Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thiols as interfacial modifiers to enhance the performance and stability of perovskite solar cells†
Jing
Cao
a,
Jun
Yin
ab,
Shangfu
Yuan
a,
Yun
Zhao
a,
Jing
Li
b and
Nanfeng
Zheng
*a
aState Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, Engineering Research Center for Nano-Preparation Technology of Fujian Province, and Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. E-mail: nfzheng@xmu.edu.cn; Fax: +86-592-2183047
bPen-Tung Sah Institute of Micro-NanoScience and Technology, Xiamen University, Xiamen 361005, China
First published on 24th April 2015
Abstract
Modifying the interfaces of CH3NH3PbI3 with TiO2 and hole transport layers using two different types of thiols leads to enhanced performance and stability of perovskite solar cells. The incorporation of HOOC–Ph–SH at the TiO2/perovskite interface facilitates electron transfer from perovskite to TiO2 and also alters the morphology of perovskite crystal growth to increase the power conversion efficiency. The modification of pentafluorobenzenethiol at the perovskite/hole transport layer interface improves the stability.
Depletion of fossil fuels has led to considerable development in many areas related to photovoltaic cells based on earth-abundant materials. Only within several years, perovskite solar cells (PSSCs) are emerging as one of the most promising candidates for the next generation of cost-effective solar cells with high efficiency.1–7 Interfacial engineering over the interfaces of the organometal trihalide perovskite layer with the hole and electron extracting layers has been demonstrated to be critical for optimizing the overall performance of PSSCs. With respect to CH3NH3PbI3, the TiO2/Spiro-OMeTAD couple with good optical transparency and perfect band alignment has become one of the most successful interfacial combinations.2–6,8–12As for PSSCs based on the mesoporous TiO2, the efficient contact of the interface between porous titania and perovskite is of paramount importance for their performance as well.
In conventional dye-sensitized solar cells (DSSCs), organic dyes bearing the anchoring groups, such as carboxylic acid moieties, can bind onto the TiO2 surface to facilitate the electron injection, improving the efficiency of DSSCs.13–15 With this motivation, organic HOOCRNH3+I− anchor molecules,16 silane self-assembled monolayers17 and 3-aminopropanoic acid SAM onto the sol–gel ZnO18 have also been successfully introduced at the TiO2/perovskite interface to optimize the overall performance of PSSCs. In most of these studies, alkyl chains are typically present between the binding sites of the anchor molecules involved. It has been well documented that the π-electron acceptors readily facilitate the better electron transfer in DSSCs.19–21 In this context, we have proposed that the interfacial modification of the TiO2/CH3NH3PbI3 interface with carboxylic acid–thiol ligands (HOOC–Ph–SH) containing a π-electron unit would provide an effective route to improve the conversion efficiency of PSSCs.
Moreover, the poor long-term stability, in particular, when subjected to environmental stress such as light-induced heat and moisture, is the main challenging issue for the commercialization of PSSCs. To address this issue, various strategies, such as controlling the crystallization process of perovskite film,22–24 introducing the insulating polymer onto the perovskite film25 and replacing the classic Spiro-OMeTAD with other hole-transports,26–28 have been investigated. However, the inherent vulnerability of methyl-ammonium lead halide lies in the highly hygroscopic methyl-ammonium cations. Moisture easily breakdowns the structure of methylammonium lead halide.25 In this work, we also report the use of hydrophobic thiols to enhance the stability of the PSSCs. Thiols containing highly hydrophobic motifs (e.g., HS–C6F5) were used to cover the perovskite surface by Pb–S coordination (Fig. 1), creating a fully hydrophobic molecular layer to inhibit the intrusion of water molecules into the perovskite film. Such a simple treatment led to much enhanced stability of PSSCs in air and under AM 1.5 G solar light illumination as well.
