIPB were used, CMSS with a small particle diameter (20 nm), concave surfaces, and a large pore size (5–8 nm) were obtained, which should be equivalent to particles formed at the initial nucleation. TIPB can enlarge the pore size and particle diameter than TMB, and the enlargement by TIPB was accomplished without the variation of the mesostructure and particle morphology. This should be ascribed to the larger size and higher hydrophobicity of TIPB than those of TMB. Besides, removal of surfactants and TAB by a dialysis process was successful for the preparation of aqueous highly dispersed colloidal mesoporous silica nanoparticles with enlarged pores. The present findings will lead to the development of siliceous materials which can accommodate more guest molecules with a larger size, and they can expand possible applications for drug delivery and bioimaging as well as catalysis and concomitantly can reduce their nanorisks.Acknowledgements
The authors thank Mr M. Fuziwara (Waseda University) for his kind assistance in TEM measurement. This work was supported in part by Grant-in-Aid for Scientific Research, MEXT (15K13809).References
- V. Valtchev and L. Tosheva, Chem. Rev., 2013, 113, 6734–6760 CrossRef CAS PubMed.
- S.-H. Wu, C.-Y. Mou and H.-P. Lin, Chem. Soc. Rev., 2013, 42, 3862–3875 RSC.
- C. E. Fowler, D. Khushalani, B. Lebeau and S. Mann, Adv. Mater., 2001, 13, 649–652 CrossRef CAS.
- S. Sadasivan, C. E. Fowler, D. Khushalani and S. Mann, Angew. Chem., Int. Ed., 2002, 41, 2151–2153 CrossRef CAS.
- H.-P. Lin and C.-P. Tsai, Chem. Lett., 2003, 1092–1093 CrossRef CAS.
- J. Fan, J. Lei, L. Wang, C. Yu, B. Tu and D. Zhao, Chem. Commun., 2003, 2140–2141 RSC.
- K. Suzuki, K. Ikari and H. Imai, J. Am. Chem. Soc., 2004, 126, 462–463 CrossRef CAS PubMed.
- K. Möller, J. Kobler and T. Bein, Adv. Funct. Mater., 2007, 17, 605–612 CrossRef.
- J. Kobler and T. Bein, ACS Nano, 2008, 2, 2324–2330 CrossRef CAS PubMed.
- A. Berggren and A. E. C. Palmqvist, J. Phys. Chem. C, 2008, 112, 732–737 CAS.
- F. Lu, S.-H. Wu, Y. Hung and C.-Y. Mou, Small, 2009, 5, 1408–1413 CrossRef CAS PubMed.
- Y.-S. Lin, N. Abadeer and C. L. Haynes, Chem. Commun., 2011, 47, 532–534 RSC.
- Y.-S. Lin, N. Abadeer, K. R. Hurley and C. L. Haynes, J. Am. Chem. Soc., 2011, 133, 20444–20457 CrossRef CAS PubMed.
- K. Ma, H. Sai and U. Wiesner, J. Am. Chem. Soc., 2012, 134, 13180–13183 CrossRef CAS PubMed.
- K. Ma, U.-W. Zwanziger, J. Zwanziger and U. Wiesner, Chem. Mater., 2013, 25, 677–691 CrossRef CAS.
- D. Douroumis, I. Onyesom, M. Maniruzzaman and J. Mitchell, Crit. Rev. Biotechnol., 2013, 33, 229–245 CrossRef CAS PubMed.
- M. Bouchoucha, R. C. Gaudreault, M.-A. Fortin and F. Kleitz, Adv. Funct. Mater., 2014, 24, 5911–5923 CrossRef CAS.
- S. M. Egger, K. R. Hurley, A. Datt, G. Swindlehurst and C. L. Haynes, Chem. Mater., 2015, 27, 3193–3196 CrossRef CAS.
- K. K. Unger, D. Kumar, M. Grün, G. Büchel, S. Lüdtke, T. Adam, K. Schumacher and S. J. Renker, J. Chromatogr., A, 2000, 892, 47–55 CrossRef CAS PubMed.
- S. H. Joo, J. Y. Park, C. K. Tsung, Y. Yamada, P. D. Yang and G. A. Somorjai, Nat. Mater., 2009, 8, 126–131 CrossRef CAS PubMed.
- Y.-S. Lin, K. R. Hurley and C. L. Haynes, J. Phys. Chem. Lett., 2012, 3, 364–374 CrossRef CAS PubMed.
