Open Access Article
Open Access ArticleCO2 controlled flocculation of microalgae using pH responsive cellulose nanocrystals†
Samuel
Eyley‡
a,
Dries
Vandamme‡
b,
Sanjaya
Lama
b,
Guy
Van den Mooter
c,
Koenraad
Muylaert
*b and
Wim
Thielemans
*a
aRenewable Materials and Nanotechnology Research Group, KU Leuven – Campus Kortrijk, Etienne Sabbelaan 53 box 7659, 8500 Kortrijk, Belgium. E-mail: wim.thielemans@kuleuven.be
bLaboratory for Aquatic Biology, KU Leuven – Campus Kortrijk, Etienne Sabbelaan 53 box 7659, 8500 Kortrijk, Belgium. E-mail: koenraad.muylaert@kuleuven.be
cDrug Delivery and Disposition, Department of Pharmaceutical and Pharmacological Sciences, KU Leuven, O & N II Herestraat 49 box 921, 3000 Leuven, Belgium
First published on 28th July 2015
Abstract
Cellulose nanocrystals were grafted with imidazole functionalities up to DS 0.06 using a one-pot functionalization strategy. The resulting nanocrystals were shown to have a pH responsive surface charge which was found to be positive below pH 6 and negative above pH 7. These imidazolyl cellulose nanocrystals were tested for flocculation of Chlorella vulgaris using CO2 to induce flocculation. Up to 90% flocculation efficiency was achieved with 200 mg L−1 dose. Furthermore, the modified cellulose nanocrystals showed good compatibility with the microalgae during cultivation, giving potential for the production of reversible flocculation systems.
Introduction
Microalgae have attracted recent interest for application in biofuels production. Discussion of biofuels always raises concerns relating to land usage causing interference with food production. Microalgae are a promising solution to this problem with no arable land usage and high oil production as a percentage of the biomass.1,2 In addition to biofuels, microalgae have a high potential in producing innovative chemicals that cannot be derived from conventional crops (e.g. specific fatty acids and natural pigments).3Unfortunately, production costs are currently high owing to large energies involved in the separation of microalgae from the growth medium due to the small size of the cells and the low concentrations during cultivation. Microalgae are also stabilized in suspension by the presence of carboxylate and phosphate groups on the microalgal cell surface.4 Flocculation and subsequent separation by flotation, filtration or sedimentation is one solution to this problem. There are a multitude of inorganic and organic flocculants presented in the literature with varying levels of performance.5 Inorganic flocculants are efficient, but generally require high doses and the resulting feedstock is contaminated with metal salts. Biodegradable organic flocculants offer an alternative that does not foul the resulting biomass and Vandamme et al. have previously reported the use of cationic starch as an organic flocculant.6 However, ideally, a flocculant could be introduced into the growth medium during cultivation of the microalgae and flocculation triggered when desired using an external input. Using pH, surface charge of flocculants can be modified by (de)protonation of surface groups or solubility of salts can be controlled as well, providing a simple method to control flocculation. The latter has been described for alkaline flocculation which allowed reversible flocculation/deflocculation of algal biomass using pH swing.7 However, repeated use of a base such as sodium hydroxide to increase the pH to induce flocculation, followed by using hydrochloric acid to re-adjust pH would lead to the net formation and accumulation of salts (in this case sodium chloride). This would increase the ionic strength of the medium and inhibit growth, especially after repeated re-use of the medium and recycling of the flocculant. We believe that this concept can be improved by using bio-based flocculants responsive to CO2 instead of mineral acids or bases in order to exclude excessive salt formation in the reaction medium.
Cellulose is the most abundant biomolecule on the planet with 1011–1012 t produced annually and provides a promising alternative to other biodegradable flocculants.8 The surface of cellulose fibres are covered with hydroxyl groups allowing facile functionalization to introduce the desired functionality and produce highly effective flocculants. Hydrolysis of this native cellulose using strong acids allows isolation of crystalline sections of the fibres, a fact known since the late 1940s.9 The crystals resulting from this hydrolysis are nano-sized rods called cellulose nanocrystals (CNCs) with size and aspect ratio dependent on the source of cellulose used for the hydrolysis as well as hydrolysis time and temperature.10,11 The high aspect-ratio of CNCs leads to a low percolation concentration which should improve floc formation over use of native fibres. Additionally, the rigidity of the CNCs prevents entanglement, reducing the risk of gelation and improving processability. Modification of cellulose nanocrystals has become a large research area, initially focussing on the compatibilization of CNCs with polymer matrices for nanocomposite applications, but more recently expanding to include more complicated modifications.12–16
Several routes to cationic cellulose nanocrystals have been described before resulting in cationic CNCs with surface charge independent of pH.17–21 While these may prove successful in the flocculation of microalgae,22 ideally CNCs for flocculation would only induce flocculation at a certain pH outside the range of the standard conditions used for cultivation, be achievable using standard equipment and occur in conditions that are not detrimental to the microalgae. As CO2 is required during the cultivation process, adjustment of CO2 saturation within the medium provides an ideal means for reducing the pH to induce flocculation. Cellulose nanocrystals with pH responsive grafts have been reported several times in the literature, including for pH controlled flocculation of CNC suspensions.23–25 Unfortunately, the materials reported thus far are either cationic in the cultivation pH range (7–10),23,25 or at a pH too low to be achievable with carbonic acid (pKa = 3.6 for H2CO3 but the apparent value for CO2 rich aqueous solutions is pKa = 6.4 due to low rates of hydration).24,26 One example of CO2 switchable CNCs was recently reported by Wang et al. based on an imidazole functionalization,27 however, in their work, the modified CNCs were consistently positively charged with switching between a highly cationic and less cationic surfaces.
