Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recombinant protein (EGFP-Protein G)-coated PbS quantum dots for in vitro and in vivo dual fluorescence (visible and second-NIR) imaging of breast tumors†
Akira
Sasaki
*ab,
Yoshikazu
Tsukasaki‡
b,
Akihito
Komatsuzaki
b,
Takao
Sakata
c,
Hidehiro
Yasuda
c and
Takashi
Jin
*bde
aBiomedical Research Institute, National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Ibaraki 305-8566, Japan. E-mail: akira.sasaki@aist.go.jp
bLaboratory for Nano-Bio Probes, Quantitative Biology Center, RIKEN, Suita, Osaka 565-0874, Japan. E-mail: tjin@riken.jp
cResearch Center for Ultra-High Voltage Electron Microscopy, Osaka University, Ibaraki, Osaka 567-0047, Japan
dWPI Immunology Frontier Research Center, Osaka University, Suita, Osaka 565-0871, Japan
eGraduate School of Frontier Biosciences, Osaka University, Suita, Osaka 565-0871, Japan
First published on 22nd December 2014
Abstract
We report a one-step synthetic strategy for the preparation of recombinant protein (EGFP-Protein G)-coated PbS quantum dots for dual (visible and second-NIR) fluorescence imaging of breast tumors at the cellular and whole-body level.
Non-invasive whole-body imaging using near-infrared (NIR) fluorescence has been broadly developed because of its low tissue absorption and scattering compared to that observed with visible fluorescence.1–3 Recently, second-NIR fluorescence (1000–1500 nm) has been used for non-invasive in vivo imaging.4–7 Second-NIR fluorescence imaging has advantages over traditional NIR fluorescence imaging (700–900 nm) such as reduced light scattering and minimal autofluorescence.5 For the second-NIR fluorescence imaging, semiconductor quantum dots (QDs) such as PbS,8–13 PbSe,10 and Ag2S14 are promising fluorescent probes because of their strong fluorescence and photostability at wavelengths in the range of 1000–1500 nm. However, fluorescence imaging using second-NIR QDs usually encounters two major difficulties. First, the preparation of the QD-based fluorescent probe needs multiple synthetic steps such as QD formation, surface modification and biomolecule conjugation.8–14 Second, the fluorescence detectors of conventional microscope systems do not support second-NIR fluorescence imaging.5,6,15,16 To overcome these problems, we have developed a dual (visible and second-NIR) emission QD probe that can be used for both cellular and non-invasive whole-body imaging. In this communication, we report a facile synthesis of recombinant protein functionalized PbS QDs with dual emission (visible and second-NIR fluorescence), which have a binding ability for antibody molecules (IgG) in aqueous solution.
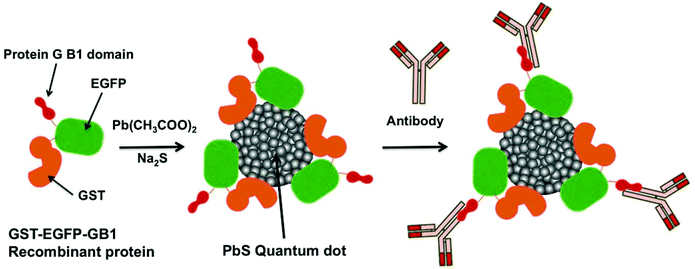
We have used an enhanced green fluorescent protein (EGFP)-Protein G B1 domain17 tagged with glutathione S-transferase (GST) as a template18,19 (GST-EGFP-GB1; 61.7 kDa, see ESI†) to grow PbS QDs in the aqueous phase (Scheme 1). The recombinant protein, GST-EGFP-GB1 was genetically designed and prepared by a conventional method for recombinant protein synthesis in Escherichia coli. Protein G is a cell surface protein of Streptococcus and its B1 domain binds to the Fc region of immunoglobulin G (IgG). It has been widely used for detecting and purifying IgG antibodies.17 Particularly in cancer molecular imaging, a receptor-targeting antibody can act as a powerful tool via the conjugation of antibodies to the QD surface. In this case, a large amount of antibodies was used for conjugation to QDs.2,11,13 Since the GST-EGFP-GB1-coated QDs contain a protein G B1 domain, they can easily bind to antibody molecules by a simple mixing of antibodies with the QD solution.20–22 The fluorescent protein EGFP is chosen to add a function of visible fluorescence emission to the second-NIR emitting PbS QDs. This dual-emitting property of the QDs enables a conduction of high-resolution cellular confocal imaging by EGFP fluorescence in addition to the deep-tissue imaging by second-NIR fluorescence. Thus the GST-EGFP-GB1-coated QDs make it possible to correlate the intracellular localization of probes with bio-distribution in the whole mouse body.
