Open Access Article
Open Access ArticleMicrowave-assisted synthesis of water-soluble, fluorescent gold nanoclusters capped with small organic molecules and a revealing fluorescence and X-ray absorption study†
C.
Helmbrecht
*a,
D.
Lützenkirchen-Hecht
*b and
W.
Frank
*a
aHeinrich-Heine-Universität Düsseldorf, Institut für Anorganische und Strukturchemie, Lehrstuhl II: Material- und Strukturforschung, Universitätsstraße 1, Düsseldorf 40225, Germany
bBergische Universität Wuppertal, Fachbereich C, Gaußstraße 20, Wuppertal 42097, Germany
First published on 12th February 2015
Abstract
Colourless solutions of blue light-emitting, water-soluble gold nanoclusters (AuNC) were synthesized from gold colloids under microwave irradiation using small organic molecules as ligands. Stabilized by 1,3,5-triaza-7-phosphaadamantane (TPA) or L-glutamine (GLU), fluorescence quantum yields up to 5% were obtained. AuNC are considered to be very promising for biological labelling, optoelectronic devices and light-emitting materials but the structure–property relationships have still not been fully clarified. To expand the knowledge about the AuNC apart from their fluorescent properties they were studied by X-ray absorption spectroscopy elucidating the oxidation state of the nanoclusters’ gold atoms. Based on curve fitting of the XANES spectra in comparison to several gold references, optically transparent fluorescent AuNC are predicted to be ligand-stabilized Au5+ species. Additionally, their near edge structure compared with analogous results of polynuclear clusters known from the literature discloses an increasing intensity of the feature close to the absorption edge with decreasing cluster size. As a result, a linear relationship between the cluster size and the X-ray absorption coefficient can be established for the first time.
Introduction
Gold nanoparticles, small as well as large representatives, in a size range from 2 to 100 nm, have been well studied and widely applied in the past.1 More recently, significantly smaller gold species with a size close to the Fermi wavelength of the conduction electrons have gained much research interest because of their intriguing properties, too.2 These species often referred to as “gold nanoclusters” (AuNC) exhibit quantum confinement effects which are extremely promising for various applications, especially for fluorescent materials.3,4 Nevertheless, some fundamental issues of the AuNC like the origin of fluorescence, the spectral broadening mechanism or the oxidation state of the nanoclusters’ gold atoms have still not been clarified.4 This is accompanied by the usage of non-uniform terms in the literature like “ultrasmall nanoparticles”, “quantum clusters” or “molecular species” and suggests their elusive character.5 Even though the term “nanoclusters” wins through and research efforts proceed, the structure–property relationships of AuNC have to be enlightened for further developments.Several photofragmentation- and microwave-assisted syntheses are described in literature,6 most of them yield red light-emitting AuNC.7 In contrast, only a few blue light-emitting gold nanoclusters have been prepared with different ligands and procedures.8 We developed a microwave-assisted procedure for the preparation of aqueous solutions of small molecule stabilized gold nanoclusters which are optically transparent and offer blue light under UV irradiation. Herein, we present a revealing fluorescence and X-ray absorption spectroscopic study of such blue light-emitting AuNC protected by the stabilizing agents 1,3,5-triaza-7-phosphaadamantane (TPA) or L-glutamine (GLU). In addition to appropriate fluorescence spectroscopic experiments we performed investigations of the X-ray absorption near edge structure (XANES) to gain information about the oxidation state of the nanoclusters’ gold atoms compared to the larger representatives which belong to the “divide and protect”-concept.9
Results and discussion
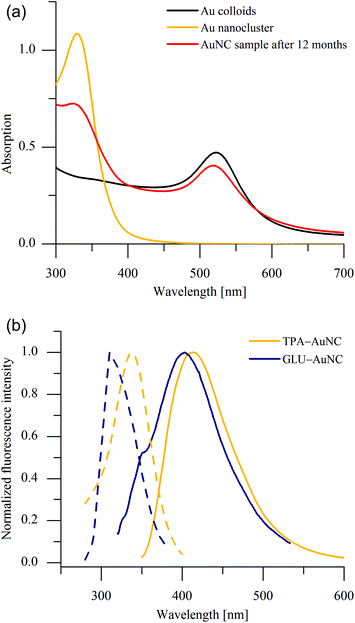