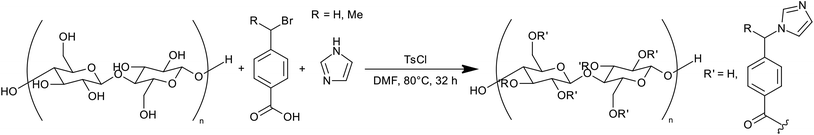
In this paper we report the synthesis of a pH responsive CNC flocculant based on imidazole functionality (pKa ∼ 7) using a modified version of the previously published one-pot procedure for cationization of cellulose nanocrystals (Fig. 1) reported by Jasmani et al.21 The flocculation efficiency of this CNC flocculant was evaluated for microalgae flocculation using Chlorella vulgaris as model species. Imidazole was chosen as a functional group due to the proximity of the pKa of substituted imidazoles to the apparent pKa of carbon dioxide in solution, allowing the use of CO2 for protonation of the resulting species, but remaining deprotonated under microalgae cultivation conditions. By using sulfuric acid hydrolysed cellulose nanocrystals, it should be possible to retain charge repulsion between the CNCs and algae at high pH during cultivation and avoid flocculation due to the sulfate groups introduced during hydrolysis.28
Results and discussion
Synthesis and characterization of ImBnOO-g-CNCs
Cellulose nanocrystals were isolated from bleached cotton wool by hydrolysis with 64% sulfuric acid at 45 °C for 60 min as reported previously.11 The nanocrystals were purified by Soxhlet extraction with ethanol prior to further modification.29 The resulting nanocrystals were modified by reaction with 4-(1-bromo-methyl)benzoic acid (producing ImBnOO-g-CNCs) activated by p-toluenesulfonyl chloride. Our modification of the procedure by Jasmani et al.21 to use imidazole as the base and nucleophile for the simultaneous esterification and nucleophilic substitution necessitated a change of solvent (as pyridine was used as base and solvent in the previous reaction). N,N-Dimethylformamide (DMF) was chosen due to the fact that cellulose nanocrystals disperse relatively well in this solvent.30 The reaction mixture was heated at 80 °C for 32 h and then the product purified by Soxhlet extraction with dichloromethane and ethanol.Successful modification of the CNCs was probed by FTIR spectroscopy (for spectra, see ESI†). Appearance of the carbonyl stretching vibration at 1720 cm−1, the C–O (ester) vibration at 1280 cm−1 and several peaks between 1620–1500 cm−1 attributed to the ring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibrations of the benzoic acid/imidazole moieties all confirm successful modification of the cellulose.
N vibrations of the benzoic acid/imidazole moieties all confirm successful modification of the cellulose.
The level of modification was assessed by elemental microanalysis. According to the percentage nitrogen in the product, the bulk degree of substitution (DS, number of hydroxyls modified per anhydro-glucose-unit) was 0.06. This corresponds with 0.64 mmol g−1 nitrogen, or 0.32 mmol g−1 basic nitrogen available for protonation in the product. Additionally, the sulfate ester content of the product (introduced during acid hydrolysis) was determined from the sulfur elemental analysis as DS = 0.02, identical to the starting material.
X-ray photoelectron spectroscopy (XPS) was used to further probe the modification of the CNCs (see ESI† for spectra and tabulated data). The carbon 1s spectrum (see ESI†) shows a new component for ester type carbon at 289.7 eV consistent with modification of the cellulose by esterification. The nitrogen 1s spectrum shows three nitrogen environments, two of which (399.1 eV and 400.8 eV) are assigned to imidazole, and one of which is assigned to imidazolium (402.0 eV). The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of imidazole to imidazolium environments shows that 2/3 of the protonatable imidazole nitrogen atoms were protonated in the isolated product. The increase in high oxidation state sulfur (2p 3/2 at 168.6 eV) compared with the starting material, and the presence of bromide (3d 5/2 at 68.1 eV) shows that p-toluenesulfonate and bromide were the counterions to the protonated imidazole. For this reason, the product was deprotonated at high pH prior to further use. A small amount of chlorine was detected in the product (0.28 at%) at a chlorine 2p 3/2 binding energy of 200.6 eV consistent with C–Cl species.31 This was most likely due to the side reaction of chlorination of cellulose by the p-toluenesulfonyl chloride.32,33
1 ratio of imidazole to imidazolium environments shows that 2/3 of the protonatable imidazole nitrogen atoms were protonated in the isolated product. The increase in high oxidation state sulfur (2p 3/2 at 168.6 eV) compared with the starting material, and the presence of bromide (3d 5/2 at 68.1 eV) shows that p-toluenesulfonate and bromide were the counterions to the protonated imidazole. For this reason, the product was deprotonated at high pH prior to further use. A small amount of chlorine was detected in the product (0.28 at%) at a chlorine 2p 3/2 binding energy of 200.6 eV consistent with C–Cl species.31 This was most likely due to the side reaction of chlorination of cellulose by the p-toluenesulfonyl chloride.32,33
X-ray diffraction (XRD) was used to assess the crystallinity of the product to ensure that the modification reaction did not cause damage to the cellulose nanocrystals and also to assess the size of the nanocrystalline product (for diffractograms, see ESI†). The crystallinity index (χc), indicative of the mass fraction of crystalline material was calculated as 0.86 for CNCs and 0.81 for ImBnOO-g-CNCs (see Experimental section for details). The mass fraction of modification in the product was calculated by dividing the percentage nitrogen in the product by the percentage nitrogen in the modification (C11H9O2N2) as 6.5%, indicating that the reduction in χc (equal to 5% mass) in the product is due to addition of an amorphous graft. The crystallite size was determined to be 6.7 nm using peak broadening calculated during the pattern fitting of cellulose Iβ. This corresponds to a specific surface area of 370 m2 g−1 and an imidazole surface coverage of 0.52 nm−2.
