Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Au nanoparticle scaffolds modulating intermolecular interactions among the conjugated azobenzenes chemisorbed on curved surfaces: tuning the kinetics of cis–trans isomerisation†
Corinna
Raimondo‡
a,
Bart
Kenens
b,
Federica
Reinders
c,
Marcel
Mayor
cd,
Hiroshi
Uji-i
be and
Paolo
Samorì
*a
aISIS & icFRC, University of Strasbourg & CNRS, 8 allée Gaspard Monge, 67000 Strasbourg, France. E-mail: samori@unistra.fr
bDepartment of Chemistry Division of Molecular Imaging and Photonics, KU Leuven – University of Leuven, Celestijnenlaan 200F, B-3001 Leuven, Belgium
cDepartment of Chemistry, University of Basel, St. Johannsring 19, 4056 Basel, Switzerland
dKarlsruhe Institute of Technology (KIT), Institute for Nanotechnology, P.O. Box 3640, 76021 Karlsruhe, Germany
eResearch Institute for Electronic Science, Hokkaido University, Japan
First published on 23rd July 2015
Abstract
π–π Intermolecular interactions among adjacent conjugated azobenzenes chemisorbed on (non-)flat Au surfaces can be tuned by varying the curvature of the Au nanoparticles. Here we show that such interactions rule the thermal cis–trans isomerization kinetics, towards a better control on the azobenzene bistability for its optimal integration as a responsive material.
Photo-switchable molecular systems have attracted great attention in the past few decades due to their tuneable physical and chemical properties paving the route towards their application as switches in (nano)devices and as key components for the fabrication of adaptable/dynamic materials.1 Among them, azobenzenes are popular scaffolds due to their efficient and reversible photo-isomerisation from a thermodynamically stable trans form to a metastable cis form. For any potential technological application of azobenzene the rate of isomerization between the two isomers is a crucial characteristic to be controlled. Such a rate is known to depend on the electronic nature of the substituents,2 the molecular geometry,3 the solvent,4 the physical state of the molecule, etc. When chemisorbed on Au planar and non-planar surfaces, electronically coupled azobenzenes have been reported not to show isomerization due to the interaction between the surface plasmon resonance (SPR), in particular in the case of gold nanoparticles, and the excited electronic states of the azobenzene, resulting in the quenching of the latter ones.5 In the case of azobenzene chromophores arranged perfectly perpendicular to the Au surface in highly ordered self-assembled monolayers (SAMs), quenching is less efficient and the optically triggered trans–cis isomerization can occur. Intermolecular interactions between neighbouring azobenzene molecules in a condensed phase can be expected to play a decisive role in their isomerization, although this aspect is still experimentally unexplored. Moreover, a major limitation for the use of azobenzene in electronics is the metastable nature of the cis isomer that can rapidly undergo back-isomerization to the thermodynamically stable trans form. Controlling the kinetics of such a back-isomerization process is therefore of great importance.
Here we show that the kinetics of the back cis to trans isomerization in azobenzene chemisorbed on Au nanoparticles with different sizes depends on the interactions between individual adjacent molecules in the condensed phase of a SAM which can be tuned by modifying the size of the Au nanoparticle acting as a structural scaffold.
In order to gain a direct insight into the role of intermolecular interactions, in particular among the first nearest neighbour molecules, in the trans–cis isomerisation, we have chosen a thiolated azobenzene derivative (AZO) able to form tightly packed SAMs on planar6 and non-planar7 Au surfaces. The AZO molecule has shown an ca. 4 times more stable cis phase when chemisorbed forming tightly packed SAMs on Au(111) when compared to solution.6 The observation of an increased stability of the cis isomers in SAMs has been rationalized by the presence of π–π interactions between neighbouring azobenzene molecules.8 A number of researchers including Grzybowski and Klajn have recently demonstrated that SAMs chemisorbed on Au nanoparticles exhibit properties which are dependent on the nanoparticle size, i.e. on the curvature of the surface.9 In the present work, we demonstrate that the AuNP scaffold can be used to regulate the kinetics of cis to trans isomerization in a fully conjugated azobenzene derivative forming a tightly packed chemisorbed SAM on its surface. Unravelling the mechanism ruling such isomerization moreover represents a fundamental step towards the understanding of the switching behaviour in azobenzene materials.
