Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hybrid van der Waals heterostructures of zero-dimensional and two-dimensional materials†
Zhikun
Zheng
*ab,
Xianghui
Zhang
a,
Christof
Neumann
a,
Daniel
Emmrich
a,
Andreas
Winter
c,
Henning
Vieker‡
a,
Wei
Liu
d,
Marga
Lensen
e,
Armin
Gölzhäuser
a and
Andrey
Turchanin
*c
aFaculty of Physics, University of Bielefeld, 33615 Bielefeld, Germany
bDepartment of Chemistry and Food Chemistry, TU Dresden, 01069 Dresden, Germany. E-mail: zhikun.zheng@tu-dresden.de
cInstitute of Physical Chemistry, Friedrich Schiller University Jena, 07743 Jena, Germany. E-mail: andrey.turchanin@uni-jena.de
dPhysical Chemistry and Center for Advancing Electronics Dresden, TU Dresden, 01069 Dresden, Germany
eInstitute of Chemistry, Technische Universität Berlin, 10623 Berlin, Germany
First published on 9th July 2015
Abstract
van der Waals heterostructures meet other low-dimensional materials. Stacking of about 1 nm thick nanosheets with out-of-plane anchor groups functionalized with fullerenes integrates this zero-dimensional material into layered heterostructures with a well-defined chemical composition and without degrading the mechanical properties. The developed modular and highly applicable approach enables the incorporation of other low-dimensional materials, e.g. nanoparticles or nanotubes, into heterostructures significantly extending the possible building blocks.
Molecular assembly of materials with precise control over their chemical composition, thickness and structure has been in the focus of physical, chemical and materials science research already for many years.1–9 This interest is motivated by engineering materials with controlled functionalities on demand. To this end, various techniques have been developed. Typical examples are Langmuir–Blodgett (LB) technique, layer-by-layer (LbL) assembly, and self-assembled monolayers (SAMs). They have been widely used to assemble zero- and one-dimensional (0D and 1D) materials such as small molecules, nanoparticles, biomolecules, polyelectrolytes and nanotubes/-wires.1–8 With the demonstration of free-standing atomically or single-molecularly thick sheets,10–12 stacking them vertically in a chosen way serves as a new technique to create designed artificial materials, the so-called van der Waals (vdW) heterostructures.9,13,14 Combination of these 2D sheets including graphene, hexagonal boron nitride or metal chalcogenides has led to mechanically stable heterostructures with high potential for applications in sensors and flexible electronics.9,15 Recent research efforts have also been focused on the development of various covalent and non-covalent functionalization approaches to combine 2D sheets with 0D and 1D materials.16–20 However, it remains a major challenge to assemble stable stacks of 0D/1D materials with 2D sheets without degrading the mechanical properties of the pristine sheets. Non-covalent interactions, such as van der Waals interactions and π–π stacking, are not strong enough to stably immobilize 0D/1D materials on 2D sheets. Thus the adhered materials can be easily removed by solvent rinsing or even with time and by environmental moisture.16,19,20 On the other hand, strong covalent interactions require a change in the integral structure of 2D sheets, which degrade their initial mechanical properties.
In this work, we present a modular and broadly applicable route to create hybrid vdW heterostructures made of individual ∼1 nm thick single molecular sheets, Janus nanomembranes (JNMs),13,21 which have well-defined anchor groups on their opposite sides, see Fig. 1, and other low-dimensional materials. JNMs are generated via electron irradiation of 4′-nitro-1,1′-biphenyl-4-thiol (NBPT) SAMs resulting in their crosslinking via formation of lateral covalent bonds and simultaneous conversion of the terminal nitro groups into amino groups22 and the subsequent release from the original substrates via the poly(methyl methacrylate) (PMMA) assisted transfer process.23,24 The upper side of the JNM has amino groups (N-side) and the lower side has sulfur species (S-side), see Fig. 1c; both sides can be independently and chemically functionalized.21 Using chemical functionalization of JNMs with the desired building blocks on their one or both faces and subsequent stacking, hybrid vdW heterostructures can be assembled. In our proof-of-concept experiments, we utilize 0D carbon – fullerene C60 – as a functional nanomaterial and covalently bind to the amino groups of JNMs; we also demonstrate functionalization of the S-side with Au nanoparticles (NPs), see Fig. 1d. We fabricate heterostructure stacks of the C60–JNM hybrid and characterize their structural, chemical and mechanical properties by optical microscopy, helium ion microscopy (HIM), X-ray photoelectron spectroscopy (XPS) and mechanical bulging tests. To make the S-side of JNMs accessible for post-modification under various experimental conditions, a universal flip-over procedure for JNMs was developed.