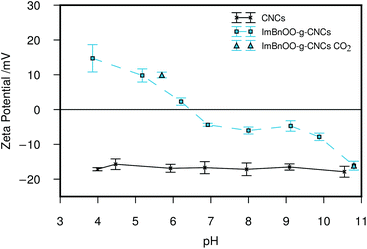
The zeta potential (ζ-potential) of the product was used to monitor the response of the nanocrystals to changes in pH. Initially, the unmodified CNCs and modifed CNCs were suspended in water and adjusted to 10.5 < pH < 11 by addition of sodium carbonate. After sonication to homogenize the suspension ζ-potential measurements were performed, then the pH was adjusted by addition of small amounts of hydrochloric acid before re-measurement of the ζ-potential. The results of the titration are shown in Fig. 2.
The results of the ζ-potential titration show that the isoelectric point of the modified cellulose nanocrystals is between pH 6.2–6.9, ideal for the intended application in flocculation of microalgae. In order to test whether the ζ-potential of ImBnOO-g-CNCs could be switched with CO2, the nanoparticles were suspended in deionized water (1 mg mL−1) and the pH adjusted to 10.8 using sodium carbonate (ζ = −16.1 ± 1.3 mV). The suspension was then purged with CO2 for 10 min. The resulting suspension had pH = 5.7 and ζ = 9.9 ± 0.8 mV confirming the ability to protonate the imidazole moieties using CO2. In contrast to previously reported imidazole grafted CNCs which consistently displayed positive zeta potential measurements,27 the negative zeta potential at high pH of ImBnOO-g-CNCs should allow co-cultivation of the microalgae above pH 6.5 followed by flocculation with pH reduction through CO2 purging.
Evaluation of flocculation efficiency for Chlorella vulgaris
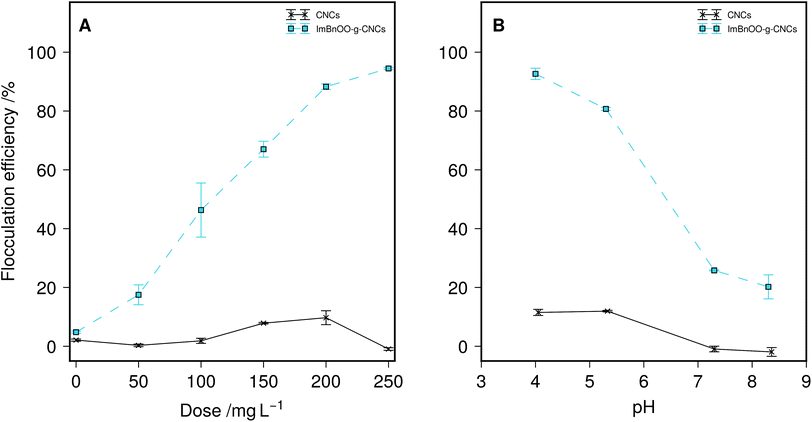
In a first flocculation experiment, dose–response of ImBnOO-g-CNCs was evaluated at pH 4 as compared with unmodified CNCs in flocculation jar tests for Chlorella vulgaris (Fig. 3A). C. vulgaris had a concentration of 0.35 g L−1, which is typical for cultivation in open raceway ponds.1 Use of unmodified CNCs resulted in weak or no flocculation (<10% flocculation efficiency). The ImBnOO-g-CNCs induced flocculation of microalgae, with flocculation efficiency increasing with increasing ImBnOO-g-CNC concentration (up to 88% at a concentration of 250 mg L−1), successfully proving that ImBnOO-g-CNCs are effective flocculants. Secondly, the pH dependence of the flocculation efficiency of ImBnOO-g-CNCs was tested using hydrochloric acid or sodium hydroxide (0.5 N) for a constant dose of 200 mg L−1 and compared with unmodified CNCs (Fig. 3B). For unmodified CNCs, the flocculation efficiency never exceeded 10%, while for ImBnOO-g-CNCs the flocculation efficiency drastically decreased as function of increasing pH: from 90% at pH 4 to 20% at pH 8. These results are consistent with the ζ-potential titration of ImBnOO-g-CNCs (Fig. 2). The ζ-potential changed from negative to positive between pH 6–7, which corresponded with a sharp decrease in flocculation efficiency in that pH region. These results also show that the interaction of negatively charged microalgae and ImBnOO-g-CNCs is mainly based on a charge neutralization mechanism.The concentration factor (CF) and aggregated volume index (AVI) were determined after sedimentation and compared for a dosage of 200 and 250 mg L−1 of ImBnOO-g-CNCs (Table 1). The CF decreased from 49 to 43 with increasing dosage of ImBnOO-g-CNCs, meaning a less concentrated biomass pellet is obtained after flocculation-sedimentation when nanocrystal dosage is increased. The AVI increased as function of dosage accordingly. ImBnOO-g-CNCs are thus effective flocculants resulting in 50-fold concentration of the biomass. This is superior compared to reported results for flocculation at high pH and using chitosan (CF = 20) but lower than reported for cationic starch (CF = 120).34
| Flocculant dose/mg L−1 | ||
|---|---|---|
| 200 | 250 | |
| CF/− | 49 ± 1 | 43 ± 3 |
| AVI/mL g−1 | 58 ± 2 | 65 ± 4 |
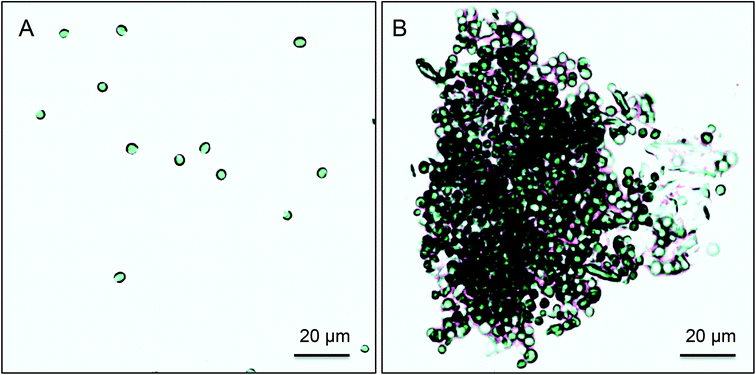
This result shows that the flocculation capacity of ImBnOO-g-CNCs is pH responsive between pH 4–8. A previous study recently reported synthesis of pH responsive polyacrylamides for harvesting microalgae.35 However, these polymers became cationic at pH 13, which implies the usage of base and acid for pH control. This will increase cost and lead to undesired salt formation. For microalgae flocculation especially, using ImBnOO-g-CNCs is particularly interesting because it allows the usage of CO2 to control pH, which is already used in the cultivation process to optimize microalgal productivity by controlling pH between 7–8. At this pH range, the ImBnOO-g-CNCs remain negatively charged and thus inactive as a flocculant. Ideally, after cultivation, the pH would be reduced by increasing CO2 saturation, causing protonation of ImBnOO-g-CNCs