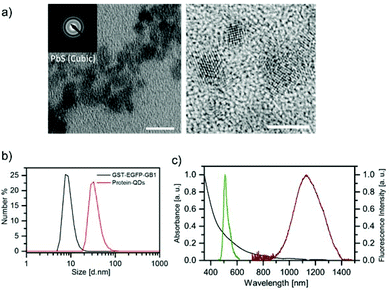
GST-EGFP-GB1-coated PbS QDs can be prepared by using a protein-mediated QD synthetic method (Scheme 1).18,19 The synthesis is carried out by reacting GST-EGFP-GB1 with lead acetate (Pb(Ac)2) under ambient conditions to promote the binding of Pb2+ to GST-EGFP-GB1 (see the Experimental section in ESI†). The addition of an aqueous solution of sodium sulfide (Na2S) immediately forms PbS QDs coated with the GST-EGFP-GB1 protein. By this synthetic method, we can easily prepare protein-coated PbS QDs within 10 minutes. To characterize the GST-EGFP-GB1-coated PbS QDs, we have analysed their transmission electron microscopic (TEM) images, dynamic light scattering (DLS), and fluorescence/absorption spectra. Fig. 1a shows TEM images of GST-EGFP-GB1-coated PbS QDs. The PbS QDs have spherical shapes (ca. 5 nm) with a selected area electron diffraction (SAED) pattern and lattice fringes, confirming the crystalline nature of the PbS QDs with a cubic structure.23 The hydrodynamic size of the PbS QDs was determined to be ca. 30 nm in diameter by DLS (Fig. 1b). This value is ca. 25 nm larger than that determined by TEM analysis. This indicates that the GST-EGFP-GB1 fusion proteins (10 nm in diameter) surround the surface of the PbS QDs. The fluorescence spectrum of the GST-EGFP-GB1-coated PbS QDs showed dual emission, with the visible emission at 515 nm and a second-NIR emission at 1150 nm, under excitation at 450 nm (Fig. 1c and S1, S2 in ESI†). The visible and NIR fluorescence emissions are from EGFP proteins and PbS QDs, respectively. The quantum yield (QY) of the second-NIR fluorescence of PbS QDs (1150 nm emission peak) was measured by a relative method with CdSeTe/CdS QDs (840 nm emission, QY = 50%) as a standard (see the Experimental section in ESI†). QY was determined to be 10% in water.