The AuNC were synthesized applying a two-step method. First ligand-stabilized gold colloids (ligand: 1,3,5-triaza-7-phosphaadamantane or L-glutamine) were obtained through a modified two-phase Brust–Schiffrin synthesis.10 In the second step the ligand-protected gold nanoparticles were redispersed in water and irradiated by microwaves in order to obtain the fluorescent nanoclusters. The UV/Vis spectrum of the ruby red gold sol shows the well-known absorption band of the plasmon resonance at 522 nm. The red colour disappears during the microwave cracking process while a new absorption band belonging to the AuNC increases at about 330 nm.It is well known that many organic, biological and polymeric species may also emit fluorescence signals in the blue-green spectral range, which may overlap with the blue emissions of our gold clusters. Therefore, it is also very important to note that the entirely new absorption band at about 330 nm and the emission band at about 405 nm cannot be detected after a microwave cracking process of the used organic ligands alone (see ESI, Fig. S1†), i.e. the observed emissions can be assigned doubtless to the gold nanoclusters. Within 12 months, the colourless solution turns red again. Some of the AuNC have agglomerated, so that both types of species, AuNC as well as colloids, coexist next to each other as can be deduced from the corresponding UV/Vis spectrum in Fig. 1a. In such a case, the microwave cracking process can be repeated obtaining AuNC again. Solutions of AuNC are colourless in ambient light, while showing blue fluorescence under UV irradiation. The fluorescence emission spectra of the TPA- and GLU-capped AuNC in solution reveal emission maxima at 413 nm and 402 nm, respectively (Fig. 1b) and the room temperature fluorescence quantum yield is estimated to be 5 and 0.5%, respectively. The much stronger fluorescence of TPA-AuNC is probably caused by the ligand contribution. In particular, the ligand's capability of donating electron density to the gold atoms leads to the quantum yield enhancement compared with GLU-AuNC. This is in agreement with the earlier proposed ligand to metal nanoparticle core charge transfer (LMNCT).11 Other AuNC with small stabilizing molecules which were known previously exhibit comparable quantum yields between 1–10%.12 In the fluorescence excitation spectra of the AuNC only a single fluorescence peak appears, so that the possibility of overlapping intrinsic and extrinsic fluorescence is ruled out.4 Thus, we take into account the scaling of the emission energy with N−1/3 as proposed by Zheng et al.13 as a measure to determine the nuclearity of the AuNC (where N is the number of gold atoms, each contributing one electron). According to this relationship the blue light-emitting AuNC are likely to consist of five gold atoms.
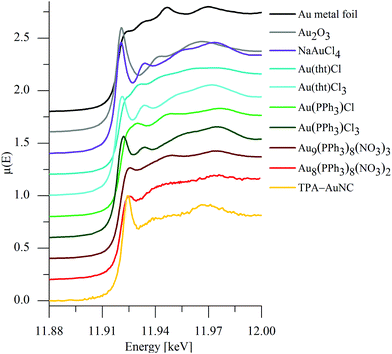
The AuNC solutions have concentrations ranging around ten ppm. Due to this strong dilution on the one hand and to the ultrasmall particle size on the other hand, the common analytical investigations are not feasible (XPS, NMR, TEM, XRD etc.). However, to obtain valence information we performed XANES experiments making use of their inherent sensitivity towards the atomic short-range order structure. Several Au reference compounds and the TPA-stabilized AuNC were investigated at the Au LIII edge using synchrotron radiation from DELTA beamlines. Due to the extremely low concentration of the element of interest the absorption spectra of the AuNC were collected in the fluorescence mode. Several spectra were added in order to obtain a reasonable signal-to-noise ratio of the near edge data. As can be seen in Fig. 2, the data quality of the TPA-AuNC spectra is sufficient for a more detailed analysis of the edge position, because white-line energy and intensity, and post edge peaks are clearly resolved. The averaged and normalized spectrum of the TPA-AuNC solution is compared to the spectra of several AuI and AuIII reference compounds in Fig. 2. Owing to the conclusions of the fluorescence spectroscopic investigations, polynuclear di- and tricationic Au8- and Au9-clusters, respectively, were chosen as references, too.