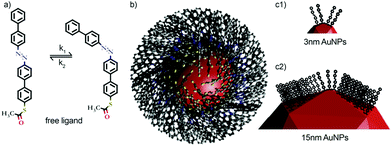
We focussed our attention on two azobenzene-modified Au nanoparticles differing in their size (3 and 15 nm, named AZOAuNP1 and AZOAuNP2, respectively; see Fig. 1) in order to explore the size-dependent properties and the dependence on the curvature, i.e. spacing between neighbouring molecules. We previously reported details on their synthesis and characterization.7 Starting from alkyl-amine AuNPs of the corresponding size as precursors, the coating molecules were substituted with AZO and the obtained particles subsequently dispersed in toluene. These particles exhibit the peculiar characteristic of being photochemically tunable between two different physical states, one featuring a large aggregation (corresponding to the trans state), and one exhibiting a colloidal dispersion in an organic medium (toluene, when in the cis state).7Fig. 1 shows a sketch of the employed systems.
Proton nuclear magnetic resonance (1H-NMR) analysis of these two differently coated nanoparticles revealed different chemical shifts of the azobenzene's aromatic protons depending on their size (Fig. S1 in the ESI†) providing evidence for their dissimilar chemical environment. This experimental result, together with the theoretical and high resolution microscopic reports by other groups,10 made it possible to model the real geometric shape of the nanoparticles not as a perfectly hairy sphere, but rather as a sphere coated with polycrystalline architectures. The size of the crystalline domains within which the molecule interacts by π–π stacking thus strongly depends on the NP size.11
Since the system undergoes simultaneous isomerisation (having a known first order kinetics) and aggregation (having unknown order kinetics) the concentration of azobenzenes on the AuNPs undergoing irradiation was kept constant. The temperature of the solution was stepwise increased up to 95–100 °C to ensure complete dissolution of the as-synthesized trans-AZOAuNPs (Fig. S2†). Samples featuring equal absorbances in the trans state were then prepared since no dependence of the extinction coefficient of the azobenzene on the curvature was found.
Different complementary techniques including 1H-NMR, UV-Vis and Surface Enhanced Raman Spectroscopy (SERS) were employed to prove the reversible and over-time reliable switch of the AZO molecules grafted on the AuNP surfaces. Albeit the UV-Vis technique allows the monitoring of the SPR band while irradiating, it does not provide easy access to the isomerisation state of the coating AZO moieties because of the spatial overlap of the peaks attributed to the AZO in the trans and cis form. On the other hand, the 1H-NMR enables the identification of the AZO isomers separately (at least for the molecules dissolved in solution) but (i) it cannot give information on the SPR of the AuNP, (ii) the integral dependence on the actual amount of molecules is still debated,12 and (iii) the spectra of the different nanoparticles depend drastically on their sizes to an extent that no isolated peaks for the smaller particles could be obtained. In addition, SERS experiments can provide evidence for the switching but, due to the different aggregation among the adjacent NPs, it cannot provide reliable integrals to be compared.
Although the use of the aforementioned techniques singularly does not offer sufficient information making it possible to achieve full characterization, the complementary use of all of them allows the full characterization of the hybrid system and enables a timescale cross-checking of the results obtained. Fig. 2 shows different spectra corresponding to the cis and the trans forms. The kinetic rate constants were evaluated via UV-Vis spectroscopy. The time required for the acquisition of each spectrum, at the chosen concentrations, was ca. 3 min.
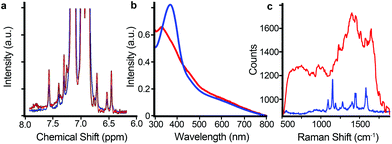 | ||
| Fig. 2 (a) 1H-NMR, (b) UV-Vis, (c) SERS for the trans (blue) and the cis (red) isomerization states of the AZO molecule when coating with AuNP1. | ||
All the systems showed a reversible first order kinetic behaviour (Fig. S6†). The reaction was monitored under UV irradiation and in the dark to evaluate the stability of the cis phase in the dark. First the kinetics of the precipitation process was studied giving overall bigger precipitation rates for larger AuNPs. The investigation of a system subjected to physical state variation is a great challenge. However, if one takes into account different aspects involved, including the concomitant azobenzene switch and aggregation/disaggregation, together with an in-depth statistical analysis, the acquisition of reliable results is possible. While irradiating different processes can occur such as (i) the sudden disaggregation of individual nanoparticles more external in the overall cluster, or (ii) the simultaneous disaggregation of smaller aggregates of AuNPs from the biggest ones, both phenomena causing great variation of UV-Vis spectral features. The same processes, but involving aggregation steps, can take place when the reversed isomerization from cis to trans is considered. The kinetics of the precipitation process being variable with the nanoparticles’ size, a normalization for the integral of the SPR was used to guarantee a comparison of an equal amount of AZO on the surface (Fig. S3–S6†), thereby ensuring an accurate determination of the cis to trans ratio in the system. From the absorbance at the maximum wavelength of the trans, which changes from sample to sample, and through the subtraction of the absorbance of the SPR at the same wavelength, it was possible to obtain the graphs of the different concentrations vs. time (Fig. S6†). From the concentration vs. time plots one can estimate the kinetic constants involved in the process (k1 and k2); in our particular case, in view of the characteristics of the system, only the back cis to trans kinetic constant (k2) could be fully determined. (Fig. S6†). The precipitation kinetics in the early stage regime show a different behaviour compared with the overall process, although the trend with the size is consistent over the whole process. Since at the early stage the system is more dependent on the actual interaction among the molecules and less subjected to other external phenomena such as temperature changes and aggregation, the early stage has been considered in this work.
