Open Access Article
Open Access ArticleMechanical tuning of conductance and thermopower in helicene molecular junctions
Jaroslav
Vacek
*a,
Jana Vacek
Chocholoušová
a,
Irena G.
Stará
a,
Ivo
Starý
a and
Yonatan
Dubi
*b
aInstitute of Organic Chemistry and Biochemistry, v.v.i., Academy of Sciences of the Czech Republic, Flemingovo nám. 2, 16610 Prague 6, Czech Republic. E-mail: vacek@uochb.cas.cz
bDepartment of Chemistry and the Ilze-Katz Institute for Nano-Scale Science and Technology, Ben-Gurion University of the Negev, Beer-Sheva 84105, Israel. E-mail: jdubi@bgu.ac.il
First published on 14th April 2015
Abstract
Helicenes are inherently chiral polyaromatic molecules composed of all-ortho fused benzene rings possessing a spring-like structure. Here, using a combination of density functional theory and tight-binding calculations, it is demonstrated that controlling the length of the helicene molecule by mechanically stretching or compressing the molecular junction can dramatically change the electronic properties of the helicene, leading to a tunable switching behavior of the conductance and thermopower of the junction with on/off ratios of several orders of magnitude. Furthermore, control over the helicene length and number of rings is shown to lead to more than an order of magnitude increase in the thermopower and thermoelectric figure-of-merit over typical molecular junctions, presenting new possibilities of making efficient thermoelectric molecular devices. The physical origin of the strong dependence of the transport properties of the junction is investigated, and found to be related to a shift in the position of the molecular orbitals.
1. Introduction
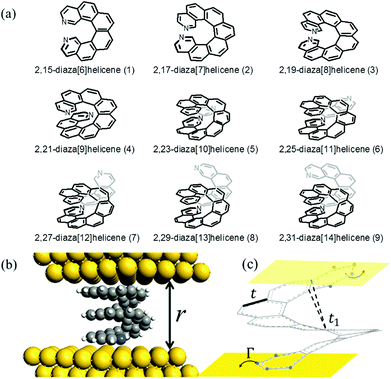
Interest in single-molecule junctions arose from their originally proposed role as functional elements in electronic devices.1 In recent years it has been realized that molecular junctions (MJs) can potentially fulfill additional functionalities ranging from opto-electronics and spintronics to thermoelectric energy conversion,2–4 which is the focus of this work. The thermopower and the thermoelectric figure of merit (FOM) are measures of a junction's ability to convert a temperature difference into electric power, and MJs were suggested as candidates for efficient and high-power thermoelectric devices5–14 due to their low dimensionality, large versatility and low thermal conductance.4,15,16 While there have already been several demonstrations of thermoelectric energy conversion in single-molecule junctions,17–24 unfortunately the typical thermopower is rather small (S ∼ 5–50 μV K−1), much smaller than that of commercially available semi-conductor-based thermoelectrics. Increasing the thermopower of molecular junctions is an essential step if MJs are ever to become relevant energy conversion technologies. The natural question that arises in this context is then: is there a way to in situ increase the thermopower, S, and the thermoelectric FOM, ZT, of MJs?In this letter, we propose to focus on helicene-based molecular junctions (HMJs), which can be used as a tool to help answer the above question. [n]Helicenes are aromatic compounds in which n benzene rings or other (hetero)aromatics are angularly annulated to give helically-shaped molecules with a spring-like structure (Fig. 1(a)). The chemistry of helicenes has been systematically explored for more than half a century (for recent reviews see, e.g.ref. 25–28), and a renewed and growing interest in helical aromatics is clearly visible within the last decade. Although the number of applications of helicenes is so far rather limited, they have already found astonishing applications to enantioselective organo- or transition metal catalysis,29,30 molecular recognition,31,32 self-assembly,33 nonlinear optical materials,34–36 chiral materials,37–39 and chiroptical electronic devices.40–42 Furthermore, as a first step towards realizing single-molecule junctions, helicenes have been placed on surfaces and probed with atomic force and scanning tunneling microscopes.43–50
We suggest that the distance r between the two metallic electrodes of a helicene molecular junction (Fig. 1(b)) can be used as an experimental control parameter that can be tuned to alter and probe the properties of the HMJ. Due to the spring-like structure of the helicene molecule, when the junction is compressed, i.e., the distance between the electrodes of the junction is reduced, the couplings between the carbon orbitals on the benzene rings increase, opening additional transport paths for the electrons and coherently changing the electronic properties of the MJ. Regulation of the electrode distance in molecular junctions has already been introduced as a means to control and measure the molecular forces and couplings51–54 (interestingly, fullerene C60 was already been demonstrated to minimize its resistance and become almost transparent for tunneling electrons if the molecule is sufficiently squeezed by an STM tip).55 However, in the studies mentioned above, the dominant origin for changes in transport is the change in the molecule–electrode coupling, while the molecular electronic structure remains largely unchanged. In a nonplanar molecule, on the other hand, pulling or stretching should affect both the molecular orbitals and the couplings. Nevertheless, there is a lack of suitable models of nonplanar molecules to systematically investigate the stretch- or compression-dependent single molecule conductance.
We demonstrate our conceptual experiment by using density-functional theory (DFT)-based transport calculations to extract the transmission function of a HMJ for different values of the inter-electrode distance, r. To demonstrate how the helicene electronic structure depends on r, we then use a tight-binding (TB) model to fit the DFT data. Next, the TB model and parameters are used to predict the thermopower and ZT of helicene molecular junctions as a function of r and helicene length, demonstrating that these can be optimized to obtain maximal thermoelectric efficiency. It is important to point out early on that we are aiming to generate qualitative rather than quantitative predictions, and therefore the use of a TB approximation is justified. However, even when using the first-principle DFT calculation, the predictions can only be qualitative due to the lack of appropriate functionals and benchmark DFT calculations for helicenes.
