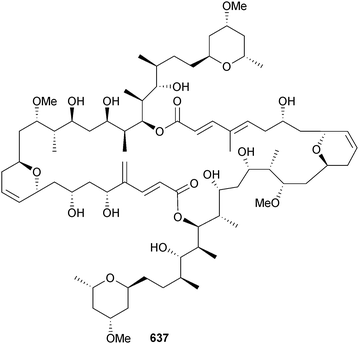
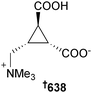
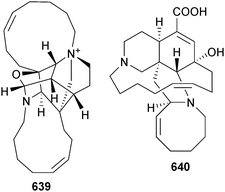
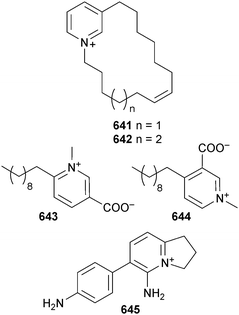
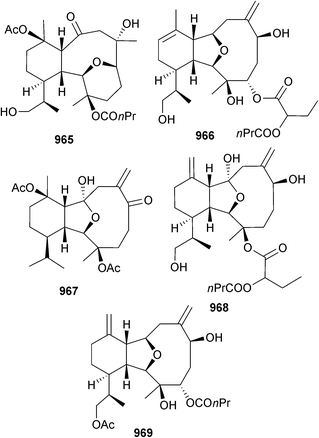
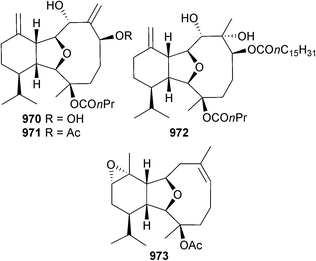
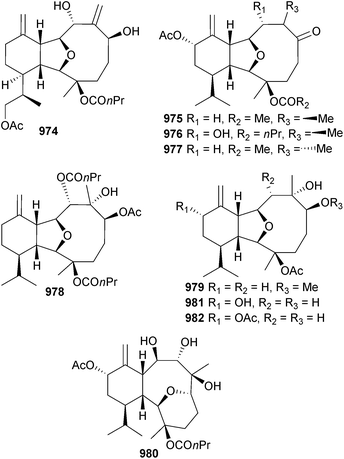
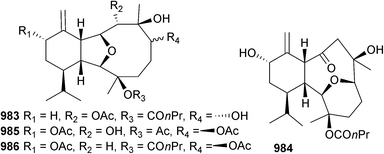
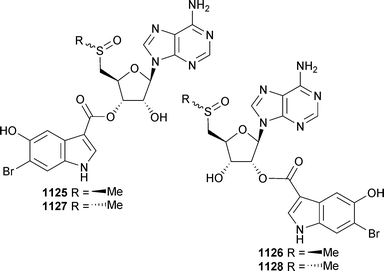
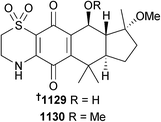
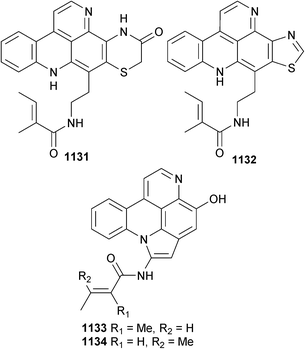
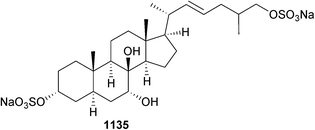
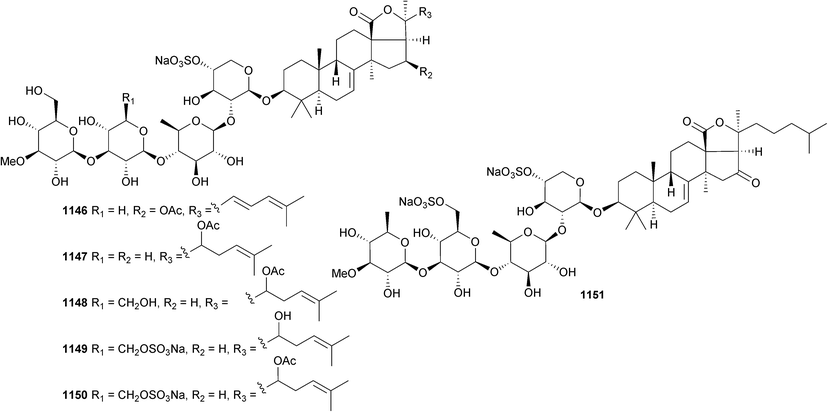
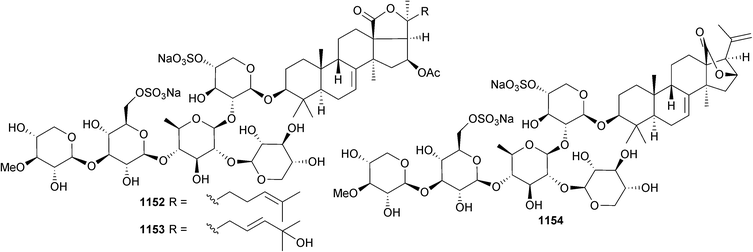
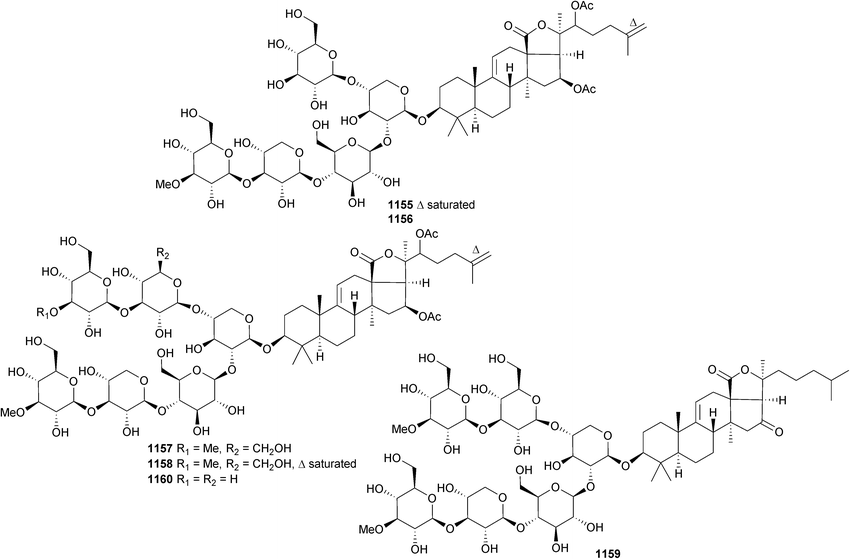
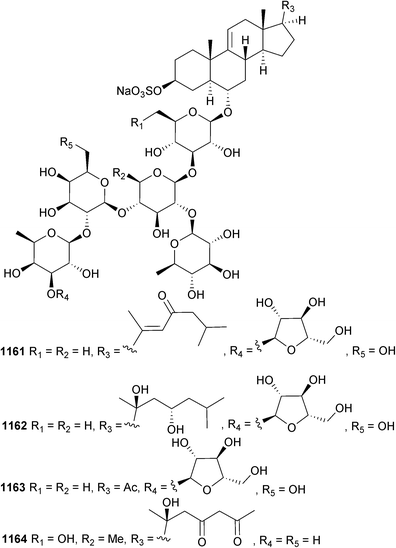
Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Marine natural products
John W.
Blunt
*a,
Brent R.
Copp
b,
Robert A.
Keyzers
c,
Murray H. G.
Munro
a and
Michèle R.
Prinsep
d
aDepartment of Chemistry, University of Canterbury, Christchurch, New Zealand. E-mail: john.blunt@canterbury.ac.nz
bSchool of Chemical Sciences, University of Auckland, Auckland, New Zealand
cCentre for Biodiscovery, and School of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, New Zealand
dChemistry, School of Science, University of Waikato, Hamilton, New Zealand
First published on 26th January 2015
Abstract
Covering: 2013. Previous review: Nat. Prod. Rep., 2014, 31, 160–258
This review covers the literature published in 2013 for marine natural products (MNPs), with 982 citations (644 for the period January to December 2013) referring to compounds isolated from marine microorganisms and phytoplankton, green, brown and red algae, sponges, cnidarians, bryozoans, molluscs, tunicates, echinoderms, mangroves and other intertidal plants and microorganisms. The emphasis is on new compounds (1163 for 2013), together with the relevant biological activities, source organisms and country of origin. Reviews, biosynthetic studies, first syntheses, and syntheses that lead to the revision of structures or stereochemistries, have been included.
1 Introduction
This review is of the literature for 2013 and describes 1163 new compounds from 379 articles, a 6% decrease in the number of compounds reported in 2012.1 As in previous reviews, the structures are shown only for new compounds, or for previously reported compounds where there has been a structural revision or a newly established stereochemistry. Previously reported compounds for which first syntheses or new bioactivities are described are referenced, but separate structures are generally not shown. Where the absolute configuration has been determined for all stereocentres in a compound, the identifying diagram number is distinguished by addition of the † symbol.2 Reviews
A selection of the many reviews on various aspects of MNP studies is listed here. A comprehensive review of MNPs reported in 2011 has appeared,2 as well as the highlights of compounds reported in 2012.3 Marine pharmacology papers for 2009–2011 have been collated,4 two reviews summarise natural products (NPs), including from marine sources, as drug leads,5,6 while another paper describes recent advances in marine drug research.7 A synopsis of the project BAMMBO for the sustainable production of biologically active molecules of marine based origin has appeared.8 General classes of compounds have been reviewed in papers on marine triterpenoids as anticancer agents,9 alkaloids from corals,10 meroterpenes from marine invertebrates,11 ‘head-to-sidechain’ cyclodepsipeptides,12 marine alkaloids containing an 1-(indol-3-yl)ethane-1,2-diamine fragment,13 tetrahydrofuran-containing macrolides,14 terpenes from Sarcophyton sp.,15,16 antimicrobial peptides from proteobacteria,17 diarrhetic shellfish toxins in Washington State.,18 di- and sesquiterpenes from Cystosiera sp.,19 and halogenated compounds from Rhodomelaceae.20 Some general reviews on various classes of compounds include data on marine compounds – anticancer steroids,21 sesterterpenoids,22 NPs containing a nitrogen–nitrogen bond,23 and muscarine, imidazole, oxazole and thiazole alkaloids.24 Reviews on various aspects of specific compounds include lamellarins N and L,25 STX,26 lyngbouilloside and related macrolides,27 thiomarinol and related dithiolopyrrolone compounds,28 okadaic acid,29 and the marinopyrroles.30 There have been many reviews covering a variety of groups of marine organisms, including bioprospecting of plankton,31 actinomycetes,32,33 S. China Sea opisthobranch molluscs,34 actinobacteria,35,36 filamentous marine cyanobacteria,37 and microbes in general.38–40 Reviews on specific organisms include Australian Dicathais orbita,41 metabolites from Osmundaria spp.,42Aspergillus spp.,43 and Bacillus spp.44 A focus on bioactivities is made in reviews on anti-inflammatory compounds,45,46 trypanocidal products,47 neuroprotective compounds,48 antitumour/anticancer agents,49–52 kinase inhibitors,53 anti-Herpes simplex agents,54 anti-HIV actives,55 angiogenesis inhibitors,56 cardioprotective peptides,57 antithrombotic peptides,58 antimicrobial peptides,59 therapeutics for Gram-negative sepsis,60 and bioactives from Antarctic and Arctic organisms.61 The chemical ecology of plankton62 and the possible ecological roles of cyanotoxins63 have been reviewed. The eighth in a companion series providing an overview of synthetic aspects of MNPs has appeared with coverage of publications from 2010.64 Further reviews of syntheses of specific compounds include marine alkyl purines,65 tetrodotoxin,66 (+)-spirastrellolide A methyl ester,67 and 'upenamide, the structure of which still remains elusive.68 A number of papers which, while not necessarily being reviews, are useful to reference here as they describe advances in techniques or approaches to discovery that are relevant to MNP studies. These include papers on novel extraction technologies for bioactives from marine algae,69 dereplication of marine actinomycetes by LCHRMS profiling,70 X-ray analysis on the nanogram to microgram scale using porous complexes,71,72 rapid screening of bioactive compounds by integrating 5-channel parallel chromatography coupled with on-line mass spectrometry and microplate based assays,73 molecular networking as a dereplication strategy,74 NMR-based metabolomic analysis of macroalgae,75 biogeography and biodiscovery hotspots of macroalgal compounds,76 and coral aquaculture to support drug discovery.77 The MarinLit database has been updated and was used as the literature source for the preparation of this present review. This database has now been transferred to the Royal Society of Chemistry from where it is available as a web-accessible version.783 Marine microorganisms and phytoplankton
MNP research effort is being increasingly directed towards marine microorganisms with 491 new compounds reported in 2013, an increase of 14% from 2012 (see 15 Conclusion). Unless otherwise stated, compounds described in this section were obtained from cultures of the named microorganisms.3.1 Marine-sourced bacteria (excluding from mangroves)
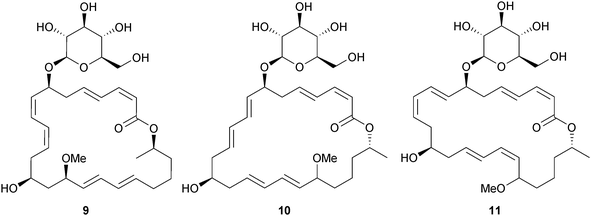
The chlorinated pyrones halomadurone A 1 and B 2 were isolated from Actinomadura sp. (ascidian Ecteinascidia turbinata, Florida Keys, U.S.A.) and with increased concentration of potassium bromide in the growth media produced the brominated analogues halomadurone C 3 and D 4. The halomadurones A–D activated the nuclear factor E2-related antioxidant response element, an indication of potential for treatment of neurodegenerative diseases.79 Discoipyrroles A–D 5–8 are alkaloids isolated from Bacillus hunanensis (sediment, Galveston Bay, Texas, U.S.A.) that inhibit the signaling pathway of the tyrosine kinase, discoidin domain receptor 2. They were each obtained as racemates and feeding experiments with several substituted benzaldehyde precursors indicated formation through a nonenzymic process, which led to a one-pot total synthesis of discoipyrrole A 5.80Three glycosylated methoxy-macrolactins 9–11 were isolated from Bacillus subtilis (B. subtilis) (sediment, Gageocho, S. Korea) and all displayed inhibition of Gram-positive and Gram-negative bacterial strains, in addition to modest antifungal activity.81 A strain of B. subtilis (sponge Haliclona simulans, Gurraig Sound, Galway, Ireland) yielded subtilomycin, a partially characterised 32-amino acid compound that was partly responsible for the observed broad spectrum antimicrobial activity of the bacterium.82
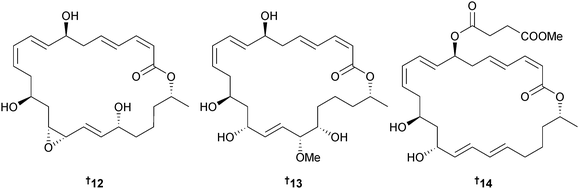
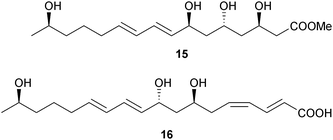
Bacillus sp. (sediment, Ieodo Reef, S. Korea)83 produced the 24-membered macrolactones macrolactin X–Z 12–14 and the unsaturated fatty acids linieodolide A 15 and B 16, all with modest antibacterial and antifungal activity.84
Two separate isolates of the myxobacterium Enhygromyxa salina yielded antibiotics. Salimyxins A 17 and B 18 were obtained from one strain (sediment, Santa Barbara, California, U.S.A.) whilst the geometric isomers enhygrolide A 19 and B 20 were isolated from another strain (sediment, Prerow, Germany). Salimyxins A 17 and B 18 are structurally very similar to demethylincisterol obtained from the sponge Homaxinella sp.,85 whilst enhygrolides A 19 and B 20 are structurally related to the nostoclides, first obtained from a Nostoc species of cyanobacterium.86 Salimyxin B 18 and enhygrolide A 19 were moderate growth inhibitors of the Gram-positive bacterium Arthrobacter cristallopoietes.87 The obligatory marine myxobacterium Enhygromxya salina (sediment, Prerow Is., Germany) was the source of the tetracyclic salimabromide 21 which was a moderate inhibitor of Arthrobacter cristallopoietes.88
Kocuria palustris (sponge Xestospongia muta, Key Largo, Florida)89 produced a thiazolyl peptide kocurin 22 with antibacterial activity including strong inhibition of methicillin-resistant Staphylococcus aureus (MRSA).90 A molecule with the same planar structure as 22 was previously isolated from Kocuria sp. in Southeast Spain91 as baringolin and is also believed to be a correction of the structure previously assigned to PM181104,92 (also obtained from a Kocuria sp.) mentioned in a patent.90 Kocurin, also produced by Kocuria marina and a Micrococcus sp. (Florida Keys),93 has been synthesised by a convergent strategy in good overall yield.94 The macrolide juvenimicin C 23 was obtained from Micromonospora sp. (sediment, Palau) and enhanced the activity of the enzymes quinone reductase I, glutathione reductase and glutathione peroxidase, suggesting potential as a cancer chemopreventive agent.95 Levantilide C 24 is a 20-membered macrolide isolated from a Micromonospora strain (Golfo Corcovaclo, Chiloe Is., Chile) with moderate antiproliferative activity against human tumour cancer cell lines (HTCLs).96 Two strains of Micromonospora (sediment, North Carolina coast, U.S.A.) yielded the polyene macrolactam micromonolactam 25, a constitutional isomer of salinilactam A97 but with a different polyene pattern and a (Z)-double bond, in contrast to the all (E)-structure of salinilactam A. Genome sequencing of one of the strains determined that 25 was derived from eleven polyketide units and a modified glutamate starter unit.98Nocardiopsis alba (deep-sea sediment, Indian Ocean) produced several diketopiperazines, including the new C-6 epimers nocazine D 26 and E 27 and the known synthetic compounds (S,Z)-3-benzylidene-6-methylpiperazine-2,5-dione and (S,Z)-3-benzylidene-6-isopropylpiperazine-2,5-dione,99 both isolated for the first time as NPs. Methoxyneihumicin was also isolated. This is a structure that had been previously reported in a conference poster100 but not in the chemical literature. Both methoxyneihumicin and the known bacterial metabolite XR334 (ref. 101) (first time marine isolate) were modestly active against HTCLs.102