Obviously, the spectrum of the TPA-AuNC substantially differs from that of the gold metal foil with regard to the edge position and the near edge structure. Furthermore, a significant photoreduction of gold can be excluded because of the relatively low photon flux of the beamlines used for DELTA.14
Moreover, the spectra of the AuI references, Au(PPh3)Cl and Au(tht)Cl (tht = tetrahydrothiophene), are different with respect to the white-line intensities and the post edge structures, most obviously seen in the feature at about 11.970 keV. The edge and white-line energies for Au2O3 and NaAuCl4 are similar to that of Au metal, while those of the other substances are located at substantially larger photon energies. In agreement to the literature dealing with Au samples, the data do not offer a linear shift between edge position and chemical oxidation state as observed for other elements.
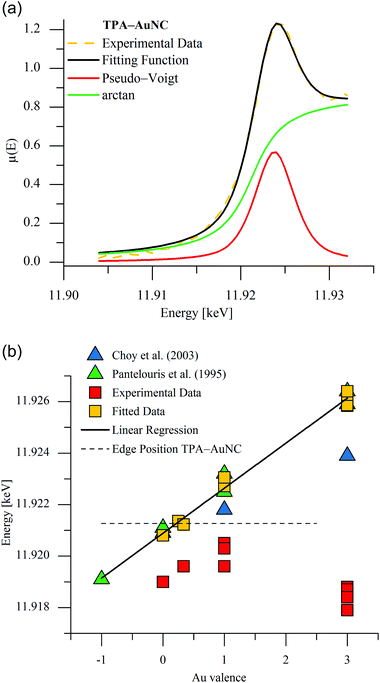
Pantelouris et al. noticed a linear shift of the absorption edge to higher energy with increasing valence at the LI edge, but not in the equivalent Au LIII XANES spectra. The latter data have to be deconvoluted because of a superimposed peak onto the absorption edge,15 that can be related to 5d–6s-hybrid orbitals according to Choy et al.16 In accordance to the literature, we thus performed an extended data analysis by applying a detailed curve fitting to the XANES spectra of the measured and background subtracted data. The white-line feature was approximated by a Pseudo-Voigt-function due to the proportion of the d-orbitals and the absorption edge by an arctan-function, respectively, as illustrated for the spectrum of the TPA-AuNC in Fig. 3a. Adopting such fitting procedures for all the measured XANES spectra, a linear relationship between the Au valence of the studied compounds and their edge energies was confirmed (Fig. 3b). The deconvoluted reference spectra are given in ESI (Fig. S2–10†). Corresponding to the linear regression line of the reference compounds with well-defined Au valences, the Au oxidation state for the TPA-AuNC could be deduced from the determined absorption edge energy at 11.9213 keV to be 0.2 (Fig. 3b). Thus, the determined oxidation state close to zero is consistent with previously published XPS studies of ligand-stabilized Au8 and Au25 nanoclusters.17 Le Guével et al. only detected binding energies for Au(I) and Au(0) in larger Au25 species. Here, the icosahedral Au13 core is capped by bridging thiolate ligands (–S–Au–S–Au–S–).18 The XPS spectrum of the smaller Au8 nanoclusters revealed only one Au 4f7/2 feature with a binding energy (84.7 eV) shifted from Au(0) (84.1 eV) to higher energies, which agrees qualitatively to our findings.
Furthermore, the AuNC produced via our microwave-assisted cracking process are obviously equal to ultrasmall transient species which were detected by Ohyama et al. in an in situ study of the AuNP growth process at the Au LII and LIII edges.19 On the one hand with regard to the energy of the absorption edge and the post edge features as determined by Ohyama et al. and the fluorescence properties on the other hand, our blue light-emitting AuNC are assumed to be predominantly Au5+ species. Due to similar fluorescence properties this cluster size is assumed for the GLU-stabilized AuNC, too.
A more detailed comparison of the X-ray absorption spectra of the investigated polynuclear clusters reveals an increasing intensity of the feature close to the absorption edge at about 11.925 keV with decreasing cluster size. Similar results were obtained for gold nanoparticles with sizes of 4.0 < 2.4 < 1.6 nm by Zhang et al.20 and cluster sizes of Au25, Au38 and Au144 by MacDonald and co-workers.21 The increasing intensity was attributed to d-band vacancies caused by an electron transfer from Au to the ligand. Taking also into account the spectra of the Au11 cluster known from literature,22 the cluster size correlates linearly with the absorption coefficient and with the integrated intensity of the Pseudo-Voigt function of the small polynuclear clusters under examination (Fig. 4). For this purpose, the relation of the X-ray absorption coefficient of KAuCl4 to the Au11 cluster was applied to the data of NaAuCl4 in our experiment in order to yield the corresponding absorption coefficient. Finally, these results confirm and extend the previous knowledge of the electronic states in small gold clusters. The correlation analysis given here seems to provide an appropriate method for estimation of the size of gold nanoclusters in general. In the present case, a cluster size of Aun with n = 5 was determined for the TPA-AuNC in perfect agreement with the analysis of the fluorescence measurements (Fig. 1b).
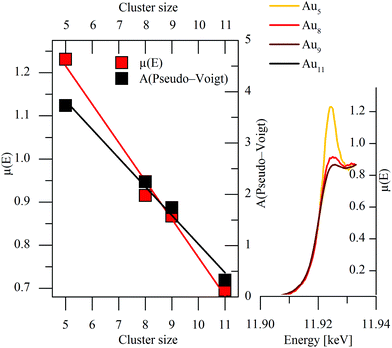 | ||
| Fig. 4 Linear relationship between cluster size and absorption coefficient and integrated area of the fitted Pseudo-Voigt-function of investigated polynuclear clusters (data of Au11 taken from Benfield et al.22). | ||
For the future, it is of fundamental interest to determine whether such a linear relationship between distinct pre-edge features and particle size exist for other metal nanoclusters like Ptn, Agn, Cun, etc. as well, and if the size dependence of those spectral features can be further supported by ab initio XANES calculations23 and in situ XANES experiments on nanocluster synthesis.19,24 The opportunity to couple X-ray absorption experiments with e.g. small angle X-ray scattering, Raman-, UV/Vis-25 and other spectroscopic methods would further foster our understanding of nanocluster synthesis and properties. Here, examinations of Au nanoclusters with bigger size (i.e. Aun with n > 11) would be very important. Furthermore, it would also be desirable to investigate the fluorescence lifetime of the AuNCs as a function of the particle size in order to prove correlations.
Materials and methods
The following reagents were used as received. Sodium tetrachloridoaurate(III) dihydrate, L-glutamine, didodecyl-dimethylammonium bromide were purchased from Alfa Aesar. Sodium tetrahydridoborate was purchased from AppliChem. Quinine sulfate was received from AcrosOrganics. 1,3,5-Triaza-7-phosphaadamantane was prepared according to literature.26 Toluene was received from AnalaR Normapur and is from analytical reagent grade. All solutions were prepared using doubly distilled water.The microwave syntheses were carried out with the single-mode microwave system CEM Discover™. The synthesis of the gold nanoclusters was performed in 10 ml Pyrex glass tubes sealed with a septum under continuously irradiation of unpulsed microwaves with a frequency of 2.45 GHz.
A Specord 210 Plus (Analytik Jena) UV/Vis-spectrophotometer was used for the UV/Vis absorption measurements. Spectra were typically measured in the range 300–800 nm. Fluorescence measurements were carried out using the fluorometer F-2700 of Hitachi. The slit widths for excitation and emission were set at 5 nm. The quantum yields (QY) of the fluorescent AuNC were measured using quinine sulfate as reference standard (QY: 60% in 0.1 M HClO4). The XANES experiments presented in this study were performed at the X-ray beamlines BL8 and BL10 at the DELTA storage ring (Dortmund, Germany) operating with 1.5 GeV electrons and 80–130 mA of stored current.27 Si(111) double-crystal monochromators with an energy resolution of about 2 eV at the energy of the Au LIII absorption edge (11.919 keV) were used, and the incident and transmitted intensities were measured by nitrogen- and argon-filled ionization chambers, respectively. The X-ray fluorescence from the samples was detected with a silicon drift diode (SDD) with a multichannel analyser (Amptek, Bedford, USA) placed 90° to the incident beam in the orbit plane of the ring. The accuracy of the energy scale was routinely checked by measuring the XANES spectra of a gold metal reference foil, and the differences observed between the scans were smaller than 0.05 eV, providing confidence in the determined edge energies of the gold samples. Several Au reference samples with different oxidation states were investigated in transmission and fluorescence mode for comparison (Fig. 2 and Fig. S2–S10 in the ESI†).
Synthetic procedures
Water-soluble, GLU- and TPA-stabilized AuNC were prepared in two steps. First, in accordance with Brust–Schiffrin 1.5 mmol sodium tetrachloridoaurate(III) dihydrate was added to a solution of 3.52 g didodecyldimethylammonium bromide (DDAB) in 200 ml toluene. The organic phase changed colour from colourless to deep purple. After separation, this stock solution was stored in the dark. To 2 ml of the stock solution diluted with 23 ml toluene 1 mmol of the ligand (GLU or TPA) was added. Under vigorous stirring 60 μl of a freshly prepared aqueous solution of sodium tetrahydridoborate (0.1 g ml−1) was slowly added. After 30 minutes the resulting AuNP were obtained by centrifugal precipitation as dark brown powder. In the second step this ligand-stabilized gold nanoparticles were dispersed in water and stirred by adding 1 mmol of TPA or GLU. Under constant stirring, the mixture was irradiated by microwaves (80 W, 60 min, 120 °C). Using a syringe filter (0.45 μm PTFE) impurities were removed from AuNC solution prior to their spectral characterization.Conclusions
Two non-thiolate stabilizing agents were successfully used to synthesize fluorescent gold nanoclusters via microwave irradiation. In particular, their water solubility and the optical transparency of the solutions in connection with the ability to emit blue light are very promising for future applications. According to the combined results of X-ray absorption and fluorescence studies, the blue light-emitting species are predicted to be ligand stabilized Au5+ nanoclusters of the same kind that exist a few seconds during the growth process of gold nanoparticles. Finally, a linear relationship between the cluster size of polynuclear clusters and their absorption coefficients was detected offering an appropriate method to estimate unknown cluster sizes in general.Acknowledgements
We would like to thank S. Balk and R. Wagner for their support at the beamline, and we gratefully acknowledge the DELTA machine group for providing an intense and stable synchrotron radiation beam.Notes and references
- (a) G. Schmid and B. Corain, Eur. J. Inorg. Chem., 2003, 2003, 3081–3098 CrossRef; (b) M. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed; (c) B. von Holt, S. Kudera, A. Weiss, T. E. Schrader, L. Manna, W. J. Parak and M. Braun, J. Mater. Chem., 2008, 18, 2728–2732 RSC; (d) A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096–2126 RSC; (e) K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739–2779 CrossRef CAS PubMed.
- (a) C. J. Lin, C. Lee, J. Hsieh, H.-H. Wang, J. K. Li, J. Shen, W. Chan, H. Yeh and W. H. Chang, J. Med. Biol. Eng., 2009, 29, 276–283 Search PubMed; (b) J. Sun and Y. Jin, J. Mater. Chem. C, 2014, 2, 8000–8011 RSC.
- (a) X. Yang, L. Gan, L. Han, D. Li, J. Wang and E. Wang, Chem. Commun., 2013, 49, 2302–2304 RSC; (b) L. Shang, F. Stockmar, N. Azadfar and G. U. Nienhaus, Angew. Chem., Int. Ed., 2013, 125, 11360–11363 CrossRef.
- X. Wen, P. Yu, Y.-R. Toh, X. Ma, S. Huang and J. Tang, Nanoscale, 2013, 5, 10251–10257 RSC.
- (a) J. R. McBride, A. D. Dukes, M. A. Schreuder and S. J. Rosenthal, Chem. Phys. Lett., 2010, 498, 1–9 CrossRef CAS PubMed; (b) Y. Lu and W. Chen, Chem. Soc. Rev., 2012, 41, 3594–3623 RSC; (c) R. Jin, H. Qian, Z. Wu, Y. Zhu, M. Zhu, A. Mohanty and N. Garg, J. Phys. Chem. Lett., 2010, 1, 2903–2910 CrossRef CAS; (d) R. Jin, Nanoscale, 2010, 2, 343–362 RSC.
- (a) A. Schäfer, Thesis, Heinrich-Heine-Universität, Düsseldorf, 2008; (b) Z. Peng, T. Walther and K. Kleinermanns, J. Phys. Chem. B, 2005, 109, 15735–15740 CrossRef CAS PubMed; (c) E. Giorgetti, A. Giusti, F. Giammanco and P. Marsili, Opt. Spectrosc., 2009, 107, 474–479 CrossRef CAS.
- (a) D. He, Y. Xiang, X. Wang and X.-F. Yu, Mater. Res. Bull., 2011, 46, 2418–2421 CrossRef CAS PubMed; (b) L. Yan, Y. Cai, B. Zheng, H. Yuan, Y. Guo, D. Xiao and M. M. F. Choi, J. Mater. Chem., 2012, 22, 1000–1005 RSC; (c) Y. Yue, T.-Y. Liu, H.-W. Li, Z. Liu and Y. Wu, Nanoscale, 2012, 4, 2251–2254 RSC; (d) L. Shang, L. Yang, F. Stockmar, R. Popescu, V. Trouillet, M. Bruns, D. Gerthsen and G. U. Nienhaus, Nanoscale, 2012, 4, 4155–4160 RSC; (e) J. Tian, L. Yan, A. Sang, H. Yuan, B. Zheng and D. Xiao, J. Chem. Educ., 2014, 91, 1715–1719 CrossRef CAS.
- (a) H. Yabu, Chem. Commun., 2011, 47, 1196–1197 RSC; (b) B. Liu, Y. Wang, M. Deng, J. Lü, C. Tong and C. Lü, RSC Adv., 2014, 4, 57245–57249 RSC; (c) J. Zheng, J. T. Petty and R. M. Dickson, J. Am. Chem. Soc., 2003, 125, 7780–7781 CrossRef CAS PubMed.
- (a) H. Häkkinen, Chem. Soc. Rev., 2008, 37, 1847–1859 RSC; (b) D. Jiang, W. Chen, R. L. Whetten and Z. Chen, J. Phys. Chem. C, 2009, 113, 16983–16987 CrossRef CAS.
- (a) M. Brust, M. Walker, D. Bethell, D. J. Schiffrin and R. Whyman, J. Chem. Soc., Chem. Commun., 1994, 801–802 RSC; (b) Y. Li, O. Zaluzhna, B. Xu, Y. Gao, J. M. Modest and Y. J. Tong, J. Am. Chem. Soc., 2011, 133, 2092–2095 CrossRef CAS PubMed.
- (a) Z. Wu and R. Jin, Nano Lett., 2010, 10, 2568–2573 CrossRef CAS PubMed; (b) F. Wang, X. Zhang, Z. Zhang and C. He, J. Mater. Chem., 2011, 21, 15167–15170 RSC; (c) H.-Y. Chang, H.-T. Chang, Y.-L. Hung, T.-M. Hsiung, Y.-W. Lin and C.-C. Huang, RSC Adv., 2013, 3, 4588–4597 RSC.
- (a) Y. Negishi, K. Nobusada and T. Tsukuda, J. Am. Chem. Soc., 2005, 127, 5261–5270 CrossRef CAS PubMed; (b) M. A. Habeeb Muhammed, S. Ramesh, S. S. Sinha, S. K. Pal and T. Pradeep, Nano Res., 2008, 1, 333–340 CrossRef; (c) C. Würth, M. Grabolle, J. Pauli, M. Spieles and U. Resch-Genger, Anal. Chem., 2011, 83, 3431–3439 CrossRef PubMed; (d) J. Zhang, Y. Fu, C. V. Conroy, Z. Tang, G. Li, R. Y. Zhao and G. Wang, J. Phys. Chem. C, 2012, 116, 26561–26569 CrossRef CAS PubMed; (e) Y. Zhang, H. Jiang, W. Ge, Q. Li and X. Wang, Langmuir, 2014, 30, 10910–10917 CrossRef CAS PubMed.
- J. Zheng, C. Zhang and R. Dickson, Phys. Rev. Lett., 2004, 93, 077402 CrossRef.
- Y. Ohkubo, T. Nakagawa, S. Seino, J. Kugai, T. A. Yamamoto, H. Nitani and Y. Niwa, J. Synchrotron Radiat., 2014, 21, 1148–1152 CrossRef CAS PubMed.
- A. Pantelouris, G. Kueper, J. Hormes, C. Feldmann and M. Jansen, J. Am. Chem. Soc., 1995, 117, 11749–11753 CrossRef CAS.
- J. Choy and Y. Kim, J. Phys. Chem. B, 2003, 107, 3348–3350 CrossRef CAS.
- X. Le Guével, B. Hötzer, G. Jung, K. Hollemeyer, V. Trouillet and M. Schneider, J. Phys. Chem. C, 2011, 115, 10955–10963 Search PubMed.
- M. Zhu, C. M. Aikens, F. J. Hollander, G. C. Schatz and R. Jin, J. Am. Chem. Soc., 2008, 130, 5883–5885 CrossRef CAS PubMed.
- (a) J. Ohyama, K. Teramura, T. Shishido, Y. Hitomi, K. Kato, H. Tanida, T. Uruga and T. Tanaka, Chem. Phys. Lett., 2011, 507, 105–110 CrossRef CAS PubMed; (b) J. Ohyama, K. Teramura, Y. Higuchi, T. Shishido, Y. Hitomi, K. Kato, H. Tanida, T. Uruga and T. Tanaka, ChemPhysChem, 2011, 12, 127–131 CrossRef CAS PubMed.
- P. Zhang and T. Sham, Phys. Rev. Lett., 2003, 90, 245502(1–4) Search PubMed.
- M. A. MacDonald, P. Zhang, H. Qian and R. Jin, J. Phys. Chem. Lett., 2010, 1, 1821–1825 CrossRef CAS.
- R. E. Benfield, D. Grandjean, M. Kröll, R. Pugin, T. Sawitowski and G. Schmid, J. Phys. Chem. B, 2001, 105, 1961–1970 CrossRef CAS.
- D. Bazin and J. J. Rehr, J. Phys. Chem. B, 2003, 107, 12398–12402 CrossRef CAS.
- H. Oyanagi, Y. Orimoto, K. Hayakawa, K. Hatada, Z. Sun, L. Zhang, K. Yamashita, H. Nakamura, M. Uehara, A. Fukano and H. Maeda, Sci. Rep., 2014, 4, 7199 CrossRef PubMed.
- (a) J. Stötzel, D. Lützenkirchen-Hecht, R. Frahm, C. V. Santilli, S. H. Pulcinelli, R. Kaminski, E. Fonda, F. Villain and V. Briois, J. Phys. Chem. C, 2010, 114, 6228–6236 CrossRef; (b) V. Briois, D. Lützenkirchen-Hecht, F. Villain, E. Fonda, S. Belin, B. Griesebock and R. Frahm, J. Phys. Chem. A, 2005, 109, 320–329 CrossRef CAS PubMed.
- E. Fluck, J. Förster, J. Weidlein and E. Hädicke, Z. Naturforsch., B: Anorg. Chem., Org. Chem., 1977, 32, 499–506 Search PubMed.
- (a) D. Lützenkirchen-Hecht, R. Wagner, U. Haake, A. Watenphul and R. Frahm, J. Synchrotron Radiat., 2009, 16, 264–272 CrossRef PubMed; (b) D. Lützenkirchen-Hecht, R. Wagner, S. Szillat, A. K. Hüsecken, K. Istomin, U. Pietsch and R. Frahm, J. Synchrotron Radiat., 2014, 21, 819–826 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The deconvoluted reference spectra are given in ESI Fig. 1–9. See DOI: 10.1039/c4nr07051h |
| This journal is © The Royal Society of Chemistry 2015 |