2. Methods
2.1. Molecular system under study
We studied diazahelicenes with the hetero-atoms in position 2 (symmetrically on both sides). These systems were selected for three reasons: (i) they are fully aromatic and highly conductive, (ii) they are synthetically accessible and were previously prepared by some of the authors56 and (iii) they provide for reasonably good binding to gold surfaces via the nitrogen functionalities.57–59 For these reasons, azamolecules such as 4,4′-bipyridine are often used in single molecule conductance experiments, for example break junction measurements.60 Based on our DFT calculations, position 2 in helicenes appears more suitable for binding than, for example, position 1, for steric reasons.To achieve conductance and thermopower tuning and switching we used two additional parameters. One is helicene length ([n]) which was varied between 6 and 14 aromatic rings and the other is helicene stretch/compression (Δr). Out of the 9 total molecular systems of different number of rings, 2,15-diaza[6]helicene (1) to 2,31-diaza[14]helicene (9), only one was completely investigated by DFT methods. This was the 2,21-diaza[9]helicene (4) (Fig. 1(a)) from the middle of the series. Here we calculated all the stretched and compressed geometries, energies, and transmission spectra. Due to the system size, the calculations were very lengthy and calculation of all 9 molecular structures would be impractical. This was the motivation to use a simple tight-binding model to study the remaining systems at much lower computational cost. The tight-binding model was, however, found to reproduce the DFT data surprisingly well. Chirality issues were not a subject of this study, and all the investigated systems were of (M) helicity.
2.2. Density-functional-theory calculation
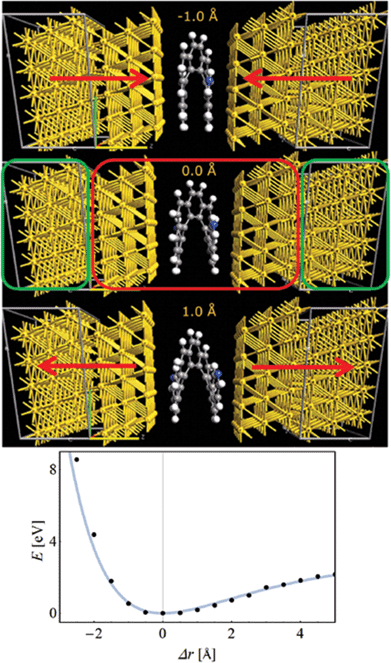
We considered a 2,21-diaza[9]-helicene (4) placed between two Au(111) electrodes. Prior to any periodic DFT calculations, all molecular structures were optimized in a vacuum at the B97D/cc-pVDZ level of theory using the G09 package.61 QuantumWise Atomistix toolkit (ATK)62–64 was then employed in all subsequent calculations described below. First, the HMJ device was constructed as a “two-probe system” within the Virtual Nano Lab (VNL) module of ATK. Here, the HMJ consists of three regions: the left electrode (bulk Au), the so-called central region and the right electrode (bulk Au), cf. Fig. 2, top panel. In the central region the helicene molecule is sandwiched between six surface layers of Au(111), i.e. with three Au layers at both sides of the molecule. The unit cell size of the left and right electrodes in the plane parallel to the gold surface was 5 × 5 Au atoms. The entire two-probe system was then optimized and the electrode separation relaxed. This resulted in our reference equilibrium (Δr = 0, corresponding to an electrode separation of r ∼8.5 Å) geometry from which we started the stretching and squeezing by just adjusting the electrode separation in 0.5 Å steps. The total compression attempted was 3.5 Å while the stretching continued until the molecule broke off the electrodes at 7.0 Å elongation. Only in the optimal configuration the unit cell was allowed to fully relax, so only limited deformations of the gold electrodes were allowed. The system was re-optimized at each step with the electrode separation constrained. DFT-PBE/SZP level of theory with 2 × 2 × 50 k-point sampling was used for all optimizations while DFT-PBE/DZP with 3 × 3 × 100 k-point sampling was used for transmission spectra evaluation. To check the numerical accuracy, optimizations with larger k-point sampling (3 × 3 × 100) were also tried with no difference in the resulting geometries. The bottom panel of Fig. 2 shows the total energy E as a function of Δr. The forces required for stretching and squeezing can be estimated from the energy curve to be ∼1 nN and ∼5 nN, respectively.The DFT results indicate that helicenes tend to lay flat on the electrode surface whenever possible.26,65 At least one aromatic ring (but more than one for most geometries) tends to form contact with the electrodes for the electrode separations used in the TB and thermopower calculations, cf. Fig. 2. Of course, the contact weakens as we stretch the molecule out but a four-point contact model appeared to be applicable throughout the stretched/compressed structures used in this paper (Fig. 1(c)).
2.3. Transport calculation within the tight-binding model
Once the Hamiltonian and self-energies are determined (eqn (1) and (2)), all the transport properties can be determined within the Landauer formalism.66–68 The Green's functions are determined via Gr,a = (E −![[script letter H]](https://www.rsc.org/images/entities/char_e142.gif) M + Σr,a)−1, where Σr,a = Σr,aT + Σr,aB, corresponding to the self-energies of the top and bottom electrodes. The transmission function is given by T(E) = Tr(ΣrTGrΣaBGa), and the transport coefficients – the conductance G, the thermopower S, and the thermal conductance κ – are determined within the Landauer formalism as G = e2L0,
M + Σr,a)−1, where Σr,a = Σr,aT + Σr,aB, corresponding to the self-energies of the top and bottom electrodes. The transmission function is given by T(E) = Tr(ΣrTGrΣaBGa), and the transport coefficients – the conductance G, the thermopower S, and the thermal conductance κ – are determined within the Landauer formalism as G = e2L0, 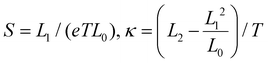 , where T is the temperature (room temperature is considered hereafter) and
, where T is the temperature (room temperature is considered hereafter) and 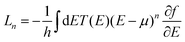 are the Landauer integrals, with h being Planck's constant, μ the chemical potential of the electrodes (set as the zero of energy hereafter), and f(E) the Fermi–Dirac distributions. The thermoelectric figure of merit ZT is given by
are the Landauer integrals, with h being Planck's constant, μ the chemical potential of the electrodes (set as the zero of energy hereafter), and f(E) the Fermi–Dirac distributions. The thermoelectric figure of merit ZT is given by 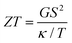 .
.