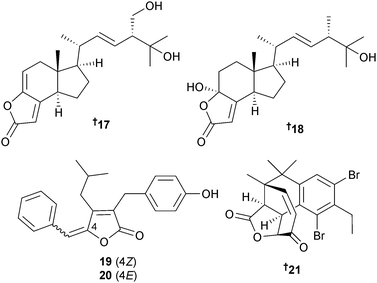
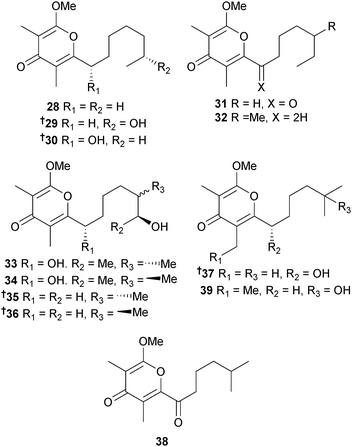
Three different groups of researchers have isolated metabolites from Nocardiopsis species and all have named them nocapyrones. To avoid confusion they are presented here in order of publication. Firstly, symbiotic Nocardiopsis alba (cone snail Conus rolani, Mactan Is., Philippines) produced the γ-pyrones nocapyrone H–Q 28–39. Of these, nocapyrone N 35/36 was isolated as a mixture of enantiomers in a 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
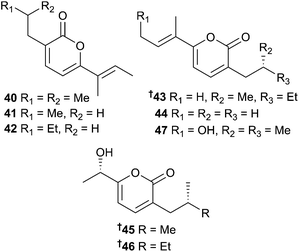
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and nocapyrone M 33/34 occurred as an inseparable mixture of diastereoisomers. Both nocapyrone H 28 and the co-isolated nocapyrone B, previously obtained from a sponge-associated Nocardiopsis strain,103 modulated nerve cell depolarisation and were active against a wide range of dorsal root ganglion neuronal cell types. Nocapyrones B and H were moderately cytotoxic to cancer cell lines.104 Secondly, three 3,6-disubstituted α-pyrones 40–42 were isolated from Nocardiopsis sp. (sediment, Ulleung Basin, Eastern sea, Korea) and named nocapyrones H–J. “Nocapyrone H” 40 inhibited pro-inflammatory factors such as nitric oxide (NO), prostaglandin E2 (PGE2) and interleukin-1β (IL-1β) (potential neuroprotective effects).105 Lastly, Nocardiopsis dassonvillei subsp. dassonvillei (sediment, Lianyungang, China) also produced α-pyrones named “nocapyrones H–N”. Of these “nocapyrone H” had the same structure as 40, “nocapyrone K” was identical to 41, while the balance, 43–47, were unique. “Nocapyrone I” 43 and “M” 46 displayed inhibition of quorum sensing (QS) controlled gene expression in Chromobacterium violaceum CV026 and Pseudomonas aeruginosa QSIS-lasI biosensors.106
1 ratio and nocapyrone M 33/34 occurred as an inseparable mixture of diastereoisomers. Both nocapyrone H 28 and the co-isolated nocapyrone B, previously obtained from a sponge-associated Nocardiopsis strain,103 modulated nerve cell depolarisation and were active against a wide range of dorsal root ganglion neuronal cell types. Nocapyrones B and H were moderately cytotoxic to cancer cell lines.104 Secondly, three 3,6-disubstituted α-pyrones 40–42 were isolated from Nocardiopsis sp. (sediment, Ulleung Basin, Eastern sea, Korea) and named nocapyrones H–J. “Nocapyrone H” 40 inhibited pro-inflammatory factors such as nitric oxide (NO), prostaglandin E2 (PGE2) and interleukin-1β (IL-1β) (potential neuroprotective effects).105 Lastly, Nocardiopsis dassonvillei subsp. dassonvillei (sediment, Lianyungang, China) also produced α-pyrones named “nocapyrones H–N”. Of these “nocapyrone H” had the same structure as 40, “nocapyrone K” was identical to 41, while the balance, 43–47, were unique. “Nocapyrone I” 43 and “M” 46 displayed inhibition of quorum sensing (QS) controlled gene expression in Chromobacterium violaceum CV026 and Pseudomonas aeruginosa QSIS-lasI biosensors.106
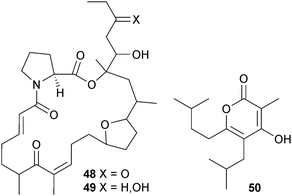
Very clearly the naming of these metabolites needs revision. Saline culture of Nocardiopsis sp. (sediment, S. Molle Is., Queensland, Australia) previously yielded norcardioazines A and B107 whilst non-saline culture of the same strain yielded nocardiopsins A and B.108 Further investigation of the strain cultivated under non-saline conditions has resulted in the isolation of the prolinyl-macrolactam polyketides nocardiopsin C 48 and D 49 and the highly substituted α-pyrone polyketide, nocardiopyrone A 50.109 It should be noted that the name nocardiopyrone A has coincidentally been given to a metabolite isolated from a terrestrial species, Nocardiopsis alkaliphila,110 and that the same CAS number appears to have been given to both compounds in error on the Scifinder database, with the terrestrial compound structure showing as corresponding to that CAS number.
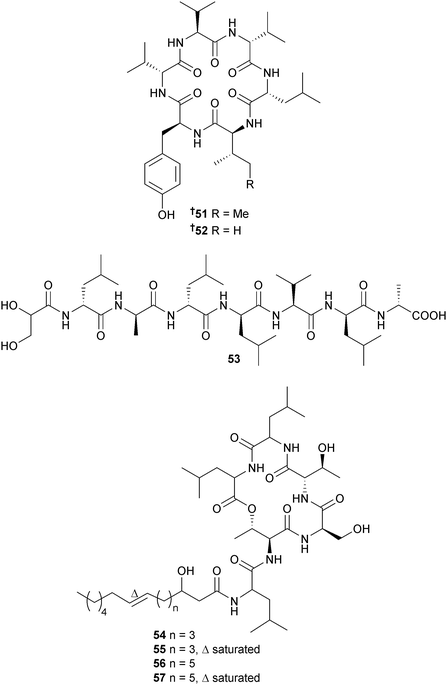
The cyclic hexapeptides nocardiamide A 51 and B 52 were isolated from Nocardiopsis sp. (La Jolla Canyon, San Diego, California, U.S.A.) and then synthesised via solid-phase peptide synthetic methods.111 A microorganism, nominally Paenibacillus profundus sp. nov., (sediment, Sea of Japan) yielded a linear glyceryl acid derived heptapeptide 53 with strong antibacterial inhibition and moderate inhibition of SK-MEL-28 cells,112 while a species of Photobacterium, closely related to P. halotolerans (mussel, Solomon Is., Pacific Ocean), was the source of the cyclodepsipeptides ngercheumicin F–I 54–57 that inhibited quorum sensing in Staphylococcus aureus.113
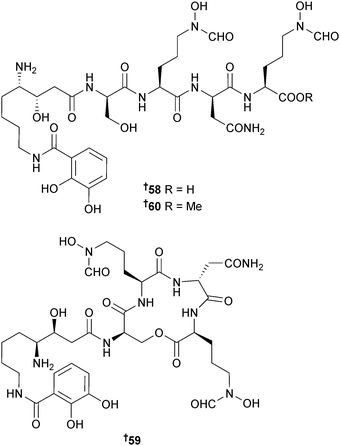
A Pseudoalteromonas sp. (oil-contaminated surface water, Gulf of Mexico after the Deepwater Horizon oil spill) yielded the siderophores lystabactin A–C 58–60 which contained the unusual nonproteinogenic amino acid 4,8-diamino-3-hydroxyoctanoic acid (LySta). Since lystabactin C is 29-methoxy lystabactin A, it may have been an artefact of isolation.114
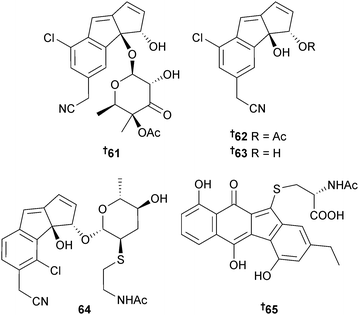
Cyanosporasides A and B are chloro- and cyano-cyclopenta[a]indene glycosides originally isolated from a Palauan Salinispora pacifica strain,115 while cyanosporasides C–E 61–63 came from investigation of another S. pacifica strain (sediment, Palau) and cyanosporasides D–F 62–64 from a Streptomyces sp. (sediment, Bahamas). Cloning, sequencing, and mutagenesis of cyanosporaside biosynthetic gene clusters from both bacteria demonstrated that the cyanosporasides are enediyne polyketides and a two-gene operon was identified which was implicated in the nitrile functionalisation of these metabolites.116 Further investigation of the strain of S. pacifica (USDA Agricultural Research Service) that produced lomaiviticins C–E117 resulted in the isolation of (−)-homoseongomycin 65. Synthesis of an isotopically-labelled derivative, homoseongomycin-d5, clarified aspects of the biosynthetic pathway.118
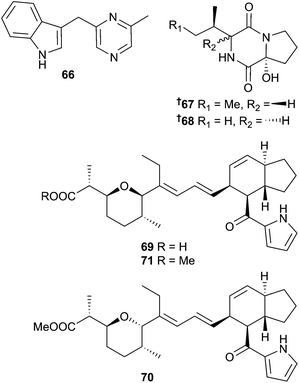
The alkaloid 66 was obtained from Serinicoccus profundi sp. nov. (deep-sea sediment, Indian Ocean) (weak activity against Staphylococcus aureus (S. aureus))119 and Staphylococcus sp. (red alga, Corallina officinalis, Nagasaki Shitsu Coast, Japan) provided the diketopiperazine derivatives staphyloamide A 67 and B 68.120Streptomyces antibioticus (sediment, source not given) yielded the indanomycin-related antibiotics 69–71 as moderate growth inhibitors of S. aureus.121
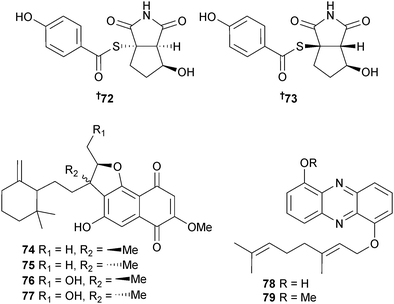
The alkaloids nitrosporeusine A 72 and B 73 with an unprecedented skeleton (benzenecarbothioc cyclopenta[c]pyrrole-1,3-dione) were isolated from S. nitrosporeus (sediment, Arctic Chukchi Sea). Both nitrosporeusines inhibited the H1N1 virus in infected MDCK cells.122 Some sesquiterpenoid naphthoquinones marfuraquinocin A–D 74–77 and the geranylated phenazines phenaziterpene A 78 and B 79 were isolated from S. niveus (sediment, S. China Sea). Marfuraquinocins A 74 and C 76 were growth inhibitors of NCI–H460 cancer cells (moderate) whilst marfuraquinocins A, C and D 77 were moderate growth inhibitors of S. aureus, with marfuraquinocins C and D also inhibitors of methicillin-resistant Staphylococcus epidermidis.123
Tetroazolemycins A 80 and B 81 are oxazole/thiazole derivatives obtained from S. olivaceus (deep-sea water, southwest Indian Ocean), both of which showed binding affinity for the metal ions Fe3+, Cu2+ and Zn2+.124S. seoulensis (shrimp gut Penasus orientalis, Qingdao, China) yielded the neuraminidase inhibitors streptoseolactone 82, limazepine G 83 and a known synthetic compound125,126 isolated for the first time as an NP, and named limazepine H.127 Endophytic S. sundarbansensis (brown alga Fucus sp., Bejaia, Algeria) provided the polyketide chromanone 84 (modest but selective activity against MRSA).128
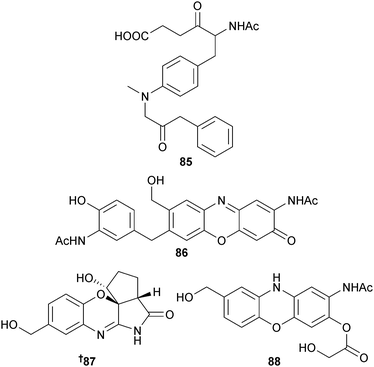
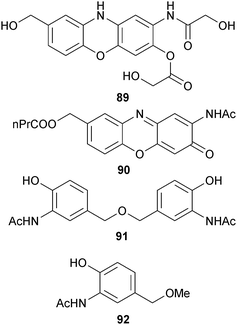
S. tateyamensis (sponge Haliclona sp., Tateyama City, Japan)129 was the source of JBIR-107 85,130 while the phenoxazine-based alkaloids venezueline A–E 86–90 and the aminophenols venezueline F 91 and G 92 were obtained from S. venezuelae (sediment, Guam) with the known analogues exfoliazone,131 chandrananimycin D132 and carboxyexfoliazone,133 all previously obtained from terrestrial Streptomyces species but now first time marine isolates. Venezueline B 87 was moderately cytotoxic towards a panel of HTCLs.134
Double mutation of a strain of S. xiamenensis (sediment, Eastern Pacific Ocean) led to production of two benzopyran derivatives xiamenmycin C 93 and D 94, which both inhibited proliferation of human lung fibroblasts (WI26),135 and Streptomyces sp. (unidentified soft coral, Weizhou Is., Guangki Province, China) was the source of the chlorinated polyketides strepchloritide A 95 and B 96 cytotoxic against MCF-7 cells (modest).136 Chlorizidine A 97, comprised of a chlorinated 2,3-dihydropyrrolizine ring attached to an unprecedented chlorinated 5H-pyrrolo[2,1-a]isoindol-5-one, was isolated from a Streptomyces strain (sediment, San Clemente, California, U.S.A.) and was moderately cytotoxic to a panel of HTCLs.137 The biosynthetic gene cluster of chlorizidine A 97 was identified and whole pathway heterologous expression and genetic manipulations were utilised to show that it is assembled by a polyketide synthase (PKS) that uniquely incorporates a fatty acid synthase-derived dichloropyrrolyl extender unit into the pyrroloisoindolone enzymic product.138
The diketopiperazine derivatives 98–102 were obtained from Streptomyces sp. (sediment, Huanghai Beach, Dalian, China) and 100 displayed modest activity against the influenza A (H1N1) virus, whilst the co-isolated fungal metabolites (3Z,6S)-3-benzylidene-6-isobutylpiperazine-2,5-dione139 and albomoursin140 displayed potent inhibition of the virus and were first time marine isolates.141
Streptomyces sp. (sediment, S. China Sea) yielded the spirotetronate lobophorin G 103, a potent inhibitor of both Mycobacterium bovis Bacillus Calmette-Guerin (BCG) and B. subtilis and a moderate inhibitor of Mycobacterium tuberculosis (M. tuberculosis).142 Sungsanpin 104 isolated from a Streptomyces sp. (deep-sea sediment, Jeju Is., S. Korea) is an example of a so-called lasso peptide, a ribosomally synthesised peptide of between 16 and 23 amino acids with an N-terminal eight- or nine-residue ring with a linear C-terminus threaded through the ring.143 Sungsanpin inhibited A549 cells in a cell invasion assay.144
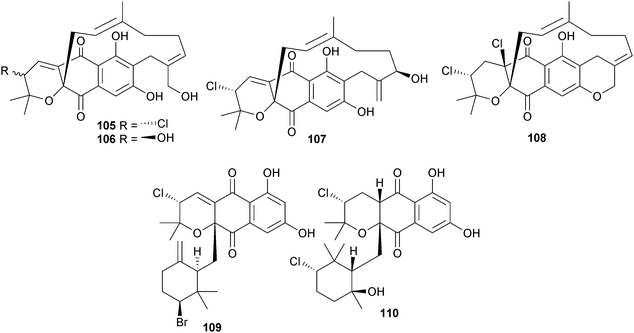
Investigation of two different Streptomyces strains identified six new napyradiomycin analogues. The Streptomyces strain CNQ-329 (sediment, San Diego, California, U.S.A.) produced napyradiomycins A–E 105–109, while strain CNH-070 (sediment, San Elijo Lagoon, Encinitas, California, U.S.A.) produced napyradiomycin F 110.