Fig. 3 displays the comparison between the obtained cis to trans kinetic constants with those obtained in the case of a SAM absorbed on flat gold. Higher variability was found for the azobenzene absorbed on flat gold (error bar not shown) and for the larger gold nanoparticles considered due to the smaller concentration of azobenzene in the optical pathway giving higher variability from measurement to measurement, and due to the high heterogeneity of the system. The error presented takes into account both the standard deviation between different samples and the fitting error. The graph reveals a correlation between the kinetic constant and the size of the nanoparticles. This means that the kinetic constant can be modulated by varying the size of the crystalline domains, therefore it depends on the spatial extension of π–π stacking between the azobenzene molecules. This proves that the higher thermal stability of the cis form for the SAM absorbed on flat surfaces can be attributed to the extended π–π stacking between the adjacent molecules due to the intrinsic nature of the molecules involved. It is also important to mention an opposite trend between different sized nanoparticles for what concern the precipitation kinetics. This opposite behaviour might be a reason why direct evaluation of the rate of the reaction results not being possible.
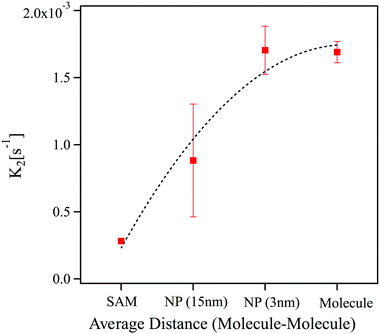 | ||
| Fig. 3 Dependence of the k2 kinetic constant on the π-stacking distance between the adjacent azobenzene molecules, from higher (SAM on flat gold) to lower (molecule solvated). | ||
The precipitation rate has also been calculated by extracting the SPR integral at each period in time to prove that in the chosen timescale the isomer state (cis vs. trans) of the AZO coating the two NP sizes is associated with the different relaxation mechanisms and not with mechanical/weight related reasons. The two precipitation processes are, in fact, absolutely equivalent in the early stage of the reactions (an example of the spectra from which the data were extracted is reported in the ESI in Fig. S3†).
Due to the complexity of the system under investigation a giant statistical analysis has been carried out. Out of the overall 25 full kinetics tested under the same conditions, 68% (17) complies with the behaviour described here. Both processes exhibit for early reaction times a linear behaviour following a first order (or pseudo first-order kinetic law). For longer times a quasi-linear behaviour is shown but with a different slope, indicating different stages in the process.
Conclusions
In summary, we have carried out a quantitative analysis that provided unambiguous evidence for the key role played by intermolecular interactions in the kinetics of the cis–trans isomerization in azobenzene SAMs exhibiting a variable size of the crystalline domains. We found a variation of the precipitation kinetics of the nanoparticles depending on their size and, most importantly, a trend for the cis state stability depending on the degree of π–π stacking. These results are particularly relevant in view of the key role played by weak interactions in physical processes such as light-switching, thereby providing additional proof of the importance of achieving full control over the self-assembly of single molecules as a route to modulate the macroscopic properties of a responsive material. Future effort in our laboratories will be addressed to the unravelling of the cooperative nature of the isomerisation process.Acknowledgements
This work was supported by the European Commission through the projects ITN iSwitch (GA no. 642196) as well as the European Research Council projects SUPRAFUNCTION (GA-257305) and PLASMHACAT (GA-280064), the EC-FP7-NMP-2012-SMALL-6 “SACS” project (GA-310651), the Agence Nationale de la Recherche through the LabEx project Chemistry of Complex Systems (ANR-10-LABX-0026_CSC), the International Center for Frontier Research in Chemistry (icFRC), the Swiss Nationals Science Foundation (SNF), and the Swiss Nanoscience Institute (SNI).Notes and references
- (a) A. C. Fahrenbach, S. C. Warren, J. T. Incorvati, A. J. Avestro, J. C. Barnes, J. F. Stoddart and B. A. Grzybowski, Adv. Mater., 2013, 25, 331–348 CrossRef CAS PubMed; (b) A. C. Coleman, J. M. Beierle, M. C. A. Stuart, B. Macia, G. Caroli, J. T. Mika, D. J. van Dijken, J. W. Chen, W. R. Browne and B. L. Feringa, Nat. Nanotechnol., 2011, 6, 547–552 CrossRef CAS PubMed; (c) M. Mas-Torrent, C. Rovira and J. Veciana, Adv. Mater., 2013, 25, 462–468 CrossRef CAS PubMed; (d) R. Klajn, J. F. Stoddart and B. A. Grzybowski, Chem. Soc. Rev., 2010, 39, 2203–2237 RSC; (e) M. M. Russew and S. Hecht, Adv. Mater., 2010, 22, 3348–3360 CrossRef CAS PubMed.