3. Results: tuning conductance and thermopower
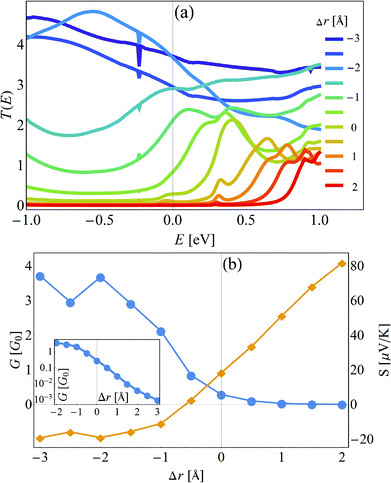
The starting point is thus a DFT-based calculation of the transmission through a helicene molecular junction, formed by 2,21-diaza[9]helicene (4) and two gold(1,1,1) electrodes. The transmission curves were calculated with the combined DFT-non-equilibrium Green's function approach.62–64Fig. 3(a) shows the transmission curves T(E) as a function of energy E for different inter-electrode distances r, incrementally changed about Δr = −3, −2.5, −2, …, 1, 1.5 Å, measured from the relaxed inter-electrode distance r (it is important to point here that Δr = 0 is the distance where the DFT calculation finds the energy minimum, but may not correspond to any experimental distance, since in any experimental setting the distance will be controlled by an external force). The most striking feature of Fig. 3(a) is the apparent closing of the transmission gap between the highest occupied and lowest unoccupied molecular orbitals (HOMO and LUMO, respectively) upon compression of the molecular junction, which transforms the MJs electronic nature from “insulating” to “metallic”, by which we mean resonant tunneling (close to a molecular level). Consequently, the conductance changes by more than three orders of magnitude. In Fig. 3(b) the conductance (in units of the conductance quantum G0 = 2e2/h) and the thermopower are plotted as a function of the distance r. The conductance shows a switching behavior with an “on”/“off” ratio of more than three orders of magnitude (inset of Fig. 3(b)), suggesting that HMJs can perform as reversible, nanoscale molecular switches.54,69–77 The thermopower shows a sign-change, a direct experimental prediction. This is an important feature, since a sign-change of the thermopower implies a change in the transport mechanism of the junction,4 yet such a change is hard to demonstrate in situ.To understand the origin of this change in the helicene electronic structure, we constructed a TB description of the system. The use of TB models to complement DFT calculations is rather common (see, e.g.ref. 78–81), and is useful for several reasons: (i) DFT calculations may become extremely computationally expensive and lengthy, (ii) the TB model allows for interpretation of the DFT calculation in terms of a simple physical picture, which is in many cases hard to build from the output of the DFT calculation (typically energies, orbitals and transmission), (iii) additional effects (such as dephasing, see below) cannot be introduced within the DFT calculation. The helicene molecule TB Hamiltonian is given by
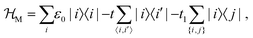 | (1) |
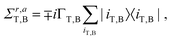 | (2) |
To obtain the TB parameters, parameters ε0, t, t1 and Γ are tuned for a best fit between the transmission functions as obtained from the tight-binding and from the DFT for a HMJ (formed by 2,21-diaza[9]helicene (4) and gold(1,1,1) electrodes) at Δr = 0. In the top inset of Fig. 4 the transmission curves of the DFT (solid blue line) and TB (dashed red line) calculations are shown, with the best fit yielding the tight-binding parameters ε0 = −0.61 eV, t = 1.98 eV, t1 = 0.279 eV and Γ = 1.78 eV. The TB curve fits the DFT data very well, even with such a small number of parameters and especially around the HOMO and LUMO resonances and below the HOMO level (note that for transport calculations, the important fit region is within kBT from the Fermi level).
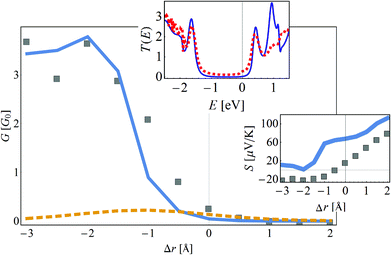
To capture the HMJ electronic behavior under compression or stretching, it is necessary to know the r-dependence of the TB parameters. It is safe to assume that the orbital energy ε0 and the nearest-neighbor hopping element t do not change substantially when the helicene molecule is stretched, and that most of the change occurs in t1 and Γ. Based on knowledge that the dependence of t1 and Γ on r originates from changes in the overlap of wave-functions, a reasonable assumption is that they depend exponentially on r, 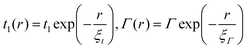 . The values of ξt and ξΓ are found by fitting the TB transmission at the Fermi energy T(εF) (which at low temperatures is proportional to the conductance67,68) to the DFT data. In Fig. 4, the conductance is plotted as a function of the change in the inter-electrode distance, Δr, obtained from the DFT data (filled gray squares) and the TB calculation (solid blue line). This fit yields the values ξt = 1.33 Å and ξΓ = 1.2 Å. To demonstrate that it is necessary to include the change in t1, the dashed orange line of Fig. 4 shows T(εF) as a function of r for the case where only Γ is dependent on r and t1 is independent of r (i.e. where the transport dependence on r comes only from the change in the contacts, while the electronic structure of the molecule itself is unchanged). The striking difference between the dashed orange line and the DFT calculation (filled gray squares) clearly shows that the internal electronic structure of the molecule is changed upon stretching (via a change in t1). The bottom inset of Fig. 4 shows the thermopower S as a function of r based on the DFT data and TB calculation (solid blue line). While some shift is visible, the overall order of magnitude and trend are similar, which is impressive considering the small number of parameters in the model and the fact that thermopower is extremely sensitive to small variations in the parameters of the molecular junction78,83 (note that the thermopower is approximately proportional to the logarithmic derivative of the transmission function).
. The values of ξt and ξΓ are found by fitting the TB transmission at the Fermi energy T(εF) (which at low temperatures is proportional to the conductance67,68) to the DFT data. In Fig. 4, the conductance is plotted as a function of the change in the inter-electrode distance, Δr, obtained from the DFT data (filled gray squares) and the TB calculation (solid blue line). This fit yields the values ξt = 1.33 Å and ξΓ = 1.2 Å. To demonstrate that it is necessary to include the change in t1, the dashed orange line of Fig. 4 shows T(εF) as a function of r for the case where only Γ is dependent on r and t1 is independent of r (i.e. where the transport dependence on r comes only from the change in the contacts, while the electronic structure of the molecule itself is unchanged). The striking difference between the dashed orange line and the DFT calculation (filled gray squares) clearly shows that the internal electronic structure of the molecule is changed upon stretching (via a change in t1). The bottom inset of Fig. 4 shows the thermopower S as a function of r based on the DFT data and TB calculation (solid blue line). While some shift is visible, the overall order of magnitude and trend are similar, which is impressive considering the small number of parameters in the model and the fact that thermopower is extremely sensitive to small variations in the parameters of the molecular junction78,83 (note that the thermopower is approximately proportional to the logarithmic derivative of the transmission function).
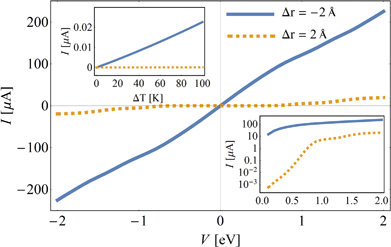
To demonstrate the potential of HMJs as switches, in Fig. 5 the I–V curve of a [9]helicene HMJ is plotted for two values of stretching distance, r = −2 Å (solid blue line) and r = 2 Å (dashed yellow line). The prominent dissimilarity between them shows that the HMJ can be mechanically switched from a “metallic” (“on” state) to an insulating (“off”) state. The bottom-right inset shows the same on a log scale, and more clearly shows the four orders of magnitude change in the current for low biases.
Besides an electronic switch, HMJs can serve as a thermoelectric mechanical switch, i.e. a junction whose thermoelectric current – the current due to a temperature difference – can change substantially. In the top-right inset of Fig. 5, the current is plotted as a function of temperature difference ΔT (at zero bias) for r = −2 Å (solid blue line) and r = 2 Å (dashed yellow line). We set the temperature of the right electrode to room temperature, TR = 300 K, and the temperature of the left electrode is TL = 300 + ΔT. The thermo-current shows ∼4 orders of magnitude difference between the two states at ΔT ∼ 50 K (in fact, since the thermopower changes sign, in principle one can have perfect thermo-electric switching because the “off” state can be tuned to have zero thermo-current).
The dramatic change of conductance upon mechanical stretching (or squeezing) is a direct prediction that is accessible to state-of-the-art experiments. In addition, since the whole effect is based on a coherent calculation of transport, it is of interest to examine the role of dephasing and decoherence in this system. Indeed, the majority of the theoretical approaches to transport and thermopower in MJs implicitly assume that the transport is coherent, and that there is no dissipation within the molecular junction itself, only at the electrodes or at the molecule–electrode interface.66,67,84 Recent experiments provide some evidence for coherent transport in the form of a zero-bias conductance dip.85–91 However, these experiments typically compare between different junctions (measured in separate experiments), and lack a control parameter that can be tuned to verify the presence of coherence in situ (except temperature in some cases92). As we demonstrate below, the dependence of conductance on the electrode distance r can be an additional experiment to show coherent (or incoherent) transport in MJs.
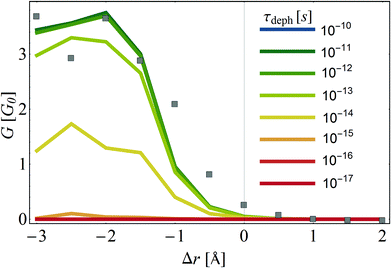
For this aim, we repeat the calculation of conductance vs. Δr (as in Fig. 4), now in the presence of dephasing. We treat dephasing in a phenomenological way, by adding to the self-energy (eqn (2)) a diagonal term Σ(r,a)deph = −iΓdeph, where Γdeph = ℏ/τdeph and τdeph is the dephasing time, i.e. the average time it takes for an electron residing on the helicene molecule to lose its phase.67,93–96 In Fig. 6, the conductance as a function of Δr is plotted for different values of dephasing time, τdeph = 10−10, 10−11, …, 10−17 s (as in Fig. 4, the squares are the data from the DFT calculation, which is purely coherent). As seen, dephasing has a substantial effect on the conductance tunability, and dephasing times of ∼10−16–10−17 are in fact detrimental to the switching behavior.
4. Results: thermoelectric figure of merit
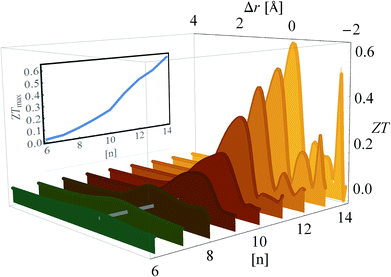
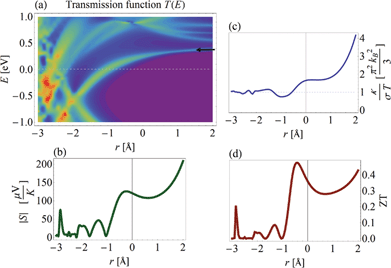
With the TB parameters (and their dependence on inter-electrode distance r) determined, we can proceed to evaluate the thermoelectric FOM of HMJs. In Fig. 7, ZT is plotted as a function of Δr for diaza[n]helicenes (1)–(9) differing in the number of all-ortho fused (hetero)aromatic rings (n = 6, 7, …, 14) and, accordingly, in their length. To calculate ZT realistically, we take also the phonon thermal conductance into account, with a typical value of κ = 20 pW K−1.10,97–99 Two conclusions can be quickly drawn from this figure: (i) ZT can be tuned by changing r, and displays an order of magnitude difference within the same junction, and (ii) the longer the helicene the better its thermoelectric performance. We find that the optimal ZT is obtained at Δr ∼ 0. In the inset the maximal ZT, ZTmax is plotted as a function of helicene length (solid line), showing that ZTmax values can be as large as ZTmax ≈ 0.6, which far exceeds current MJ values.To understand the origin of this strong ZT dependence on r we focus on the 2,27-diaza[12]helicene (7) HMJ ([n] = 12) as an example (but the results are qualitatively similar for all [n]), and study how the different elements that constitute ZT depend on Δr. In Fig. 8(a) the transmission function T(E) is color-plotted as a function of E and Δr. The black arrow indicates the position of the LUMO peak, and it can be seen that upon compression of the junction (which increases the inter-stack hopping term t1), the position of the LUMO changes, shifting down toward the Fermi level (dashed line). Once the LUMO crosses the Fermi level, the HOMO–LUMO gap is no longer visible and the HMJ becomes “metallic” (resonant tunneling). The shift in the LUMO position implies that the filling of the gap cannot be viewed as due to the emergence of an additional conduction channel (the π-stacking), but rather due to a change in the electronic properties of the helicene, induced by enhanced hopping along the stacks.
The thermopower S, whose absolute value is plotted in Fig. 8(b), reflects the behavior of T(E) (since it is approximately equal to the logarithmic derivative of T(E) at the Fermi level4,67). The peak in |S| at Δr ∼ −0.5 Å is a result of the Fermi level being close to (but not at) the LUMO resonance, where the slope of T(E) is maximal. Surprisingly, |S| increases dramatically when the junction is stretched, although the LUMO resonance shifts away from the Fermi level. This is due to the decrease in Γ, which typically increases the slope of the transmission function. Note that the thermopower can reach values of S ∼ 100–200 μV K−1 (and larger for longer HMJs), which is more than one order of magnitude larger than values typically observed in molecular junctions.17–21
In Fig. 8(c) the ratio between the thermal and charge conductances κ/σT is plotted as a function of Δr. In metallic systems, that ratio is expected to be ∼1 in accordance with the Wiedemann–Franz (WF) law, and this is indeed seen for the compressed HMJ, where the molecule is “metallic”. However, upon stretching the molecule there is a significant deviation from the WF law. Thus, the dependence of ZT on Δr, plotted in Fig. 8(d), can be summarized as follows: the peak near Δr ∼ 0 Å is due to the enhanced slope of the transmission function caused by the shift of the LUMO resonance toward the Fermi level. The peak at large Δr values is due both to the increase in S (originating in the decrease in Γ) and to the violation of the WF law.
5. Conclusion
In summary, we have shown that helicene-based molecular junctions can be used as a benchmark tool to investigate and mechanically tune transport and thermopower in molecular junctions. Using a combination of DFT and tight-binding calculations, we have shown that by compressing or stretching the molecular junction, its electronic properties can be controlled, leading to drastic changes in the transport (from a so-called “insulating” to “metallic” resonant-tunneling behavior) and possible substantial increase in the thermopower and thermoelectric efficiency. The origin of these effects is the helical spring-like structure of the helicene molecule; due to this structure, stretching and compressing leads to changes in the tunneling matrix elements between vertically-neighboring atoms (Fig. 1). Control over the helicene length and number of rings was shown to lead to more than an order of magnitude increase in the thermoelectric figure of merit of HMJs, and to a typical thermopower which is an order of magnitude larger than that of more common molecular junctions. The idea of a mechanically controllable conductance and thermopower in molecular junctions should apply to other molecules with non-planar geometry, for instance cycloparaphenylenes,100–102 cyclacenes,103 ball-like molecules,104,105 carbon cages,106–108 tailored fullerenes,109 fullerene cages,110–112 and short DNA molecules (already demonstrated as elements in molecular junctions),113,114 paving the way towards in situ mechanical control of transport properties of molecular junctions.Acknowledgements
The authors thank Prof. P. Reddy from the University of Michigan for valuable discussions and comments. Y.D. acknowledges funding from the University of Michigan–Ben-Gurion University of the Negev Joint Research Collaboration. This work was financially supported by a Czech Science Foundation grant (P207/10/2207) and by the Institute of Organic Chemistry and Biochemistry, Academy of Sciences of the Czech Republic (RVO: 61388963).References
- A. Aviram and M. A. Ratner, Chem. Phys. Lett., 1974, 29, 277–283 CrossRef CAS.
- S. V. Aradhya and L. Venkataraman, Nat. Nanotechnol., 2013, 8, 399–410 CrossRef CAS PubMed.
- M. Tsutsui and M. Taniguchi, Sensors, 2012, 12, 7259–7298 CrossRef CAS PubMed.
- Y. Dubi and M. Di Ventra, Rev. Mod. Phys., 2011, 83, 131 CrossRef CAS.
- P. Murphy, S. Mukerjee and J. Moore, Phys. Rev. B: Condens. Matter, 2008, 78, 161406 CrossRef.
- J. P. Bergfield and C. A. Stafford, Nano Lett., 2009, 9, 3072–3076 CrossRef CAS PubMed.
- J. P. Bergfield, M. A. Solis and C. A. Stafford, ACS Nano, 2010, 4, 5314–5320 CrossRef CAS PubMed.
- C. M. Finch, V. M. Garcia-Suarez and C. J. Lambert, Phys. Rev. B: Condens. Matter, 2009, 79, 033405 CrossRef.
- O. Karlström, H. Linke, G. Karlström and A. Wacker, Phys. Rev. B: Condens. Matter, 2011, 84, 113415 CrossRef.
- D. Nozaki, H. Sevinçli, W. Li, R. Gutiérrez and G. Cuniberti, Phys. Rev. B: Condens. Matter, 2010, 81, 235406 CrossRef.
- R. Stadler and T. Markussen, J. Chem. Phys., 2011, 135, 154109 CrossRef PubMed.
- H. Nakamura, T. Ohto, T. Ishida and Y. Asai, J. Am. Chem. Soc., 2013, 135, 16545–16552 CrossRef CAS PubMed.
- V. M. García-Suárez, C. J. Lambert, D. Z. Manrique and T. Wandlowski, Nanotechnology, 2014, 25, 205402 CrossRef PubMed.
- C. Evangeli, K. Gillemot, E. Leary, M. T. Gonzalez, G. Rubio-Bollinger, C. J. Lambert and N. Agrait, Nano Lett., 2013, 13, 2141–2145 CrossRef CAS PubMed.
- G. Mahan and J. Sofo, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 7436–7439 CrossRef CAS.
- M. Paulsson and S. Datta, Phys. Rev. B: Condens. Matter, 2003, 67, 241403 CrossRef.
- J. Malen, P. Doak, K. Baheti, T. Tilley, A. Majumdar and R. Segalman, Nano Lett., 2009, 9, 3406–3412 CrossRef CAS PubMed.
- J. Malen, P. Doak, K. Baheti, T. Tilley, R. Segalman and A. Majumdar, Nano Lett., 2009, 9, 1164–1169 CrossRef CAS PubMed.
- J. Malen, S. Yee, A. Majumdar and R. Segalman, Chem. Phys. Lett., 2010, 491, 109–122 CrossRef CAS PubMed.
- P. Reddy, S. Jang, R. Segalman and A. Majumdar, Science, 2007, 315, 1568–1571 CrossRef CAS PubMed.
- J. R. Widawsky, P. Darancet, J. B. Neaton and L. Venkataraman, Nano Lett., 2012, 12, 354–358 CrossRef CAS PubMed.
- W. B. Chang, C.-K. Mai, M. Kotiuga, J. B. Neaton, G. C. Bazan and R. A. Segalman, Chem. Mater., 2014, 26, 7229–7235 CrossRef CAS.
- Y. Kim, W. Jeong, K. Kim, W. Lee and P. Reddy, Nat. Nanotechnol., 2014, 9, 881–885 CrossRef CAS PubMed.
- S. K. Lee, T. Ohto, R. Yamada and H. Tada, Nano Lett., 2014, 14, 5276–5280 CrossRef CAS PubMed.
- Y. Shen and C.-F. Chen, Chem. Rev., 2011, 112, 1463–1535 CrossRef PubMed.
- M. Gingras, Chem. Soc. Rev., 2013, 42, 1051–1095 RSC.
- M. Gingras, Chem. Soc. Rev., 2013, 42, 968–1006 RSC.
- M. Gingras, G. Felix and R. Peresutti, Chem. Soc. Rev., 2013, 42, 1007–1050 RSC.
- M. J. Narcis and N. Takenaka, Eur. J. Org. Chem., 2014, 21–34 CrossRef CAS PubMed.
- K. Yavari, P. Aillard, Y. Zhang, F. Nuter, P. Retailleau, A. Voituriez and A. Marinetti, Angew. Chem., Int. Ed., 2014, 53, 861–865 CrossRef CAS PubMed.
- K.-i. Shinohara, Y. Sannohe, S. Kaieda, K.-i. Tanaka, H. Osuga, H. Tahara, Y. Xu, T. Kawase, T. Bando and H. Sugiyama, J. Am. Chem. Soc., 2010, 132, 3778–3782 CrossRef CAS PubMed.
- M. Li, H.-Y. Lu, R.-L. Liu, J.-D. Chen and C.-F. Chen, J. Org. Chem., 2012, 77, 3670–3673 CrossRef CAS PubMed.
- M. Shcherbina, X.-b. Zeng, T. Tadjiev, G. Ungar, S. Eichhorn, K. Phillips and T. Katz, Angew. Chem., Int. Ed., 2009, 48, 7837–7840 CrossRef CAS PubMed.
- T. Verbiest, S. V. Elshocht, M. Kauranen, L. Hellemans, J. Snauwaert, C. Nuckolls, T. J. Katz and A. Persoons, Science, 1998, 282, 913–915 CrossRef CAS.
- T. Verbiest, S. V. Elshocht, A. Persoons, C. Nuckolls, K. E. Phillips and T. J. Katz, Langmuir, 2001, 17, 4685–4687 CrossRef CAS.
- T. J. Wigglesworth, D. Sud, T. B. Norsten, V. S. Lekhi and N. R. Branda, J. Am. Chem. Soc., 2005, 127, 7272–7273 CrossRef CAS PubMed.
- E. Anger, M. Srebro, N. Vanthuyne, L. Toupet, S. Rigaut, C. Roussel, J. Autschbach, J. Crassous and R. Reau, J. Am. Chem. Soc., 2012, 134, 15628–15631 CrossRef CAS PubMed.
- L. Adriaenssens, L. Severa, D. Koval, M. M. Císařová, I. Belmonte, E. C. Escudero-Adán, P. Novotná, P. Sázelová, J. Vávra, R. Pohl, D. Šaman, M. Urbanová, V. Kašička and F. Teplý, Chem. Sci., 2011, 2, 2314–2320 RSC.
- S. Graule, M. Rudolph, N. Vanthuyne, J. Autschbach, C. Roussel, J. Crassous and R. Reau, J. Am. Chem. Soc., 2009, 131, 3183–3185 CrossRef CAS PubMed.
- Y. Yang, R. C. da Costa, M. J. Fuchter and A. J. Campbell, Nat. Photonics, 2013, 7, 634–638 CrossRef CAS.
- M. J. Fuchter, J. Schaefer, D. K. Judge, B. Wardzinski, M. Weimar and I. Krossing, Dalton Trans., 2012, 41, 8238–8241 RSC.
- L. Shi, Z. Liu, G. Dong, L. Duan, Y. Qiu, J. Jia, W. Guo, D. Zhao, D. Cui and X. Tao, Chem. – Eur. J., 2012, 18, 8092–8099 CrossRef CAS PubMed.
- M. Stöhr, S. Boz, M. Schär, M.-T. Nguyen, C. A. Pignedoli, D. Passerone, W. B. Schweizer, C. Thilgen, T. A. Jung and F. Diederich, Angew. Chem., Int. Ed., 2011, 50, 9982–9986 CrossRef PubMed.
- T. Balandina, M. W. van der Meijden, O. Ivasenko, D. Cornil, J. Cornil, R. Lazzaroni, R. M. Kellogg and S. De Feyter, Chem. Commun., 2013, 49, 2207–2209 RSC.
- J. S. Prauzner-Bechcicki, S. Godlewski, J. Budzioch, G. Goryl, L. Walczak, P. Sehnal, I. G. Stará, I. Starý, F. Ample, C. Joachim and M. Szymonski, ChemPhysChem, 2010, 11, 3522–3528 CrossRef CAS PubMed.
- P. Sehnal, I. G. Stará, D. Šaman, M. Tichỳ, J. Míšek, J. Cvačka, L. Rulíšek, J. Chocholoušová, J. Vacek, G. Goryl, M. Szymonski, I. Císařová and I. Starý, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 13169–13174 CrossRef CAS PubMed.
- S. Godlewski, J. S. Prauzner-Bechcicki, J. Budzioch, L. Walczak, I. G. Stará, I. Starý, P. Sehnal and M. Szymonski, Surf. Sci., 2012, 606, 1600–1607 CrossRef CAS PubMed.
- C. M. Hauke, P. Rahe, M. Nimmrich, J. Schutte, M. Kittelmann, I. G. Stara, I. Stary, J. Rybaceek and A. Kuhnle, J. Phys. Chem. C, 2012, 116, 4637–4641 CAS.
- R. Fasel, M. Parschau and K.-H. Ernst, Nature, 2006, 439, 449–452 CrossRef CAS PubMed.
- A. Shchyrba, M.-T. Nguyen, C. Włckerlin, S. Martens, S. Nowakowska, T. Ivas, J. Roose, T. Nijs, S. Boz, M. Schur, M. Sthr, C. A. Pignedoli, C. Thilgen, F. Diederich, D. Passerone and T. A. Jung, J. Am. Chem. Soc., 2013, 135, 15270–15273 CrossRef CAS PubMed.
- J. Zhou, S. Samanta, C. Guo, J. Locklin and B. Xu, Nanoscale, 2013, 5, 5715–5719 RSC.
- Z. Huang, F. Chen, R. D'agosta, P. A. Bennett, M. Di Ventra and N. Tao, Nat. Nanotechnol., 2007, 2, 698–703 CrossRef CAS PubMed.
- J. Zhou, G. Chen and B. Xu, J. Phys. Chem. C, 2010, 114, 8587–8592 CAS.
- I. Diez-Perez, J. Hihath, T. Hines, Z.-S. Wang, G. Zhou, K. Müllen and N. Tao, Nat. Nanotechnol., 2011, 6, 226–231 CrossRef CAS PubMed.
- C. Joachim, J. K. Gimzewski, R. R. Schlittler and C. Chavy, Phys. Rev. Lett., 1995, 74, 2102–2105 CrossRef CAS.
- J. Míšek, F. Teplỳ, I. G. Stará, M. Tichỳ, D. Šaman, I. Císařová, P. Vojtíšek and I. Starỳ, Angew. Chem., Int. Ed., 2008, 47, 3188–3191 CrossRef PubMed.
- A. Bilic, J. R. Reimers and N. S. Hush, J. Phys. Chem. B, 2002, 106, 6740–6747 CrossRef CAS.
- D.-Y. Wu, B. Ren and Z.-Q. Tian, ChemPhysChem, 2006, 7, 619–628 CrossRef CAS PubMed.
- D. Wu, M. Hayashi, C. Chang, K. Liang and S. Lin, J. Chem. Phys., 2003, 118, 4073–4085 CrossRef CAS PubMed.
- S. Y. Quek, M. Kamenetska, M. L. Steigerwald, H. J. Choi, S. G. Louie, M. S. Hybertsen, J. Neaton and L. Venkataraman, Nat. Nanotechnol., 2009, 4, 230–234 CrossRef CAS PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09 Revision D.01, Gaussian Inc., Wallingford, CT, 2009 Search PubMed.
- M. Brandbyge, J.-L. Mozos, P. Ordejón, J. Taylor and K. Stokbro, Phys. Rev. B: Condens. Matter, 2002, 65, 165401 CrossRef.
- J. M. Soler, E. Artacho, J. D. Gale, A. García, J. Junquera, P. Ordejón and D. Sánchez-Portal, J. Phys.: Condens. Matter, 2002, 14, 2745 CrossRef CAS.
- K. Stokbro, D. E. Petersen, S. Smidstrup, A. Blom, M. Ipsen and K. Kaasbjerg, Phys. Rev. B: Condens. Matter, 2010, 82, 075420 CrossRef.
- J. Seibel, L. Zoppi and K.-H. Ernst, Chem. Commun., 2014, 50, 8751–8753 RSC.
- S. Datta, Electronic Transport in Mesoscopic Systems, Cambridge University Press, 1997 Search PubMed.
- M. Di Ventra, Electrical Transport in Nanoscale Systems, Cambridge University Press, 2008 Search PubMed.
- U. Peskin, J. Phys. B: At., Mol. Opt. Phys., 2010, 43, 153001 CrossRef.
- L. Cai, M. A. Cabassi, H. Yoon, O. M. Cabarcos, C. L. McGuiness, A. K. Flatt, D. L. Allara, J. M. Tour and T. S. Mayer, Nano Lett., 2005, 5, 2365–2372 CrossRef CAS PubMed.
- A. J. Kronemeijer, H. B. Akkerman, T. Kudernac, B. J. van Wees, B. L. Feringa, P. W. M. Blom and B. de Boer, Adv. Mater., 2008, 20, 1467–1473 CrossRef CAS PubMed.
- C. N. Lau, D. R. Stewart, R. S. Williams and M. Bockrath, Nano Lett., 2004, 4, 569–572 CrossRef CAS.
- S. J. van der Molen and P. Liljeroth, J. Phys.: Condens. Matter, 2010, 22, 133001 CrossRef PubMed.
- O. Y. Loh and H. D. Espinosa, Nat. Nanotechnol., 2012, 7, 283–295 CrossRef CAS PubMed.
- Y. Li Huang, Y. Lu, T. C. Niu, H. Huang, S. Kera, N. Ueno, A. T. S. Wee and W. Chen, Small, 2012, 8, 1423–1428 CrossRef PubMed.
- C. A. Martin, R. H. Smit, H. S. van der Zant and J. M. van Ruitenbeek, Nano Lett., 2009, 9, 2940–2945 CrossRef CAS PubMed.
- C. A. Martin, R. H. Smit, R. van Egmond, H. S. van der Zant and J. M. van Ruitenbeek, Rev. Sci. Instrum., 2011, 82, 053907 CrossRef PubMed.
- Y. Kim, H. Song, F. Strigl, H.-F. Pernau, T. Lee and E. Scheer, Phys. Rev. Lett., 2011, 106, 196804 CrossRef.
- Y. Dubi, J. Chem. Phys., 2013, 138, 114706 CrossRef PubMed.
- O. Karlström, M. Strange and G. C. Solomon, J. Chem. Phys., 2014, 140, 044315 CrossRef PubMed.
- J. K. Viljas, F. Pauly and J. C. Cuevas, Phys. Rev. B: Condens. Matter, 2008, 77, 155119 CrossRef.
- F. Pauly, J. K. Viljas and J. C. Cuevas, Phys. Rev. B: Condens. Matter, 2008, 78, 035315 CrossRef.
- C. J. O. Verzijl, J. S. Seldenthuis and J. M. Thijssen, J. Chem. Phys., 2013, 138, 094102 CrossRef CAS PubMed.
- Y. Dubi, New J. Phys., 2013, 15, 105004 CrossRef.
- E. Scheer and J. C. Cuevas, Molecular electronics: an introduction to theory and experiment, World Scientific, 2010, vol. 1 Search PubMed.
- S. V. Aradhya, J. S. Meisner, M. Krikorian, S. Ahn, R. Parameswaran, M. L. Steigerwald, C. Nuckolls and L. Venkataraman, Nano Lett., 2012, 12, 1643–1647 CrossRef CAS PubMed.
- C. R. Arroyo, S. Tarkuc, R. Frisenda, J. S. Seldenthuis, C. H. M. Woerde, R. Eelkema, F. C. Grozema and H. S. J. van der Zant, Angew. Chem., Int. Ed., 2013, 125, 3234–3237 CrossRef PubMed.
- D. Fracasso, H. Valkenier, J. C. Hummelen, G. C. Solomon and R. C. Chiechi, J. Am. Chem. Soc., 2011, 133, 9556–9563 CrossRef CAS PubMed.
- C. M. Guédon, H. Valkenier, T. Markussen, K. S. Thygesen, J. C. Hummelen and S. J. van der Molen, Nat. Nanotechnol., 2012, 7, 305–309 CrossRef PubMed.
- W. Hong, H. Valkenier, G. Mészáros, D. Z. Manrique, A. Mishchenko, A. Putz, P. M. García, C. J. Lambert, J. C. Hummelen and T. Wandlowski, Beilstein J. Nanotechnol., 2011, 2, 699–713 CrossRef PubMed.
- V. Rabache, J. Chaste, P. Petit, M. L. Della Rocca, P. Martin, J.-C. Lacroix, R. L. McCreery and P. Lafarge, J. Am. Chem. Soc., 2013, 135, 10218–10221 CrossRef CAS PubMed.
- A. Batra, J. S. Meisner, P. Darancet, Q. Chen, M. L. Steigerwald, C. Nuckolls and L. Venkataraman, Faraday Discuss., 2014, 174, 79–89 CAS.
- T. Markussen and K. S. Thygesen, Phys. Rev. B: Condens. Matter, 2014, 89, 085420 CrossRef.
- J. L. D'Amato and H. M. Pastawski, Phys. Rev. B: Condens. Matter, 1990, 41, 7411–7420 CrossRef.
- M. Krems, M. Zwolak, Y. V. Pershin and M. Di Ventra, Biophys. J., 2009, 97, 1990–1996 CrossRef CAS PubMed.
- D. Nozaki, C. Gomes da Rocha, H. M. Pastawski and G. Cuniberti, Phys. Rev. B: Condens. Matter, 2012, 85, 155327 CrossRef.
- P. P. Pal and R. Pati, J. Phys. Chem. C, 2011, 115, 17564–17573 CAS.
- J. Malen, S. Yee, A. Majumdar and R. Segalman, Chem. Phys. Lett., 2010, 491, 109–122 CrossRef CAS PubMed.
- W.-L. Ong, S. Majumdar, J. A. Malen and A. J. McGaughey, J. Phys. Chem. C, 2014, 118, 7288–7295 CAS.
- D. Segal, A. Nitzan and P. Hänggi, J. Chem. Phys., 2003, 119, 6840–6855 CrossRef CAS PubMed.
- R. Jasti, J. Bhattacharjee, J. B. Neaton and C. R. Bertozzi, J. Am. Chem. Soc., 2008, 130, 17646–17647 CrossRef CAS PubMed.
- T. J. Sisto, M. R. Golder, E. S. Hirst and R. Jasti, J. Am. Chem. Soc., 2011, 133, 15800–15802 CrossRef CAS PubMed.
- J. Xia and R. Jasti, Angew. Chem., Int. Ed., 2012, 51, 2474–2476 CrossRef CAS PubMed.
- H. S. Choi and K. S. Kim, Angew. Chem., Int. Ed., 1999, 38, 2256–2258 CrossRef CAS.
- E. Kayahara, T. Iwamoto, H. Takaya, T. Suzuki, M. Fujitsuka, T. Majima, N. Yasuda, N. Matsuyama, S. Seki and S. Yamago, Nat. Commun., 2013, 4, 3694 Search PubMed.
- X.-J. Kong, L.-S. Long, Z. Zheng, R.-B. Huang and L.-S. Zheng, Acc. Chem. Res., 2009, 43, 201–209 CrossRef PubMed.
- W. Wang, Y.-X. Wang and H.-B. Yang, Org. Chem. Front., 2014, 1, 1005–1009 RSC.
- K. Matsui, Y. Segawa and K. Itami, J. Am. Chem. Soc., 2014, 136, 16452–16458 CrossRef CAS PubMed.
- T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino and S. Yamago, Angew. Chem., Int. Ed., 2011, 50, 8342–8344 CrossRef CAS PubMed.
- N. B. Shustova, I. V. Kuvychko, D. V. Peryshkov, J. B. Whitaker, B. W. Larson, Y.-S. Chen, L. Dunsch, K. Seppelt, A. A. Popov, S. H. Strauss and O. V. Boltalina, Chem. Commun., 2011, 47, 875–877 RSC.
- B. Skwara, R. W. Góra, R. Zalesny, P. Lipkowski, W. Bartkowiak, H. Reis, M. G. Papadopoulos, J. M. Luis and B. Kirtman, J. Phys. Chem. A, 2011, 115, 10370–10381 CrossRef CAS PubMed.
- V. A. Pomogaev, P. V. Avramov, A. A. Kuzubov and V. Y. Artyukhov, Int. J. Quantum Chem., 2015, 115, 239–244 CrossRef CAS PubMed.
- Y. H. Hu and E. Ruckenstein, J. Am. Chem. Soc., 2005, 127, 11277–11282 CrossRef CAS PubMed.
- K. Wang, J. M. Hamill, B. Wang, C. Guo, S. Jiang, Z. Huang and B. Xu, Chem. Sci., 2014, 5, 3425–3431 RSC.
- N. Kang, A. Erbe and E. Scheer, Appl. Phys. Lett., 2010, 96, 023701 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2015 |