Four of the napyradiomycins A, D–F were cytotoxic (moderate) to HCT-116 cells whilst napyradiomycins A and B inhibited MRSA (moderate).
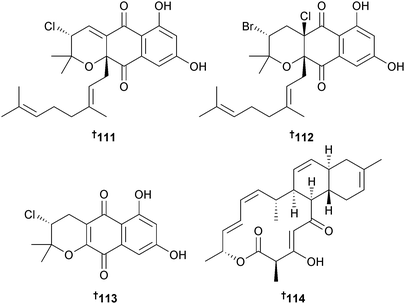
Also isolated were napyradiomycins B2–B4; B3 (ref. 145) and B4 (ref. 146) as first time marine isolates.147 Three napyradiomycins, 4-dehydro-4a-dechloronapyradiomycin A1 111, 3-dechloro-3-bromonapyradiomycin A1 112 and 3-chloro-6,8-dihydroxy-8-α-lapachone 113 isolated from a Streptomyces species (sediment, Xieyang Is., Beihai, Guangxi Province, China) displayed moderate inhibition of several Gram-positive bacteria while 3-dechloro-3-bromonapyradiomycin A1 112 was moderately active against several HTCLs.148 A Streptomyces sp. (sediment, Santa Barbara, California, U.S.A.) yielded the antibiotic anthracimycin 114, significantly active against Bacillus anthracis. Early in vivo results indicated that 114 also provided significant protection against MRSA cell lines. The planar structure of anthracimycin 114 may have been published in a 2011 patent149 but insufficient detail was given to permit a full comparison.150
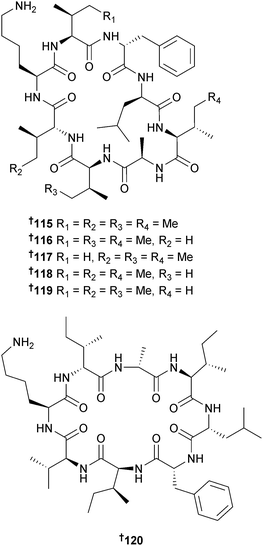
Surugamides A–E 115–119, cyclic octapeptides with four D-amino acid residues, were obtained from Streptomyces sp. (deep-sea sediment, Kinko Bay, Japan) and were modest inhibitors of the protease enzyme bovine cathepsin B.151 Three strains of S. champavatii (sediment, Gotland Deep and Kiel Bight, Baltic Sea and Urania Basin, Eastern Mediterranean) produced the octapeptide champacyclin 120, an inhibitor of the bacterium Erwinia amylovora, the causative agent of fire blight disease in certain plants. Champacyclin 120 has the same planar structure as surugamide A 115 but different configurations at two amino acid residues. Champacyclin was also prepared by solid-phase peptide synthesis.152
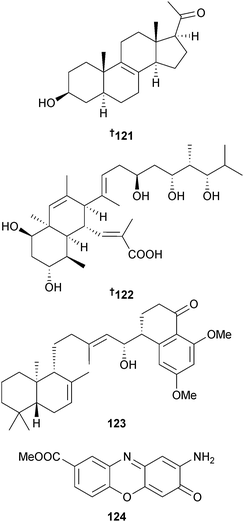
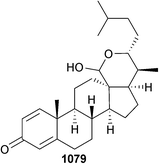
Streptomyces sp. (sediment, S. China Sea) yielded the pregnene steroid 3219A 121 with a rare Δ8,9-double bond in the skeleton,153 and the polyketide nahuoic acid A 122 was obtained from a Streptomyces sp. (sediment, Padana Nahua, Papua New Guinea) as a selective SAM-competitive inhibitor of the histone methyltransferase enzyme SETD8.154 A meroterpenoid actinoranone 123 was isolated from a bacterium, likely a Streptomyces species (sediment, San Diego, California, U.S.A.)155 as a moderate cytotoxin of HCT-116156 and Streptomyces sp. (sediment, Marsa Matruh city, Egypt) was the source of maroxazinone 124, moderately cytotoxic to several HTCLs.157
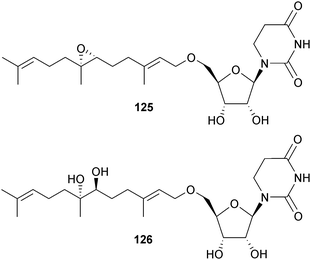
Farnesides A 125 and B 126, linear sesquiterpenoids connected by ether linkages to a ribose dihydrouracil nucleoside, came from Streptomyces sp. (sediment, Nacula Is., Yasawa Is., Fiji) with farneside A modestly active against Plasmodium falciparum (P. falciparum).158
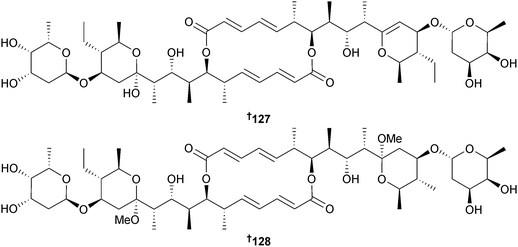
A PCR-based genetic screening experiment targeting the dTDP-glucose-4,6-dehydratase gene was used to identify that a Streptomyces sp. (sediment, Heishijiao Bay, Dalian, China) could potentially produce glycosidic antibiotics. Further investigation of this strain yielded the 6-deoxyhexose-containing antibiotics, 11′,12′-dehydroelaiophylin 127 and 11,11′-O-dimethyl-14′-deethyl-14′-methylelaiophylin 128, of which 127 was an inhibitor of MRSA and vancomycin-resistant Enterococci pathogens. The elaiophylin derivative 128 might be an artefact resulting from methanolysis during the isolation procedure.159
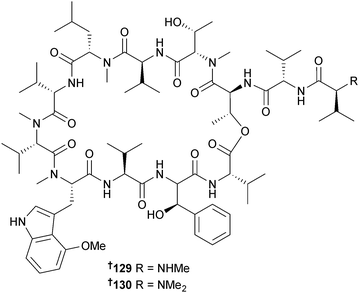
The cyclic peptides ohmyungsamycin A 129 and B 130 were isolated from a Streptomyces sp. (sand, Shinyang Beach, Jeju Is., S. Korea). During determination of configurations a new method to determine the absolute configuration of N,N-dimethylvaline was developed which utilises phenylglycine methyl ester derivatisation coupled with chromatographic analysis and provides a general and convenient method for determination of the configurations of amino acids with fully substituted amine groups. Ohmyungsamycins A 129 and B 130 inhibited growth of several HTCLs and of Gram-positive and Gram-negative bacteria with ohmyungsamycin A 129 being much more potent than B 130.160
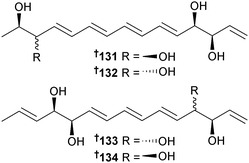
Separacenes A–D 131–134 are polyene polyols obtained from Streptomyces sp. (sediment, Jeju Is., S. Korea). Separacenes A 131 and B 132 are C-3 epimers whilst separacenes C 133 and D 134 are C-12 epimers. Separacene A 131 was a modest inhibitor of Candida albicans (C. albicans) isocitrate lyase and two HTCLs.161
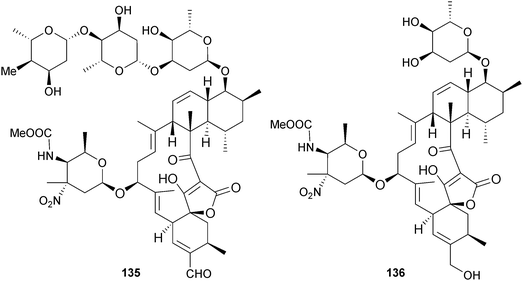
Streptomyces sp. (deep-sea sediment, S. China Sea) was the source of lobophorins H 135 and I 136 of which lobophorin H 135 exhibited significant inhibition of B. subtilis and moderate inhibition of S. aureus while lobophorin I 136 was much less active.162
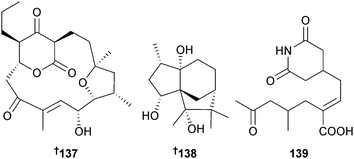
The polycyclic polyketide akaeolide 137 was isolated from a Streptomyces sp. (sediment, Miyazaki Harbour, Japan) as a modest cytotoxin to 3Y1 rat fibroblasts.163 Strepsesquitriol 138, a caged sesquiterpene isolated from Streptomyces sp. (sediment, Bay of Bengal, Indian Ocean), was a moderate inhibitor of lipopolysaccharide-induced TNFα production in RAW264.7 macrophages,164 while cycloheximide acid A 139 was obtained from Streptomyces sp. (seawater, E. China Sea, Wenzhou, Zhejiang Province, China).165
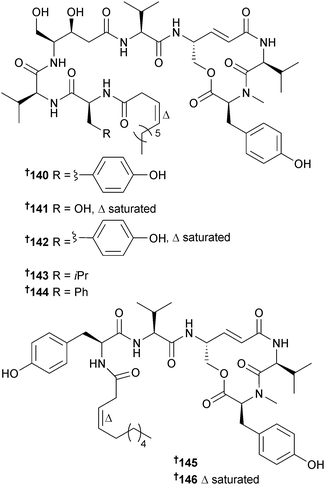
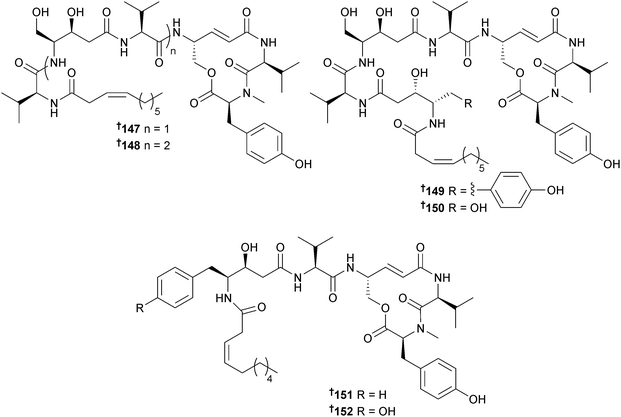
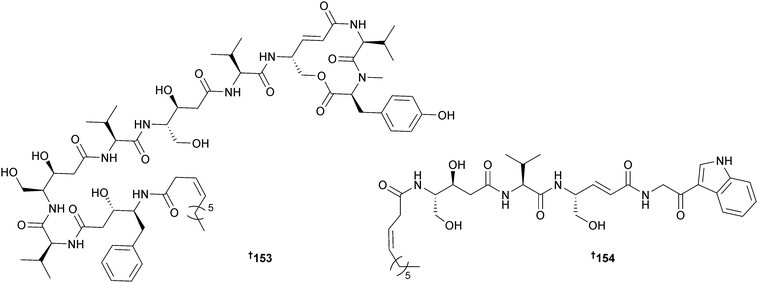
The immunosuppressant cyclic lipopeptides thalassospiramides A and B were originally obtained from the α-proteobacterium Thalassospira sp.166 Reinvestigation of the original producer, a second strain of Thalassospira (source not given), Tistrella mobilis (Red Sea167) and Tistrella bauzanensis (Pacific Ocean167) led to the isolation of fourteen analogues thalassospiramides A1–A5 140–144, C 145 and C1 146, E 147 and E1 148, B1 149 and B2 150, D 151 and D1 152 and thalassospiramide F 153 that have been subdivided into six structural classes with variations in the length and composition of the acyl peptide side chain. The planar structures of 149 and 152 were described in a patent as metabolites of another α-proteobacterium, Oceanospirillum sp.168 and potent inhibitors of the cysteine protease calpain 1. In the current study selected thalassospiramides (A, A1, B, C, D1 and E1) were tested and all displayed potent activity against calpain 1. Biosynthetic gene clusters for all four bacterial strains were characterised revealing some atypical NRPS biochemical features such as intrasynthetase trans A domain activation, module skipping and multimodule iteration which likely yield the structural diversity.169Thalassospira sp. (brown alga Rosenvingea sp., Bahamas) yielded a further member of the thalassospiramide family of peptides, thalassospiramide G 154. The co-isolated thalassospiramides A166 and D169 were moderate inhibitors of NO production in lipopolysaccharide (LPS)-stimulated mouse macrophage RAW 264.7 cells.170
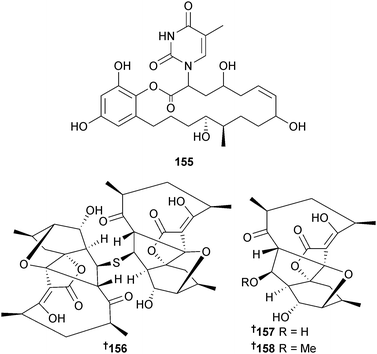
The 18-membered macrolide macplocimine A 155 was obtained from the filamentous sulfur bacterium Thioploca sp. (benthic microbial mat, Chile).171Verrucosispora sp. (deep-sea sediment, S. China Sea) was the source of three further abyssomicin polyketides abyssomicin J–L 156–158. Abyssomicin C172 was also isolated and converted to abyssomicin J 156. In vitro and cell-based analytical studies were then used to show that abyssomicin J 156 can act as a prodrug which, upon oxidative activation, will be selectively transformed to atrop-abyssomicin C,173 an anti-TB antibiotic.174
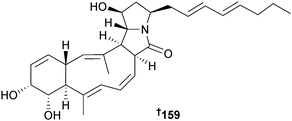
Heronamide A, a polyketide macrolactam originally obtained from an Australian, sediment-derived Streptomyces sp.,175 was reisolated from a Streptomyces sp. (sediment, Uranouchi Bay, Kochi Prefecture, Japan). Detailed NMR analysis of heronamide A and derivatives resulted in configurational reassignment of heronamide A to 159 and the suggestion that the configurations of heronamides B175 and C175 should be reinvestigated.176
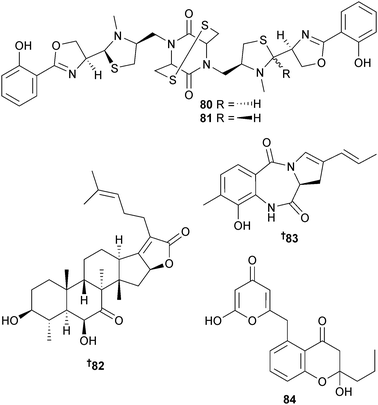
The configuration of the α-methylserine residue in the tetrapeptides JBIR-34 and JBIR-35 and in the trichostatin analogue JBIR-111, originally obtained from a sponge-derived Streptomyces sp.177,178 have been corrected from (R) to (S).179,180Tenacibaculum mesophilum (unidentified sponge, Republic of Palau) yielded a siderophore bisucaberin B. This is an open form of the known macrocyclic dimer bisucaberin181,182 that has been reported as a degradation product of desferrioxamine B183 but not as a product of de novo biosynthesis.184
3.2 Bacteria from mangroves
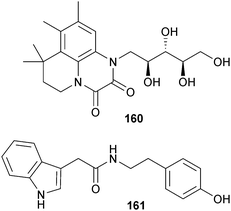