- K. C.-W. Wu and Y. Yamauchi, J. Mater. Chem., 2012, 22, 1251–1256 RSC.
- X. Li, J. C. Barnes, A. Bosoy, J. F. Stoddart and J. I. Zink, Chem. Soc. Rev., 2012, 41, 2590–2605 RSC.
- J. L. Vivero-Escoto, R. C. H. Phillips and W. Lin, Chem. Soc. Rev., 2012, 41, 2673–2685 RSC.
- P. Yang, S. Gai and J. Lin, Chem. Soc. Rev., 2012, 41, 3679–3698 RSC.
- P. Nadrah, O. Planinšek and M. Gaberšček, J. Mater. Sci., 2014, 49, 481–495 CrossRef CAS.
- X. Huang, N. P. Young and H. E. Townley, Nanomater. Nanotechnol., 2014, 4, 1–15 Search PubMed.
- L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed.
- Y. Chen, H. Chen and J. Shi, Expert Opin. Drug Delivery, 2014, 11, 917–930 CrossRef CAS PubMed.
- P. Xu, S. Guo, H. Yu and X. Li, Small, 2014, 10, 2404–2412 CrossRef CAS PubMed.
- X. Wang, H. Chen, K. Zhang, M. Ma, F. Li, D. Zeng, S. Zheng, Y. Chen, L. Jiang, H. Xu and J. Shi, Small, 2014, 10, 1403–1411 CrossRef CAS PubMed.
- R. Roggers, S. Kanvinde, S. Boonsith and D. Oupický, AAPS PharmSciTech., 2014, 15, 1163–1171 CrossRef CAS PubMed.
- I. Sierra, D. P. Quintanilla, S. Morante and J. Gañán, J. Chromatogr., A, 2014, 1363, 27–40 CrossRef CAS PubMed.
- S. A. Jadhav, Inorg. Chem. Front., 2014, 1, 735–739 RSC.
- C. Argyo, V. Weiss, C. Brauchle and T. Bein, Chem. Mater., 2014, 26, 435–451 CrossRef CAS.
- Y. Zhang, B. Y. W. Hsu, C. Ren, X. Li and J. Wang, Chem. Soc. Rev., 2015, 44, 315–335 RSC.
- N. Song and Y.-W. Yang, Chem. Soc. Rev., 2015, 44, 3474–3504 RSC.
- M. Wang, Z. Sun, Q. Yue, J. Yang, X. Wang, Y. Deng, C. Yu and D. Zhao, J. Am. Chem. Soc., 2014, 136, 1884–1892 CrossRef CAS PubMed.
- Q. Qu, G. Zhou, Y. Ding, S. Feng and Z. Gu, J. Non-Cryst. Solids, 2014, 405, 104–115 CrossRef CAS.
- X. Du and S. Z. Qiao, Small, 2015, 11, 392–413 CrossRef CAS PubMed.
- X. Ma, K. Hahn and S. Sanchez, J. Am. Chem. Soc., 2015, 137, 4976–4979 CrossRef CAS PubMed.
- H. Ishii, T. Ikuno, A. Shimojima and T. Okubo, J. Colloid Interface Sci., 2015, 448, 57–64 CrossRef CAS PubMed.
- S. Rashi, S. K. Prajapati and D. Singh, World J. Pharm. Pharm. Sci., 2015, 4, 332–347 CAS.
- G. Chen, Z. Teng, X. Su, Y. Liu and G. Lu, J. Biomed. Nanotechnol., 2015, 11, 1–8 CrossRef.
- Y. Chen, H. Chen and J. Shi, Adv. Healthcare Mater., 2015, 4, 158–165 CrossRef CAS PubMed.
- Z. Ma, J. Bai, Y. Wang and X. Jiang, ACS Appl. Mater. Interfaces, 2014, 6, 2431–2438 CAS.
- S. B. Hartono, M. Yu, W. Gu, J. Yang, E. Strounina, X. Wang, S. Qiao and C. Yu, Nanotoxicology, 2014, 25, 1–12 Search PubMed.
- D. Niu, Z. Liu, Y. Li, X. Luo, J. Zhang, J. Gong and J. Shi, Adv. Mater., 2014, 26, 4947–4953 CrossRef CAS PubMed.
- Z. Gao and I. Zharov, Chem. Mater., 2014, 26, 2030–2037 CrossRef CAS.
- J. Peng, J. Liu, J. Liu, Y. Yang, C. Li and Q. Yang, J. Mater. Chem. A, 2014, 2, 8118–8125 CAS.