and activation of flocculation. In a subsequent experiment, we have therefore monitored Chlorella vulgaris during cultivation in the presence of 200 mg L−1 of ImBnOO-g-CNCs. Growth of Chlorella was monitored daily for 10 days of cultivation and comparison made between cultures co-cultivated with modified CNCs, unmodified CNCs and without CNCs present (Fig. 4). The growth curve of Chlorella in the presence of unmodified CNCs was very similar to the control without nanocrystals. For cultures of Chlorella in the presence of ImBnOO-g-CNCs growth seemed slightly negatively affected, especially in the first days of cultivation. However at day 10, all treatments had a comparable biomass density based on absorbance. At day 10, the pH was decreased using CO2 to 3.5 and the flocculation efficiency was evaluated for all treatments (Table 2). This resulted in a flocculation efficiency of 90% for Chlorella cultures in the presence of ImBnOO-g-CNCs, while this remained below 5% for cultures containing unmodified CNCs. These flocculation efficiencies are similar to those obtained in the first experiment and confirm that flocculation of Chlorella using ImBnOO-g-CNCs can be controlled efficiently using CO2. Upon flocculation induced by CO2, stable flocs settled easily after 10 min (Fig. 5).
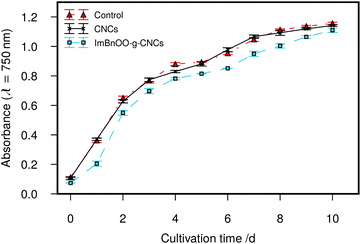 | ||
| Fig. 4 Growth curve of Chlorella vulgaris co-cultivated with 200 mg L−1 of nanocrystals. Lines are provided as a guide only. | ||
| Chlorella + CO2 | Concentration/mg L−1 | Flocculation efficiency/% |
|---|---|---|
| ImBnOO-g-CNCs | 200 | 90.4 ± 0.4 |
| CNCs | 200 | 4.9 ± 0.5 |
| Control | — | 0.65 ± 0.06 |
Finally deflocculation was attempted by increasing pH to 8.8 using sodium hydroxide. Deflocculation could result in CNC release allowing reuse of the flocculant. However, increasing the pH resulted in a decrease of flocculation efficiency of only 10–15%, suggesting that deflocculation in this particular case is potentially sterically hindered when cell coagulation has been established.36 Further study is needed to optimize flocculation reversibility to optimize flocculant reuse.
These results show that nanocrystals with imidazole functionalization can be co-cultivated with Chlorella without severe inhibition of growth. Using CO2 for pH control, which is also used for optimal cultivation of microalgae, surface charge of ImBnOO-g-CNCs could be reversed from net negative to positive, which resulted in efficient flocculation. In this way, cumulative usage of acids and base for pH control, such as hydrochloric acid and sodium hydroxide, is avoided. The required dosage of ImBnOO-g-CNCs seemed to be higher than that needed for traditional flocculants such as chitosan (80 mg L−1) or cationic starch (150 mg L−1).34 However, the degree of substitution of charges on the cationic starch is approximately 3 times higher than determined for ImBnOO-g-CNCs, but still requires a mass addition that is 3/4 of the amount needed for ImBnOO-g-CNCs to get the same degree of flocculation. This indicates more efficient flocculation behaviour for ImBnOO-g-CNCs on a per charge basis. Therefore, with increased DS on ImBnOO-g-CNCs, we may expect to further increase the flocculation effectiveness and also achieve a lower dosage than is needed for traditional flocculants. In addition, ImBnOO-g-CNC flocculation also gave a higher concentration factor after sedimentation.
Conclusions
Imidazole grafted cellulose nanocrystals with a DS of 0.06 were successfully synthesized using a one-pot modification strategy based on previously published procedures. The resulting nanocrystals were shown to have a pH dependent surface charge which was positive below pH 6.2 and negative above pH 6.9. By adjusting the amount of CO2 present in the system, the surface charge of the nanocrystals was successfully switched from negative to positive through protonation of the imidazole moieties. The flocculation efficiency of the modified CNCs was tested with Chlorella vulgaris and found to be pH dependent, in line with surface charge measurements. Maximum flocculation efficiency achieved at pH 3.5 with CO2 purging was greater than 90% with a dose of 200 mg L−1. The effect of CO2 responsive nanocrystals on Chlorella vulgaris cultivation was found to be minimal.Despite the large dose required for effective flocculation, improvement in substitution of the CNCs by optimization of the grafting conditions has the potential to yield CO2 switchable flocculants with flocculation efficiency in-line with previously reported permanently charged species with the benefit of reversibility of the flocculation and compatibility with the cultivation process.