Bacillus hunanensis (sediment, Trinity Bay, Galveston Texas, U.S.A.) yielded hunanamycin A 160, the first NP with a pyrido[1,2,3-de]quinoxaline-2,3-dione core, which also displayed modest inhibition of Salmonella enterica.185 Hunanamycin A was subsequently synthesised via a simple and scaleable method from 6,7-dimethyl-1,4-dihydroquinoxaline-2,3-dione.186 An indole alkaloid 161 was obtained from Pantoea agglomerans (mangrove Ceriops tagal, Zhanjiang, Guangdong, China) along with two phenylethylamine derivatives, 3-(p-hydroxy)benzoyl indole187 and 1,2-di(1H-indol-3-yl)ethane,188 both known synthetic compounds but now isolated for the first time as MNPs.189
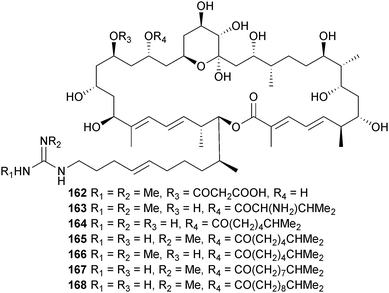
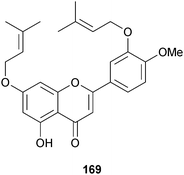
Streptomyces sp. (mangrove rhizosphere soil Heritiera globosa, Wenchang, China)190 was the source of a series of azalomycin F analogues 162–168 which were all broad-spectrum antimicrobials and inhibitors of HCT-116 cells.191 The di-O-prenylated flavone 169 was isolated from an endophytic Streptomyces sp. (mangrove root Myoporum bontioides, Leizhou Peninsula, Guangdong Province, China) and was a moderate inhibitor of the plant pathogenic fungi, Colletotrichum musae, Gibberella zeae and Penicillium citrinum.192
3.3 Marine-sourced fungi (excluding from mangroves)
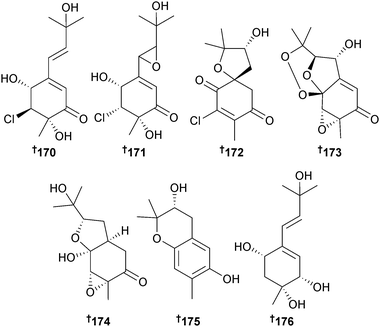
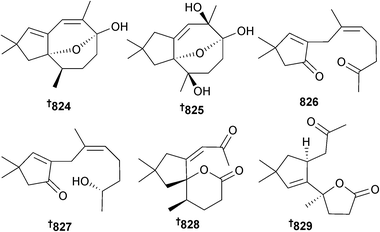
Several acremine metabolites, 5-chloroacremine A 170, 5-chloroacremine H 171 and acremines O–R 172–175, together with the known terrestrial fungal metabolite acremine F,193 were isolated from Acremonium persicinum (sponge Anomoianthella rubra, Gneering Reef, S. E. Queensland, Australia). The configuration of acremine F was determined as 176 and this was the first isolation as an MNP.194
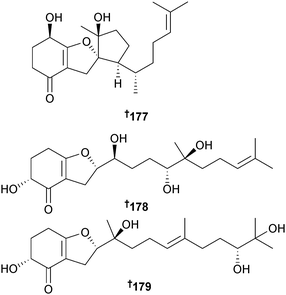
Alternaria sp. (sponge Callyspongia sp., Sanya, Hainan Is., China) was the source of a variety of meroterpenoids including tricycloalternarene A 177, the hydrogenated benzofurans, bicycloalternarene A–D 178–181, the hydrogenated chromans, bicycloalternarene E 182 and F 183, and the hydrogenated cyclopenta-[b]-chromans, tricycloalternarene B 184 and C 185. Four additional monocyclic meroterpenoids monocycloalternarene A195186 and monocycloalternarene B–D 187–189 were obtained when the putative precursors sodium 3,4-dihydroxybenzoic acid or shikimic acid were fed to the fungus, reinforcing the proposed shikimate-isoprenoid hybrid biosynthetic pathway. All the metabolites except bicycloalternarenes E 182 and F 183 were weak to moderate inhibitors of NF-κB in RAW264.7 cells.196
The sesquiterpene ascotrichic acid 190 was isolated from Ascotricha sp. (coastal mud, Fenghua County, Zhejiang, China).197 The benzoquinone derivatives aculeatusquinone A–D 191–194 were isolated from Aspergillus aculeatus (sediment, Langqi Is., Fujian, China) and of these aculeatusquinones B and D were moderately cytotoxic to several HTCLs.198
An oxepin-containing alkaloid 195, a quinazolinone-containing alkaloid 196 and a dihydrobenzofuran derivative 197 were obtained from A. carneus (brown alga Laminaria sachalinensis, Kunachir Is., Russia).199 Clavatustides A 198 and B 199, cyclodepsipeptides with an unusual anthranilic acid dimer and a D-phenyllactic acid residue, were isolated from A. clavatus (hydrothermal vent crab Xenograpsus testudinatus, Kueishantao, Taiwan) and suppressed proliferation of HTCLs.200
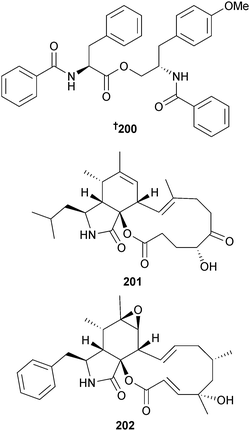
A. elegans (soft coral Sarcophyton sp., Weizhou coral reef, S. China Sea) produced the phenylalanine derivative 4′-methoxyasperphenamate 200 and the cytochalasins aspochalasin A1 201 and cytochalasin Z24 202, in addition to a number of known cytochalasin analogues. 4′-Methoxyasperphenamate 200 was modestly active against Staphylococcus epidermidis while the known cytochalasins aspochalasin I,201 J,201 D202,203 and H204 displayed strong antifouling activity against larval settlement of the barnacle Balanus amphitrite (B. amphitrite). Aspochalasins I, J and H, previously isolated from terrestrial Aspergillus species, are first time MNPs.205
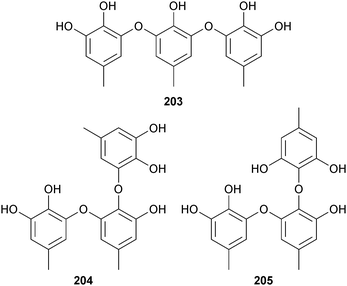
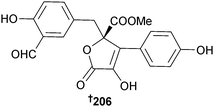
Of the tris-pyrogallol ethers sydowiol A–C 203–205 from A. sydowii (sediment, E. China Sea), sydowiols A 203 and C 205 inhibited M. tuberculosis protein tyrosine phosphatase A (PTPA).206A. terreus, var. boedijnii (Blochwitz) (red alga Laurencia ceylanica, Arugam Bay, Sri Lanka) produced a new butyrolactone 206 which was a strong inhibitor of the enzyme β-glucuronidase.
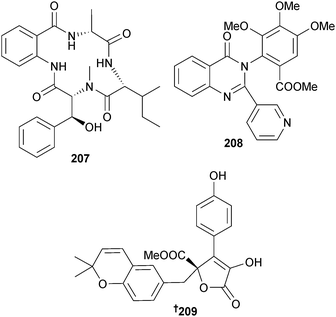
A number of known compounds were also isolated which included (+)-asterrelenin,207 a moderate inhibitor of β-glucuronidase, (3R,4R)-6,7-dimethoxy-4-hydroxymellin208 and (+)-territonin A,207 all reported as first time MNPs.209 The cyclic tetrapeptide asperterrestide A 207, the alkaloid terremide C 208 and an aromatic butenolide aspernolide E 209 were obtained from A. terreus (gorgonian Echinogorgia aurantiaca, Sanya, Hainan Province, China). Asperterrestide A 207 inhibited influenza virus strains H1N1 and H3N2 and was cytotoxic to HTCLs.210
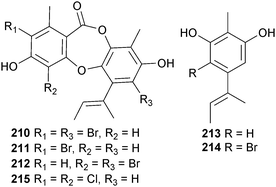
Cultivation of A. unguis (unidentified sponge, Tub-La-Mu Bay, Pang-nga Province, Thailand) in media containing different halogen salts led to the production of “unnatural natural” depsidones. Growth in media containing KBr produced the brominated depsidones aspergillusidone D–F 210–212 and the orcinol derivatives aspergillusidone A 213 and B 214, whilst culture in KI produced another new depsidone 2,4-dichlorounguinol 215. Of these, aspergillusidones D–F 210–212 inhibited aromatase, a therapeutic target for breast cancer treatment.211
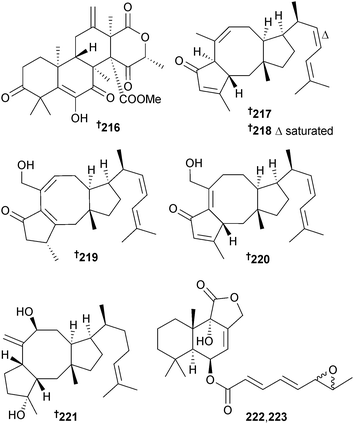
A large number of terpenes were sourced from A. ustus (green alga Codium fragile, Zhoushan Is., Zhejiang Province, China) and included the meroterpene 1,2-dihydroterretonin F 216, the sesterterpenes (6α)-21-deoxyophiobolin G 217, (6α)-16,17-dihydro-21-deoxyophiobolin G 218, ophiobolins U–W 219–221 and the diasteroisomeric sesquiterpenes, (6-strobilactone-B) esters of (E,E)-6,7-epoxy-2,4-octadienoic acids 222 and 223 as new compounds. Ophiobolin F212 was obtained from the marine environment for the first time. Ophiobolin U 219 and the co-isolated known (5α,6α)-ophiobolin H213 moderately inhibited growth of E. coli.214
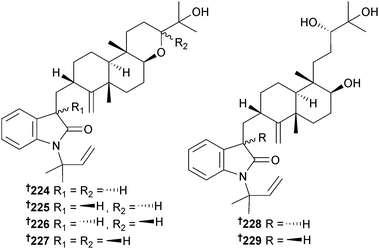
Anthcolorins A–F 224–229, tetrahydropyran diterpene metabolites containing an oxoindoline moiety were isolated from A. versicolor (sea urchin Anthocidaris crassispana, Tanabe Bay, Wakayama, Japan), as three sets of epimeric pairs with moderate growth inhibition (P388) noted for anthcolorins B–D 225–227.215,216
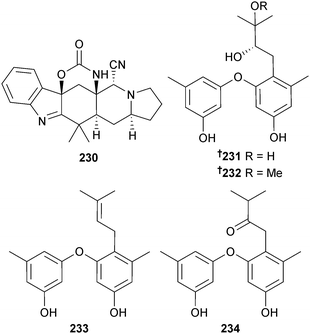
Aspeverin 230 isolated from A. versicolor (green alga Codium fragile, Dalian, China) was a moderate growth inhibitor of the phytoplankton Heterosima akashiwo.217 Four prenylated diphenyl ethers diorcinol B–E 231–234 were obtained from A. versicolor (mud, Yellow Sea),218 of which two, diorcinols D and E were toxic to HTCLs.219
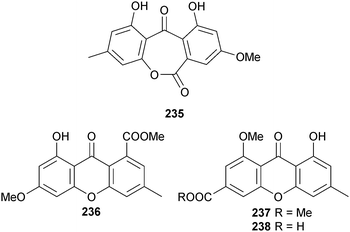
Endophytic A. wentii (brown alga Sargassum sp., no location specified) produced wentiquinone A 235, along with another secoanthraquinone derivative which was claimed as new and named wentiquinone B. A compound of this structure had already been isolated as guepinone from the terrestrial fungus Pestalotiopsis guepinii,220 but this was the first isolation from the marine environment.221 The xanthone derivatives yicathin A–C 236–238 were isolated from endophytic A. wentii (red alga Gymnogongrus flabelliformis, Pingtan Is., China). Yicathins B and C had antimicrobial activities.222
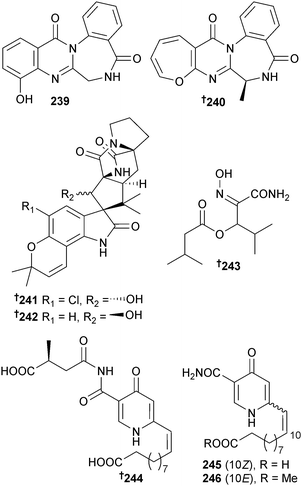
A. westerdijkiae (deep-sea sediment, S. China Sea) was the source of the benzodiazepine alkaloids circumdatin K 239 and L 240, the prenylated indole alkaloids 5-chlorosclerotiamide 241 and 10-epi-sclerotiamide 242 and the amide aspergilliamide B 243 (ref. 223) whilst Aspergillus sp. (mussel Mytilus edulis, Toyama Bay, Japan Sea)224 produced himeic acids E–G 244–246.225
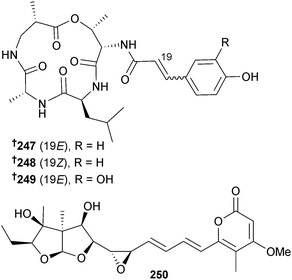
The cyclic tetrapeptides aspergillipeptide A–C 247–249 and asteltoxin B 250 were isolated from Aspergillus sp. (gorgonian Melitodes squamata, Sanya, Hainan province, China) with aspergillipeptide C 249 showing strong antifouling activity against Bugula neritina (B. neritina) larvae settlement.226
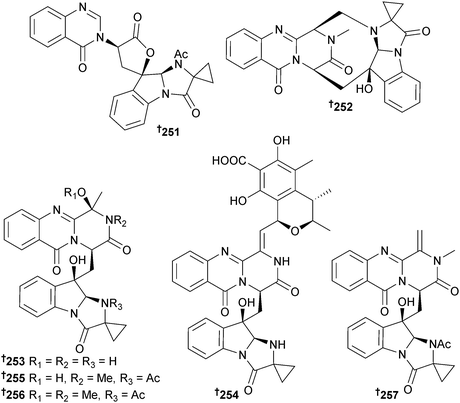
Aspergillus sp. (sponge Tethya aurantium, Limski canal, N. Adriatic Sea, Croatia) produced seven new alkaloids, tryptoquivaline K 251 and fumiquinazolines K–P 252–257, the latter group containing the rare 1-aminocyclopropane-1-carboxylic acid residue.227
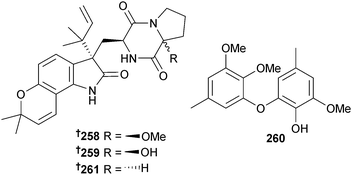
The prenylated indole alkaloids 17-epi-notoamide Q 258 and M 259 and the phenyl ether derivative cordyol D 260 were obtained from Aspergillus sp. (gorgonian Dichotella gemmacea, Xisha Is., S. China Sea). A further phenyl ether was isolated and claimed as new but had already been reported from the mangrove-associated fungus Penicillium expansum.228 The synthetic compound dehydronotoamide C229 was obtained for the first time as an NP and the fungal metabolite notoamide C230 was also reisolated and the absolute configuration previously proposed231 for this metabolite proven as 261.232 As a consequence the configurations of the Aspergillus-derived notoamides J,233 Q234 and M,235 have been corrected from (3S) to (3R) for notoamide J235,236 and from (3R) to (3S) for notoamides Q and M.237,238