To assemble C60–JNM heterostructures, we synthesized JNMs on gold substrates (JNM/Au), immobilized C60 onto the N-side of the JNMs, and stacked the C60–JNM hybrids on top of each other (see ESI† for details). The successful immobilization of C60 onto JNM/Au was confirmed by XPS. Fig. 2a shows the XP spectra of a JNM/Au. The C1s signal consists of several peaks with binding energies (BEs) at 284.2 eV and 285.3 eV, which are due to the aromatic carbon and the C–S/C–N bonds, respectively; and the aromatic shake-up satellites at 287–290 eV.24 The N1s signal at 399.3 eV is characteristic for amino groups. The S2p signal shows the presence of two sulfur species with the S2p3/2 BEs at 162.0 and 163.2 eV, which are due to thiolates and sulfides/disulfides formed upon irradiation,25 respectively. The effective thickness of a JNM calculated from the attenuation of the Au4f7/2 signal is about 1.1 nm.26 In Fig. 2b, the XP spectra of a JNM with C60 grafted to the amino groups are presented.27 The successful grafting is confirmed by the corresponding changes of the respective XP signals. The total intensity of the C1s signal increases by ∼30% and a new N1s peak at ∼400.3 eV appears due to the formation of C–N bonds.27–29 The intensity ratio between this peak and the total N1s intensity is ∼30%, which indicates the percentage of amino groups on forming chemical bonds with C60. Intensity of the S2p signal decreases showing an increase of the hybrid thickness (see Table S1† for thickness change).
To demonstrate that C60–JNMs can be released from their original substrate and further used for fabrication of the heterostructures, we tested their transfer onto 285 nm SiO2/Si substrates by the PMMA assisted process.24 These substrates were chosen as they enable the observation of JNMs by optical interference (see Fig. S1a†).14Fig. 2c shows XP spectra of the transferred C60–JNM. The characteristics of the XP signals are similar to those of the pristine C60–JNM/Au. A slight increase of the C1s peak at ∼288.3 eV is observed, which is most likely due to the presence of some PMMA residuals after the transfer.30 The S2p3/2 signal at 162.0 eV disappears and the intensity of the S2p3/2 signal at 163.2 eV increases significantly due to the transformation of thiolates into sulfides/disulfides or into unbound thiols during the transfer process.21 A new doublet appears with S2p3/2 at 167.2 eV caused by the oxidation of thiolates/sulfides/disulfides into the sulfonic group.31 Note that the sulfonic group is negatively charged in water, which can be used for the functionalization of the S-side of JNMs by electrostatic interactions.
For the fabrication of cm2-sized patterned C60–JNM heterostructures on 285 nm SiO2/Si, a simple method was applied. C60–JNM sheets on Au/mica substrates were cut with scissors into rectangular stripes of ∼0.5 cm width and then the sheets were transferred onto the Si-wafer by putting them on top of each other in different orientations. This procedure leads to the formation of regions with either no, one, two or three sheets (Fig. S1a†). Note that the uniform contrast within the areas with varying numbers of C60–JNM sheets reflects their homogeneous thickness.
As only the electron irradiated areas of NBPT SAMs are converted into JNMs and there is no lateral crosslinking between molecules in the non-irradiated areas, it is possible by selective electron irradiation to pattern JNMs, functionalize them with C60 and then transfer the patterned C60–JNM onto a new substrate (Fig. S1b†). Such a procedure makes it possible to produce the JNM-based hybrids in any shape without additional resist materials either by using electron irradiation through stencil masks, as in this experiment, or by standard electron beam lithography.
The assembled heterostructure stacks were characterized by XPS. We found no significant changes in the shapes of C1s, S2p and N1s signals for the bi-layer and the tri-layer of C60–JNM (Fig. 2d and e), which indicates that each layer has a similar chemical composition. The obtained effective thickness of the bi-layer and tri-layer stacks is ∼3.7 nm and ∼5.4 nm, respectively, which confirms further homogeneous contributions of each layer to the total structure. This homogeneity benefits from the high reproducibility by functionalization of mechanically stable JNMs, which is difficult to achieve employing the layer-by-layer growth on conventional SAMs.32–34
To quantitatively characterize the mechanical properties of individual C60–JNMs and their heterostructures, they were studied by mechanical bulge tests. To this end, the sheets were transferred onto a silicon substrate with an array of square shaped orifices. Fig. 3a shows a helium ion microscopy (HIM) image of a C60–JNM hybrid. An orifice with the spanned C60–JNM is observed (marked “freestanding”), which indicates that the hybrid can support its own weight and preserve its mechanical integrity. Apart from the large homogeneous area, some wrinkles or ruptures (Fig. 3b and c) are observed, which are typical for mechanically robust nanosheets.10Fig. 3d shows a homogeneous freestanding JNM/(C60–JNM)3 heterostructure spanning over an orifice with dimensions of 40 × 44 μm2. The heterostructure in Fig. 3e shows some wrinkles, which increase the imaging contrast and help to identify the freestanding membrane by HIM. Note that only the homogeneous structures as in Fig. 3d were employed for bulge tests with an atomic force microscope (AFM). To ensure that the interaction between a (C60–JNM)n and an AFM tip is the same as that for an individual JNM, a JNM was placed on top of the respective heterostructures forming the JNM/(C60–JNM)n stacks. The testing was performed by adjusting an AFM tip to the nanomembrane center,35,36 as schematically shown in Fig. 3f. Different N2 pressures are applied beneath the membrane, and its corresponding deflection is then recorded by AFM. Fig. 3g shows a typical pressure-deflection curve for a JNM/(C60–JNM)2 heterostructure (see Fig. S2† for JNM, C60–JNM and JNM/(C60–JNM)n, n = 1 and 3). By fitting multiple curves to a pressure-deflection equation for rectangular/square membranes,35,36 Young's modulus (EYoung) can be extracted (Fig. 3h and Table S1†). First, we compare the mechanical properties of pristine JNM with C60–JNM. To this end, the in-plane elastic modulus (E2d) has to be considered.37–39 It is equal to EYoung multiplied by the thickness of the sheet. The obtained E2d values for JNM (9.9 ± 1.7 N m−1) and C60–JNM (11.6 ± 1.7 N m−1) are of the same magnitude, which shows that the mechanical robustness of a JNM is not diminished upon its covalent functionalization. Next, we compare the mechanical properties of multilayered stacks considering the respective EYoung values.37EYoung for JNM/(C60–JNM)1, JNM/(C60–JNM)2 and JNM/(C60–JNM)3 are 7.5 ± 1.8 GPa, 7.2 ± 1.3 GPa and 8.7 ± 1.5 GPa, respectively (Fig. 3h). It can be clearly seen that within the measurement accuracy the Young's moduli of the JNM/(C60–JNM)n (n = 1, 2 and 3) heterostructures have similar values demonstrating that the mechanical properties are not degraded upon the assembly of the hybrid JNMs into stacks.
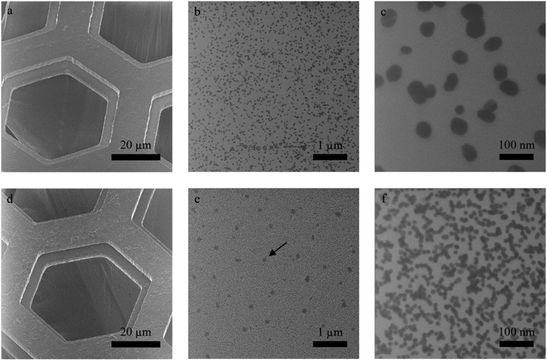
To show that for the assembly of hybrid heterostructures the S-side of JNMs can also be employed, we studied its functionalization with Au NPs (Fig. 1c and Fig. 4). Negatively charged Au NPs with sizes of ∼55 and 16 nm were used to this end. As the S-side of a JNM due to the presence of sulfonic groups is also negatively charged, a positively charged adhesive polyelectrolyte layer of poly(diallyldimethylammonium chloride) was added to immobilize the negatively charged Au NPs. We employed scanning transmission ion microscopy (STIM) to image the hybrid nanomembranes suspending hexagonal grids (∼25 μm) with the functionalized S-side oriented upwards. From statistical analysis we estimate the average coverage of the 55 and 16 nm-sized Au NPs on JNMs to be ∼15% and ∼50%, respectively, showing that the coverage correlates with the NP size. As a wide variety of materials can be immobilized by electrostatic interactions,2 this strategy provides a flexible route to incorporate different materials into JNM-based hybrids. The modification of the S-side of JNMs with the above-described method needs PMMA as a protection layer, which restricts the modification to conditions compatible with this layer. In case such a layer cannot be employed, the JNM can also be flipped over and transferred onto a new solid substrate where the originally bottom S-side becomes the terminal one (Fig. S3–5†).
Conclusions
In summary, we have presented a modular and highly applicable approach for the assembly of cm2-sized vdW hybrid heterostructures. This approach is based on the utilization of ∼1 nm thick bifacial and mechanically stable JNMs, their chemical functionalization and subsequent stacking into layered heterostructures. In the present study the heterostructures of JNMs with C60 and gold nanoparticles were investigated. The possibility of bifacial chemical functionalization of JNMs paves the way to hybrid vdW heterostructures with a variety of other 0D and 1D materials.Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (SPP “Graphene” and Heisenberg Programme), the Volkswagenstiftung and the Alexander von Humboldt Foundation (Sofja Kovalevskaja Award to MCL). Z. Z. and X. Z. contributed equally to this work. We thank Prof. Xinliang Feng (TU Dresden) for fruitful discussions.Notes and references
- K. B. Blodgett, J. Am. Chem. Soc., 1934, 56, 495 CrossRef CAS.
- G. Decher, Science, 1997, 277, 1232 CrossRef CAS.
- K. Ariga, J. P. Hill and Q. Ji, Phys. Chem. Chem. Phys., 2007, 9, 2319 RSC.
- S. Srivastava and N. A. Kotov, Acc. Chem. Res., 2008, 41, 1831 CrossRef CAS PubMed.
- Z. Tang, Y. L. Wang, P. Podsiadlo and N. A. Kotov, Adv. Mater., 2006, 18, 3203 CrossRef CAS.
- J. Sagiv, J. Am. Chem. Soc., 1980, 102, 92 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103 CrossRef CAS PubMed.
- A. Ulman, Introduction to Ultrathin Organic Films, Academic, San Diego, CA, 1991 Search PubMed.
- A. K. Geim and I. V. Grigorieva, Nature, 2013, 499, 419 CrossRef CAS PubMed.
- K. S. Novoselov, D. Jiang, F. Schedin, T. J. Booth, V. V. Khotkevich, S. V. Morozov and A. K. Geim, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 10451 CrossRef CAS PubMed.
- J. Sakamoto, J. van Heijst, O. Lukin and A. D. Schluter, Angew. Chem., Int. Ed., 2009, 48, 1030 CrossRef CAS PubMed.
- W. Eck, A. Küller, M. Grunze, B. Völkel and A. Gölzhäuser, Adv. Mater., 2005, 17, 2583 CrossRef CAS.
- (a) M. Woszczyna, A. Winter, M. Grothe, A. Willunat, S. Wundrack, R. Stosch, T. Weimann, F. Ahlers and A. Turchanin, Adv. Mater., 2014, 26, 4831 CrossRef CAS PubMed; (b) C. T. Nottbohm, A. Turchanin, A. Beyer, R. Stosch and A. Gölzhäuser, Small, 2011, 7, 874 CrossRef CAS PubMed.
- (a) H. Yin, S. Zhao, K. Zhao, A. Muqsit, H. Tang, L. Chang, H. Zhao, Y. Gao and Z. Tang, Nat. Commun., 2015, 6, 6430 CrossRef CAS PubMed; (b) H. Tang, J. Wang, H. Yin, H. Zhao, D. Wang and Z. Tang, Adv. Mater., 2015, 27, 1117 CrossRef CAS PubMed; (c) H. Yin, S. Zhao, J. Wan, H. Tang, L. Chang, L. He, H. Zhao, Y. Gao and Z. Tang, Adv. Mater., 2013, 25, 6270 CrossRef CAS PubMed.
- T. Georgiou, R. Jalil, B. D. Belle, L. Britnell, R. V. Gorbachev, S. V. Morozov, Y.-J. Kim, A. Gholinia, S. J. Haigh, O. Makarovsky, L. Eaves, L. A. Ponomarenko, A. K. Geim, K. S. Novoselov and A. Mishchenko, Nat. Nanotechnol., 2013, 8, 100 CrossRef CAS PubMed.
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156 CrossRef CAS PubMed.
- D. Voiry, A. Goswami, R. Kappera, C. C. C. Silva, D. Kaplan, T. Fujita, M. Chen, T. Asefa and M. Chhowalla, Nat. Chem., 2015, 7, 45 CrossRef CAS PubMed.