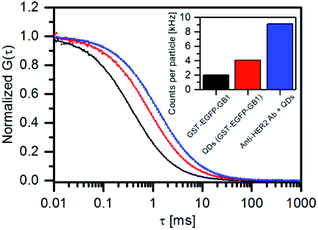
The binding ability of GST-EGFP-GB1-coated QDs towards antibody was confirmed using fluorescence correlation spectroscopy (FCS).23,24Fig. 2 shows fluorescence autocorrelation curves for the GST-EGFP-GB1 protein, GST-EGFP-GB1-coated QD, and the mixture of anti-HER2 (human epidermal growth factor 2) antibody + GST-EGFP-GB1-coated QDs. Fluorescence autocorrelation curves are fitted according to the one-component diffusion model,23,24 allowing spherical shape assumption for all three samples. Binding between the Protein G B1 domain and the antibody was confirmed by the change in the diffusion time of the GST-EGFP-GB1-coated QDs (0.82 ms) and a mixture of anti-HER2 antibody + GST-EGFP-GB1-coated QDs (1.26 ms). Based on the Einstein–Stokes equation, hydrodynamic diameters were estimated to be 23 nm for GST-EGFP-GB1-coated QDs and 35 nm for the anti-HER2 antibody + GST-EGFP-GB1-coated QDs complex. This result confirms the binding of anti-HER2 antibody to the GST-EGFP-GB1-coated QDs. It is also confirmed by the analysis of the photon counts per particle (CPP), expressing the fluorescence brightness of the single-particle. The CPP value of the GST-EGFP-GB1-coated QDs is two times higher than that of the free GST-EGFP-GB1 protein (inset of Fig. 2), indicating that at least two GST-EGFP-GB1 molecules are included in one PbS QD. It should also be noted that the CPP value of the mixture of anti-HER2 antibody + GST-EGFP-GB1-coated QDs is four times higher than that of the free GST-EGFP-GB1 protein. This indicates that the complex of antibody + QDs contain two GST-EGFP-GB1-coated QDs due to the inter-particle binding of the GB1 domain by their respective QDs.
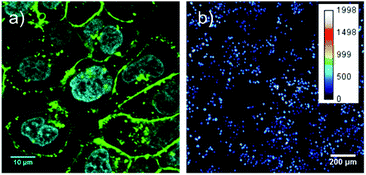
To demonstrate the capability of GST-EGFP-GB1-coated QDs, we first performed the fluorescence imaging of breast tumor cells in the visible and second-NIR regions. For fluorescence imaging, we have used a human breast tumor cell line (KPL-4)25 over-expressing HER2 on its cell membrane. Fig. 3a shows a high-resolution confocal fluorescence image (excitation: 473 nm, emission: 515 nm, at 60× magnification) of KPL-4 cells stained with anti-HER2 antibody and GST-EGFP-GB1-coated QDs. Green fluorescence of the EGFP is clearly observed on the cell surface, indicating that GST-EGFP-GB1-coated QDs bind to the HER2 receptors via anti-HER2 antibody on the KPL-4 cell surface (Fig. 3a and S3 in ESI†). For the second-NIR fluorescence imaging, we have used a home-built wide-field dissecting microscope equipped with an InGaAs detector (see the Experimental section in ESI†). Fig. 3b shows a second-NIR fluorescence image of KPL-4 cells stained with anti-HER2 antibody and GST-EGFP-GB1-coated QDs. The second-NIR fluorescence (excitation: 670 nm, emission: 1300 nm, 6.3× magnification) is clearly observed after a 30 min incubation of KPL-4 cells with anti-HER2 antibody and GST-EGFP-GB1-coated QDs (Fig. 3b). Each bright spot represents a single-cell. These results show that the GST-EGFP-GB1-coated QDs act as a dual-emission molecular targeting probe with visible and second-NIR fluorescence. GST-EGFP-GB1-coated QDs showed low cytotoxicity in HeLa cells at the concentration (<250 nM) used for in vitro and in vivo fluorescence imaging (Fig. S4 in ESI†).