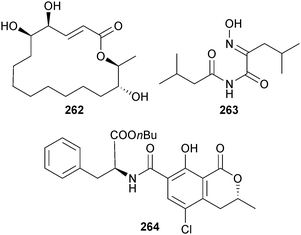
Aspergillide D 262, a 16-membered macrolide, was isolated from Aspergillus sp. (gorgonian Melitodes squamata, Sanya, Hainan Province, China).239 Co-isolated were two known sesquiterpenoid nitrobenzoyl esters, 9α,14-dihydroxy-6β-p-nitrobenzoylcinnamolide240 and 7α,14-dihydroxy-6β-p-nitrobenzoylconfertifolin,240 moderate inhibitors of H1N1.239 Two aspergillic acid group toxins aspergilliamide 263 and ochratoxin A butyl ester 264 were obtained from Aspergillus sp. (gorgonian Melitodes squamata, Sanya, Hainan Province, China), both modestly toxic to brine shrimp (Artemia salina). Co-isolated was the known neoaspergillic acid241 and, surprisingly, the aluminium and zirconium salts of the acid.242
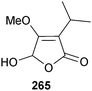
A racemic mixture of a γ-lactone derivative 265 was isolated from Aspergillus sp. (gorgonian Melitodes squamata, Sanya, Hainan Province, China) with significant toxicity to brine shrimp.243 A lactam derivative was also obtained and the structure proposed as a dehydrated pyrrolyl 1-isoquinoline alkaloid,243 a structure originally proposed for marinamide, but which was subsequently revised to that of the dehydrated quinoline alkaloid penicinoline (in section 3.4 below this same problem is addressed with respect to two unidentified microorganisms grown in co-culture).244,245 While it might be possible that these two compounds have very similar NMR data, X-ray crystallography of this new marinamide is required to resolve the doubt.
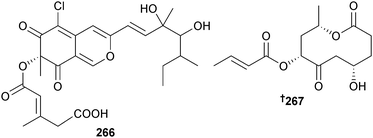
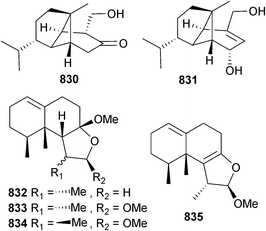
Bartalinia robillardoides (sponge Tethya aurantium, Limsky Channel, Croatia) was the source of the chloroazaphilone helicusin E 266 and the pentaketide bartanolide 267. Isochromophilones X246 and XI246 were also isolated and claimed as new but are known terrestrial fungal metabolites. Isochromophilone XI,246 along with other known fungal metabolites helicusin A247 and deacetylsclerotiorin,248 had a variety of moderate to weak antimicrobial activities.249
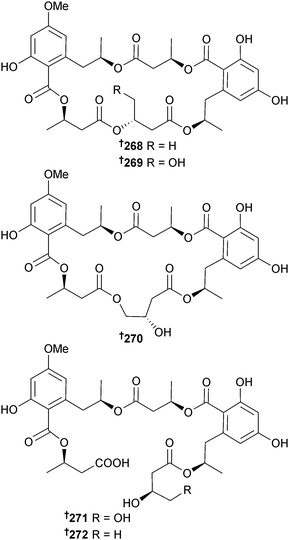
Calcarisporium sp. (seawater, Wadden Sea, Germany) generated macrocyclic and linear polyesters including calcarides A–E 268–272, out of which calcarides A–C 268–270 and the co-isolated analogues 15G256α and 15G256β, previously obtained from the marine fungus Hypoxylon oceanicum,250 inhibited growth of Staphylococcus epidermidis and Xanthomonas campestris while the linear ester 15G256π inhibited growth of Propionibacterium acnes.251
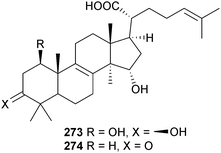
Two lanostanes 273 and 274, with the latter previously reported in the patent literature as a metabolite of the mushroom Fomitopsis pinicola,252 were obtained from endophytic Ceriporia lacerate (starfish Acanthaster planci, Hainan Sanya National Coral Reef Reserve, China).253 Although a further lanostane, 3β-acetoxy-15α-hydroxylanosta-8,24-dien-21-oic acid was also claimed as new, it had previously been isolated from a fungal endophyte of a traditional Chinese medicinal plant Huperzia serrata.254
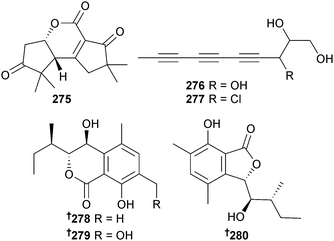
Chondrostereum sp. (soft coral Sarcophyton tortuosum, Hainan Sanya National Coral Reef Reserve, China), previously the source of chondrosterins A–E,255 produced further chondrosterins F–H 275–277. The terrestrial fungal metabolites incarnal256 and arthrosporone,257 and the plant metabolite (2E)-decene-4,6,8-triyn-1-ol,258 were also all isolated for the first time as MNPs.259 The benzolactone metabolites chrysoarticulin A–C 278–280 were isolated from Chrysosporium articulatum, (unidentified dictyoceratid sponge, Gagu-do, S. Korea) with chrysoarticulin C 280 active against the bacterial transpeptidase enzyme sortase A.260
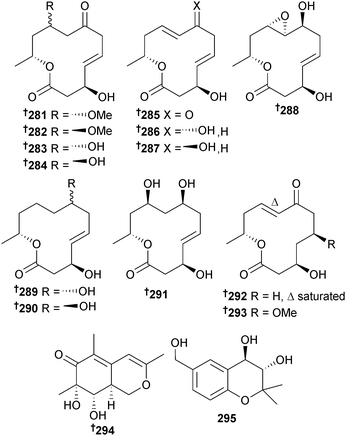
Dendrodochium sp. (sea cucumber Holothuria nobilis, S. China Sea) produced the 12-membered macrolides dendrodolide A–M 281–293 (dendrodolides A–E, G–I, K and L had modest inhibitory activity against two HTCLs),261 while the polyketides 294 and 295 were obtained from Eutypella scoparia (sediment, S. China Sea).262
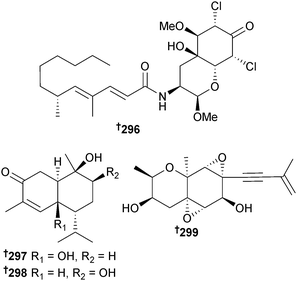
Gymnascella dankaliensis (sponge Halichondria japonica, Osaka Bay, Japan)263 provided dankastatin C 296, a polyketide tyrosine derivative with potent growth inhibition of P388 cells.264Hypocreales sp. (sponge Gelliodes carnosa, S. China Sea) was the source of the cadinane-type sesquiterpenes hypocreaterpene A 297 and B 298. The known terrestrial plant metabolites, (1R,6R,7R,10S)-10-hydroxy-4(5)-cadinen-3-one265 and (R)-5,6-dihydro-6-pentyl-2H-pyran-2-one266 were also isolated for the first time as MNPs and both had moderate anti-inflammatory activity (inhibition of NO production).267 Oxirapentyn E 299, a highly oxidised chromene was isolated from Isaria felina (sediment, Vietnam) as a growth stimulant of corn (Zea mays L.) and barley (Hordeum vulgare L.) rootlets.268
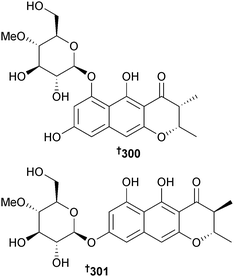
Metarhizium anisopliae (unidentified sponge, Naozhou Is., Guangxi, China) generated two naphtho-γ-pyrone glycosides indigotide G 300 and H 301. The known compounds isochaetochromin B2 (ref. 269) and ustilaginoidin D270 were obtained for the first time from a marine source and displayed modest inhibition of Mycobacterium phlei.271
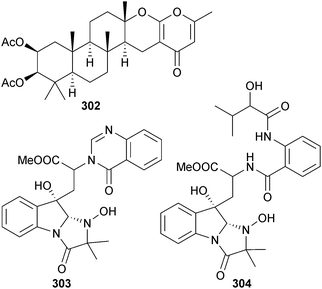
Sartorypyrone B 302, a moderate inhibitor of HTCLs, was obtained from Neosartorya tsunodae (sponge Aka coralliphaga, Similan Is., Phagna Province, Thailand),272 while tryptoquivalines R 303 and S 304 are indole alkaloids obtained from Neosartorya sp. (intertidal mud, Hainan Province, China),273 previously the producer of tryptoquivalines P and Q.274
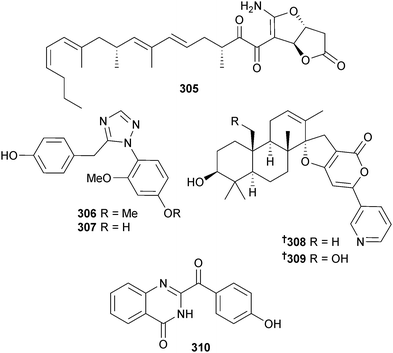
Paecilomyces sp., (unspecified sponge, Tinggi Is., Malaysia) was the source of the dione 305,275 while the chrysotriazoles A 306 and B 307 were obtained from endophytic Penicillium chrysogenum (brown alga Sargassum palladium, Fujian, China).276P. oxalicum (sediment, Bohai Bay, Liaoning Province, China) produced decaturins E 308 and F 309,277 and 2-(4-hydroxybenzoyl) quinazolin-4(3H)-one 310 was isolated from P. oxalicum (strain 0312F1, Genbank accession no. EU926977) as a moderate inhibitor of tobacco mosaic virus (TMV) and the human gastric cancer cell line SGC-7901.278
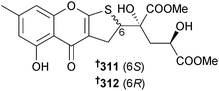
The dihydrothiophene-condensed chromones oxalicumone A 311 and B 312 were obtained from P. oxalicum (gorgonian Muricella flexuosa, Sanya, China) with oxalicumone A 311 moderately cytotoxic to HTCLs.279 A further chromone was also claimed as new and named as oxalicumone C but while isolated from a natural source for the first time, is a known reaction product of chloromonilicin, a metabolite of the cherry rot fungus Monilinia fructicola.280
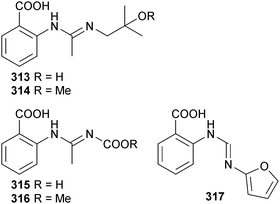
The anthranilic acid derivatives penipacid A–C 313–315, E 316 and G 317 were isolated from P. paneum (sediment, S. China Sea) together with a known analogue, 2-[(1-methyl-2-oxopropylidene)aminobenzoic acid,281 previously synthesised but now isolated as an NP. Penipacids A 313 and E 316 inhibited human colon cancer RKO cells and 2-[(1-methyl-2-oxopropylidene)aminobenzoic acid was cytotoxic to HeLa cells.282
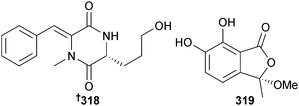
P. pinophilum (sediment, Pearl River Estuary, S. China Sea) yielded pinodiketopiperazine A 318 and 6,7-dihydroxy-3-methoxy-3-methylphthalide 319 and the known synthetic compounds, alternariol 2,4-dimethyl ether283,284 and L-5-oxoproline methyl ester285 as first time NPs. The phthalide 319 displayed potent cytotoxicity to brine shrimp and pinodiketopiperazine A 318, alternariol 2,4-dimethyl ether283,284 and the co-isolated known metabolites N-methylphenyldehydroalanyl-L-proline-anhydride286 and rubralide C287 all exhibited moderate inhibition of E. coli growth.288
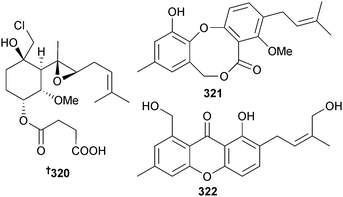
The chlorinated sesquiterpenoid ligerin 320 came from a Penicillium strain (seawater, La Prée, Loire Atlantique, France) and strongly inhibited the osteosarcoma cell line POS1,289 while another Penicillium sp. (sediment, Jiaozhou Bay, China) yielded prenpenicillide 321 and prenxanthone 322.290
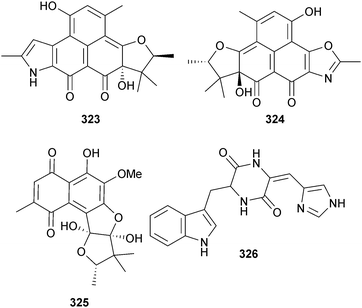
The polyaromatic metabolites herqueiazole 323, herqueioxazole 324 and herqueidiketal 325 were obtained from Penicillium sp. (sediment, Gagu-do, S. Korea). Herqueidiketal 325 was moderately cytotoxic to A549 cells and significantly inhibitory against sortase A.291Penicillium sp. (gorgonian Dichotella gemmacea, Sanya, Hainan Province, China) produced the indolyl diketopiperazine penilloid A 326 in addition to a number of known indole alkaloids.
