Open Access Article
Open Access ArticleA light-driven supramolecular nanowire actuator†
Junho
Lee
a,
Seungwhan
Oh
b,
Jaeyeon
Pyo
a,
Jong-Man
Kim
*bc and
Jung Ho
Je
 *a
*a
aX-ray Imaging Center, Department of Materials Science and Engineering, Pohang University of Science and Technology, Pohang 790-784, Republic of Korea. E-mail: jhje@postech.ac.kr
bDepartment of Chemical Engineering, Hanyang University, Seoul 133-791, Republic of Korea
cInstitute of Nano Science and Technology, Hanyang University, Seoul 133-791, Republic of Korea. E-mail: jmk@hanyang.ac.kr
First published on 16th March 2015
Abstract
A single photomechanical supramolecular nanowire actuator with an azobenzene-containing 1,3,5-tricarboxamide derivative is developed by employing a direct writing method. Single nanowires display photoinduced reversible bending and the bending behavior follows first-order kinetics associated with azobenzene photoisomerization. A wireless photomechanical nanowire tweezers that remotely manipulates a single micro-particle is also demonstrated.
Photomechanical actuators,1–6 which convert light energy directly into physical motion, have clear advantages over conventional electrostatic actuators7,8 because they can be remotely controlled in the absence of invasive wires or electrodes. An additionally attractive feature is that actuation of photomechanical actuators can be readily tuned through control of the wavelength, direction and intensity of input light. Owing to these properties, a wide variety of photoresponsive actuating materials and systems, including photochromic crystals,1,6,9–11 azobenzene derivatives,2,5,12,36 and supramolecular hydrogel,3 have been developed thus far. However, the development of photomechanical nanowire actuators, which are in critical demand because of their use as active actuating components in nanodevices, has been for the most part unexplored. In this study, we developed a single photomechanical molecular nanowire actuator that employs an azobenzene-containing supramolecule as a supramolecular nanowire scaffold. Single photomechanical nanowires undergo bending upon irradiation with UV light that is reversed upon visible light irradiation, and the bending behavior follows first-order kinetics associated with azobenzene photoisomerization. A wireless photomechanical nanowire tweezers that remotely manipulates a single micro-particle was also developed. The strategy employed to design the single photomechanical nanowires could very well serve as the foundation for the development of other novel molecular based light-driven nanowire actuators.
Photoinduced three-dimensional (3D) motion comprised of bending,13 twisting,14,15 and rotation16 has highly promising practical applications. In particular, one-dimensional (1D)-like photomechanical actuators are beneficial to 3D motion due to its high degree of freedom motion.17 The development of photomechanical nanowire actuators are in critical demand for their use as active actuating components in nanodevices. However, current approaches, such as recrystallization,1,6,9–11,13–15 inkjet printing,4 melt-and-draw method17,18 only afford micron-sized actuators and they suffer from the lack of dimensional control. Irie et al.19 (or Ikeda et al.12) demonstrated photo-responsive nanoactuating in a bulk crystal (or nanoparticles), which was, however, restricted to one-dimension and showed very limited actuating displacements (<10 nm). The template-assisted solvent annealing method20,21 by Al-Kaysi et al. provided photomechanical nanowires (∼35 nm), which were randomly dispersed on a substrate and therefore showed a motion limitation in two-dimensions. Accordingly, a reliable method for photomechanical nanowire actuators with omnidirectional actuating in nanoscale and large (>μm) actuating displacements has yet to be developed.
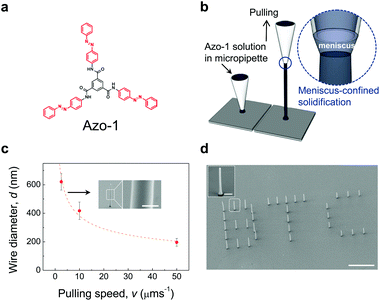
Herein we report a new single photomechanical nanowire actuator that shows three-dimensional (3D) actuating in nanoscale for the first time, by vertically growing the azobenzene containing supramolecule, tris(4-((E)-phenyldiazenyl)phenyl)-benzene-1,3,5-tricarboxamide (Azo-1) (Fig. 1(a)). A meniscus-guided solidification method,21 which has proven to be an effective bottom-up strategy for organic nanowires, was employed. The key feature of the method is that it relies on confined solidification of an organic solution within a nanoscale meniscus (Fig. 1(b)). Single vertical nanowires can be generated at room temperature by guiding the meniscus upward using a micropipette. The azobenzene containing benzenetricarboxamide (BTC) derivative, Azo-1, was selected as the nanowire material owing to its photoresponsive shape change property as well as its intriguing C3-symmetrical structure that enables facile formation of columnar supramolecules.26 The Azo-1 nanowires fabricated by the meniscus-guided method have a monoclinic crystal structure26 (Fig. S1, ESI†). In general, it is very difficult to control the sizes and shapes of 1D nanostructures of self-assembled supramolecules.27 In contrast, owing to its direct writing capability using organic materials, the meniscus-guided solidification method enables ready growth of 1D nanostructure.22–25 In fact, this approach can be utilized to grow vertical Azo-1 nanowires that have smooth surfaces in high-aspect-ratios and diameters (d) in the range of 200 to 1000 nm that are pulling speed governed (Fig. 1(c)). Furthermore, 3D writing ability and tunable pulling time property of the method enables exact positioning and alignment of individual Azo-1 nanowires, as demonstrated by the precisely controlled formation of the letters ‘B’, ‘T’, and ‘C’ comprised of nanowire arrays with respective lengths of 20, 15, 10 μm (Fig. 1(d)).
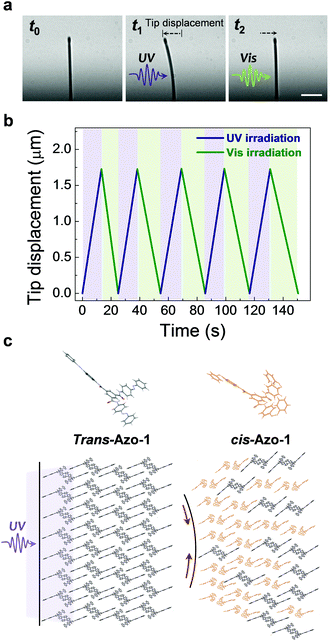
Importantly, vertically grown Azo-1 nanowires undergo reversible photo-induced mechanical motion (Fig. S2 in ESI† for experimental set-up). This is demonstrated by the observation that bending and unbending of a single Azo-1 nanowire (d ∼ 500 nm and l (length) ∼ 20 μm) take place upon respective UV (λ ∼ 365 nm, 1.5 mW cm−2; t0 to t1) and visible (λ ∼ 455 nm, 4 mW cm−2; t1 to t2) light irradiation (Fig. 2(a)). Upon UV light irradiation, a single nanowire bends toward the light source and returns to its initial position upon visible light irradiation from the same direction. Importantly, single nanowires display reversible 3D bending motion depending on the position of the UV and visible light sources (ESI Movie S1 and Fig. S3 in ESI†). Finally, the reversible bending process takes place repeatedly by alternating the UV and visible light irradiation without fragmentation (Fig. 2(b), see also ESI Movie S2, and Fig. S4 in ESI†).
The bending phenomenon experienced by the nanowire (d ∼ 500 nm) is likely a consequence of photoisomerization about the azo π-bond in the component Azo-1 supramolecules (Fig. 2(c)).28 UV light irradiation of azobenzenes typically promotes trans-to-cis isomerization, a chemical process that is accompanied by a significant decrease in the overall length of the molecule from ca. 9.0 Å in the trans form to ca. 5.5 Å in the cis form. The molecular geometry change caused by the photoisomerization reaction is known cause a macroscopic shape change.2 For instance, polymer networks, cross-linked with azobenzene moieties, undergo contraction when irradiated with UV light.29
It should be noted that absorption of 365 nm UV light by the Azo-1 nanowire occurs only within ∼100 nm from the surface of the nanowire, as estimated by fitting a theoretical model (dashed red line) for photoinduced bending based on Beer's law30 (Fig. 3(a)). Here the bending curvature (1/R; R = the bending radius) of the Azo-1 nanowire was measured as a function of d after irradiation with UV light for 10 s (Fig. 3(b)). The small attenuation length (∼100 nm), compared to that of liquid-crystal elastomer (∼1 μm),31,32 is attributed mostly to high density of the azobenzene chromophores.26 In fact, we experimentally confirmed the contraction of the Azo-1 nanowire at the surface region (<100 nm) upon UV irradiation, based on using a real-time grazing incidence X-ray diffraction (GIXD) method (Fig. S5, ESI†). Thus, the length contraction that takes place at the surface of the nanowire is responsible for the photoinduced bending (Fig. 4(a)).
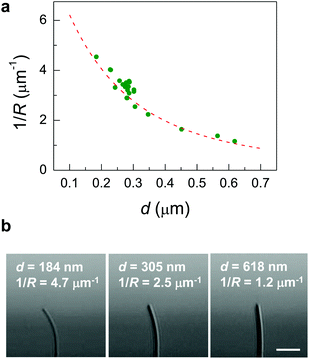 | ||
| Fig. 3 (a) The bending curvature (1/R; R = the bending radius) of the Azo-1 nanowire, measured as a function of d after UV light irradiation for 10 s. The decrease in 1/R with d (closed green circles) is well matched with a theoretical model (dashed red line), 1/R ∼ λ/w2[(λ/w + 0.5)e−w/L − λ/w+0.5],30 where w is the thickness of a solid plate and λ is the attenuation length, when λ is 100 nm. This indicates that the length contraction takes place at the surface of the nanowire. The depth profile of the photoisomerization is described by ccis(x) = ccis(0) × {exp(−x/λ)}, where ccis is the cis fraction of Azo-1 supramolecules and λ is the attenuation length, based on the theoretical model by Beer's law.30 (b) Representative optical microscope images of the nanowires (l (length) = 22.5 μm) with different diameters after UV light irradiation for 10 s. Scale bar, 10 μm. | ||
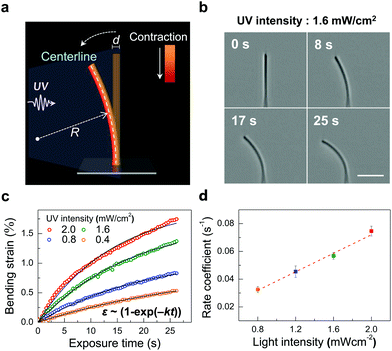
In order to gain quantitative information on the bending behavior of the Azo-1 nanowires, the kinetics of the photoinduced bending were investigated. Specifically, the bending of an Azo-1 nanowire (d ∼ 450 nm) promoted by a given UV intensity was determined with time (Fig. 4(b)). The bending strain (ε = (d/2)/R) of the Azo-1 nanowire was then estimated by using the bending radius R (Fig. 4(a)). By taking advantage of an image-processing and MATLAB-based circle fitting algorithm, the bending curvatures (1/R) of a nanowire at various exposure times were calculated (Fig. S6, ESI†). The results show that bending strain exponentially increases with exposure time in accord with the relationship 1 − exp(−kt), where k is the rate coefficient (Fig. 4(c)). The time dependence of the strain corresponds to first-order kinetics of the trans → cis photoisomerization in azobenzene, (ccis ∝ [1 − exp(−kt)]), where ccis is the cis fraction of chromophores (Fig. S7, ESI†). The bending strain and the degree of photoisomerization ([1 − exp(−kreactt)] × 100 (%)) showed almost a linear relationship (Fig. S7(c), ESI†), as reported.33 The result represents that the bending motion of the Azo-1 nanowire is directly linked to the molecular level transition of the azobenzene stereoisomeric forms. The rate coefficient (k) (Fig. 4(d)), estimated from fitting the strain data based on ε ∼ (1 − exp(−kt)) given in Fig. 4(c), is linearly proportional to the UV light intensity (I0). This finding confirms that the kinetics of the photoinduced bending of the nanowires parallels the first-order kinetics of the azobenzene photoisomerization process. It is noteworthy that irradiation using a low intensity (0.8 mW cm−2) of UV light for a few seconds is sufficient to induce bending of the nanowires, (Fig. 4(c)). This light intensity level meets the accepted safety standard.34 (ACGIH 2005, 1.0 mW cm−2 for periods lasting <1000 s), suggesting that the system is potentially suitable for use in biomedical devices.
Remarkably, the Azo-1 nanowire actuators display a very high thermal stability. The bending strains of the Azo-1 nanowires, measured as a function of time at a given temperature (25, 60, and 90 °C) after irradiation with UV light (1.6 mW cm−2, 10 s), display little variations up to one month (Fig. S8, ESI†). This high thermal stability is attributed to the fact that the rate of the thermal back-isomerization (cis → trans) reaction of the Azo-1 supramolecules is exceptionally low, as a consequence presumably of strong intermolecular interactions between the cis forms.
The final phase of current investigation focused on a demonstration that the photo-induced bending phenomenon could be used to design a photomechanical nanowire tweezers, which is controlled remotely without the need for invasive wires or electrodes that are required in conventional electrostatic actuators.7,8 As illustrated schematically in Fig. 5, bending of an Azo-1 nanowire actuator arm (yellow) against a fixed polystyrene (PS) nanowire arm (black), the latter of which does not experience a photomechanical response, would allow the device to be employed to grip a micro-object (gray sphere) upon UV light irradiation. To explore this design strategy, both Azo-1 (d ∼ 500 nm) and PS nanowire (d ∼ 500 nm) arms were individually integrated on the tip of a glass microcapillary tube using the meniscus-guided method. The resulting tweezers, in fact, was successfully manipulated to pick, lift, and move a PS microparticle (d ∼ 4 μm) on a silicon substrate upon irradiation with UV light (1.5 mW cm−2 for 20 s) (1 → 2 → 3 in Fig. 5) (ESI Movie S2†). Based on the low UV dose required to activate the nanowire arm, we anticipate that the new photomechanical tweezers is applicable to biomechanical systems.
Conclusions
In conclusion, the effort described above led to the development of a method to fabricate a single photomechanical nanowire actuator, which relies on the use of a technique in which solidification of Azo-1 supramolecules takes place within a nanoscale meniscus. Single Azo-1 nanowires were observed to exhibit reversible omnidirectional bending and unbending with large actuating displacements (≤1.7 μm) when alternately irradiated with UV and visible light. In addition, bending of the photomechanical nanowires displays high thermal stability. The results of the studies also reveal that the bending behavior follows first-order kinetics associated with an azobenzene photoisomerization reaction,35 implying that bending is linked to the molecular level phase transition of Azo-1 molecules (trans–cis isomerization of azobenzene). Finally, we have constructed a new photomechanical nanowire tweezers, designed using this novel photoactuation process, and have shown that it can be used to manipulate a single micro-particle remotely. Significantly, the strong possibility exists that light-driven nanowire actuators of the type described above might serve as new nanorobotic devices for manipulation of micro/nano-objects that have general utility in biomedical science and engineering.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (no. 2006-0050683 and 2014R1A2A1A01005862) and by Brain Korea 21 PLUS project for Center for Creative Industrial Materials.Notes and references
- S. Kobatake, S. Takami, H. Muto, T. Ishikawa and M. Irie, Nature, 2007, 446, 778–781 CrossRef CAS PubMed.
- H. Yu and T. Ikeda, Adv. Mater., 2011, 23, 2149–2180 CrossRef CAS PubMed.
- Y. Takashima, S. Hatanaka, M. Otsubo, M. Nakahata, T. Kakuta, A. Hashidzume, H. Yamaguchi and A. Harada, Nat. Commun., 2012, 3, 1270 CrossRef PubMed.
- C. L. van Oosten, C. W. M. Bastiaansen and D. J. Broer, Nat. Mater., 2009, 8, 677–682 CrossRef CAS PubMed.
- Z. Jiang, M. Xu, F. Li and Y. Yu, J. Am. Chem. Soc., 2013, 135, 16446–16453 CrossRef CAS PubMed.
- O. S. Bushuyev, T. A. Singleton and C. J. Barrett, Adv. Mater., 2013, 25, 1796–1800 CrossRef CAS PubMed.
- O. Kim, T. J. Shin and M. J. Park, Nat. Commun., 2013, 4, 2208 Search PubMed.
- E. Detsi, P. Onck and J. T. M. De Hosson, ACS Nano, 2013, 7, 4299–4306 CrossRef CAS PubMed.
- H. Koshima, R. Matsuo, M. Matsudomi, Y. Uemura and M. Shiro, Cryst. Growth Des., 2013, 13, 4330–4337 CAS.
- F. Terao, M. Morimoto and M. Irie, Angew. Chem., Int. Ed., 2012, 51, 901–904 CrossRef CAS PubMed.
- P. Naumov, J. Kowalik, K. M. Solntsev, A. Baldridge, J.-S. Moon, C. Kranz and L. M. Tolbert, J. Am. Chem. Soc., 2010, 132, 5845–5857 CrossRef CAS PubMed.
- S. Tsoi, J. Zhou, C. Spillmann, J. Naciri, T. Ikeda and B. Ratna, Macromol. Chem. Phys., 2013, 214, 734–741 CrossRef CAS.
- M. Morimoto and M. Irie, J. Am. Chem. Soc., 2010, 132, 14172–14178 CrossRef CAS PubMed.
- D. Kitagawa, H. Nishi and S. Kobatake, Angew. Chem., Int. Ed., 2013, 52, 9320–9322 CrossRef CAS PubMed.
- L. Zhu, R. O. Al-Kaysi and C. J. Bardeen, J. Am. Chem. Soc., 2011, 133, 12569–12575 CrossRef CAS PubMed.
- M. Yamada, M. Kondo, J.-I. Mamiya, Y. Yu, M. Kinoshita, C. J. Barrett and T. Ikeda, Angew. Chem., Int. Ed., 2008, 47, 4986–4988 CrossRef CAS PubMed.
- T. Yoshino, M. Kondo, J.-I. Mamiya, M. Kinoshita, Y. Yu and T. Ikeda, Adv. Mater., 2010, 22, 1361–1363 CrossRef CAS PubMed.
- W. Deng, M.-H. Li, X. Wang and P. Keller, Liq. Cryst., 2009, 36, 1023–1029 CrossRef CAS.
- M. Irie, S. Kobatake and M. Horichi, Science, 2001, 291, 1769–1772 CrossRef CAS PubMed.
- R. O. Al-Kaysi, A. M. Müller and C. J. Bardeen, J. Am. Chem. Soc., 2006, 128, 15938–15939 CrossRef CAS PubMed.
- R. O. Al-Kaysi and C. J. Bardeen, Adv. Mater., 2007, 19, 1276–1280 CrossRef CAS.
- J. T. Kim, S. K. Seol, J. Pyo, J. S. Lee, J. H. Je and G. Margaritondo, Adv. Mater., 2011, 23, 1968–1970 CrossRef CAS PubMed.
- J. Yoo, J. Pyo and J. H. Je, Nanoscale, 2014, 6, 3557–3560 RSC.
- J. Pyo, J. T. Kim, J. Yoo and J. H. Je, Nanoscale, 2014, 6, 5620–5623 RSC.
- J. Yoo, S. Jeong, S. Kim and J. H. Je, Adv. Mater., 2015 DOI:10.1002/adma.201404945.
- S. Lee, S. Oh, J. Lee, Y. Malpani, Y.-S. Jung, B. Kang, J. Y. Lee, K. Ozasa, T. Isoshima, S. Y. Lee, M. Hara, D. Hashizume and J.-M. Kim, Langmuir, 2013, 29, 5869–5877 CrossRef CAS PubMed.
- L. C. Palmer and S. I. Stupp, Acc. Chem. Res., 2008, 41, 1674–1684 CrossRef CAS PubMed.
- C. J. Barrett, J.-I. Mamiya, K. G. Yager and T. Ikeda, Soft Matter, 2007, 3, 1249–1261 RSC.
- C. D. Eisenbach, Polymer, 1980, 21, 1175–1179 CrossRef CAS.
- M. Warner and L. Mahadevan, Phys. Rev. Lett., 2004, 92 CrossRef CAS , 134302.
- M. Petr and P. T. Hammond, Macromolecules, 2011, 44, 8880–8885 CrossRef CAS.
- M. Saphiannikova and D. Neher, J. Phys. Chem. B, 2005, 109, 19428–19436 CrossRef CAS PubMed.
- T. Kim, L. Zhu, L. J. Mueller and C. J. Bardeen, J. Am. Chem. Soc., 2014, 136, 6617–6625 CrossRef CAS PubMed.
- American Conference of Governmental Industrial Hygienists (ACGIH). (2005). TLVs and BEIs based on the documentation of the threshold limit values for chemical substances and physical agents and biological exposure indices. Cincinnati: author.
- I. Mita, K. Horie and K. Hirao, Macromolecules, 1989, 22, 558–563 CrossRef CAS.
- T. Muraoka, K. Kinbara, Y. Kobayashi and T. Aida, J. Am. Chem. Soc., 2003, 125, 5612–5613 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, X-ray powder diffraction (XRD) patterns of solution-crystallized sample, meniscus-guided microwires, and freeze-dried sample of Azo-1, Schematic of experimental set-up, 3D bending motion of Azo-1 nanowire, FE-SEM image of a bent Azo-1 nanowire after UV irradiation, real-time grazing incidence X-ray diffraction (GIXD) for an Azo-1 microwire, Imaging analyses, Absorption spectra of an Azo-1 film, and thermostability of Azo-1 nanowire. See DOI: 10.1039/c5nr01118c |
| This journal is © The Royal Society of Chemistry 2015 |