- S. Jiang, S. Butler, E. Bianco, O. D. Restrepo, W. Windl and J. E. Goldberger, Nat. Commun., 2014, 5, 1 Search PubMed.
- H. Y. Mao, Y. H. Lu, J. D. Lin, S. Zhong, A. T. S. Wee and W. Chen, Prog. Surf. Sci., 2013, 88, 132 CrossRef CAS.
- J. Du, S. Pei, L. Ma and H.-M. Cheng, Adv. Mater., 2014, 26, 1958 CrossRef CAS PubMed.
- Z. Zheng, C. T. Nottbohm, A. Turchanin, H. Muzik, A. Beyer, M. Heilemann, M. Sauer and A. Gölzhäuser, Angew. Chem., Int. Ed., 2010, 49, 8493 CrossRef CAS PubMed.
- W. Eck, V. Stadler, W. Geyer, M. Zharnikov, A. Gölzhäuser and M. Grunze, Adv. Mater., 2000, 12, 805 CrossRef CAS.
- A. Turchanin and A. Gölzhäuser, Prog. Surf. Sci., 2012, 87, 108 CrossRef CAS.
- A. Turchanin, A. Beyer, C. T. Nottbohm, X. Zhang, R. Stosch, A. S. Sologubenko, J. Mayer, P. Hinze, T. Weimann and A. Gölzhäuser, Adv. Mater., 2009, 21, 1233 CrossRef CAS.
- A. Turchanin, D. Käfer, M. El-Desawy, C. Wöll, G. Witte and A. Gölzhäuser, Langmuir, 2009, 25, 7342 CrossRef CAS PubMed.
- M. Schnietz, A. Turchanin, C. T. Nottbohm, A. Beyer, H. H. Solak, P. Hinze, T. Weimann and A. Gölzhäuser, Small, 2009, 5, 2651 CrossRef CAS PubMed.
- P. J. Moriarty, Surf. Sci. Rep., 2010, 65, 175 CrossRef CAS.
- K. M. Chen, W. B. Caldwell and C. A. Mirkin, J. Am. Chem. Soc., 1993, 115, 1193 CrossRef CAS.
- W. B. Caldwell, K. Chen, C. A. Mirkin and S. J. Babinec, Langmuir, 1993, 9, 1945 CrossRef CAS.
- A. Pirkle, J. Chan, A. Venugopal, D. Hinojos, C. W. Magnuson, S. McDonnell, L. Colombo, E. M. Vogel, R. S. Ruoff and R. M. Wallace, Appl. Phys. Lett., 2011, 99, 122108 CrossRef.
- A. P. Terzyk, Colloids Surf., A, 2001, 177, 23 CrossRef CAS.
- S. Heid, F. Effenberger, K. Bierbaum and M. Grunze, Langmuir, 1996, 12, 2118 CrossRef CAS.
- N. Tillman, A. Ulman and T. L. Penner, Langmuir, 1989, 5, 101 CrossRef CAS.
- H. Sugimura, H. Yonezawa, S. Asai, Q. W. Sun, T. Ichii, K. H. Lee, K. Murase, K. Noda and K. Matsushige, Colloids Surf., A, 2008, 321, 249 CrossRef CAS.
- X. H. Zhang, A. Beyer and A. Gölzhäuser, Beilstein J. Nanotechnol., 2011, 2, 826 CrossRef CAS PubMed.
- X. H. Zhang, C. Neumann, P. Angelova, A. Beyer and A. Gölzhäuser, Langmuir, 2014, 30, 8221 CrossRef CAS PubMed.
- C. Lee, X. Wie, J. W. Kysar and J. Hone, Science, 2008, 521, 385 CrossRef PubMed.
- Z. Zheng, C. S. Ruiz-Vargas, T. Bauer, A. Rossi, P. Payamyar, F. Schiffmann, J. VandeVondele, A. Stemmer, J. Sakamoto and A. D. Schlüter, Macromol. Rapid Commun., 2013, 34, 1670 CrossRef CAS PubMed.
- Z. Zheng, L. Opilik, F. Schiffmann, W. Liu, G. Bergamini, P. Ceroni, L. Lee, A. Schütz, J. Sakamoto, R. Zenobi, J. VandeVondele and A. D. Schlüter, J. Am. Chem. Soc., 2014, 136, 6103 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nr03475b |
| ‡ Current address: CNM Technologies, 33602 Bielefeld, Germany. |
| This journal is © The Royal Society of Chemistry 2015 |