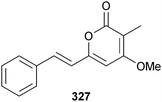
Aspergillus sydowii (gorgonian Verrucella umbraculum, Sanya, Hainan Province, China) also yielded additional known indole alkaloids including fumiquinazoline D,292 cyclotryprostatin B293 and fumiquinazoline G,294 which in addition to (E)-3-(1H-imidazol-4-ylmethylene)-6-(1H-indol-3-ylmethyl)-2,5-piperazinedione,295 meleagrin,296 roquefortine C,297 and 11α-methoxy roquefortine C298 from the Penicillium sp. exhibited significant antifouling activity towards B. amphitrite and/or B. neritina larvae. Meleagrin also exhibited moderate activity against the larvae settlement-inducing bacterium Micrococcus luteus.299 Penstyrylpyrone 327 and the known terrestrial fungal metabolite anhydrofulvic acid (first time MNP)300 were obtained from Penicillium sp. (unidentified sponge. Jeju Is., S. Korea) as inhibitors of protein tyrosine phosphatase 1B (PTP1B) activity. Furthermore, penstyrylpyrone 327 suppressed production of pro-inflammatory mediators via the NF-κB pathway through expression of the anti-inflammatory enzyme, heme oxygenase.301
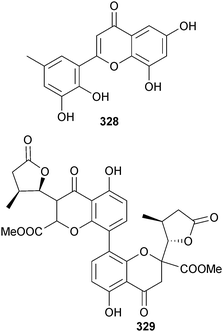
Penicillium sp. (gorgonian coral Dichotella gemmacea, Sanya, Hainan, China) was the source of the polyketides 328 and paecilin C 329, and some known analogues. 6,8,5′,6′-Tetrahydroxy-3′-methylflavone 328, emodin,302 citreorosein302 and isorhodoptilometrin303 exhibited significant antifouling activity against B. amphitrite larvae settlement while penicillixanthone A304 was moderately antibacterial.305
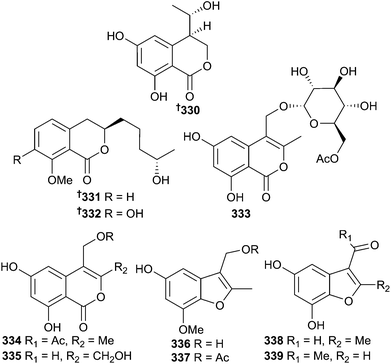
Endophytic Penicillium sp. (unidentified sponge, Weizhou, S. China Sea) was the source of the hydroisocoumarins penicimarin A–C 330–332, the isocoumarins penicimarin D–F 333–335 and the benzofurans penicifuran A–D 336–339, out of which only penicifuran A 336 was cytotoxic to Staphylococcus albus (moderate).306
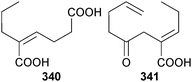
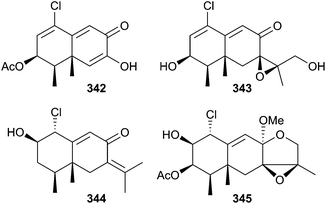
Co-cultivation of Penicillium sp. (sponge Mycale angulosa, Toque-Toque Is., Brazil)307 and Trichoderma sp. (sponge Geodia corticostylifera, same location)307 led to the unusual polyketides, (Z)-2-ethylhex-2-enedioic acid 340 and (E)-4-oxo-2-propylideneoct-7-enoic acid 341.308 A chloro-trinoreremophilane sesquiterpene 342 and three chlorinated eremophilane sesquiterpenes 343–345 were isolated from Penicillium sp. (deep-sea sediment, Prydz Bay, Antarctica). Just 342 was cytotoxic to HTCLs (moderate).309
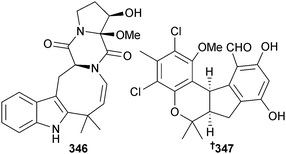
The isoaustamide alkaloid 346 was obtained from Penicillium sp. (unidentified sponge, Jeju Is., S. Korea). Also isolated for the first time as an NP was deoxydihydroisoaustamide, previously reported as an intermediate in the total synthesis of (+)-deoxyisoaustamide.310,311Pestalotiopsis sp. (soft coral Sarcophyton sp., Yongxing Is., S. China Sea) was the source of the chlorinated benzophenone derivative (±)-pestalachloride D 347 (moderate antibacterial activity against several Gram-positive strains). Co-isolated was (±)-pestalachloride C, known as a metabolite of terrestrial endophytic Pestalotiopsis adusta312 but now a first time MNP.313
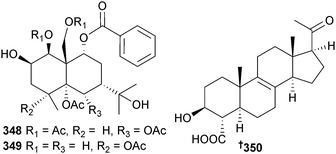
The eudesmane sesquiterpenes 348 and 349 were produced by Pestalotiopsis sp. (brown alga Sargassum horneri, Wenzhou, China) in response to abiotic stress elicitation by addition of CuCl2 to the growth media and both were both potent inhibitors of tyrosinase.314 Endophytic Phaeosphaeria spartinae (red alga Ceramium sp., North Sea, Büsum, Germany) was the source of spartopregnenolone 350.315
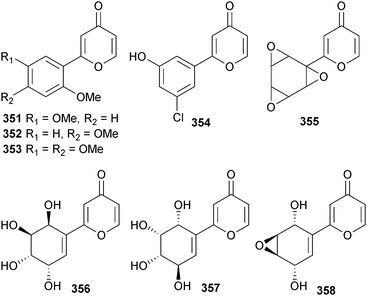
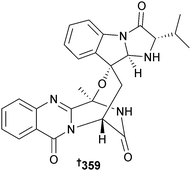
Polyporapyranones A–H 351–358 were isolated from two Polyporales species (seagrass Thalassia hemprichii, location unspecified, presumably Thailand). Polyporapyranones A 351 and D 354 exhibited moderate and weak inhibition of the Vero cell line respectively.316Scopulariopsis sp. (gorgonian Carijoa sp., Weizhou, S. China Sea) was the source of fumiquinazoline L 359, an alkaloid with a heptacyclic skeleton.317
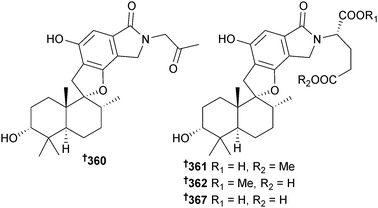
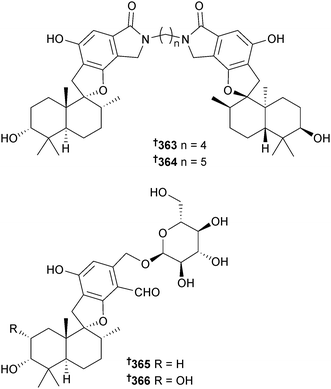
Stachybotrys chartarum (sponge Xestospongia testudinaris, Xisha Is., China) yielded new phenylspirodrimanes stachybotrin D–F 360–362, stachybocin E 363 and F 364 and stachyboside A 365 and B 366, of which stachybotrin D 360 inhibited replication of HIV-1 by targeting reverse transcriptase and blocked non-nucleoside reverse transcriptase inhibitors-resistant strains as well. The absolute configuration of a co-isolated known terrestrial sesquiterpenoid 367 (S. chortarum318) was determined.319
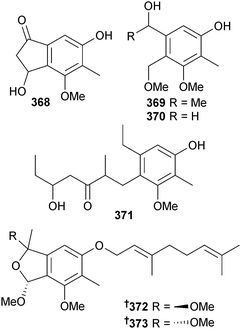
Several polyketides were obtained from Stachylidium sp. (sponge, Callyspongia sp. cf. C. flammea, Bear Is., Sydney, Australia) including cyclomarinone 368, maristachone A–E 369–373 and marilactone.320 Due to rotation values being close to zero, racemic mixtures were assumed for cyclomarinone 368, maristachone A 369 and the epimers 372 and 373. Marilactone320 is a known synthetic compound but now a first time NP. From a biosynthetic perspective, all of the isolated compounds are unusual due to the presence of an additional carbon atom over the basic polyketide skeleton.321
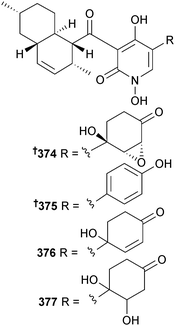
Stagonosporopsis cucurbitacearum (unidentified sponge, Atami-shi, Shizuoka Prefecture, Japan) yielded the alkaloids didymellamides A–D 374–377. Didymellamide A 374 inhibited growth of several pathogenic fungi including azole-resistant C. albicans.322
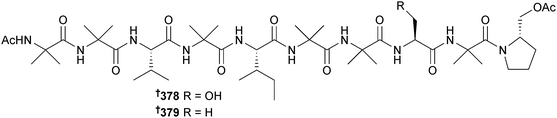
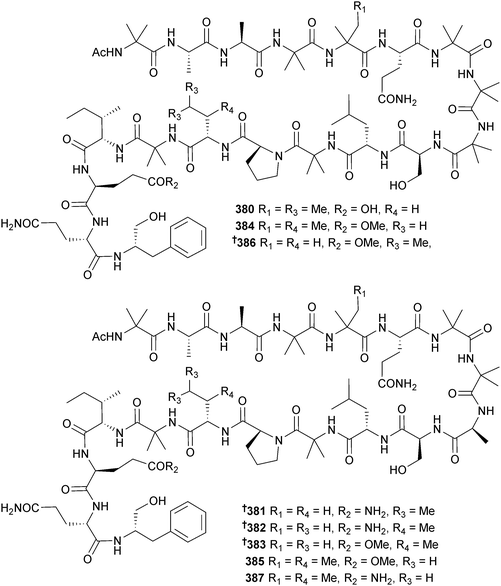
The peptaibols aspereline G 378 and H 379 were obtained from Trichoderma asperellum (sediment, Langqi Is., Fujian, China),323 while asperelines G-Z13 are thirty-two new short peptaibols detected from T. asperellum (sediment, Penguin Is., Antarctica) by ultrahigh pressure liquid chromatography in combination with electrospray-ionisation tandem mass spectrometry (UHPLC-ESIMS/MS).324 Several strains of marine-derived T. atroviride (University of Nantes culture collection) produced two series of 17-residue peptaibiotics with a common C-terminus325 and eight new peptaibols 380–387, trichorzianine 1938, 1909, 1895, 1896, 1924, 1910, 1924A and 1909A, linear 19-residue hydrophobic peptides were obtained from T. atroviride (Axinellid sponge, Akhziv, Mediterranean coast, Israel).326
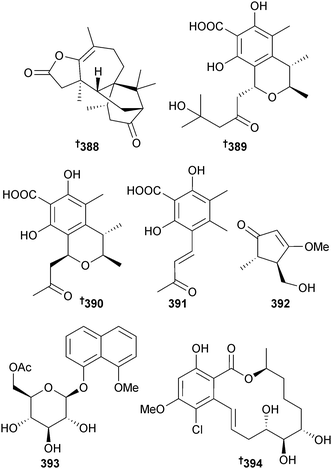
The diterpenoid lactone trichodermaerin 388 was isolated from endophytic T. erinaceum (sea star Acanthaster planci, Hainan Sanya National Coral Reef Reserve, China).327 A Xylariaceae sp. (gorgonian coral Melitodes squamata, S. China Sea) produced a number of polyketides including penicitrinol F 389, 7-carboxypenicitrinol C 390 and 391–393. Several known polyketides were also isolated and of these, dihydrocitrinin328 and phenol acid A328 strongly inhibited settlement of B. neritina larvae with dihydrocitrinin328 also an inhibitor of the enzymes SHP2 and IMPDH. Phenol acid A328 and dihydrocitrinone329 inhibited cathepsin B and (3R,4S)-(+)-4-hydroxy-6-deoxyscytalone330 inhibited the enzymes SHP2, PTP1B and IMPDH and is a first time MNP.331 There is considerable confusion surrounding this report: the name penicitrinol F has been given previously to a citrinin derivative obtained from a Penicllium sp.332 so 389 should be renamed. Also, for 7-carboxypenicitrinol C 390 there is a discrepancy between the configuration in the diagram and in the text. The text gives (1R) but the diagram gives (1S). If the diagram is correct, this is a known compound from both terrestrial333 and marine334,335 fungi. The configuration of cochliomycin C, a resorcylic acid lactone obtained from the gorgonian-derived fungus Cochliobolus lunatus336 has been corrected to 394.337
Addition of sodium bromide to a culture of Aspergillus ochraceus (red alga Chondria crassicualis, Yokji Is., Kyeongnam Province, S. Korea) resulted in medium-induced production of (R)-(−)-5-bromomellein as a modest radical scavenger (against 1,1-diphenyl-2-picrylhydrazyl (DPPH)). Both the racemate338 and antipode339 have been previously synthesised but this is the first report of their isolation as NPs.340 The sesquiterpene helminthosporic acid has been reported previously as a semi-synthetic derivative of the fungal metabolite helminthosporol aldehyde341 but has been isolated for the first time as an NP from Drechslera sp. (green alga Ulva sp., Tönning, North Sea).342 Also as a first time MNP was the terrestrial fungal metabolite epiepoformin343 isolated from an endophytic Penicillium sp. (brown alga Fucus spiralis, Bridge End, Shetland Is., U.K.).344
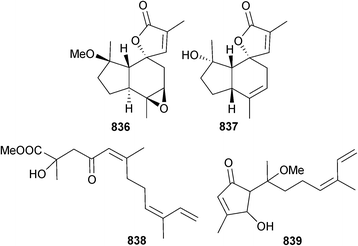
3.4 Fungi from mangroves
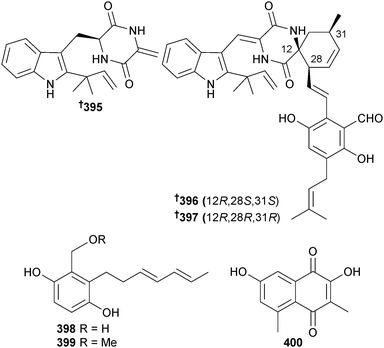
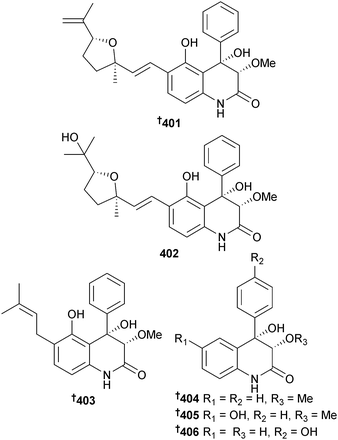
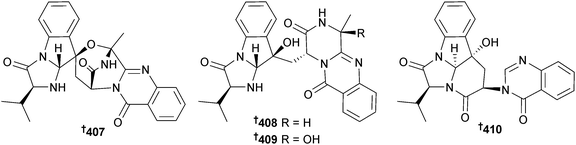
Aspergillus effuses (rhizosphere soil, unidentified mangrove, Fujian Province, China) produced the prenylated indole diketopiperazine alkaloid dihydroneochinulin B 395 and the enantiomeric spiro-polyketide-diketopiperazine hybrids cryptoechinuline D 396 and 397. The latter compound has been isolated previously from terrestrial345 and marine346 fungi but in this study was resolved into enantiomers and absolute configurations assigned.347 The benzyl derivatives aspergentisyl A 398 and aspergentisyl B 399 and a naphthoquinone derivative aspergiodiquinone 400 were isolated from A. glaucus (mangrove sediment, unspecified species, Fujian Province, China). Aspergentisyls A 398 and B 399 were strong radical-scavengers (DPPH).348Some 4-phenyl-3,4-dihydroquinolone derivatives were obtained from A. nidulans (mangrove leaves Rhizophora stylosa, source not given, presumably China), namely aniduquinolone A–C 401–403, 6-deoxyaflaquinolone E 404, isoaflaquinolone E 405 and 14-hydroxyaflaquinolone F 406. Of these, aniduquinolones B 402 and C 403 and the co-isolated aflaquinolone A349 were moderately toxic to brine shrimp. Aflaquinolone A, previously obtained from a terrestrial Aspergillus sp., was obtained for the first time as an MNP.350