- L. Miller, G. Winter, B. Baur, B. Witulla, C. Solbach, S. Reske and M. Lindén, Nanoscale, 2014, 6, 4928–4935 RSC.
- X. Ye, J. Wang, Y. Xu, L. Niu, Z. Fan, P. Gong, L. Ma, H. Wang, Z. Yang and S. Yang, J. Appl. Polym. Sci., 2014, 131, 41173 Search PubMed.
- M. Wu, Q. Meng, Y. Chen, Y. Du, L. Zhang, Y. Li, L. Zhang and J. Shi, Adv. Mater., 2015, 27, 215–222 CrossRef CAS PubMed.
- N. Ž. Knežević and J.-O. Durand, Nanoscale, 2015, 7, 2199–2209 RSC.
- H. Vallhov, S. Gabrielsson, M. Strømme, A. Scheynius and A. E. G. Bennett, Nano Lett., 2007, 7, 3576–3582 CrossRef CAS PubMed.
- D. Napierska, C. J. Thomassen, V. Rabolli, D. Lison, L. Gonzalez, M. K. Volders, J. A. Martens and P. H. Hoet, Small, 2009, 5, 846–853 CrossRef CAS PubMed.
- F. Lu, S.-H. Wu, Y. Hung and C.-Y. Mou, Small, 2009, 5, 1408–1413 CrossRef CAS PubMed.
- Q. He, Z. Zhang, Y. Gao, J. Shi and Y. Li, Small, 2009, 5, 2722–2729 CrossRef CAS PubMed.
- D. Napierska, L. C. J. Thomassen, D. Lison, J. Martens and P. H. Hoet, Part. Fibre Toxicol., 2010, 7, 39–70 CrossRef CAS PubMed.
- V. Rabolli, L. C. J. Thomassen, C. Princen, D. Napierska, L. Gonzalez, M. K. Volders, P. H. Hoet, F. Huaux, C. E. A. Kirschhock, J. A. Martens and D. Lison, Nanotoxicology, 2010, 4, 307–318 CrossRef CAS PubMed.
- Y.-S. Lin and C. L. Haynes, J. Am. Chem. Soc., 2010, 132, 4834–4842 CrossRef CAS PubMed.
- F. Zhao, Y. Zhao, Y. Liu, X. Chang, C. Chen and Y. Zhao, Small, 2011, 7, 1322–1337 CrossRef CAS PubMed.
- D. Shen, J. Yang, X. Li, L. Zhou, R. Zhang, W. Li, L. Chen, R. Wang, F. Zhang and D. Zhao, Nano Lett., 2014, 14, 923–932 CrossRef CAS PubMed.
- M. Varache, I. Bezverkhyy, L. Saviot, F. Bouyer, F. Baras and F. Bouyer, J. Non-Cryst. Solids, 2015, 408, 87–97 CrossRef CAS.
- A. B. D. Nandiyanto, S.-G. Kim, F. Iskandar and K. Okuyama, Microporous Mesoporous Mater., 2009, 120, 447–453 CrossRef CAS.
- Y. Hoshikawa, H. Yabe, A. Nomura, T. Yamaki, A. Shimojima and T. Okubo, Chem. Mater., 2010, 22, 12–14 CrossRef CAS.
- M.-H. Kim, H.-K. Na, Y.-K. Kim, S.-R. Ryoo, H. S. Cho, K. E. Lee, H. Jeon, R. Ryoo and D.-H. Min, ACS Nano, 2011, 5, 3568–3576 CrossRef CAS PubMed.
- V. Polshettiwar, D. Cha, X. Zhang and J. M. Basset, Angew. Chem., Int. Ed., 2010, 49, 9652–9656 CrossRef CAS PubMed.
- S.-M. Lai, H.-Y. Lai and M.-Y. Chou, Microporous Mesoporous Mater., 2014, 196, 31–40 CrossRef CAS.
- Q. Cai, Z.-S. Luo, W.-Q. Pang, Y.-W. Fan, X.-H. Chen and F.-Z. Cui, Chem. Mater., 2001, 13, 258–263 CrossRef CAS.
- T. Yokoi, T. Karouji, S. Ohta, J. N. Kondo and T. Tatsumi, Chem. Mater., 2010, 22, 3900–3908 CrossRef CAS.
- J. Wang, A. S. Narutaki, A. Shimojima and T. Okubo, J. Colloid Interface Sci., 2012, 385, 41–47 CrossRef CAS PubMed.