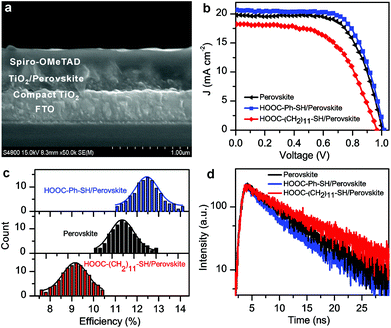
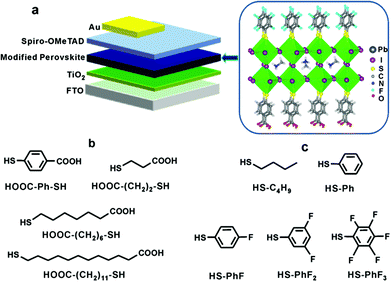 | ||
| Fig. 1 (a) The schematic structure of the perovskite solar cell modified by HOOC–R–SH and HS–R′ whose molecular structures are shown in (b) and (c), respectively. | ||
In this work, the entire fabrication procedure of perovskite solar devices was carried out in open air at a common relative humidity of 45%. As shown in Fig. 1a, the fabricated device adopted the commonly used mesoporous configuration, with thiol-modifications at the interfaces of mesoporous-TiO2/perovskite and perovskite/Spiro-OMeTAD as well. In a typical procedure, a compact titania layer was deposited on a FTO (fluorine doped tin oxide) conducting glass substrate, which was followed by the deposition of a mesoporous TiO2 layer of 200 nm thickness to enhance electron extraction and transport. Our idea of introducing ligands bearing both carboxylic acid and thiol groups (HOOC–Ph–SH as presented in Fig. 1b) at the interface between porous titania and perovskite layers was to enhance their contact, thus enhancing the electron transfer. Experimentally, the as-obtained TiO2 mesoporous films were treated with HOOC–R–SH before the deposition of the perovskite layer. The deposited perovskite film was then treated with thiols having hydrophobic groups (HS–R′, as shown in Fig. 1c) to create a hydrophobic molecular layer. The main purpose of such a layer is to block the entrance of water molecules into the perovskite film, thus enhancing its stability against humidity. Finally, a layer of p-type organic hole-conductor Spiro-OMeTAD and the gold cathode were deposited on the top to complete the fabrication. The detailed procedures are given in the ESI.† The structures of the fabricated perovskite devices were characterized by XRD (Fig. S1†), UV-Vis (Fig. S2†) and SEM (Fig. 2a, S3 and S4†). All these data indicated that the perovskite structures were well-formed in all of the fabricated solar cells.29,30
To confirm the successful anchoring of carboxylic acid–thiol between mesoporous TiO2 and perovskite, FTIR spectra of films during the fabrication of devices were recorded (Fig. S5†). In the case where HOOC–Ph–SH was used as a molecular solder for the mesoporous TiO2 and perovskite, the HOOC–Ph–SH/perovskite film displayed an absorption peak at 1697 cm−1 which can be assigned to COO stretching. The disappearance of the absorption peak at 2581 cm−1 and the appearance of the absorption peak at 435 cm−1 suggests the coordination of SH-groups to Pb on the perovskite. The peak at 435 cm−1 can be assigned to the Pb–S coordination-function.31 These results demonstrated the successful anchoring of carboxylic acid–thiol between TiO2 and perovskite with –COOH bound to TiO2 and –SH bound to Pb on the perovskite.
In order to demonstrate the effect of carboxylic acid–thiol linkers on the electron transfer at the TiO2/perovskite interface, the overall performance of the FTO/TiO2/HOOC–R–SH/perovskite/Spiro-OMeTAD devices (Fig. 2a) was measured under AM 1.5, 100 mW cm−2 light illumination. The incident photon-to-current conversion efficiency spectra (IPCE) of the cells spanned from the UV region to 800 nm (Fig. S6†), matching well with their UV-Vis absorption spectra (Fig. S2†). As illustrated in Fig. 2b, c, S7 and Table S1,† the incorporation of HOOC–Ph–SH at the TiO2/perovskite interface did enhance the overall performance of the solar cells. The best efficiency of 14.1% was observed from the solar cells fabricated with HOOC–Ph–SH. Measurements over 100 fabricated cells gave an average efficiency of 12.4 ± 1.8%. In comparison, 100 devices fabricated without HOOC–Ph–SH offered an average efficiency of 11.6 ± 1.6% with the best device reaching a power-conversion efficiency of 13.2%. The best device was fabricated with the HOOC–Ph–SH ligand bearing the π-electron motif with the short-circuit current density (Jsc), open-circuit voltage (Voc) and fill factor (FF) being 20.66 mA cm−2, 1.02 V and 0.67, respectively.