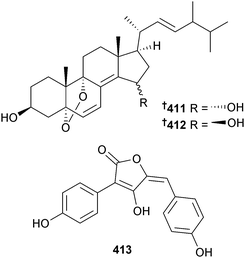
Aniquinazolines A–D 407–410 are quinazolinone alkaloids from the endophytic A. nidulans (mangrove leaves Rhizophora stylosa, unspecified location, presumably China) and were all strongly cytotoxic to brine shrimp.351 The nigerasterols A 411 and B 412 were obtained from endophytic A. niger (mangrove Avicennia marina, Hainan, China) as relatively potent inhibitors of the HTCLs HL60 and A549.352 The butenolide isoaspulvinone E 413 came from A. terreus (mangrove rhizospehere soil, Fujian Province, China) along with the known butenolides aspulvinone E353 and pulvic acid.354 All exhibited significant H1N1 virus inhibition but only isoaspulvinone E inhibited H1N1 viral neuraminidase. Pulvic acid was a first time MNP.355
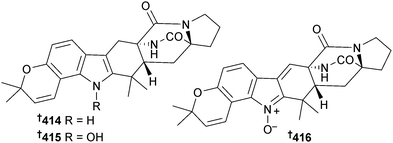
A. taichungensis (mangrove root soil Acrostichum aureum, no location given)356 was the source of the prenylated indole alkaloids 6-epi-stephacidin A 414, N-hydroxy-6-epi-stephacidin A 415 and 6-epi-avrainillanide 416, and of these 415 and 416 were cytotoxic to two HTCLs. On exposure to light and air 415 converted to a complex mixture of analogues, including (+)-versicolamide B,357 a mixture of two compounds (here named versicolamide C) and 416, which suggested that 416 may be an artefact. 6-Epi-stephacidin A 414 was stable under the same conditions.358
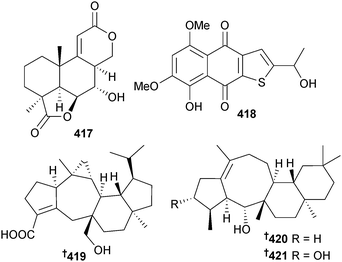
Botryosphaerin F 417 was obtained from endophytic A. terreus (mangrove branch Bruguiera gymnorhiza, Guangxi, China) and inhibited growth of HTCLs.359 Several known compounds were also isolated including LL-Z1271β,360 which although reported as active against the HL60 cell line used here, had previously been reported as being inactive against a number of other HTCLs.361,362 Endophytic A. terreus (mangrove branch Bruguiera gymnorhiza, Guangxi province, China) was the source of a thiophene compound 418. The co-isolated 6-ethyl-5-hydroxy-3,7-dimethoxynaphthoquinone,363 a known synthetic compound, was a first time NP.364 Asperterpenoid A 419, a sesterterpenoid with a new carbon skeleton, was isolated from endophytic Aspergillus sp. (mangrove species not specified, no location given) and displayed inhibitory activity against M. tuberculosis protein tyrosine phosphatase B (mPTPB).365 Asperterpenols A 420 and B 421 are sesterterpenoids with an unusual 5/8/6/6 tetracyclic ring skeleton. Both were acetylcholinesterase inhibitors and were obtained from endophytic Aspergillus sp. (mangrove, S. China Sea).366
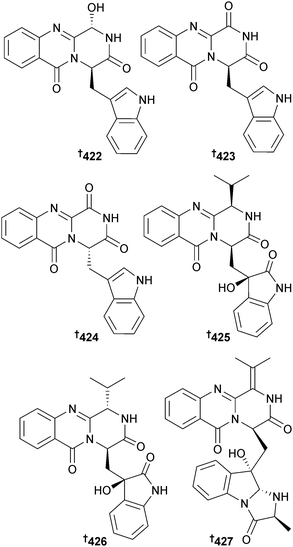
Cladosporium sp. (mangrove soil, Guangzhou, China) was the source of a number of indole alkaloids including five glyantrypine derivatives; 3-hydroxyglyantrypine 422, oxoglyantrypine 423, 424, cladoquinazoline 425 and epi-cladoquinazoline 426 and a pyrazinoquinazoline derivative norquinadoline A 427. Of these alkaloids, oxoglyantrypine 424 and norquinadoline A 427, together with the co-isolated known terrestrial Aspergillus alkaloid metabolites, deoxynortryptoquivaline,367 deoxytryptoquivaline,367 tryptoquivaline368 and quinadoline B369 had significant activities against H1N1. The latter four were also obtained for the first time as MNPs. Over time, a solution of oxoglyantrypine 423 partially converted into 424, leading to the proposal that 424 was an artefact.370
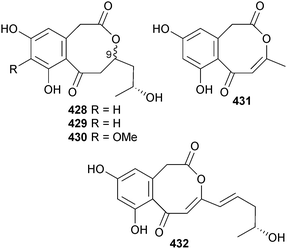
Further investigation of endophytic Corynespora cassiicola (mangrove leaf Laguncularia racemosa, Hainan Is., China), which originally yielded some decalactone derivatives,371 yielded some minor metabolites coryoctalactone A–E 428–432, of which coryoctalactones A 428 and B 429 were assumed to be C-9 epimers.372
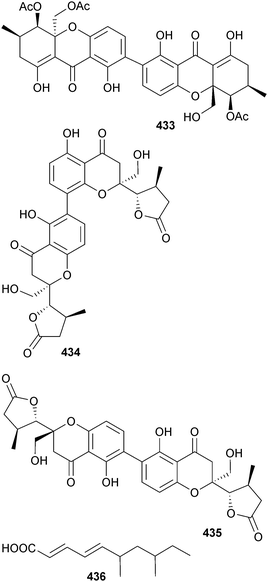
As part of a screening programme for new antimalarial compounds, four metabolites were obtained from several species of Chinese mangrove endophytic fungi from either Mai Po Nature Reserve, Hong Kong or Hainan Is., Taiwan. Despite lack of a tight correlation between location and source microorganism, this study described the isolation of a dimeric tetrahydroxanthone dicerandrol D 433 from a Diaporthe sp., diaporthochromes A 434 and B 435 from another Diaporthe sp. and the lipid 436 was obtained from Xylaria sp. Dicerandrol D 433 exhibited potent activity against P. falciparum with relatively low toxicity to A549 cells.373
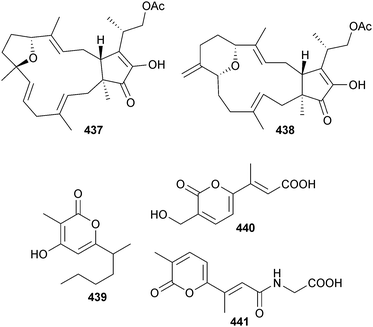
Endophytic Fusarium proliferatum (mangrove Bruguiera sexangula, Hainan Is., China) produced the tricyclic sesterterpenes fusaprolifin A 437 and B 438 and the 2H-pyran-2-one derivatives prolipyrone A–C 439–441. Fusaprolifins A and B had modest activity against brine shrimp.374
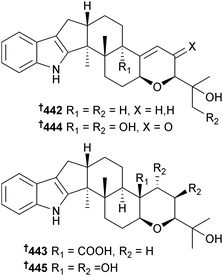
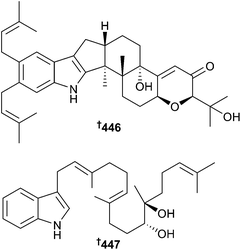
Penicillium camemberti (mangrove soil Rhizophora apiculata, Wenchang, Hainan Province, China) produced the indole diterpenoids 442–447, as well as some known analogues. Of these, emindole SB,375 21-isopentenylpaxilline,376 paspaline,377,378 and paxilline379 displayed significant activity against H1N1 as did indole diterpenoids 442–444, 446 and 447. 21-Isopentenylpaxilline376 and dehydroxypaxilline380 were obtained for the first time as MNPs.381
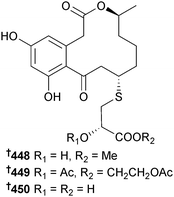
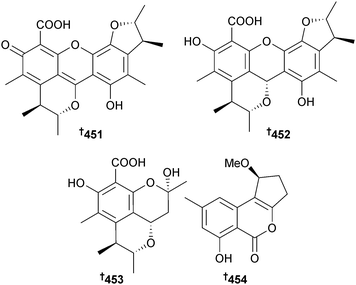
Penicillium sumatrense (mangrove rhizosphere Lumnitzera racemosa, WenChang, Hainan Is., China) yielded sumalarins A–C 448–450, sulfur-containing curvularin derivatives which were cytotoxic to several HTCLs.382 The planar structure of sumalarin C 450 had previously been reported as part of several compound libraries.383–385 The citrinin dimers penicitrinone E 451 and penicitrinol J 452 and the citrinin monomers penicitrinol K 453 and citrinolactone D 454 were isolated from Penicillium sp. (mangrove sediment, Fu Gong, Long Hai, Taiwan Strait, China).386
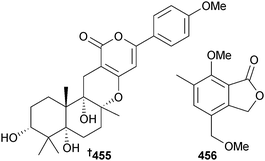
Arisugacin I 455, an α-pyrone meroterpene, was obtained from endophytic Penicillium sp. (mangrove leaves Kandelia candel, Shankou, Guangxi Province, China) as an inhibitor of acetylcholinesterase.387 The known fungal metabolite arisugacin F388 was also obtained for the first time from the marine environment.387 Endophytic Penicillium sp. (mangrove leaves Avicennia sp., Dong Sai, Hainan, China) yielded the isobenzofuranone 456 which was moderately cytotoxic to KB and KBV200 cells.389
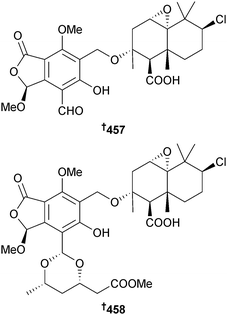
Pestaliopens A 457 and B 458, hybrid sesquiterpene–cyclopaldic acid metabolites with an unusual carbon skeleton, were isolated from endophytic Pestalotiopsis sp. (mangrove leaves Rhizophora mucronata, Hainan Is. China). Pestaliopen A 457 exhibited modest inhibition of E. faecalis.390
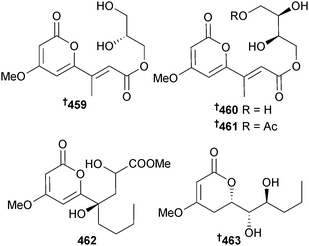
P. virgatula (mangrove leaf Sonneratia caseolaris, Dong Zhai Gang mangrove garden, Hainan Is., China) yielded the α-pyrone derivatives pestalotiopyrone I–L 459–462 as well as (6S,1′S,2′S)-hydroxypestalotin 463,391 a diastereoisomer of a metabolite isolated from a plant associated Penicillium sp.392
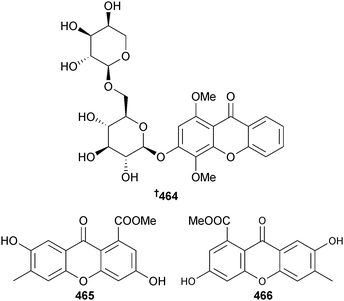
The xanthones O-glycoside 464 isolated from endophytic Phomopsis sp. (mangrove stem Excoecaria agallocha, Dong Zai, Hainan, China)393 and 465 and 466 isolated from Phomopsis sp. (mangrove sediment, Shankou, Hainan, China)394,395 were moderate inhibitors of HEp-2 and HepG2 cells.
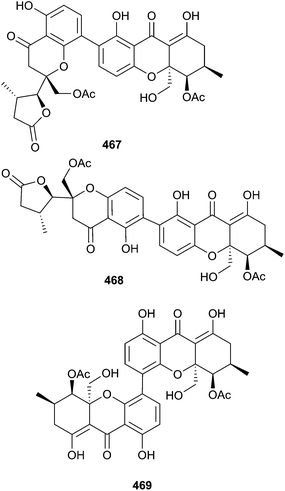
Three new phomoxanthone compounds phomolactonexanthone A 467, B 468 and deacetyl-phomoxanthone C 469 were obtained from Phomopsis sp. (mangrove branch Acanthus ilicifolius, Hainan, S. China Sea) along with five phomoxanthones known as endophytic metabolites of terrestrial fungi, namely dicerandrol A,396 dicerandrol B,396 dicerandrol C,396 deacetylphomoxanthone B397 and penexanthone A,398 all isolated as first time MNPs.399
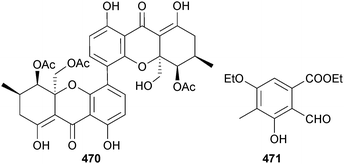
Phomopsis sp. (mangrove plant Rhizhopora mucronata, Muara Angke, Jakarta, Indonesia) was the producer of the dimeric tetrahydroxanthone 12-O-deacetyl-phomoxanthone A 470 which exhibited moderate inhibition of several Gram-positive bacteria.400 A polysubstituted benzaldehyde derivative 471 was isolated from co-culture of two unidentified mangrove fungi (S. China Sea coast).401
Marinamide and the methyl ester, methyl-marinamide were originally isolated from a co-culture of two mangrove endophytic fungi from the S. China Sea Coast and assigned as pyrrolyl 1-isoquinolone alkaloids.402 Subsequently, the fungus Auxarthron reticulatum (sponge Ircinia variabilis) yielded the quinolinone methyl-penicinoline, shown to be identical to methyl-marinamide requiring structural revision.244 The revised structure of marinamide is identical to that of penicinoline, previously obtained from a mangrove endophytic fungus.403 This problem has already been addressed above in Section 3.3. Both marinamide/penicinoline403 and its methyl ester245 displayed potent cytotoxicity to several HTCLs.