- K. Gille, H. Knoll and K. Quitzsch, Int. J. Chem. Kinet., 1999, 31, 337–350 CrossRef CAS.
- K. Janus, J. Sworakowski and E. Luboch, Chem. Phys., 2002, 285, 47–54 CrossRef CAS.
- J. Garcia-Amoros, A. Sanchez-Ferrer, W. A. Massad, S. Nonell and D. Velasco, Phys. Chem. Chem. Phys., 2010, 12, 13238–13242 RSC.
- R. Klajn, Pure Appl. Chem., 2010, 82, 2247–2279 CrossRef.
- M. Elbing, A. Blaszczyk, C. von Haenisch, M. Mayor, V. Ferri, C. Grave, M. A. Rampi, G. Pace, P. Samorì, A. Shaporenko and M. Zharnikov, Adv. Funct. Mater., 2008, 18, 2972–2983 CrossRef CAS PubMed.
- C. Raimondo, F. Reinders, U. Soydaner, M. Mayor and P. Samorì, Chem. Commun., 2010, 46, 1147–1149 RSC.
- G. Pace, V. Ferri, C. Grave, M. Elbing, C. von Hanisch, M. Zharnikov, M. Mayor, M. A. Rampi and P. Samorì, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 9937–9942 CrossRef CAS PubMed.
- (a) K. P. Browne and B. A. Grzybowski, Langmuir, 2011, 27, 1246–1250 CrossRef CAS PubMed; (b) T. Zdobinsky, P. S. Maiti and R. Klajn, J. Am. Chem. Soc., 2014, 136, 2711–2714 CrossRef PubMed.
- (a) B. R. Cuenya, Thin Solid Films, 2010, 518, 3127–3150 CrossRef CAS PubMed; (b) A. Mayoral, H. Barron, R. Estrada-Salas, A. Vazquez-Duran and M. Jose-Yacaman, Nanoscale, 2010, 2, 335–342 RSC; (c) J. Patel, L. Nemcova, P. Maguire, W. G. Graham and D. Mariotti, Nanotechnology, 2013, 24, 245606 CrossRef PubMed.
- (a) O. Balmes, J. O. Malm, N. Pettersson, G. Karlsson and J. O. Bovin, Microsc. Microanal., 2006, 12, 145–150 CrossRef CAS PubMed; (b) M. T. Kumara, B. C. Tripp and S. Muralidharan, Chem. Mater., 2007, 19, 2056–2064 CrossRef CAS; (c) V. Petkov, Y. Peng, G. Williams, B. H. Huang, D. Tomalia and Y. Ren, Phys. Rev. B: Condens. Matter, 2005, 72, 195402 CrossRef.
- (a) A. Badia, W. Gao, S. Singh, L. Demers, L. Cuccia and L. Reven, Langmuir, 1996, 12, 1262–1269 CrossRef CAS; (b) O. Kohlmann, W. E. Steinmetz, X. A. Mao, W. P. Wuelfing, A. C. Templeton, R. W. Murray and C. S. Johnson, J. Phys. Chem. B, 2001, 105, 8801–8809 CrossRef CAS; (c) A. C. Templeton, S. W. Chen, S. M. Gross and R. W. Murray, Langmuir, 1999, 15, 66–76 CrossRef CAS; (d) R. H. Terrill, T. A. Postlethwaite, C. H. Chen, C. D. Poon, A. Terzis, A. D. Chen, J. E. Hutchison, M. R. Clark, G. Wignall, J. D. Londono, R. Superfine, M. Falvo, C. S. Johnson, E. T. Samulski and R. W. Murray, J. Am. Chem. Soc., 1995, 117, 12537–12548 CrossRef CAS; (e) X. Liu, M. Yu, H. Kim, M. Mameli and F. Stellacci, Nat. Commun., 2012, 3, 1182 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Physico-chemical characterization of the different sizes of nanoparticles, UV-Vis, Surface Enhanced Raman Spectroscopy (SERS), materials and methods. See DOI: 10.1039/c5nr03688g |
| ‡ Present address: Northwestern University, 2145 Sheridan rd. 60201 Evasnton, IL, USA. |
| This journal is © The Royal Society of Chemistry 2015 |