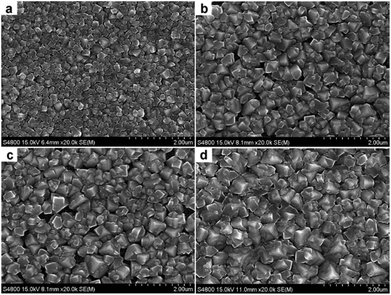
To further confirm the enhanced electron transfer in the HOOC–R–SH modified perovskite solar cells, the transient photoluminescence (PL) measurements were also carried out using the steady state and time-resolved fluorescence method (Fig. 2d). Interestingly, compared to the perovskite-only film with a decay time of τ = 9.3 ns, the film with the HOOC–Ph–SH modification exhibited a relatively short transient decay time of 7.6 ns. While, the film with HOOC–(CH2)11–SH had a longer decay time of 11.9 ns. The longer lifetime for the HOOC–(CH2)11–SH modified cell may have been caused by the weak electron transfer ability due to the presence of the long alkyl chain between –COOH and –SH motifs, therefore leading to a faster electron recombination. The relatively short lifetime for the cells having HOOC–Ph–SH bound at the interface between the TiO2 and CH3NH3PbI3 layers suggested a π-electron transport-method to retard the charge recombination, resulting in an overall enhanced photovoltaic performance. It should also be noted that the introduction of HOO–R–SH binders altered the growth behavior of perovskites. As shown in the top-view SEM images of the as-prepared HOOC–R–SH modified perovskite films (Fig. 3), the CH3NH3PbI3 nanocrystals supported on the TiO2 are evidently larger in size, compared to that of the perovskite-only sample. At this point, it is still unclear how the HOOC–R–SH modification promotes the electron transfer from perovskite to TiO2. More work is still needed to determine whether the overall improved efficiency originated from the electronic effect, the morphological effect or both.
The long-term stability is among the biggest remaining challenges in perovskite solar cells. Water, which is a Lewis base, readily combines with CH3NH3PbI3 and removes the ammonium from the perovskite structure, leading to an easy breakdown of the perovskite framework. While the thiol modification at the TiO2/perovskite interface helped to promote the electron transfer to some extent, such a modification did not enhance cells’ stability (Fig. S8†). Owing to the strong coordination of thiol on Pb2+, employing hydrophobic thiols to create a molecular protection layer on perovskite might be an effective method to stabilize perovskite solar cells against humidity. Following this idea, we applied a series of HS–R′ ligands (Fig. 1c) to decorate the perovskite films before the deposition of Spiro-OMeTAD. With such a modification, the surface of the perovskite nanocrystallites was slightly eroded (Fig. 3d). The successful modification of thiol on the perovskite surface was confirmed by the disappearance of the IR absorption peak of –SH at 2581 cm−1 and the absorption peak of Pb–S at 435 cm−1 (Fig. S5†).
As shown in the I–V and IPCE curves of the most-efficient cells (Fig. 4, S9 and S10†), the features of photocurrent responses in the range from 300 nm to 800 nm with the maximum efficiency of over 80% roughly resemble their respective absorption spectra (Fig. S2†). The modification of hydrophobic thiols on perovskite did not alter the average performance of the cells (Fig. 4b and S10†), although the best efficiencies slightly decreased. Compared to the cells without the HS–R′ modification, the cells with the modification exhibited poorly fill factors and low voltages, which might be explained by the fact that the surface modification of thiols on the perovskite surface retarded the efficient contact between the perovskite and hole transport layers.
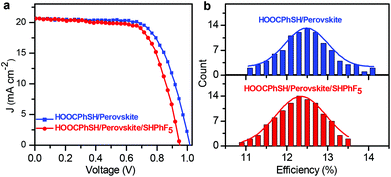 | ||
| Fig. 4 (a) The I–V characteristics of the best cells and (b) the performance distributions of 100 individual devices of HOOC–Ph–SH/perovskite and HOOC–Ph–SH/perovskite/HS–R′. | ||
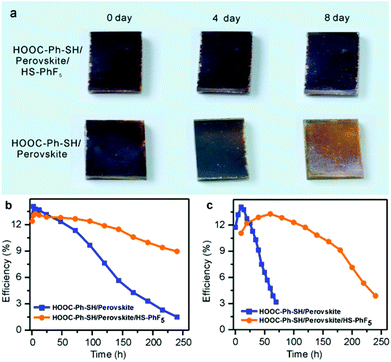
Without causing much deleterious effects to the cells’ overall performance, the surface modification of perovskite by “water-resistant” HS–R′ ligands offers an effective protection method to improve the stability of perovskite solar cells. As shown in Fig. 5a, when stored in air at room temperature with a humidity of about 45%, the perovskite solar cells with the encapsulation of HS–PhF5 showed no obvious color change in 8 days. In comparison, in the same period, the cells without the thiol encapsulation decayed to a great extent and the color faded from dark to yellow. Accordingly, the HS–R′ modification experienced a marked increase in stability, especially the HS–PhF5 ligand bearing hydrophobic fluorine units (Fig. 5b and S11†). The devices with the HS–PhF5 modification retained over 80% of their original efficiency after 10-day storage in air with a relative humidity of ∼45%. In contrast, under the same storage conditions, the cells with thiol modification lost ∼80% of their efficiency after 10 days. The cells with thiol modification exhibited much enhanced stability under illumination in humid air as well. As under illumination at AM 1.5 G (Fig. 5c and S12†), the cells without thiol modification lost 50% of their activities within 50 min. In comparison, the time for the cells with the HS–PhF5 to lose 50% of their original efficiencies was extended beyond 200 min. These results nicely demonstrate that the deposition of hydrophobic thiol at the interface between perovskite and hole transport layers can efficiently inhibit the escape of methylammonium and entrance of water molecules, providing an effective molecular sealing approach to enhance the stability of perovskite solar cells.
Conclusions
In conclusion, an effective interfacial modification route using thiols has been developed to improve both performance and stability of perovskite solar cells. The chemical modification at the TiO2/perovskite interface by HOOC–Ph–SH facilitated the growth of bigger perovskite crystallites and enhanced the transfer of photo-generated electrons from perovskite to TiO2 as well. With such a modification, the best photon-to-current conversion efficiency of 14.1% was observed for the perovskite solar cells fabricated under ambient conditions. Furthermore, modifying the perovskite/Spiro-OMeTAD interface with hydrophobic thiols, especially HS–PhF5 remarkably improved the stability of PSSCs in air or under AM 1.5 G solar light illumination. The developed interfacial modification approach should be effective to enhance both conversion efficiency and stability of perovskites having other architectures.Acknowledgements
We thank the MOST of China (2011CB932403) and the NSFC of China (21420102001, 21131005, 21390390) for financial support.Notes and references
- J.-H. Im, C.-R. Lee, J.-W. Lee, S.-W. Park and N.-G. Park, Nanoscale, 2011, 3, 4088–4093 RSC.
- H.-S. Kim, C.-R. Lee, J.-H. Im, K.-B. Lee, T. Moehl, A. Marchioro, S.-J. Moon, R. Humphry-Baker, J.-H. Yum, J. E. Moser, M. Grätzel and N.-G. Park, Sci. Rep., 2012, 2, 1–7 Search PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- M. M. Lee, J. Teuscher, T. Miyasaka, T. N. Murakami and H. J. Snaith, Science, 2012, 338, 643–647 CrossRef CAS PubMed.
- M. Liu, M. B. Johnston and H. J. Snaith, Nature, 2013, 501, 395–398 CrossRef CAS PubMed.
- W. Nie, H. Tsai, R. Asadpour, J.-C. Blancon, A. J. Neukirch, G. Gupta, J. J. Crochet, M. Chhowalla, S. Tretiak and M. A. Alam, Science, 2015, 347, 522–525 CrossRef CAS PubMed.
- H. Zhou, Q. Chen, G. Li, S. Luo, T.-b. Song, H.-S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Science, 2014, 345, 542–546 CrossRef CAS PubMed.
- S. Dharani, H. K. Mulmudi, N. Yantara, P. T. Thu Trang, N. G. Park, M. Grätzel, S. Mhaisalkar, N. Mathews and P. P. Boix, Nanoscale, 2014, 6, 1675–1679 RSC.
- M. A. Green, A. Ho-Baillie and H. J. Snaith, Nat. Photonics, 2014, 8, 506–514 CrossRef CAS PubMed.
- S. Kazim, M. K. Nazeeruddin, M. Grätzel and S. Ahmad, Angew. Chem., Int. Ed., 2014, 53, 2812–2824 CrossRef CAS PubMed.
- P. Qin, A. L. Domanski, A. K. Chandiran, R. Berger, H. J. Butt, M. I. Dar, T. Moehl, N. Tetreault, P. Gao, S. Ahmad, M. K. Nazeeruddin and M. Grätzel, Nanoscale, 2014, 6, 1508–1514 RSC.