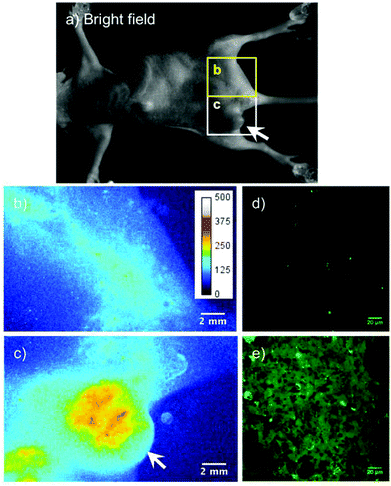
Next, we performed a second-NIR fluorescence imaging of the breast tumor implanted KPL-4 cells in a nude mouse. An aqueous solution (200 μL) of GST-EGFP-GB1-coated QDs (1 μM) mixed with anti-HER2 antibody was injected into the tail vein of a live mouse, and second-NIR fluorescence images (excitation: 670 nm, emission: 1300 nm) were taken after 48 hours of QD injection (Fig. 4). As shown in Fig. 4c, intense second-NIR fluorescence from the breast tumor is clearly observed, compared to the control without QD injection (Fig. S5 in ESI†). This indicates the accumulation of anti-HER2 antibody binding QDs in the tumor. In contrast, there is no significant second-NIR fluorescence in the area without tumors (Fig. 4b). To confirm actual accumulation of the QDs in the breast tumor cells, we carried out ex vivo fluorescence imaging of an isolated tumor at a higher magnification by a confocal microscope and a second-NIR microscope (Fig. 4d, e, and S6 in ESI†). Compared with the negative control without QDs injection (Fig. 4d), intense fluorescence of EGFP emitting from the anti-HER2 antibody-conjugated QDs was observed in the breast tumor (Fig. 4e). This finding indicates that the conjugates of anti-HER2 antibody and GST-EGFP-GB1-coated QDs pass through the blood vessels of the breast tumor and bind to the HER2 receptors on the tumor cells.
In summary, we have demonstrated a one-step facile method for preparing GST-EGFP-GB1 protein-coated PbS QDs with a dual emission of visible (515 nm) and second-NIR fluorescence (1150 nm). The PbS QDs have two molecules of GST-EGFP-GB1 protein per QD particle, and they can easily bind IgG antibodies via their GB1 domain. We have demonstrated that the GST-EGFP-GB1-coated PbS QDs have a dual-color probe capability for the fluorescence molecular imaging of breast tumors at the cellular and whole-body level. An important feature of this work is that the GST-EGFP-GB1-coated QDs make it possible to correlate the cellular distribution of QD probes with their bio-distribution in the whole body. These dual emission PbS QDs with antibody binding ability can be extended to a variety of in vitro and in vivo tumor imaging techniques by constructing conjugates between specific antibodies for tumors and the GST-EGFP-GB1 protein-coated PbS QDs.
Acknowledgements
We thank Dr Scott Gradia (University of California, Berkeley) for making the Addgene plasmid 29713 available through Addgene. All experiments were performed in compliance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Osaka University Animal Care and Use Committee.Notes and references
- (a) R. Weissleder, Nat. Biotechnol., 2001, 19, 316–317 CrossRef CAS PubMed; (b) J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626–634 CrossRef CAS PubMed; (c) X. Gao, Y. Cui, R. M. Levenson, L. W. K. Chung and S. Nie, Nat. Biotechnol., 2004, 22, 969–976 CrossRef CAS PubMed; (d) S. Kim, Y. T. Lim, E. G. Soltesz, A. M. De Grand, D. J. Lee, A. Nakayama, J. A. Parker, T. Mihaljevic, R. G. Laurence, D. M. Dor, L. H. Cohn, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2004, 22, 93–97 CrossRef CAS PubMed; (e) X. Gao, L. Yang, J. A. Petros, F. F. Marshall, J. W. Simons and S. Nie, Curr. Opin. Biotechnol., 2005, 16, 63–72 CrossRef CAS PubMed; (f) C. L. Amiot, S. Xu, S. Liang, L. Pan and J. X. Zhao, Sensors, 2008, 8, 3082–3105 CrossRef CAS PubMed.
- X. He, J. Gao, S. S. Gambhir and Z. Cheng, Trends Mol. Med., 2010, 16, 574–583 CrossRef CAS PubMed.
- J. H. Ryu, M. Shin, S. A. Kim, S. Lee, H. Kim, H. Koo, B. S. Kim, H. K. Song, S. H. Kim, K. Choi, I. C. Kwon, H. Jeon and K. Kim, Biomaterials, 2013, 34, 9149–9159 CrossRef CAS PubMed.