- K.-C. Kao and C.-Y. Mou, Microporous Mesoporous Mater., 2013, 169, 7–15 CrossRef CAS.
- G. Sponchia, R. Marin, I. Freris, M. Marchiori, E. Moretti, L. Storaro, P. Canton, A. Lausi, A. Benedetti and P. Riello, J. Nanopart. Res., 2014, 16, 1–14 CrossRef.
- A. B. Fuertes, P. V. Vigón and M. Sevilla, J. Colloid Interface Sci., 2010, 349, 173–180 CrossRef CAS PubMed.
- J. S. Beck, J. C. Vartuli, W. J. Roth, M. E. Leonowicz, C. T. Kresge, K. D. Schmitt, C. T. W. Chu, D. H. Olson, E. W. Sheppard, S. B. McCullen, J. B. Higgins and J. L. Schlenker, J. Am. Chem. Soc., 1992, 114, 10834–10843 CrossRef CAS.
- N. Ulagappan and C. N. R. Rao, Chem. Commun., 1996, 2759–2760 RSC.
- M. Luechinger, G. D. Pirngruber, B. Lindlar, P. Laggner and R. Prins, Microporous Mesoporous Mater., 2005, 79, 41–52 CrossRef CAS.
- Y. Zhang, H. Zhang, E. Che, L. Zhang, J. Han, Y. Yang, S. Wang, M. Zhang and C. Gao, Colloids Surf., B, 2015, 128, 77–85 CrossRef CAS PubMed.
- H. Yamada, C. Urata, H. Ujiie, Y. Yamauchi and K. Kuroda, Nanoscale, 2013, 5, 6145–6153 RSC.
- C. Urata, Y. Aoyama, A. Tonegawa, Y. Yamauchi and K. Kuroda, Chem. Commun., 2009, 5094–5096 RSC.
- H. Yamada, C. Urata, Y. Aoyama, S. Osada, Y. Yamauchi and K. Kuroda, Chem. Mater., 2012, 24, 1462–1471 CrossRef CAS.
- H. Yamada, C. Urata, S. Higashitamori, Y. Aoyama, Y. Yamauchi and K. Kuroda, ACS Appl. Mater. Interfaces, 2014, 6, 3491–3500 CAS.
- C. Urata, H. Yamada, R. Wakabayashi, Y. Aoyama, S. Hirosawa, S. Arai, S. Takeoka, Y. Yamauchi and K. Kuroda, J. Am. Chem. Soc., 2011, 133, 8102–8105 CrossRef CAS PubMed.
- E. Yamamoto, M. Kitahara, T. Tsumura and K. Kuroda, Chem. Mater., 2014, 26, 2927–2933 CrossRef CAS.
- H. Ujiie, A. Shimojima and K. Kuroda, Chem. Commun., 2015, 51, 3211–3214 RSC.
- H. Yamada, C. Urata, E. Yamamoto, S. Higashitamori, Y. Yamauchi and K. Kuroda, ChemNanoMat, 2015, 1, 194–202 CrossRef.
- M. Tiemann, V. Goletto, R. Blum, F. Babonneau, H. Amenitsch and M. Lindén, Langmuir, 2002, 18, 10053–10057 CrossRef CAS.
- J. L. Blin and B. L. Su, Langmuir, 2002, 18, 5303–5308 CrossRef CAS.
- H. Kunieda, K. Ozawa and K. L. Huang, J. Phys. Chem. B, 1998, 102, 831–838 CrossRef CAS.
- A. Fukuoka, I. Kikkawa, Y. Sasaki, A. Shimojima and T. Okubo, Langmuir, 2009, 25, 10992–10997 CrossRef CAS PubMed.
- D.-S. Moon and J.-K. Lee, Langmuir, 2012, 28, 12341–12347 CrossRef CAS PubMed.
- M. F. Ottaviani, A. Moscatelli, D. D. Giscard, F. D. Renzo, P. J. Kooyman, B. Alonso and A. Galarneau, J. Phys. Chem. B, 2004, 108, 12123–12129 CrossRef CAS.
- J. P. Gallivan and D. A. Dougherty, J. Am. Chem. Soc., 2000, 122, 870–874 CrossRef CAS.
- M. J. Hollamby, D. Borisova, P. Brown, J. Eastoe, I. Grillo and D. Shchukin, Langmuir, 2012, 28, 4425–4433 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr04465k |
| ‡ H. Yamada and H. Ujiie contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |