Synthesis of heterodimer radionuclide nanoparticles for magnetic resonance and single-photon emission computed tomography dual-modality imaging†
Jing
Zhu‡
a,
Bin
Zhang‡
b,
Jian
Tian
c,
Jiaqing
Wang
a,
Yu
Chong
c,
Xin
Wang
d,
Yaoyao
Deng
a,
Minghua
Tang
e,
Yonggang
Li
d,
Cuicui
Ge
*c,
Yue
Pan
*a and
Hongwei
Gu
*a
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science & Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, 215123, China. E-mail: hongwei@suda.edu.cn; panyue@suda.edu.cn
bDepartment of Nuclear Medicine, The First Affiliated Hospital of Soochow University, Suzhou 215006, China
cSchool for Radiological and Interdisciplinary Sciences (RAD-X) & Collaborative Innovation Center of Radiation Medicine of Jiangsu Higher Education Institutions, Soochow University, Suzhou, China. E-mail: ccge@suda.edu.cn
dDepartment of Radiology, The First Affiliated Hospital of Soochow University, Soochow University, Suzhou 215006, China
eAnalysis and Testing Center, Soochow University, Suzhou, China
First published on 5th January 2015
Abstract
We report a facile synthesis of bifunctional Fe3O4-Ag125I heterodimers for use as dual-modality imaging agents in magnetic resonance (MR) and single-photon emission computed tomography (SPECT). We introduced 125I, which is a clinically used radioisotope, as a SPECT reporter, into Fe3O4-Ag heterodimer nanoparticles to provide a new type of bifunctional contrast agent for MRI and SPECT imaging.
In recent years non-invasive imaging techniques, such as magnetic resonance imaging (MRI),1 single-photon emission computed tomography (SPECT),2 computed tomography (CT),3 photoacoustic tomography (PAT),4 and optical imaging,5 have become indispensible clinical methods for diagnosing various diseases. However, a single imaging modality is rarely sufficient to obtain all the demanded information.6 Using bimodal imaging probes and combining two techniques can synergize the advantages and/or mitigate the disadvantages of individual techniques. In the past few years, various nanocomposites have been developed with functional combinations including CT/MRI, MRI/PET, MRI/SPECT, CT/PAT, and SPECT/CT.7–11 Our group has also developed heterostructured nanocomposites for CT/MRI.7 Compared with CT, MRI offers higher resolution of soft tissues without any associated radiation risks. Encouraged by the successful combination of SPECT and CT,11 SPECT and MRI (SPECT/MRI) have been actively pursued by the imaging community in recent years.9 MRI has high spatial resolution with lower sensitivity, while SPECT has an outstanding sensitivity but lower resolution. MRI/SPECT dual-modal contrast agents can enable a hybrid imaging modality that takes advantage of these features. There is a demand for multimodal contrast agents for MRI/SPECT pre-clinically and clinically. One approach to achieve these nanomaterials is by labeling metal nanoparticles used in MRI, with radionuclides used in SPECT.
Magnetic iron oxide nanoparticles are known as T2-relaxing contrast agents that decrease the MRI signal intensity by shortening the T2 relaxation times of nearby protons, resulting in dark areas in images.1 Iodine-125 (125I) is a candidate single-photon emitting radionuclide with good potential in SPECT imaging application because of its suitable long half-life (t1/2 = 61.14 days). Additionally, the production of this radioisotope is simple and cost-effective.
We hypothesized that 125I could be inserted into a suitable nanocomposite functionalized by PEG to form a MRI/SPECT contrast agent. In this communication, Fe3O4-Ag heterostructured nanoparticles were selected for this purpose. Radiolabeling of the Fe3O4-Ag heterodimer nanoparticles was achieved by the rapid reaction of iodine with the Ag component of the nanoparticles. Scheme 1 illustrates the general synthetic strategy towards Fe3O4-Ag125I nanoparticles. Firstly, the synthesis of Fe3O4-Ag nanoparticles was performed according to a reported procedure with some slight modifications.12 In brief, the monodisperse Fe3O4 nanoparticles were synthesized through a thermal decomposition of an iron-oleate complex13 and then the Ag component was grown onto the Fe3O4 nanoparticles by adding the silver acetate into the reaction system. Secondly, the Fe3O4-Ag heterostructured nanoparticles were functionalized by a hydrophilic mPEG-LA polymer and transferred from hexane to water.14 The as-synthesized PEGylated Fe3O4-Ag heterostructured nanoparticles were found to be highly stable in water with no visible aggregation over one month. Finally, the Ag component of the heterostructured nanoparticles was reacted with sodium iodine-125, and stirred at room temperature to produce the radionuclide labeled Fe3O4-Ag125I heterodimer nanoparticles (Scheme 1 and ESI†). The effectiveness of the heterodimer radionuclide nanoparticles as MRI contrast agents was evaluated via an in vitro MRI relaxivity measurement. The 125I radiolabeled heterodimer nanoparticles were further tested as SPECT contrast agents via in vivo experiments.
Transmission electron microscope (TEM) was used to follow the synthesis procedure and to characterize the heterodimer nanoparticles. Fe3O4 nanoparticles with uniform morphology are shown in Fig. 1A. The average size of Fe3O4 nanoparticles was about 14 nm. The heterostructured Fe3O4-Ag nanoparticles are shown in Fig. 1B, which are well-dispersed in hexane. A well-established phase transfer process using the mPEG-LA polymer efficiently allowed the dispersion of the Fe3O4-Ag nanoparticles in an aqueous phase through metal–thiol bonds.14 A TEM image (Fig. S1†) confirmed that they still retained the heterodimer nanostructures in morphology after phase transfer. After coating with mPEG-LA, these Fe3O4-Ag nanoparticles (PEG-Fe3O4-Ag) were easily dispersed in water (Fig. S2†). No precipitates were observed even after one month. As shown in Fig. 1C, the size of the Ag125I spherical component in heterodimer nanoparticles was about 9 nm. The addition of a radionuclide into the Fe3O4-Ag nanoparticles did not change the morphology of the samples. Furthermore, the energy dispersive X-ray spectroscopy (EDS) analysis (Fig. 1D) confirmed the presence of silver, iron, and iodine elements in the synthesized heterostructured nanoparticles.
Representative X-ray diffraction (XRD) patterns of the Fe3O4-Ag and Fe3O4-Ag125I nanoparticles are shown in Fig. 2A, pointing out the occurrence of crystalline phases of both AgI and Fe3O4 in the heterostructures. Representative X-ray photoelectron spectroscopy (XPS) spectra of Fe3O4-Ag125I nanoparticles (Fig. 2B and C) also showed the presence of Ag and I with the peak of binding energy occurring at 367.9 eV (for Ag 3d5/2) and 618.4 eV (for I 3d5/2), respectively.
After characterization, we obtained T2-MRI images of a phantom at various concentrations of Fe3O4-Ag125I nanoparticles dispersed in deionized water using a GE 1.5 T Signa Excite MRI scanner. As the nanoparticle concentration increased, the signal intensity decreased significantly (Fig. 3, inset). The measured r2 value was 139.8 mM−1 s−1 (Fig. 3) obtained by plotting 1/T2 against the iron concentration ([Fe]) in the solution, which was close to that of a commercial MRI contrast agent.15 The T2 contrast effect of the nanoparticles was saturated at a concentration of 0.34 mmol L−1 and no signal attenuation was observed above this concentration. Since MRI is very sensitive, a sufficient T2 contrast effect was observed even at low concentrations.
 | ||
| Fig. 3 Plots of 1/T2versus iron concentration. Transverse (r2) relaxivity was determined by linear flitting of the plots (inset: T2-weighted MR images of the same phantom). | ||
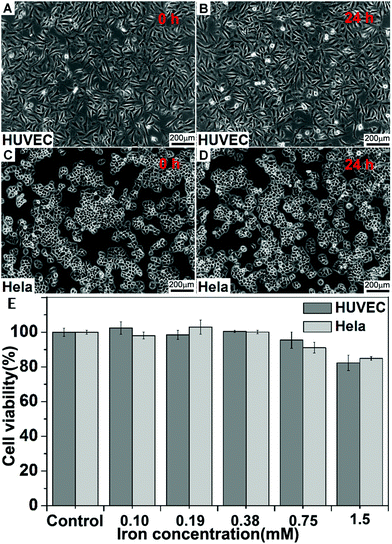
We assessed the biocompatibility of the Fe3O4-Ag125I nanoparticles toward mammalian cells by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay using a HUVEC (normal) and HeLa cell line, respectively. The PEG-Fe3O4-Ag125I nanoparticles were tested over a dosage range of 0–1.5 mM of Fe3O4-Ag125I nanoparticles at 37 °C. The MTT assay (Fig. 4E) showed no appreciable cytotoxicity (cell viability > 85%) detected up to a concentration of 1.5 mM after 24 h of exposure. This high biocompatibility is a great advantage of Fe3O4-Ag125I nanoparticles for further clinical use. We further demonstrated the cytocompatibility of Fe3O4-Ag125I nanoparticles through optical microscopy. The HUVEC and HeLa cells were incubated with the Fe3O4-Ag125I nanoparticles (1.5 mM) at 37 °C for 24 h, respectively. As shown in Fig. 4A–D, there was no obvious change in the cellular morphology after treated with the Fe3O4-Ag125I nanoparticles, which agreed with the MTT data.
To confirm the 125I labeling yield, various concentrations of Na125I were added into PEG-Fe3O4-Ag heterostructured nanoparticles (2 mL, 1 mmol Fe L−1). Free radioisotopes were removed from the radiolabeled nanoparticles by filtering through a sterile membrane. The content of the free iodine was less than 1%, as estimated by analysis of the activity of the supernatant. The radiolabeling yield of Fe3O4-Ag125I heterostructured nanoparticles was found to be above 80% after 30 min of stirring (Fig. 5A). A mixture of free 125I and water showed a nearly zero labeling yield, confirming the successful labeling of 125I to Fe3O4-Ag heterostructured nanoparticles.
To demonstrate the feasibility of Fe3O4-Ag125I heterostructured radionuclide nanoparticles for in vivo SPECT imaging, a heterostructured radionuclide nanoparticle solution (100 μL, 120 μCi) was intravenously injected into Kunming mice (n = 5) via the tail vein. A solution of Na125I with the same radioactivity was used as a comparison. In the whole-body SPECT imaging of mice, liver and spleen were all clearly visualized at 1 h post-injection (Fig. 5B left), but little or no accumulation in kidney, muscle or other major organs. Upon injection of Na125I at 1 h (Fig. 5B, right), nanoparticles showed darkening of the kidney and the bladder, but nearly no liver and spleen uptake. Dominant liver (31.98 ± 2.44% ID g−1) and spleen (41.87 ± 4.41% ID g−1) uptake of Fe3O4-Ag125I nanoparticles was observed at 1 h post-injection (Table S1†). These results suggest that such heterostructured radionuclide nanoparticles show potential capability in tumor imaging of mice. Besides, the heterostructured nanoparticles demonstrate a new type of dual-modality probe for MRI/SPECT imaging through compromising their capabilities in high spatially-resolved MRI and sensitive SPECT imaging from Fe3O4 nanoparticles and 125I-radiolabelled nanoparticles, respectively. More specific research studies on targeting and combination of other therapies are under investigation.
Conclusions
In conclusion, we have developed Fe3O4-Ag125I heterostructured radionuclide nanoparticles as novel dual-modality imaging agents for MRI and SPECT. Combining Fe3O4-Ag nanoparticles with radioisotopes (125I) may provide new synthetic routes for dual-modality contrast agents. The Fe3O4-Ag125I heterostructured radionuclide nanoparticles showed low cell toxicity, clearly reduced T2-MRI signal intensity and high radiolabeling efficiency. Furthermore in vivo SPECT images of mice showed strong uptake of this nanomaterial by liver and spleen. We anticipate that these heterodimer radionuclide nanoparticles will have great potential in biological and medical imaging.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21373006, 51402203, 21207164), the National Basic Research Program of China (973 Program 2014CB931900), the Natural Science Foundation of Jiangsu Province for Young Scholars (BK20140326), the Natural Science Foundation of Jiangsu Higher Education Institutions (14KJB430021), the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Application Fundamental Research Program of Suzhou City (SYS201224). We thank Prof. Chunying Chen at the National Center for Nanoscience and Technology (NCNST) China for helpful discussions. We also thank Prof. Kai Yang and Ms Xiaoyan Zhong for radionuclide assistance.Notes and references
- C. Xu and S. Sun, Adv. Drug Delivery Rev., 2013, 65, 732 CrossRef CAS PubMed; G. Hadjipanayis, J. Bonder, S. Balakrishnan, X. Wang, H. Mao and C. Hadjipanayis, Small, 2008, 4, 1925 CrossRef PubMed; J. Zeng, L. Jing, Y. Hou, M. Jiao, R. Qiao, Q. Jia, C. Liu, F. Fang, H. Lei and M. Gao, Adv. Mater., 2014, 26, 2694 CrossRef PubMed; Y. Li, Y. Lu, H. Hong, Y. Chen, X. Ma, L. Guo, Z. Wang, J. Chen, M. Zhu, J. Ni, H. Gu, J. Lu and J. Ying, Chem. Commun., 2011, 47, 6320 RSC; Y. Pan, X. Du, F. Zhao and B. Xu, Chem. Soc. Rev., 2012, 41, 2912 RSC; Z. Zhao, Z. Zhou, J. Bao, Z. Wang, J. Hu, X. Chi, K. Ni, R. Wang, X. Chen, Z. Chen and J. Gao, Nat. Commun., 2013, 4, 2266 Search PubMed; A. Chrastina and J. Schnitzer, Int. J. Nanomed., 2010, 5, 653 Search PubMed.
- M. Perrier, M. Busson, G. Massasso, J. Long, V. Boudousq, J. Pouget, S. Peyrottes, C. Perigaud, C. Porredon-Guarch, J. de Lapuente, M. Borras, J. Larionova and Y. Guari, Nanoscale, 2014, 6, 13425 RSC.
- D. Huo, J. He, H. Li, H. Yu, T. Shi, Y. Feng, Z. Zhou and Y. Hu, Colloids Surf., B, 2014, 117, 29 CrossRef CAS PubMed; K. Ai, Y. Liu, J. Liu, Q. Yuan, Y. He and L. Lu, Adv. Mater., 2011, 23, 4886 CrossRef PubMed.
- L. Cheng, J. Liu, X. Gu, H. Gong, X. Shi, T. Liu, C. Wang, X. Wang, G. Liu, H. Xing, W. Bu, B. Sun and Z. Liu, Adv. Mater., 2014, 26, 1886 CrossRef CAS PubMed; Z. Li, S. Yin, L. Cheng, K. Yang, Y. Li and Z. Liu, Adv. Funct. Mater., 2014, 24, 2312 CrossRef.
- Z. Zhang, J. Wang and C. Chen, Adv. Mater., 2013, 25, 3869 CrossRef CAS PubMed.
- A. Louie, Chem. Rev., 2010, 110, 3146 CrossRef CAS PubMed; N. Bertrand, J. Wu, X. Xu, N. Kamaly and C. Farokhzad, Adv. Drug Delivery Rev., 2014, 66, 2 CrossRef PubMed; N. A. Frey, S. Peng, K. Cheng and S. Sun, Chem. Soc. Rev., 2009, 38, 2532 RSC; D. Ho, X. Sun and S. Sun, Acc. Chem. Res., 2011, 44, 875 CrossRef PubMed.
- J. Zhu, Y. Lu, Y. Li, J. Jiang, L. Cheng, Z. Liu, L. Guo, Y. Pan and H. Gu, Nanoscale, 2014, 6, 199 RSC.
- L. Zhou, X. Zheng, Z. Gu, W. Yin, X. Zhang, L. Ruan, Y. Yang, Z. Hu and Y. Zhao, Biomaterials, 2014, 35, 7666 CrossRef CAS PubMed; X. Cui, S. Belo, D. Krüger, Y. Yan, R. Rosales, M. Jauregui-Osoro, H. Ye, S. Su, D. Mathe, N. Kovács, I. Horváth, M. Semjeni, K. Sunassee, K. Szigeti, M. Green and P. Blower, Biomaterials, 2014, 35, 5840 CrossRef PubMed; R. Sharma, Y. Xu, S. Kim, M. Schueller, D. Alexoff, S. Smith, W. Wang and D. Schlyer, Nanoscale, 2013, 5, 7476 RSC; L. Wang, H. Xing, S. Zhang, Q. Ren, L. Pan, K. Zhang, W. Bu, X. Zheng, L. Zhou, W. Peng, Y. Hua and J. Shi, Biomaterials, 2013, 34, 3390 CrossRef PubMed; J. Gao, G. Liang, B. Zhang, Y. Kuang, X. Zhang and B. Xu, J. Am. Chem. Soc., 2007, 129, 142 Search PubMed; K. Dong, Z. Liu, J. Liu, S. Huang, Z. Li, Q. Yuan, J. Ren and X. Qu, Nanoscale, 2014, 6, 2211 RSC.
- M. Suchy, R. Bartha and R. Hudson, RSC Adv., 2013, 3, 3249 RSC.
- C. Hadjipanayis, M. Bonder, S. Balakrishnan, X. Wang, H. Mao, S. Gambhir, A. Ahmad, I. Uddin, V. Dube and S. Kumar, J. Nucl. Med., 2011, 52, 1613 Search PubMed.
- Y. Shao, S. Cherry, K. Farahani, K. Meadors, S. Siegel, R. Silverman and P. Marsden, Phys. Med. Biol., 1997, 42, 1965 CrossRef CAS; M. Judenhofer, H. Wehrl, D. Newport, C. Catana, S. Siegel, M. Becker, A. Thielscher, M. Kneilling, M. Lichy, M. Eichner, K. Klingel, G. Reischl, S. Widmaier, M. Rocken, R. Nutt, H. Machulla, K. Uludag, S. Cherry, C. Claussen and B. Pichler, Nat. Med., 2008, 14, 459 CrossRef PubMed; H. Herzog, Z. Med. Phys., 2012, 22, 281 CrossRef PubMed.
- J. Jiang, H. Gu, H. Shao, E. Devlin, G. Papaefthymiou and J. Ying, Adv. Mater., 2008, 20, 4403 CrossRef CAS.
- J. Park, K. An, Y. Hwang, J. Park, H. Noh, J. Kim, J. Park, N. Hwang and T. Hyeon, Nat. Mater., 2004, 3, 891 CrossRef CAS PubMed.
- L. Cheng, K. Yang, Y. Li, J. Chen, C. Wang, M. Shao, S. Lee and Z. Liu, Angew. Chem., Int. Ed., 2011, 50, 7385 CrossRef CAS PubMed.
- M. Wang, C. Wang, K. Young, L. Hao, M. Medved, T. Rajh, H. Fry, L. Zhu, G. Karczmar, C. Watson, J. Jiang, N. Markovic and V. Stamenkovic, Chem. Mater., 2012, 24, 2423 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of general experimental procedures, TEM image. See DOI: 10.1039/c4nr07255c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2015 |