Experimental
Materials
Cotton wool, hydrochloric acid (37%, extra pure), sodium hydroxide (99%, p.a., ISO, in pellets), pyridine (99%, for synthesis) and dichloromethane (99.5%, for synthesis) were purchased from Carl Roth. Sulfuric acid (95%, RECTAPUR), 4-(bromomethyl)benzoic acid (97%, Alfa Aesar), p-toluenesulfonyl chloride (98%, Alfa Aesar) and ethanol (absolute, EMPLURAR ) were purchased from VWR International. N,N-Dimethylformamide (99.8%, Extra Dry over Molecular Sieves, AcroSeal®), Imidazole (99%), potassium bromide (IR spectroscopy grade) and Amberlite® MB-6113 (for ion chromatography, mixed resin) were purchased from Acros Organics.Characterization
Infrared spectra were recorded on a Bruker Alpha FTIR spectrometer in transmission mode. The samples were dispersed in potassium bromide and pressed into a disc before acquisition. Data was recorded between 4000–400 cm−1 over 16 scans with 4 cm−1 resolution. The resulting peaks for cellulose were assigned using data published by Maréchal and Chanzy on the assignment of the FTIR spectrum for Cellulose Iβ.37Elemental analysis (C, H, N, S) data was collected on a Thermo Flash 2000 elemental analyser using methionine as a calibration standard with linear calibration. Carbon, hydrogen, nitrogen and sulfur contents were reported as mass percentage of the sample. Degree of substitution (DS) was calculated using an iterative procedure whereby the empirical formula of the graft added to cellulose was added to the empirical formula of the anhydroglucose unit until the percentage nitrogen was equal in the calculated elemental analysis results and the actual results. Nitrogen was chosen due to the absence of this element in the starting material and importance in the subsequent use of the material as a flocculant. Water content of each of the samples was determined by thermogravimetric analysis (TGA) performed on a TA instruments AutoTGA 2950HR under nitrogen. Samples were heated at 10 °C min−1 to 120 °C, followed by an isothermal period for 1 h, then continued heating at 10 °C min−1 to 600 °C. Water content was determined as mass loss at 125 °C and used to correct the calculated elemental analysis data.
Zeta potential titration was performed by suspending 50 mg of each sample in 50 mL deionized water followed by addition of sodium carbonate solution (1 M) until the resulting pH was between 10–11. The suspension was then sonicated for 5 min prior to commencement of the zeta potential measurements. Zeta potential measurements were carried out on a Brookhaven Instruments NanoBrook Omni in phase analysis light scattering (PALS) mode. Equilibration for 5 min was allowed before collection and zeta potential was averaged over five measurements of 30 cycles each. pH was adjusted using hydrochloric acid (1 M) between sets of measurements.
X-ray diffraction data was collected on a PANalytical X'Pert Pro multi-purpose diffractometer (MPD) in Bragg-Brentano parafocusing geometry, with Cu Kα (45 kV, 40 mA) radiation, automated divergence and receiving slits (10 mm illuminated length), 10 mm beam mask and a step size of 0.02°. Samples were analysed on a silicon “zero-background” sample holder as a loose powder with the packing density influencing the amount of sample in the beam (freeze dried samples are less aggregated). The sample stage was rotated during acquisition to reduce preferred orientation effects in the plane of the stage. The empty sample holder was scanned and used as an instrumental background. Profex with the BGMN backend was used to perform fitting of the cellulose Iβ pattern from Nishiyama et al.38 and a polynomial background to the experimental data using a Rietveld refinement algorithm. Crystallite size was determined from peak broadening of the (200) reflection. For the resulting fitted data, the crystallinity index (χc) of the cellulose was calculated as described by Thygesen et al. using eqn (2) where s is the scattering vector described by eqn (1), Ic(s) is the intensity at a particular vector due to crystalline material and I(s) is the total intensity at a particular vector.39 The limits of the integration were chosen so that s0 is the scattering vector at 2θ = 10° (0.23 Å−1) and s1 is the scattering vector at 2θ = 40° (0.83 Å−1).
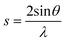 | (1) |
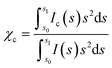 | (2) |
X-ray photoelectron spectroscopy data was collected at the University of Nottingham Nanotechnology and Nanoscience Centre. Spectra were recorded on a Kratos Axis Ultra X-ray Photoelectron Spectrometer employing a monochromated Al Kα (hν = 1486.6 eV, 120 W) X-ray source, hybrid (magnetic/electrostatic) optics (300 × 700 μm aperture), hemispherical analyser, multichannel plate and delay line detector (DLD) with a take-off angle of 90° and an acceptance angle of 30°. The analyser was operated in fixed analyser transmission (FAT) mode with survey scans taken with a pass energy of 80 eV and high resolution scans with a pass energy of 20 eV. All scans were acquired under charge neutralization conditions using a low energy electron gun within the field of the magnetic lens. The resulting spectra were processed using CasaXPS software. Binding energy was referenced to adventitious carbon at 285 eV. High resolution spectra were fitted with Voigt type peaks with some asymmetry correction based on the vibrational modes of the cellulose molecule, which cause peak broadening due to some photon energy being absorbed by vibrational transitions in the bonds connected to the atom of interest.40,41 This results in small peaks at higher binding energy associated with the main photoelectron line. The separation of these peaks from the main peak are determined by the energy of the vibrational transition.41 By considering the FTIR spectrum of the compound and converting the energy of the vibrational modes to electron volts, the asymmetry was set for each component peak in the high resolution spectrum, based on the vibrational modes present in the bonds connected to that atom.41
Synthesis of cellulose nanocrystals (CNCs)
Sulfuric acid (550 mL, 10.1 M) was heated at 45 °C and cotton wool (50 g) added slowly with mechanical stirring. Stirring and heating was continued for 60 min, before addition of deionized water (800 mL) to quench the reaction. The cellulose was separated from the acidic reaction media by centrifugation (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 °C) for 20 min. The solid roduct was washed with two successive centrifugations (20
000g, 10 °C) for 20 min. The solid roduct was washed with two successive centrifugations (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 10 °C) for 35 min, before dialysing against running deionized water for 48 h in a regenerated cellulose dialysis tube (MWCO 12–14 kDa). The nanocrystal suspension was homogenized using a sonication horn before filtration through a porosity 2 fritted glass filter to separate aggregates. The resulting homogeneous suspension was mixed with Amberlite® MB-6113 mixed bed ion exchange resin (100 g) for 12 h, separated, frozen in liquid nitrogen, then freeze dried. The cellulose nanocrystals were subsequently Soxhlet extracted with ethanol (48 h) in a cellulose extraction thimble to remove organic surface contaminants and dried in vacuo. Found: C, 42.95; H, 6.04; N, <0.1; S, 0.37%. Calc. for (C6H10O5.06S0.02)n (DS(OSO3 H) = 0.02 with 2.51% adsorbed water): C, 49.92; H, 6.28; S, 0.37%.
000g, 10 °C) for 35 min, before dialysing against running deionized water for 48 h in a regenerated cellulose dialysis tube (MWCO 12–14 kDa). The nanocrystal suspension was homogenized using a sonication horn before filtration through a porosity 2 fritted glass filter to separate aggregates. The resulting homogeneous suspension was mixed with Amberlite® MB-6113 mixed bed ion exchange resin (100 g) for 12 h, separated, frozen in liquid nitrogen, then freeze dried. The cellulose nanocrystals were subsequently Soxhlet extracted with ethanol (48 h) in a cellulose extraction thimble to remove organic surface contaminants and dried in vacuo. Found: C, 42.95; H, 6.04; N, <0.1; S, 0.37%. Calc. for (C6H10O5.06S0.02)n (DS(OSO3 H) = 0.02 with 2.51% adsorbed water): C, 49.92; H, 6.28; S, 0.37%. ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) max(KBr)/cm−1 3343 ν(O–H), 2901 ν(C–H), 1640 δ(H2 O), 1430 δ(C–O–H), 1337 δ(C–O–H), 1318 δ(C–O–H), 1204 ν(C–O–C, glycosidic), 1163 ν(C–O–C, glycosidic, asym.), 1113 ν(C2–OH), 1059 ν(C3–OH), 1034 ν(C6–OH), 707 ω/τ(C–OH), 667 ω/τ(C–OH).
max(KBr)/cm−1 3343 ν(O–H), 2901 ν(C–H), 1640 δ(H2 O), 1430 δ(C–O–H), 1337 δ(C–O–H), 1318 δ(C–O–H), 1204 ν(C–O–C, glycosidic), 1163 ν(C–O–C, glycosidic, asym.), 1113 ν(C2–OH), 1059 ν(C3–OH), 1034 ν(C6–OH), 707 ω/τ(C–OH), 667 ω/τ(C–OH).
Synthesis of cellulose 4-(1-imid-azo-lyl-methyl)benzoate nano-crystals (ImBnOO-g-CNCs)
Cellulose nanocrystals (1.04 g), 4-bromomethylbenzoic acid (1.48 g, 6.9 mmol), imidazole (1.2 g, 18 mmol) and p-toluenesulfonyl chloride (1.19 g, 6.2 mmol) were suspended in dry N,N-dimethylformamide (50 mL) under an atmosphere of argon and heated at 80 °C for 24 h. The resulting suspension was filtered though a cellulose extraction thimble and Soxhlet extracted with dichloromethane (24 h) and ethanol (48 h) before drying in vacuo to yield a cream coloured solid product. Found: C, 45.54; H, 6.03; N, 0.90; S, 0.35%. Calc. for C6.62H10.45O5.11N0.11S0.02 (DS (Imidazole) = 0.06, DS(OSO3 H) = 0.02 with 1.13% adsorbed H2 O): C, 45.17; H, 6.10; N, 0.90; S, 0.35%.![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) max(KBr)/cm−1 3344 ν(O–H), 2902 ν(C–H), 1721 ν(C
max(KBr)/cm−1 3344 ν(O–H), 2902 ν(C–H), 1721 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1637 δ(H2 O), 1617 ν(C
O), 1637 δ(H2 O), 1617 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1579 ν(C
N), 1579 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1550 ν(C
N), 1550 ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C
C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), 1429 δ(C–O–H), 1337 δ(C–O–H), 1317 δ(C–O–H), 1281 ν(C–O, ester), 1205 ν(C–O–C, glycosidic), 1163 ν(C–O–C, glycosidic, asym.), 1113 ν(C2–OH), 1059 ν(C3–OH), 1034 ν(C6–OH), 707 ω/τ(C–OH), 667 ω/τ(C–OH).
N), 1429 δ(C–O–H), 1337 δ(C–O–H), 1317 δ(C–O–H), 1281 ν(C–O, ester), 1205 ν(C–O–C, glycosidic), 1163 ν(C–O–C, glycosidic, asym.), 1113 ν(C2–OH), 1059 ν(C3–OH), 1034 ν(C6–OH), 707 ω/τ(C–OH), 667 ω/τ(C–OH).
Cultivation of Chlorella vulgaris
The green freshwater microalgae Chlorella vulgaris 211-11b (SAG) was selected as model species and it was cultivated in dechlorinated deionized water enriched with inorganic nutrients according to the concentration of the Wright's cryptophyte medium.42 Bubble column photobioreactors (30 L) were used to cultivate the microalgae. The system was mixed by sparging with 0.2 μm filtered air (5 L min−1) and pH was controlled at 8.5 by addition of 2–3% CO2 using a pH-stat system. Growth of the microalgae was monitored by measuring the absorbance at 750 nm. Microalgal dry weight was determined gravimetrically by filtration using Whatman glass fibre filters (Sigma–Aldrich) and drying until constant weight at 105 °C. Flocculation experiments were performed in the early stationary phase at a biomass concentration of 0.35 g L−1.Flocculation experiments
Flocculation of the microalgal suspensions in the presence of CNCs was investigated using jar test experiments (n = 2). These experiments were carried out in 50 mL beakers that were stirred using a magnetic stirrer. CNCs were suspended in MQ-water at 5 g L−1 and adjusted to pH 10.8 using sodium carbonate (1 M). The microalgal suspension was mixed intensively (1000 rpm) for 10 min during CNCs addition. Then, the suspensions were mixed gently (250 rpm) for another 20 min, after which they were allowed to settle for 30 min. Dose–response was evaluated by testing 6 doses (0, 50, 100, 150, 200 and 250 mg L−1). Prior to CNCs addition, pH was adjusted to 4. Subsequently, the relative importance of pH was evaluated by testing 4 pH levels (4.0, 5.3, 7.3 and 8.4) at a constant CNCs concentration of 200 mg L−1. pH of the microalgal suspensions was adjusted prior to addition of CNCs using hydrochloric acid or sodium hydroxide solutions (0.5 N). The flocculation efficiency was calculated based on changes in the optical density (measured at 750 nm) prior to flocculant addition (ODi) and after settling (ODf). The flocculation efficiency (ηa), or the percentage of microalgae biomass removed from suspension, was calculated as: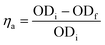 | (3) |
Floc characterization
After 30 min of sedimentation, the suspension was allowed to settle an additional 15 min to determine the concentration factor (CF) and the aggregated volume index (AVI). Both parameters are related to each other and provide information about the residual water content of the particulate phase.34 The CF was determined by dividing the total volume of 50 mL by the volume of the particulate phase after 30 min of sedimentation. The AVI is defined as the volume in milliliters occupied by 1 g of algal suspension in the particulate phase after settling and is calculated as: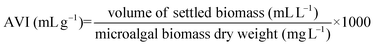 | (4) |
Cultivation of Chlorella vulgaris in presence of CNCs
The effect of CNCs on the growth of Chlorella vulgaris 211-11b (SAG) was tested in a separate batch cultivation experiment in 1L photobioreactors using identical growth medium as ascribed above. The growth of Chlorella was compared to cultures in presence of 200 mg L−1 ImBnOO-g-CNCs and cultures with and without 200 mg L−1 unmodified CNCs (n = 2). Microalgal growth was monitored daily for 10 days of cultivation by measuring light absorbance at 750 nm. At day 10 of cultivation, pH of the cultures was lowered by addition of CO2 until pH was dropped below 4. Flocculation was assessed as previously described and flocculation efficiency was calculated using eqn (3). Single-cell conditions of Chlorella were analysed using light microscopy (Olympus BX51; 200× and Infinity 2 USB camera Luminera corp.) and compared to the control treatment. Afterwards, the pH of the flocculated microalgae suspension was increased to 8.8 using sodium hydroxide solution (0.5 N) while the suspension was mixed intensively (1000 rpm) for 10 min. Then, the suspensions were mixed gently (250 rpm) for another 20 min, after which they were allowed to settle for 30 min prior to flocculation efficiency assessment as previously described.Acknowledgements
S. E. and W. T. would like to thank the Research Foundation – Flanders (FWO) for funding under the Odysseus grant (G.0C60.13N) and KU Leuven for grant OT/14/072. The authors thank Kevin Hendrickx in the Department of Mechanical Engineering, KU Leuven for performing TGA and Emily Smith at the Nottingham Nanotechnology and Nanoscience Centre (University of Nottingham) for XPS analysis. D. V. is a Postdoctoral Researcher funded by the Research Foundation – Flanders (FWO) (12D8914N). K. M. is funded by the Research Foundation – Flanders (FWO) research grant 1509513N and KU Leuven grant OT/14/065.References
- R. H. Wijffels and M. J. Barbosa, Science, 2010, 329, 796–799 CrossRef CAS PubMed.
- E. Stephens, I. L. Ross, Z. King, J. H. Mussgnug, O. Kruse, C. Posten, M. a. Borowitzka and B. Hankamer, Nat. Biotechnol., 2010, 28, 126–128 CrossRef CAS PubMed.
- G. Markou and E. Nerantzis, Biotechnol. Adv., 2013, 31, 1532–1542 CrossRef CAS PubMed.
- S. Hadjoudja, V. Deluchat and M. Baudu, J. Colloid Interface Sci., 2010, 342, 293–299 CrossRef CAS PubMed.
- D. Vandamme, I. Foubert and K. Muylaert, Trends Biotechnol., 2013, 31, 233–239 CrossRef CAS PubMed.
- D. Vandamme, I. Foubert, B. Meesschaert and K. Muylaert, J. Appl. Physiol., 2010, 22, 525–530 Search PubMed.
- D. Vandamme, A. Beuckels, G. Markou, I. Foubert and K. Muylaert, BioEnergy Res., 2015, 8, 716–725 CrossRef CAS.
- H. Zhao, J. H. Kwak, Z. C. Zhang, H. M. Brown, B. W. Arey and J. E. Holladay, Carbohydr. Polym., 2007, 68, 235–241 CrossRef CAS PubMed.