- L. Zhu, J. Xiao, J. Shi, J. Wang, S. Lv, Y. Xu, Y. Luo, Y. Xiao, S. Wang, Q. Meng, X. Li and D. Li, Nano Res., 2015 DOI:10.1007/s12274-12014-10592-y.
- P. Wang, S. M. Zakeeruddin, J. E. Moser, M. K. Nazeeruddin, T. Sekiguchi and M. Grätzel, Nat. Mater., 2003, 2, 402–407 CrossRef CAS PubMed.
- Z. Yao, M. Zhang, H. Wu, L. Yang, R. Li and P. Wang, J. Am. Chem. Soc., 2015, 137, 3799–3802 CrossRef CAS PubMed.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and G. Michael, Science, 2011, 334, 629–633 CrossRef CAS PubMed.
- Y. Ogomi, A. Morita, S. Tsukamoto, T. Saitho, Q. Shen, T. Toyoda, K. Yoshino, S. S. Pandey, T. Ma and S. Hayase, J. Phys. Chem. C, 2014, 118, 16651–16659 CAS.
- L. Liu, A. Mei, T. Liu, P. Jiang, Y. Sheng, L. Zhang and H. Han, J. Am. Chem. Soc., 2015, 137, 1790–1793 CrossRef CAS PubMed.
- L. Zuo, Z. Gu, T. Ye, W. Fu, G. Wu, H. Li and H. Chen, J. Am. Chem. Soc., 2015, 137, 2674–2679 CrossRef CAS PubMed.
- W. M. Campbell, A. K. Burrell, D. L. Officer and K. W. Jolley, Coord. Chem. Rev., 2004, 248, 1363–1379 CrossRef CAS PubMed.
- S. Fukuzumi and T. Kojima, J. Mater. Chem., 2008, 18, 1427 RSC.
- Q. Wang, W. M. Campbell, E. E. Bonfantani, K. W. Jolley, D. L. Officer, P. J. Walsh, K. Gordon, R. Humphry-Baker, M. K. Nazeeruddin and M. Grätzel, J. Phys. Chem. B, 2005, 109, 15397–15409 CrossRef CAS PubMed.
- S. Bai, Z. Wu, X. Wu, Y. Jin, N. Zhao, Z. Chen, Q. Mei, X. Wang, Z. Ye, T. Song, R. Liu, S.-t. Lee and B. Sun, Nano Res., 2014, 7, 1749–1758 CrossRef CAS.
- L. Chen, F. Tang, Y. Wang, S. Gao, W. Cao, J. Cai and L. Chen, Nano Res., 2015, 8, 263–270 CrossRef CAS PubMed.
- F. X. Xie, D. Zhang, H. Su, X. Ren, K. S. Wong, M. Grätzel and W. C. H. Choy, ACS Nano, 2015, 1, 639–646 CrossRef PubMed.
- S. N. Habisreutinger, T. Leijtens, G. E. Eperon, S. D. Stranks, R. J. Nicholas and H. J. Snaith, Nano Lett., 2014, 14, 5561–5568 CrossRef CAS PubMed.
- S. Lv, L. Han, J. Xiao, L. Zhu, J. Shi, H. Wei, Y. Xu, J. Dong, X. Xu, D. Li, S. Wang, Y. Luo, Q. Meng and X. Li, Chem. Commun., 2014, 50, 6931–6934 RSC.
- A. Mei, X. Li, L. Liu, Z. Ku, T. Liu, Y. Rong, M. Xu, M. Hu, J. Chen and Y. Yang, Science, 2014, 345, 295–298 CrossRef CAS PubMed.
- Z. Wu, S. Bai, J. Xiang, Z. Yuan, Y. Yang, W. Cui, X. Gao, Z. Liu, Y. Jin and B. Sun, Nanoscale, 2014, 6, 10505–10510 RSC.
- J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M. K. Nazeeruddin and M. Grätzel, Nature, 2013, 499, 316–319 CrossRef CAS PubMed.
- Y. Wu, A. Islam, X. Yang, C. Qin, J. Liu, K. Zhang, W. Peng and L. Han, Energy Environ. Sci., 2014, 7, 2934–2938 CAS.
- R. A. Nyquist, C. L. Putzig, R. O. Kagel and M. A. Leugers, Infrared spectra of inorganic compounds, Academic Press, New York, 1971 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details of the XRD, UV-vis spectra, cross-sectional SEM images and the EQE spectra of the cells. See DOI: 10.1039/c5nr01820j |
| This journal is © The Royal Society of Chemistry 2015 |