- A. M. Smith, M. C. Mancini and S. Nie, Nat. Nanotechnol., 2009, 4, 710–711 CrossRef CAS PubMed.
- N. Won, S. Jeong, K. Kim, J. Kwag, J. Park, S. G. Kim and S. Kim, Mol. Imaging, 2012, 11, 338–352 CAS.
- L. A. Sordillo, Y. Pu, S. Pratavieira, Y. Budansky and R. R. Alfano, J. Biomed. Opt., 2014, 19, 056004 CrossRef PubMed.
- (a) Z. Liu, W. Cai, L. He, N. Nakayama, K. Chen, X. Sun, X. Chen and H. Dai, Nat. Nanotechnol., 2007, 2, 47–52 CrossRef CAS PubMed; (b) K. Welsher, Z. Liu, D. Daranciang and H. Dai, Nano Lett., 2008, 8, 586–590 CrossRef CAS PubMed; (c) K. Welsher, Z. Liu, S. P. Sherlock, J. T. Robinson, Z. Chen, D. Daranciang and H. Dai, Nat. Nanotechnol., 2009, 4, 773–780 CrossRef CAS PubMed; (d) K. Welsher, S. P. Sherlock and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 8943–8948 CrossRef CAS PubMed; (e) G. Hong, J. C. Lee, J. T. Robinson, U. Raaz, L. Xie, N. F. Huang, J. P. Cooke and H. Dai, Nat. Med., 2012, 18, 1841–1846 CrossRef CAS PubMed; (f) J. T. Robinson, G. Hong, Y. Liang, B. Zhang, O. K. Yaghi and H. Dai, J. Am. Chem. Soc., 2012, 134, 10664–10669 CrossRef CAS PubMed; (g) Z. Tao, G. Hong, C. Shinji, C. Chen, S. Diao, A. L. Antaris, B. Zhang, Y. Zou and H. Dai, Angew. Chem., Int. Ed., 2013, 125, 13240–13244 CrossRef; (h) D. J. Naczynski, M. C. Tan, M. Zevon, B. Wall, J. Kohl, A. Kulesa, S. Chen, C. M. Roth, R. E. Riman and P. V. Moghe, Nat. Commun., 2013, 4, 2199, DOI:10.1038/ncomms3199.
- E. H. Sargent, Adv. Mater., 2005, 17, 515–522 CrossRef CAS.
- M. A. Hines and G. D. Scholes, Adv. Mater., 2003, 15, 1844–1849 CrossRef CAS.
- (a) B. R. Hyun, H. Chen, D. A. Rey, F. W. Wise and C. A. Batt, J. Phys. Chem. B, 2007, 111, 5726–5730 CrossRef CAS PubMed; (b) J. M. Pietryga, D. J. Werder, D. J. Williams, J. L. Casson, R. D. Schaller, V. I. Klimov and J. A. Hollingsworth, J. Am. Chem. Soc., 2008, 130, 4879–4885 CrossRef CAS PubMed.
- Y. Nakane, Y. Tsukasaki, T. Sakata, H. Yasuda and T. Jin, Chem. Commun., 2013, 49, 7584–7586 RSC.
- Y. Tsukasaki, M. Morimatsu, G. Nishimura, T. Sakata, H. Yasuda, A. Komatsuzaki, T. M. Watanabe and T. Jin, RSC Adv., 2014, 4, 41164–41171 RSC.
- Y. Tsukasaki, A. Komatsuzaki, Y. Mori, Q. Ma, Y. Yoshioka and T. Jin, Chem. Commun., 2014, 50, 14356–14359 RSC.
- (a) Y. Du, B. Xu, T. Fu, M. Cai, F. Li, Y. Zhang and Q. Wang, J. Am. Chem. Soc., 2010, 132, 1470–1471 CrossRef CAS PubMed; (b) G. Hong, J. T. Robinson, Y. Zhang, S. Diao, A. L. Antaris, Q. Wang and H. Dai, Angew. Chem., Int. Ed., 2012, 124, 9956–9959 CrossRef; (c) P. Jiang, Z. Q. Tian, C. N. Zhu, Z. L. Zhang and D. W. Pang, Chem. Mater., 2012, 24, 3–5 CrossRef CAS; (d) Y. Zhang, G. Hong, Y. Zhang, G. Chen, F. Li, H. Dai and Q. Wang, ACS Nano, 2012, 6, 3695–3702 CrossRef CAS PubMed; (e) P. Jiang, C. N. Zhu, Z. L. Zhang, Z. Q. Tian and D. W. Pang, Biomaterials, 2012, 33, 5130–5135 CrossRef CAS PubMed; (f) H. Y. Yang, Y. W. Zhao, Z. Y. Zhang, H. M. Xiong and S. N. Yu, Nanotechnology, 2013, 24, 055706 CrossRef PubMed; (g) Y. Zhang, Y. Zhang, G. Hong, W. He, K. Zhou, K. Yang, F. Li, G. Chen, Z. Liu, H. Dai and Q. Wang, Biomaterials, 2013, 34, 3639–3646 CrossRef CAS PubMed; (h) Y. Zhang, Y. Liu, C. Li, X. Chen and Q. Wang, J. Phys. Chem. C, 2014, 118, 4918–4923 CrossRef CAS; (i) R. Gui, A. Wan, X. Liu, W. Yuan and H. Jin, Nanoscale, 2014, 6, 5467–5473 RSC; (j) C. Li, Y. Zhang, M. Wang, Y. Zhang, G. Chen, L. Li, D. Wu and Q. Wang, Biomaterials, 2014, 35, 393–400 CrossRef CAS PubMed; (k) G. Chen, F. Tian, Y. Zhang, Y. Zhang, C. Li and Q. Wang, Adv. Funct. Mater., 2014, 24, 2481–2488 CrossRef CAS.
- G. Wang, J. Ji and X. Xu, J. Mater. Chem. C, 2014, 2, 1977–1981 RSC.
- S. K. Patel, M. J. Patrick, J. A. Pollock and J. M. Janjic, J. Biomed. Opt., 2013, 18, 101312 CrossRef PubMed.
- A. M. Gronenborn, D. R. Filpula, N. Z. Essig, A. Achari, M. Whitlow, P. T. Wingfield and G. M. Clore, Science, 1991, 253, 657–661 CAS.
- N. Ma, A. F. Marshall and J. Rao, J. Am. Chem. Soc., 2010, 132, 6884–6885 CrossRef CAS PubMed.
- X. He, L. Gao and N. Ma, Sci. Rep., 2013, 3, 2825 Search PubMed.
- R. Elshafey, A. C. Tavares, M. Siaj and M. Zourob, Biosens. Bioelectron., 2013, 50, 143–149 CrossRef CAS PubMed.
- T. Niide, K. Shimojo, R. Wakabayashi, M. Goto and N. Kamiya, Langmuir, 2013, 29, 15596–15605 CrossRef CAS PubMed.
- X. Sun, G. Zhang, R. S. Keynton, M. G. O'Toole, D. Patel and A. M. Gobin, Nanomedicine, 2013, 9, 1214–1222 CrossRef CAS PubMed.
- T. Jin, F. Fujii, Y. Komai, J. Seki, A. Seiyama and Y. Yoshioka, Int. J. Mol. Sci., 2008, 9, 2044–2061 CrossRef CAS PubMed.
- A. Sasaki, H. Sakata and M. Kinjo, Curr. Pharm. Biotechnol., 2010, 11, 117–121 CAS.
- J. Kurebayashi, T. Otsuki, C. K. Tang, M. Kurosumi, S. Yamamoto, K. Tanaka, M. Mochizuki, H. Nakamura and H. Sonoo, Br. J. Cancer, 1999, 79, 707–717 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4nr06480a |
| ‡ Present address: University of Texas Health Science Center Northeast, Tyler, Texas 75708-3154, USA. |
| This journal is © The Royal Society of Chemistry 2015 |