- B. G. Rånby, Acta Chem., Scand., 1949, 3, 649–650 CrossRef.
- S. Beck-Candanedo, M. Roman and D. G. Gray, Biomacromolecules, 2005, 6, 1048–1054 CrossRef CAS PubMed.
- S. Elazzouzi-Hafraoui, Y. Nishiyama, J. Putaux, L. Heux, F. Dubreuil and C. Rochas, Biomacromolecules, 2008, 9, 57–65 CrossRef CAS PubMed.
- S. J. Eichhorn, A. Dufresne, M. Aranguren, N. E. Marcovich, J. R. Capadona, S. J. Rowan, C. Weder, W. Thielemans, M. Roman, S. Renneckar, W. Gindl, S. Veigel, J. Keckes, H. Yano, K. Abe, M. Nogi, A. N. Nakagaito, A. Mangalam, J. Simonsen, A. S. Benight, A. Bismarck, L. A. Berglund and T. Peijs, J. Mater. Sci., 2010, 45, 1–33 CrossRef CAS.
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941–3994 RSC.
- D. Klemm, F. Kramer, S. Moritz, T. Lindström, M. Ankerfors, D. Gray and A. Dorris, Angew. Chem., Int. Ed., 2011, 50, 5438–5466 CrossRef CAS PubMed.
- Y. Habibi, Chem. Soc. Rev., 2014, 43, 1519–1542 RSC.
- S. Eyley and W. Thielemans, Nanoscale, 2014, 6, 7764–7779 RSC.
- M. Hasani, E. D. Cranston, G. Westman and D. G. Gray, Soft Matter, 2008, 4, 2238–2244 RSC.
- S. Eyley and W. Thielemans, Chem. Commun., 2011, 47, 4177–4179 RSC.
- H. de la Motte, M. Hasani, H. Brelid and G. Westman, Carbohydr. Polym., 2011, 85, 738–746 CrossRef CAS PubMed.
- M. Zaman, H. Xiao, F. Chibante and Y. Ni, Carbohydr. Polym., 2012, 89, 163–170 CrossRef CAS PubMed.
- L. Jasmani, S. Eyley, R. Wallbridge and W. Thielemans, Nanoscale, 2013, 5, 10207–10211 RSC.
- D. Vandamme, S. Eyley, G. Van den Mooter, K. Muylaert and W. Thielemans, Bioresour. Technol., 2015, 194, 270–275 CrossRef CAS PubMed.
- A. E. Way, L. Hsu, K. Shanmuganathan, C. Weder and S. J. Rowan, ACS Macro Lett., 2012, 1, 1001–1006 CrossRef CAS.
- K. H. M. Kan, J. Li, K. Wijesekera and E. D. Cranston, Biomacromolecules, 2013, 14, 3130–3139 CrossRef CAS PubMed.
- L. Chen, W. Cao, N. Grishkewich, R. M. Berry and K. C. Tam, J. Colloid Interface Sci., 2015, 450, 101–108 CrossRef CAS PubMed.
- N. N. Greenwood and A. Earnshaw, Chemistry of the Elements, Elsevier Butterworth-Heinemann, Oxford, 2nd edn, 2005 Search PubMed.
- H.-D. Wang, P. G. Jessop, J. Bouchard, P. Champagne and M. F. Cunningham, Cellulose, 2015 DOI:10.1007/s10570-015-0690-3.
- X. M. Dong, J.-F. Revol and D. G. Gray, Cellulose, 1998, 5, 19–32 CrossRef CAS.
- M. Labet and W. Thielemans, Cellulose, 2011, 18, 607–617 CrossRef CAS.
- D. Viet, S. Beck-Candanedo and D. G. Gray, Cellulose, 2007, 14, 109–113 CrossRef CAS.
- XPS of Polymers Database, ed. G. Beamson and D. Briggs, SurfaceSpectra, Manchester, 2000 Search PubMed.
- T. Teshirogi, H. Yamamoto, M. Sakamoto and H. Tonami, Sen'i Gakkaishi, 1978, 34, T510–T515 CrossRef CAS.
- C. L. McCormick, T. R. Dawsey and J. K. Newman, Carbohydr. Res., 1990, 208, 183–191 CrossRef CAS.
- D. Vandamme, K. Muylaert, I. Fraeye and I. Foubert, Bioresour. Technol., 2014, 151, 383–387 CrossRef CAS PubMed.
- K. L. Morrissey, C. He, M. H. Wong, X. Zhao, R. Z. Chapman, S. L. Bender, W. D. Prevatt and M. P. Stoykovich, Biotechnol. Bioeng., 2014, 1–32 Search PubMed.
- J. Gregory, Encyclopedia of Colloid and Interface Science, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 459–491 Search PubMed.
- Y. Maréchal and H. Chanzy, J. Mol. Struct., 2000, 523, 183–196 CrossRef.
- Y. Nishiyama, P. Langan and H. Chanzy, J. Am. Chem. Soc., 2002, 124, 9074–9082 CrossRef CAS PubMed.
- A. Thygesen, J. Oddershede, H. Lilholt, A. B. Thomsen and K. Ståhl, Cellulose, 2005, 12, 563–576 CrossRef CAS.
- Surface Analysis by Auger and X-ray Photoelectron Spectroscopy, ed. D. Briggs and J. T. Grant, IM Publications, Manchester, 2003 Search PubMed.
- J. Walton, P. Wincott, N. Fairley and A. Carrick, Peak Fitting with CasaXPS, Acolyte Science, Knutsford, 2010 Search PubMed.
- D. Vandamme, I. Foubert, I. Fraeye, B. Meesschaert and K. Muylaert, Bioresour. Technol., 2012, 105, 114–119 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Spectra for all products. See DOI: 10.1039/C5NR03853G |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |