Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
J-aggregation, its impact on excited state dynamics and unique solvent effects on macroscopic assembly of a core-substituted naphthalenediimide†
Haridas
Kar
a,
Dominik W.
Gehrig
b,
Frédéric
Laquai
*b and
Suhrit
Ghosh
*a
aPolymer Science Unit, Indian Association for the Cultivation of Science, 2A & 2B Raja S. C. Mullick Road, Kolkata, India-700032. E-mail: psusg2@iacs.res.in
bMax Planck Research Group for Organic Optoelectronics, Max Planck Institute for Polymer Research, Ackermannweg 10, D-55128 Mainz, Germany. E-mail: laquai@mpip-mainz.mpg.de
First published on 12th March 2015
Abstract
Herein we reveal a straightforward supramolecular design for the H-bonding driven J-aggregation of an amine-substituted cNDI in aliphatic hydrocarbons. Transient absorption spectroscopy reveals sub-ps intramolecular electron transfer in isolated NDI molecules in a THF solution followed by a fast recombination process, while a remarkable extension of the excited state lifetime by more than one order of magnitude occurred in methylcyclohexane likely owing to an increased charge-separation as a result of better delocalization of the charge-separated states in J-aggregates. We also describe unique solvent-effects on the macroscopic structure and morphology. While J-aggregation with similar photophysical characteristics was noticed in all the tested aliphatic hydrocarbons, the morphology strongly depends on the “structure” of the solvents. In linear hydrocarbons (n-hexane, n-octane, n-decane or n-dodecane), formation of an entangled fibrillar network leads to macroscopic gelation while in cyclic hydrocarbons (methylcyclohexane or cyclohexane) although having a similar polarity, the cNDI exhibits nanoscale spherical particles. These unprecedented solvent effects were rationalized by establishing structure-dependent specific interactions of the solvent molecules with the cNDI which may serve as a general guideline for solvent-induced morphology-control of structurally related self-assembled materials.
Introduction
Conjugated π-systems provide diverse opportunities for creating functional supramolecular assemblies1 with tunable photophysical properties2 and an enhanced charge-transporting ability3 as a result of the through space π–π interaction which is of considerable interest in miniaturized organic electronic devices4 as well as in biological applications.5 In this context, core-substituted naphthalenediimides (cNDIs)6 have been explored for a large number of applications7 including organic solar cells, electron transport in field effect transistors, anion transport, photo-induced electron transport, fluorescence resonance energy transfer, bio-sensing and in donor–acceptor type conjugated polymers8 owing to their substitution-dependent tunable absorption spanning over almost the entire visible range, their superior fluorescence properties, variable π-acidity and tunable electrochemical properties. Unlike the bay-substituted perylene dyes, the cNDIs retain the planarity of the aromatic ring even after core substitution and thus π-stacking is not hindered. Nevertheless, in contrast to a large number of reports on supramolecular assembly of core unsubstituted NDIs9–11 such examples on cNDIs are still rare,12 possibly due to the lack of a facile synthetic route for their preparation until a recent surge in this field.13 However, even those examples do not adequately elucidate the precise nature of the inter-chromophoric interactions or the impact of self-assembly on photophysical properties. Considering the structural and functional diversities in this class of π-systems, we envisioned that the supramolecular assembly of cNDIs would be worth investigating to examine the effects of structural nuances on self-assembly, morphology, and photophysical properties.14 Amongst various substituted derivatives, amine-substituted NDIs are particularly interesting because of their fascinating redox properties (both di-cation and di-anion formation are possible) and photo-induced charge-separation.15 Herein we reveal the first report of J-aggregation16 of a core-substituted NDI-derivative and unprecedented solvent effects on macroscopic assembly and their excited state dynamics.Results and discussion
H-bonding driven J-aggregation
The structure of the amine-substituted NDI derivative cNDI-1 is shown in Scheme 1. It was synthesized in a few steps (Scheme S1†) from naphthalenetetracarboxylic acid dianhydride and isolated as a deep blue waxy material17 in an overall yield of 34%. The NDI core was substituted by secondary amines and further connected to trialkoxybenzamide (TB) wedges to promote H-bonding (among the amides) driven π-stacking. The control NDI derivative (cNDI-2) that is devoid of the structure-directing TB-wedge18 was also prepared following a similar procedure (Scheme S1†).UV/vis absorption studies reveal strong solvent effects on self-assembly of cNDI-1. In a “good” solvent such as CHCl3 or THF, sharp absorption bands are noticed (Fig. 1a) at 353 and 370 nm (S0–S1) due to π–π* transitions and also a broad charge transfer band at 600 nm.6 In methylcyclohexane (MCH), the π–π* absorption bands exhibit a significant bathochromic shift of ∼11.0 nm in contrast to the control cNDI-2 which does not exhibit such solvent dependent spectral changes (Fig. S1a†). The oscillator strength of the lowest energy π–π* transition band in THF and MCH was estimated (see ESI† for details) to be 14.1 and 10, respectively. Furthermore, photoluminescence spectra show (Fig. 1b) sharp emission bands in MCH exhibiting a mirror-image-like symmetry with the absorption bands with a smaller Stokes shift (20 nm) compared to that in THF (Stokes shift ∼34 nm) suggesting J-aggregation of cNDI-1 in MCH.19 Although the quantum yield of cNDI-1 was found to be low in both solvents, in MCH it was marginally higher (0.153%) than THF (0.07%) corroborating with literature precedence on J-aggregation.16,19 For the control cNDI-2, the values were almost identical in THF (0.07%) and MCH (0.09%). 1H NMR studies (Fig. 1c) further support self-assembly. In CDCl3, both cNDI-1 and cNDI-2 exhibit sharp peaks at ∼8.4 ppm corresponding to the aromatic proton of the NDI ring while in a nonpolar medium (MCH–C6D6: 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the peak disappears only for cNDI-1 suggesting its very high relaxation time as a result of firm self-assembly. It may also be related to a line broadening as a consequence of strong inter-chromophoric interactions. However, for cNDI-2, only an upfield shift is noticed which can be attributed to mere solvent dielectric effects. Thus taking into account these observations, it is conceivable that the TB-wedge dictates the J-aggregation of cNDI-1 possibly by synergistic H-bonding interactions among the amide groups which were directly verified by solvent dependent FT-IR studies. Fig. 2a shows selected regions of the FT-IR spectra of cNDI-1 in MCH and THF. To avoid confusion in peak assignments FT-IR spectra of cNDI-2 were also recorded which show (inset, Fig. 2a) the peak positions of the imide carbonyls marked with blue arrows, but they are not of concern in the context of H-bonding of amides. In addition to those, characteristic peaks are noticed at 3387 cm−1, 1650 cm−1 and 1585 cm−1 (marked with red arrow) in THF solution of cNDI-1 for the N–H stretch, C
1), the peak disappears only for cNDI-1 suggesting its very high relaxation time as a result of firm self-assembly. It may also be related to a line broadening as a consequence of strong inter-chromophoric interactions. However, for cNDI-2, only an upfield shift is noticed which can be attributed to mere solvent dielectric effects. Thus taking into account these observations, it is conceivable that the TB-wedge dictates the J-aggregation of cNDI-1 possibly by synergistic H-bonding interactions among the amide groups which were directly verified by solvent dependent FT-IR studies. Fig. 2a shows selected regions of the FT-IR spectra of cNDI-1 in MCH and THF. To avoid confusion in peak assignments FT-IR spectra of cNDI-2 were also recorded which show (inset, Fig. 2a) the peak positions of the imide carbonyls marked with blue arrows, but they are not of concern in the context of H-bonding of amides. In addition to those, characteristic peaks are noticed at 3387 cm−1, 1650 cm−1 and 1585 cm−1 (marked with red arrow) in THF solution of cNDI-1 for the N–H stretch, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch and amide-11, respectively, of the amide group. These peaks show pronounced solvent effects and appear at 3250 cm−1, 1625 cm−1 and 1575 cm−1, respectively, providing unequivocal evidence in support of inter-molecular H-bonding among the neighboring amide groups in J-aggregated cNDI-1.20 Interestingly in the presence of even a trace amount of the H-bond competing solvent MeOH, the UV/vis spectrum of cNDI-1 in MCH exhibits a significant blue shift (Fig. 2b) and almost resembles the monomeric spectrum in THF further ascertaining the decisive role of H-bonding on J-aggregation.
O stretch and amide-11, respectively, of the amide group. These peaks show pronounced solvent effects and appear at 3250 cm−1, 1625 cm−1 and 1575 cm−1, respectively, providing unequivocal evidence in support of inter-molecular H-bonding among the neighboring amide groups in J-aggregated cNDI-1.20 Interestingly in the presence of even a trace amount of the H-bond competing solvent MeOH, the UV/vis spectrum of cNDI-1 in MCH exhibits a significant blue shift (Fig. 2b) and almost resembles the monomeric spectrum in THF further ascertaining the decisive role of H-bonding on J-aggregation.
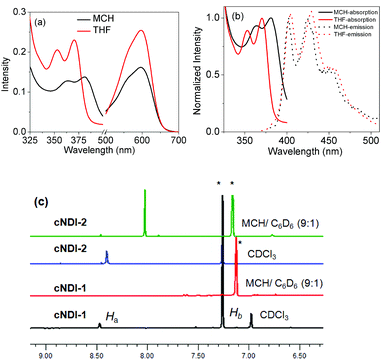 | ||
Fig. 1 (a) UV/Vis (l = 1.0 mm) and (b) intensity normalized absorption and emission (λex = 350 nm) spectra of cNDI-1 in THF and MCH. (c) 1H NMR spectra (selected region) in CDCl3 and MCH–C6D6 (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). *Indicates solvent peaks; C = 0.2 mM (UV), 2.0 mM (NMR); T = 25 °C. UV/Vis spectra of cNDI-1 in benzene (Fig. S2†) do not reflect any specific interaction with the NDI chromophore and thus the presence of 10% C6D6 is not expected to cause any significant perturbation in the self-assembly process. 1). *Indicates solvent peaks; C = 0.2 mM (UV), 2.0 mM (NMR); T = 25 °C. UV/Vis spectra of cNDI-1 in benzene (Fig. S2†) do not reflect any specific interaction with the NDI chromophore and thus the presence of 10% C6D6 is not expected to cause any significant perturbation in the self-assembly process. | ||
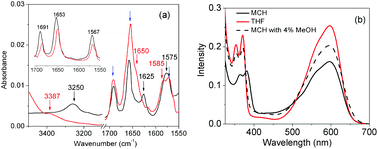 | ||
| Fig. 2 (a) FT-IR spectra of cNDI-1 (inset, cNDI-2) in THF (red line) and MCH (black line). C = 0.2 mM; T = 25 °C. (b) Effect of MeOH on the UV/vis spectra. | ||
Solvent effects on macroscopic assembly and gelation
To understand the solvent effects on self-assembly, we compared the UV/Vis spectra of cNDI-1 in a few other organic solvents (Fig. S2†) with varying polarity. In fact, apart from MCH, pronounced red-shifted spectra are observed only in n-decane which strongly favors H-bonding due to its low polarizability. However, cNDI-1 shows a spontaneous macroscopic gelation21,22 (for gelation test results in other solvents, see Table S1†) in n-decane (inset, Fig. 3b) with an entangled fibrillar structure (length: several micrometers, width: 30–40 nm) as revealed by high resolution transmission electron microscopy (HRTEM) images (Fig. 3b, S3†). In sharp contrast, spherical nanoparticles are seen (Fig. 3a, S3†) in the HRTEM images of cNDI-1 in MCH and the solution does not turn to a gel (inset, Fig. 3a) even at a very high (10.0 mM) concentration. Atomic force microscopy (AFM) studies also suggest a similar contrast in morphology revealing spherical particles (width = 135 ± 15 nm, height = 12 ± 2 nm) and entangled fibers in MCH (Fig. 3c) and n-decane (Fig. 3d), respectively. However, virtually identical absorption and PL spectra (Fig. 3e) in n-decane and MCH confirm similar J-aggregation and MeOH-induced disassembly (Fig. S4†) confirms the involvement of H-bonding in both solvents. Similar changes in the 1H NMR spectrum in decane (Fig. S5†) as observed in the case of MCH (Fig. 1c) suggest rigidification of the chromophore.Thus, it becomes evident that the difference lies in the macroscopic assembly and not in the inter-chromophoric interaction. Furthermore, very similar spectral features in the gel state compared to a dilute solution in n-decane (Fig. 3f) suggest analogous inter-chromophoric interactions in the gel and the sol state. Then, why do such contrasting macroscopic structures exist in these two solvents of almost identical polarity? Powder X-ray diffraction (XRD) studies (Fig. 4b) provide valuable information to rationalize this intriguing observation. XRD data of the dried gel in n-decane show a sharp peak at 2θ = 1.99° (d = 45 Å) nearly matching with the length of the cNDI-1 along the y-direction (Fig. 4a) and also show successive peaks corresponding to d/2 and d/3 suggesting a lamellar packing. Interestingly, XRD patterns of a dried solution from MCH reveal an additional sharp peak at 2θ = 5.3° (d = 16 Å). Such an intense peak is unlikely due to the d/2 (as in the case of n-decane). Interestingly, this distance matches closely with the length of the molecule along the x-direction. Thus, it appears that in MCH, the growth happens in all three directions by (H-bonding + π–π interactions), van der Waals interactions among the peripheral alkyl chains of the TB-wedge and C-8 chains connected to the imide position, which understandably leads to spherical structures as observed by HRTEM and AFM microscopy. However, in n-decane, the absence of an intense peak corresponding to the molecular length along the x-direction suggests no long range growth along this direction. This can be attributed to the capping of the C-8 chains by the linear hydrocarbon solvent molecules by effective van der Waals interactions. Thus instead of a 3D spherical structure, in this case fibrillar morphology is noticed.
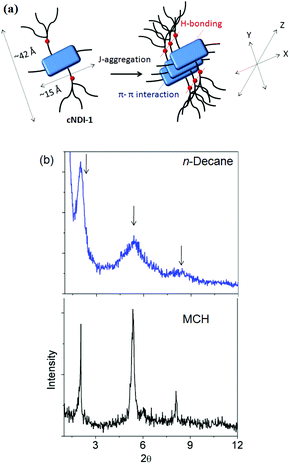 | ||
| Fig. 4 (a) Proposed model for self-assembly; (b) XRD pattern of the dropcast sample from J-aggregated cNDI-1 gel (n-decane) and sol (MCH). | ||
To substantiate such possibility of a specific interaction of the extended C10 chains of n-decane with the alkyl chains23 of the gelator, the possibility of external chiral induction24 to the J-aggregated cNDI-1 was examined by using two different chiral solvents, namely (S)-citronellyl bromide and (S)-limonene, which can be considered closely similar to n-decane and MCH, respectively, in terms of their linear and cyclic structures. The CD spectra of cNDI-1 show (Fig. 5) intense bisignated Cotton effects in (S)-citronellyl bromide, but not in (S)-limonene. Interestingly, the CD band in (S)-citronellyl bromide disappears after the addition of trace amounts of trifluoroacetic acid as it destroys H-bonding and thus disrupts self-assembly. In fact, the chromophore itself does not contain any chiral centre and thus the observed Cotton effect has to be attributed to induction of chirality by the solvent molecules, which itself is only effective in the case of the straight chain chiral solvent due to a more efficient van der Waals interaction with the peripheral alkyl chains of the gelator.
 | ||
| Fig. 5 Solvent dependent CD spectra of cNDI-1. The chemical structures of the solvents are shown on the left. | ||
In this scenario one can expect tunable gelation depending on the length of the hydrocarbon solvent, as this will determine the efficacy of interaction. To test this hypothesis, we studied gelation in n-hexane (C6), n-octane (C8), n-decane (C10) and n-dodecane (C12) solvents in which the gelation properties are indeed very different (Table 1). For instance, in n-dodecane a gel was formed within ∼15 min, while it took ∼18 h in n-hexane. This is also reflected in the viscoelastic properties of the gels as revealed by stress-amplitude sweep measurements in different solvents. At a lower value of applied stress, storage modulus (G′) > loss modulus (G′′) is observed (Table 1) for all samples suggesting the existence of a typical gel phase. The value of G′–G′′ which indicates that the elastic nature shows almost one order of magnitude increase for the n-dodecane gel compared to the n-hexane gel (Fig. 6), while the values in the n-octane and n-decane gel (Fig. S6†) lie in-between (Table 1).
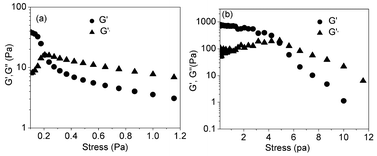 | ||
| Fig. 6 Stress amplitude sweep measurements results for cNDI-1 gel (C = 10 mM) in (a) n-hexane and (b) n-dodecane. | ||
| Properties | n-Hexane | n-Octane | n-Decane | n-Dodecane |
|---|---|---|---|---|
| Time (min) | 1100 | 480 | 60 | 15 |
| T g/°C | 38 | 46 | 64 | 76 |
| CGC (mM) | 2.8 | 2.0 | 1.2 | 0.8 |
| G′ (Pa) | 38 | 74 | 92 | 794 |
| G′′ (Pa) | 8.0 | 10 | 13 | 88 |
| σ y (Pa) | 0.2 | 1.0 | 1.7 | 4.9 |
Furthermore, the yield-stress (σy) (the critical value where G′ becomes less than G′′), a parameter that indicates robustness, also follows the same trend confirming this unprecedented impact of the chain length of the aliphatic hydrocarbon solvents on gelation. It is noteworthy that the solubility parameters of these solvents25 are almost comparable and slightly increases (Table S2†) with increasing chain length. Thus as per literature precedence26 the CGC should have increased going from n-hexane to n-dodecane, but we noticed the opposite trend. Furthermore the solubility parameters of MCH and n-dodecane are almost the same (Table S2†) but we find no gelation in MCH while strongest gelation in n-dodecane. Thus the solvent dependent gelation in the present system cannot be rationalized based on mere solubility parameters of the solvents. Intriguingly the absorption spectra of cNDI-1, measured soon after solution preparation, look almost identical (Fig. S7†) in all cases, indicating spontaneous J-aggregation irrespective of the chain length of the solvent molecules. The question arises as to why gelation is so slow? To answer this question, we examined the morphology of cNDI-1 in n-octane by AFM microscopy at different time intervals (Fig. 7) which shows a very interesting morphology evolution during aging.27 For a freshly prepared solution, spherical particles are noticed even in this case (like in MCH) which gradually convert to fibrils leading to gelation as a result of van der Waals interactions between solvent molecules and the alkyl chains of the gelator. It is conceivable that the efficacy of such an interaction increases with the length of the alkyl chain which eventually determines the time required for gelation and in addition the physical properties of the gels (Table 1). On the other hand, cyclic hydrocarbons such as MCH or cyclohexane, lacking such propensity to interact with the alkyl chains of the gelator, are unable to produce a macroscopic gel, even though similar J-aggregation was noticed.
Impact of J-aggregation on excited state dynamics
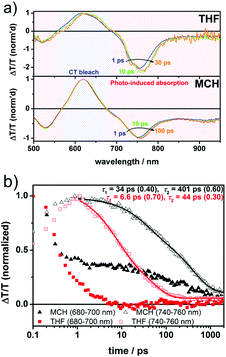
Finally, we performed ultrafast transient absorption pump–probe spectroscopy to elucidate the excited state dynamics of cNDI-1 in different solvents on the picosecond to nanosecond timescale as depicted in Fig. 8 and 9. The measurements reveal a strong dependence of the excited state lifetimes on the polarity of the solvent. THF was used as a “good” solvent, in which mainly monomeric states of cNDI-1 exist (Fig. 1). The transient spectra after 1 ps shown in Fig. 8a exhibit a ground-state bleach (GSB) originating from the depopulation of the ground state at the position of the charge transfer absorption band and moreover photo-induced absorption (PIA) features below 558 nm and above 688 nm. The spectral features remain virtually the same after 1 ps. However, on the sub-ps timescale we observed spectral changes (Fig. 9) indicating the transition from the primary photoexcitation to an intramolecular charge-transfer (CT) state. | ||
| Fig. 9 Sub-ps spectra of cNDI-1 in MCH (upper panel) and dynamics at various wavelength regions (lower panel). | ||
This implies ultrafast electron transfer from the electron rich amine wedge to the NDI core within the temporal resolution of our TA setup of ∼200 fs. The ps–ns decay dynamics of the PIA peak around 750 nm is shown in Fig. 8b (open symbols). The excited state decay in THF can be described by a biexponential function consisting of a short-lived component with an inverse decay rate of 6.6 ps and a relative amplitude of 70% and a longer-lived component exhibiting an inverse decay rate of 44 ps with a relative amplitude of 30%. We assign the short component to a fast decay of excited states, while the slow component is assigned to the recombination of intramolecular CT states. This is in line with previous reports, for instance, such decay dynamics on the timescale of tens of picoseconds have been reported earlier for nitrogen-substituted NDIs.28cNDI-1 was also measured in MCH in which it shows J-aggregation. MCH was particularly chosen as the solvent for TA experiments as it avoids scattering of pump and probe beams on the one hand, while maintaining a sufficient degree of supramolecular organization on the other hand. cNDI-1 excited states exhibit distinct differences in MCH compared to THF. Interestingly, the ratio of the peaks of GSB and PIA is altered. While the measurement in THF resulted in a peak ratio of GSB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PIA ∼ 1
PIA ∼ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, it was only 1
2, it was only 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in MCH. The PIA peak at 750 nm is assigned to NDI anions according to the literature.9 We note, that a similar change has been reported for perylenediimide (PDI) derivatives.29 We speculate that the higher NDI anion absorption in THF can be explained by a stronger localization of the charge on a single chromophore unit resulting in a more pronounced charge-induced absorption peak. In contrast, in MCH delocalization is enabled by aggregation and therefore the relative intensity of the spectral feature is reduced. Furthermore, a feature around 900 nm evolves, which is assigned to aggregates, as previously also observed for PDI derivatives. In line with the spectral changes, a prolongation of excited state lifetimes was observed. A biexponential fit to the decay dynamics obtained from TA measurements in MCH yielded inverse decay rates of 34 ps and 401 ps with relative amplitudes of 40% and 60%, respectively. The considerable extension of the excited state lifetime by more than one order of magnitude is likely caused by an increased separation of charges enabled by the aggregation of chromophores and hence an increased delocalization30 of charge separated states.
1 in MCH. The PIA peak at 750 nm is assigned to NDI anions according to the literature.9 We note, that a similar change has been reported for perylenediimide (PDI) derivatives.29 We speculate that the higher NDI anion absorption in THF can be explained by a stronger localization of the charge on a single chromophore unit resulting in a more pronounced charge-induced absorption peak. In contrast, in MCH delocalization is enabled by aggregation and therefore the relative intensity of the spectral feature is reduced. Furthermore, a feature around 900 nm evolves, which is assigned to aggregates, as previously also observed for PDI derivatives. In line with the spectral changes, a prolongation of excited state lifetimes was observed. A biexponential fit to the decay dynamics obtained from TA measurements in MCH yielded inverse decay rates of 34 ps and 401 ps with relative amplitudes of 40% and 60%, respectively. The considerable extension of the excited state lifetime by more than one order of magnitude is likely caused by an increased separation of charges enabled by the aggregation of chromophores and hence an increased delocalization30 of charge separated states.
Conclusions
In summary, we have demonstrated for the first time the design of a supramolecular building block based on an amine-substituted NDI-chromophore for H-bonding driven J-aggregation in non-polar solvents and its effect on excited state dynamics. Unprecedentedly, the lifetime of the charge-separated state could be prolonged by more than one order of magnitude by J-aggregation, which appears highly promising for various applications including efficient photo-catalysis and energy conversion.31,32 We have also shown intriguing solvent effects on self-assembly. While the polarity, which influences H-bonding, dictated the J-aggregation, the macroscopic structure and gelation showed a strong dependence on the structure of the solvent molecules. Linear and cyclic hydrocarbons produced contrasting morphologies (fibrillar and spherical, respectively), leading to macroscopic gelation only in the former cases. While several reports have correlated solvent effects on gelation33 by different physical parameters such as Hildebrand, Hansen and Kamlet–Taft parameters, polarity (Et(30)) or dielectric constant, this is the first example to the best of our knowledge demonstrating that even a variation in the chain length or structure, namely linear vs. cyclic, in a series of aliphatic hydrocarbon solvents with identical polarity can impose such a remarkable influence on morphology.Acknowledgements
HK thanks CSIR, India for a research fellowship. SG thanks SERB, India for funding (Project no: SR/S1/OC-18/2012). FL thanks the Max Planck Society for funding the Max Planck Research Group and gratefully acknowledges a Kekulé scholarship of the Fonds der Chemischen Industrie (FCI).Notes and references
- F. J. M. Hoeben, P. Jonkheijm, E. W. Meijer and A. P. H. J. Schenning, Chem. Rev., 2005, 105, 1491 CrossRef CAS PubMed; Z. Chen, A. Lohr, C. R. Saha-Möller and F. Würthner, Chem. Soc. Rev., 2009, 38, 564 RSC; T. Nakanishi, Chem. Commun., 2010, 46, 3425 RSC; T. F. A. De Greef, M. M. J. Smulders, M. Wolffs, A. P. H. J. Schenning, R. P. Sijbesma and E. W. Meijer, Chem. Rev., 2009, 109, 5687 CrossRef PubMed; A. Ajayaghosh and V. K. Praveen, Acc. Chem. Res., 2007, 40, 644 CrossRef PubMed; S. Yagai and A. Kitamura, Chem. Soc. Rev., 2008, 37, 1520 RSC; A. Mishra, C.-Q. Ma and P. Bauerle, Chem. Rev., 2009, 109, 1141 CrossRef PubMed; D. G. Rodríguez and A. P. H. J. Schenning, Chem. Mater., 2011, 23, 310 CrossRef; F. Würthner, Chem. Commun., 2004, 1564 RSC; M. R. Molla and S. Ghosh, Phys. Chem. Chem. Phys., 2014, 16, 26672 RSC; A. Das and S. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 2038 CrossRef PubMed.
- L. Magginia and D. Bonifazi, Chem. Soc. Rev., 2012, 41, 211 RSC; S. S. Babu, S. Prasanthkumar and A. Ajayaghosh, Angew. Chem., Int. Ed., 2012, 51, 1766 CrossRef CAS PubMed.
- A. Saeki, Y. Koizumi, T. Aida and S. Seki, Acc. Chem. Res., 2012, 45, 1193 CrossRef CAS PubMed.
- A. W. Hains, Z. Liang, M. A. Woodhouse and B. A. Gregg, Chem. Rev., 2010, 110, 6689 CrossRef CAS PubMed; J. Mei, Y. Diao, A. L. Appleton, L. Fang and Z. Bao, J. Am. Chem. Soc., 2013, 135, 6724 CrossRef PubMed.
- For a review on supramolecular biomaterials see: K. P. Milroy, M. H. Sonntag and L. Brunsveld, Chem. – Eur. J., 2013, 19, 10786 CrossRef PubMed.
- For a review on cNDI see: N. Sakai, J. Mareda, E. Vauthey and S. Matile, Chem. Commun., 2010, 46, 4225 RSC.
- For representative examples on cNDI based organic materials see: T. Earmme, Y.-J. Hwang, N. M. Murari, S. Subramaniyan and S. A. Jenekhe, J. Am. Chem. Soc., 2013, 135, 14960 CrossRef CAS PubMed; B. C. Popere, A. M. Della Pelle and S. Thayumanavan, Macromolecules, 2011, 44, 4767 CrossRef; B. A. Jones, A. Facchetti, T. J. Marks and M. R. Wasielewski, Chem. Mater., 2007, 19, 2703 CrossRef; J. H. Oh, S.-L. Suraru, W.-Y. Lee, M. Könemann, H. W. Höffken, C. Röger, R. Schmidt, Y. Chung, W.-C. Chen, F. Würthner and Z. Bao, Adv. Funct. Mater., 2010, 20, 2148 CrossRef; R. Bhosale, R. S. K. Kishore, V. Ravikumar, O. Kel, E. Vauthey, N. Sakai and S. Matile, Chem. Sci., 2010, 1, 357 RSC; K. Baeg, D. Khim, S. Jung, M. Kang, I. You, D. Kim, A. Facchetti and Y. Noh, Adv. Mater., 2012, 24, 5433 CrossRef PubMed; Y. A. Getmanenko, S. Singh, B. Sandhu, C. Wang, T. Timofeeva, B. Kippelen and S. R. Marder, J. Mater. Chem. C, 2014, 2, 124 RSC; C. Röger, Y. Miloslavina, D. Brunner, A. R. Holzwarth and F. Würthner, J. Am. Chem. Soc., 2008, 130, 5929 CrossRef PubMed; S. Bevers, S. Schutte and L. W. McLaughlin, J. Am. Chem. Soc., 2000, 122, 5905 CrossRef; S. Chopin, F. Chaignon, E. Blart and F. Odobel, J. Mater. Chem., 2007, 17, 4139 RSC; R. Fernando, Z. Mao, E. Muller, F. Ruan and G. Sauvé, J. Phys. Chem. C, 2014, 118, 3433 Search PubMed.
- For a review on conjugated polymers based on NDI see: J. Wang and T. Higashihara, Polym. Chem., 2013, 4, 5518 RSC.
- S. V. Bhosale, C. H. Jani and S. J. Langford, Chem. Soc. Rev., 2008, 37, 331 RSC.
- P. Mukhopadhyay, Y. Iwashita, M. Shirakawa, S.-i. Kawano, N. Fujita and S. Shinkai, Angew. Chem., Int. Ed., 2006, 45, 1592 CrossRef CAS PubMed; G. D. Pantos, P. Pengo and J. K. M. Sanders, Angew. Chem., Int. Ed., 2007, 46, 194 CrossRef PubMed; J. Zheng, W. Qiao, X. Wan, J. P. Gao and Z. Y. Wang, Chem. Mater., 2008, 20, 6163 CrossRef; H. Shao and J. R. Parquette, Chem. Commun., 2010, 46, 4285 RSC; S. Tu, S. H. Kim, J. Joseph, D. A. Modarelli and J. R. Parquette, J. Am. Chem. Soc., 2011, 133, 19125 CrossRef PubMed; M. Kumar and S. J. George, Chem. Sci., 2014, 5, 3025 RSC; M. B. Avinash, E. Verheggen, C. Schmuck and T. Govindaraju, Angew. Chem., Int. Ed., 2012, 51, 10324 CrossRef PubMed.
- In the past few years we have reported various supramolecular strategies for H-bonding driven self-assembly of NDI-building blocks in organic and water media and also in macromolecular systems. For representative examples see: M. R. Molla and S. Ghosh, Chem. Mater., 2011, 23, 95 CrossRef CAS; M. R. Molla and S. Ghosh, Chem. – Eur. J., 2012, 18, 9849 CrossRef PubMed; A. Das and S. Ghosh, Macromolecules, 2013, 46, 3939 CrossRef; A. Das and S. Ghosh, Angew. Chem., Int. Ed., 2014, 53, 1092 CrossRef PubMed.
- I. D. Cat, C. Röger, C. C. Lee, F. J. M. Hoeben, M. J. Pouderoijen, A. P. H. J. Schenning, F. Würthner and S. De Feyter, Chem. Commun., 2008, 5496 RSC; S. V. Bhosale, C. Jani, C. H. Lalander and S. J. Langford, Chem. Commun., 2010, 46, 973 RSC; S. V. Bhosale, C. H. Jani, C. H. Lalander, S. J. Langford, I. Nerush, J. G. Shapter, D. Villamaina and E. Vauthey, Chem. Commun., 2011, 8226 RSC.
- S.-L. Suraru and F. Würthner, Angew. Chem., Int. Ed., 2014, 53, 7428 CrossRef CAS PubMed.
- S. S. Babu, K. K. Kartha and A. Ajayaghosh, J. Phys. Chem. Lett., 2010, 1, 3413 CrossRef CAS.
- S. Bhosale, A. L. Sisson, P. Talukdar, A. Fürstenberg, N. Banerji, E. Vauthey, G. Bollot, J. Mareda, C. Röger, F. Würthner, N. Sakai and S. Matile, Science, 2006, 313, 84 CrossRef CAS PubMed.
- For a recent review on J-aggregation see: F. Würthner, T. E. Kaiser and C. R. Saha-Möller, Angew. Chem., Int. Ed., 2011, 50, 3376 CrossRef PubMed.
- The waxy nature of such a high molecular weight material although can be counterintuitive but recent reports suggest that alkyl chain crowding may induce such amorphous nature even for pi-conjugated systems. For details see: S. S. Babu, M. J. Hollamby, J. Aimi, H. Ozawa, A. Saeki, S. Seki, K. Kobayashi, K. Hagiwara, M. Yoshizawa, H. Möhwald and T. Nakanishi, Nat. Commun., 2013, 4, 1969–1961 Search PubMed.
- H. Kar, M. R. Molla and S. Ghosh, Chem. Commun., 2013, 49, 4220 RSC.
- For very similar spectral changes in the case of J-aggregating perylene dyes see: X.-Q. Li, X. Zhang, S. Ghosh and F. Würthner, Chem. – Eur. J., 2008, 14, 8074 CrossRef CAS PubMed . For examples on J-aggregation of unsubstituted NDI see: H. Shao, T. Nguyen, N. C. Romano, D. A. Modarelli and J. R. Parquette, J. Am. Chem. Soc., 2009, 131, 16374 CrossRef PubMed; M. R. Molla, D. Gehrig, L. Roy, V. Kamm, A. Paul, F. Laquai and S. Ghosh, Chem. – Eur. J., 2014, 20, 760 CrossRef PubMed; S. Choi, J. Bouffard and Y. Kim, Chem. Sci., 2014, 5, 751 RSC.
- S. Cantekin, T. F. A. de Greefab and A. R. A. Palmans, Chem. Soc. Rev., 2012, 41, 6125 RSC; S. Diring, F. Camerel, B. Donnio, T. Dintzer, S. Toffanin, R. Capelli, M. Muccini and R. Ziessel, J. Am. Chem. Soc., 2009, 131, 18177 CrossRef CAS PubMed.
- For a recent review on pi-gelators see: S. S. Babu, V. K. Praveen and A. Ajayaghosh, Chem. Rev., 2014, 114, 1973 CrossRef CAS PubMed.
- For general reviews on small molecule gelators see: L. A. Estroff and A. D. Hamilton, Chem. Rev., 2004, 104, 1201 CrossRef CAS PubMed; N. M. Sangeetha and U. Maitra, Chem. Soc. Rev., 2005, 34, 821 RSC; L. E. Buerkle and S. J. Rowan, Chem. Soc. Rev., 2012, 41, 6089 RSC.
- M. J. Hollamby, M. Karny, P. H. H. Bomans, N. A. J. M. Sommerdijk, A. Saeki, S. Seki, H. Minamikawa, I. Grillo, B. R. Pauw, P. Brown, J. Eastoe, H. Möhwald and T. Nakanishi, Nat. Chem., 2014, 6, 690 CrossRef CAS.
- For representative examples on chiral induction see: E. Yashima and K. Maeda, Macromolecules, 2008, 41, 3 CrossRef CAS; H. Goto, Y. Okamoto and E. Yashima, Macromolecules, 2002, 35, 4590 CrossRef; L. Brunsveld, J. A. J. M. Vekemans, J. H. K. K. Hirschberg, R. P. Sijbesma and E. W. Meijer, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4977 CrossRef PubMed; A. P. H. J. Schenning, J. v. Herrikhuyzen, P. Jonkheijm, Z. Chen, F. Würthner and E. W. Meijer, J. Am. Chem. Soc., 2002, 124, 10252 CrossRef PubMed; M. R. Molla, A. Das and S. Ghosh, Chem. Commun., 2011, 47, 8934 RSC.
- A. F. M. Barton, Chem. Rev., 1975, 75, 731 CrossRef CAS.
- K. Hanabusa, M. Matsumoto, M. Kimura, A. Kakehi and H. Shirai, J. Colloid Interface Sci., 2000, 224, 231 CrossRef CAS PubMed.
- A. Das, B. Maity, D. Koley and S. Ghosh, Chem. Commun., 2013, 5757 RSC; C. Rest, A. Martin, V. Stepanenko, N. K. Allampally, D. Schmidt and G. Fernández, Chem. Commun., 2014, 50, 13366 RSC.
- U. Megerle, I. Pugliesi, C. Schriever, C. F. Sailer and E. Riedle, Appl Phys B: Lasers Opt., 2009, 96, 215 CrossRef CAS PubMed; I. Pugliesi, U. Megerle, S.-L. Suraru, F. Würthner, E. Riedle and S. Lochbrunner, Chem. Phys. Lett., 2011, 504, 24 CrossRef PubMed; S. Chopin, F. Chaignon, E. Blart and F. Odobel, J. Mater. Chem., 2007, 17, 4139 RSC.
- J. M. Lim, P. Kim, M. Yoon, J. Sung, V. Dehm, Z. Chen, F. Würthner and D. Kim, Chem. Sci., 2013, 4, 388 RSC.
- For recent reports on delocalization effects on the NDI radical anion see: A. Takai, T. Yasuda, T. Ishizuka, T. Kojima and M. Takeuchi, Angew. Chem., Int. Ed., 2013, 52, 9167 CrossRef CAS PubMed; Y. Wu, M. Frasconi, D. M. Gardner, P. R. McGonigal, S. T. Schneebeli, M. R. Wasielewski and J. F. Stoddart, Angew. Chem., Int. Ed., 2014, 53, 9476 CrossRef PubMed.
- S. Fukuzumi, Electron Transfer of p-Functional Systems and Applications, Functional Organic Materials, Wiley-VCH, 2007, pp. 465–510 Search PubMed.
- For a very recent report on visible light induced catalysis using perylene dyes, see: I. Ghosh, T. Ghosh, J. I. Bardagi and B. König, Science, 2014, 346, 725 CrossRef CAS PubMed.
- P. Curcio, F. Allix, G. Pickaert and B. J. Grégoire, Chem. – Eur. J., 2011, 17, 13603 CrossRef CAS PubMed; A. R. Hirst and D. K. Smith, Langmuir, 2004, 20, 10851 CrossRef PubMed; W. Edwards, C. A. Lagadec and D. K. Smith, Soft Matter, 2011, 7, 110 RSC; Y. Jeong, K. Hanabusa, H. Masunaga, I. Akiba, K. Miyoshi, S. Sakurai and K. Sakurai, Langmuir, 2005, 21, 586 CrossRef PubMed; P. Zhu, X. Yan, Y. Su, Y. Yang and J. Le, Chem. – Eur. J., 2010, 16, 3176 CrossRef PubMed; T. Pinault, B. Isare and L. Bouteiller, ChemPhysChem, 2006, 7, 816 CrossRef PubMed; A. Aggeli, M. Bell, N. Boden, J. N. Keen, P. F. Knowles, T. C. B. McLeish, M. Pitkeathly and S. E. Radford, Nature, 1997, 386, 259 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization, additional spectroscopic and microscopic data on self-assembly and gelation test details in various solvents. See DOI: 10.1039/C5NR00483G |
| This journal is © The Royal Society of Chemistry 2015 |