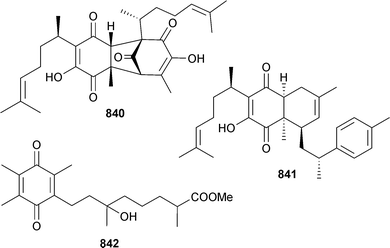
3.5 Cyanobacteria
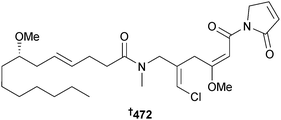
There has been a marked drop in the number of new metabolites reported from cyanobacteria, continuing the downward trend from 2012. The lipopeptide malyngamide 4 472 was isolated from Moorea producens (Red Sea, Jeddah, Saudi Arabia) as a moderate inhibitor of several HTCLs.404
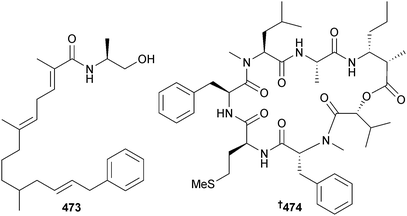
M. producens (La Parguera, Puerto Rico) was the source of the lipopeptides parguerene 473 and precarriebowmide 474. Studies of the stability of precarriebowmide 474 to atmospheric oxygen indicated that carriebowmide405 and carriebowmide sulfone,406 previously isolated from Lyngbya polychroa and Lyngbya majuscula respectively, may in fact be isolation artefacts of precarriebowmide 474.407
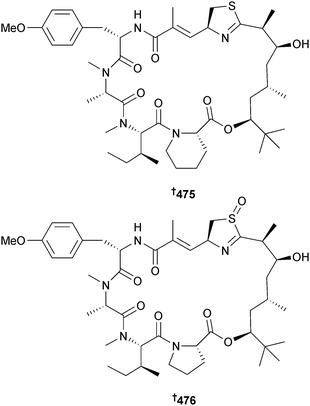
Two new aprataoxin analogues, apratoxin H 475 and apratoxin A sulfoxide 476, were obtained from M. producens, (Nabq Mangroves, Gulf of Aqaba, Red Sea) and both exhibited cytotoxicity to NCI-H460 lung cancer cells, but apratoxin H 475 was much more potent than apratoxin A sulfoxide 476.408
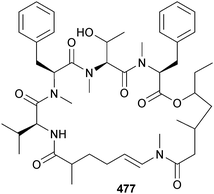
M. bouillonii (New Britain, Papua New Guinea) was the source of bouillonamide 477, a cyclic depsipeptide which contained two unique polyketide-derived moieties, a 2-methyl-6-methylamino-hex-5-enoic acid residue and a unit of 3-methyl-5-hydroxy-heptanoic acid. Bouillonamide 477 displayed moderate toxicity to neuron 2a mouse neuroblastoma cells.409
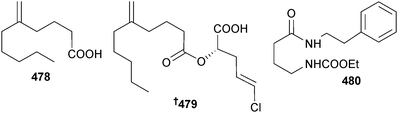
A cyanobacterium of similar morphology to Lyngbya sp. (Piti Bay, Guam) produced the lipids pitinoic acid A 478 and B 479. Pitinoic acid A 478 inhibited quorum sensing in Pseudomonas aeruginosa and pitinoic acid B 479 exhibited anti-inflammatory activity, inhibiting production of pro-inflammatory cytokine expression. Pitinoic acid B 479 has been synthesised.410 A species resembling the genus Symploca (Santa Cruz Is., Coiba National Park, Panama) yielded santacruzamate A 480, a potent and specific inhibitor of histone deacetylase 4 and cytotoxic to several HTCLs. Santacruzamate A 480 was synthesised from γ-aminobutyric acid.411
3.6 Dinoflagellates
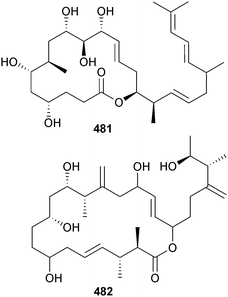
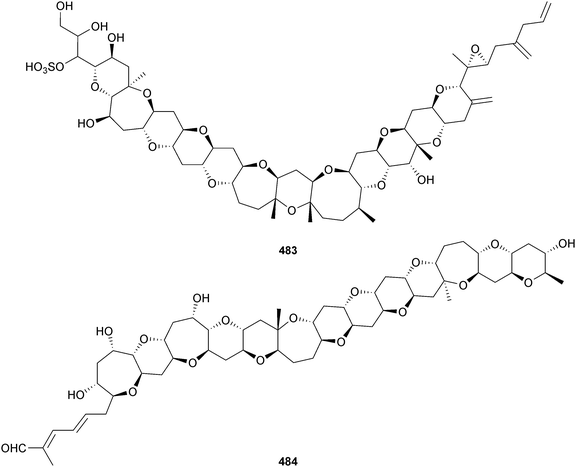
An Amphidinium sp. (sediment, Iriomote Is., Japan) was the producer of iriomoteolides-4a 481 and -5a 482, which displayed moderate cytotoxicity against human B lymphocyte DG-75 cells.412An epoxy polyether with twelve contiguous trans-fused ether rings, gambieroxide 483 was obtained from Gambierdiscus toxicus (Papeete, Tahiti, French Polynesia).413 Gymnocin-A2 484 was isolated from Karenia (formerly Gymnodinium) mikimotoi (Kushimoto Bay, Wakayama, Japan) as a moderate cytotoxin to P388 cells, along with the known synthetic analogue, gymnocin-A carboxylic acid414 (first isolation from a natural source).415
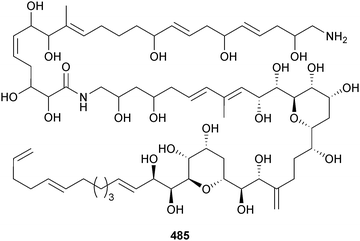
The epiphytic, benthic dinoflagellate Ostreopsis cf. ovata (Jeju Is., S. Korea) was the source of ostreol A 485, significantly cytotoxic to brine shrimp,416 whilst the IK2 strain of O. ovata (Ikei Is., Okinawa, Japan) produced ovatoxins-a, -d and -e, each tentatively assigned by negative fast-atom bombardment collision-induced tandem mass spectrometry (FAB CID MS/MS).417
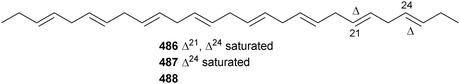
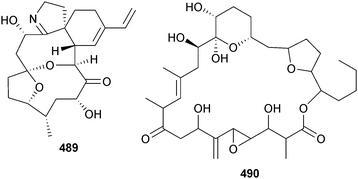
Pyrocystis lunula (University of Texas Culture Collection) yielded three polyunsaturated C27 hydrocarbons; n-heptacosa-3,6,9,12,15,18-hexaene (C27:6) 486, (approx. 0.7 ng per sheathed cell), n-heptacosa-3,6,9,12,15,18,21-heptaene (C27:7) 487 and n-heptacosa-3,6,9,12,15,18,21,24-octaene (C27:8) 488.418 The benthic dinoflagellate Vulcanodinium rugosum (Northland, New Zealand) yielded portimine 489, a polycyclic ether toxin containing a five-membered imine ring, which exhibited potent toxicity to P388 cells, in addition to activation of caspases, as an indication of apoptotic activity.419 The structure of amphidinolide N, the most potent cytotoxic macrolide isolated from Amphidinium sp. to date420 has been revised to 490 (and the relative configuration has been assigned).421
3.7 Microalgae
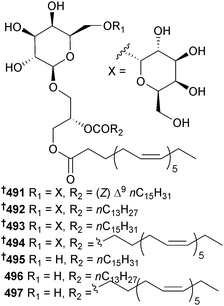
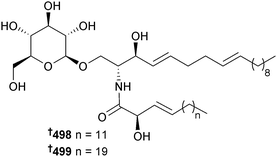
The microalga Nannochloropsis granulata (Provasoli-Guillard National Centre for Culture of Marine Phytoplankton, West Boothbay Harbour, Maine) was the source of the digalactosyldiacylglycerols 491, 492 and the known 493 (ref. 422) and 494,422 whose configurations were determined. Also isolated were the monogalactosyl analogues 495,422496 (ref. 423) (first time as an NP) and 497.423 All of the isolated metabolites exhibited strong NO inhibitory activity against LPS-induced NO production in RAW264.7 macrophage cells suggesting potential as anti-inflammatory agents.424 The green microalga Tetraselmis sp. (National Institute of Technology and Evaluation Biological Resource Centre, Chiba, Japan) was a producer of the glycosylceramides GT1 498 and GT2 499.4253.8 Synthetic aspects
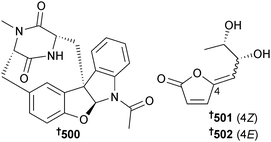
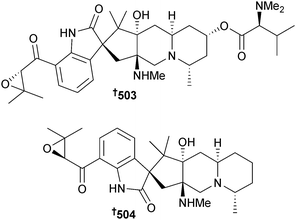
Synthesis of acremolin, originally isolated from sponge-associated Acremonium strictum and assigned as containing a 1H-aziridine moiety426 proved that the alternative structure independently proposed (an isomeric, substituted N2,3-ethenoguanine)427 was indeed correct.428 Total synthesis of ent-(−)-azonazine utilising a hypervalent iodine-mediated biomimetic oxidative cyclisation to construct the core, has resulted in revision of the absolute configuration of natural (+)-azonazine, originally obtained from Hawaiian Aspergillus insulicola429 to 500,430 while syntheses of versicolactones A and B, lactones originally isolated from coral-associated Aspergillus versicolor,431 have resulted in revision of the absolute configurations of the NPs to (4Z,6R,7S)-501 and (4E,6R,7S)-502 respectively.432 It should be noted that the names versicolactones A and B have also been used to refer to unrelated sesquiterpene lactones isolated from the plant Aristolochia versicolor.433,434Citrinadins A and B are pentacyclic alkaloids, originally obtained from a red alga-associated strain of Penicillium citrinum.435,436 An enantioselective total synthesis of (−)-citrinadin A has been achieved in twenty steps from commercially available materials which featured an asymmetric vinylogous Mannich addition of a dienolate to a chiral pyridinium salt to set the initial chiral centre. The synthesis led to revision of the core stereochemistry of the citrinadins and thus of citrinadin A to 503.437 An enantioselective total synthesis of (+)-citrinadin B featuring a stereoselective intermolecular nitrone cycloaddition reaction as a key step, similarly led to revision of the configuration of citrinadin B to (+)-504.438
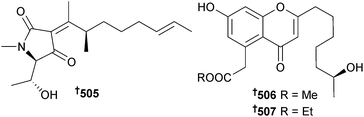
Trichodermatide A, a polyketide isolated from the fungus Trichoderma reesei,439 has been synthesised from L-tartaric acid utilising an intramolecular ketal formation reaction to construct the core of the molecule.440 The total syntheses of the putative structures of (±)-trichodermatides B and C featuring the oxa-[3 + 3] annulation strategy have also been accomplished but mismatch of spectroscopic data between the synthetic and NP samples has indicated that the structural assignments of these metabolites may need revision.441 Syntheses of two diastereoisomers of penicillenol C1, originally obtained from an endophytic, mangrove-associated Penicillium species,442 have led to reassignment of the absolute configuration as 505 (5S,6R,9S).443 The absolute configurations of the endophytic mangrove Pestalotiopsis sp. metabolites, pestalotiopsones D and E444 were determined through total syntheses as 506 and 507 respectively.445
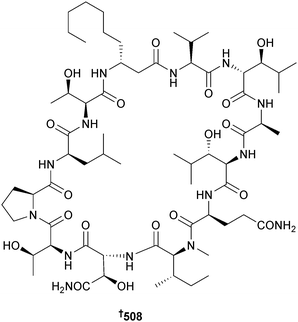
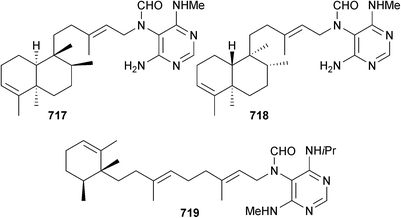
Synthesis of the reported structure of xylopyridine A, a DNA-binding agent originally obtained from a mangrove-associated Xylaria sp.,446 has indicated that the reported structure is incorrect and requires revision.447 Total synthesis of laxaphycin B, a metabolite of terrestrial Anabaena laxa448 and of marine L. majuscula,449 was achieved through stepwise automated solid-phase peptide synthesis, which led to revision of configuration to 508. The related L. majuscula metabolite lyngbyacyclamide A450 was also synthesised by a similar procedure.451
The dolastatin 14 analogue, malevamide E, was originally obtained from the cyanobacterium Symploca laete-viridis and the stereochemistry of the peptidic portion assigned.452 Convergent synthesis of (29S,37S)-malevamide E involving Julia-Kocienski olefination, Urpi acetal aldol and Shiina macrolactonisation reactions has been achieved but a mismatch of the NMR data between the synthetic and natural samples indicated that the originally assigned configurations of some of the amino acids need revision.453 A stereoselective synthesis of the C-43–C-67 fragment of amphidinol 3, originally obtained from the dinoflagellate Amphidinium klebsii,454 revised the originally assigned configuration at C-51 from (R) to (S).455 Ieodomycins A and B are antimicrobial fatty acids originally obtained from a Bacillus sp.456 The first457 of several total syntheses of these published in 2013,458–460 has been achieved in fifteen steps via the chiral pool approach from D-glucose.457 Syntheses of the glycolipopeptides ieodoglucomide A and B, originally obtained from Bacillus licheniformis,461 have been accomplished via a method involving β-glycosylation and Grubbs olefin cross-metathesis as key steps. The syntheses highlighted that the optical rotation values were originally misreported as being of opposite sign to their actual values and the authors of the isolation paper had noted this also.462,463 Syntheses of marinacarbolines A–D, antimalarial β-carboline alkaloids originally obtained from Marinactinospora thermotolerans464 were achieved in four steps from methyl 1-chloro-β-carboline-3-carboxylate465 and the cyclic peptide urukthapelstatin A, originally isolated from the bacterium Mechercharimyces asporophorigenens466,467 has been synthesised via a convergent strategy.468 Trioxacarcin A, a structurally complex glycosidic metabolite of terrestrial469 and marine470Streptomyces species, has been synthesised via a method which utilised late-stage stereoselective glycosylation reactions of aglycon substrates.471 Indoxamycins A, C, and F, cytotoxic tricyclic polypropionates originally obtained from a Streptomyces sp.472 and whose stereochemistry has also been revised as a result of a synthesis of indoxamycin B,473 have been synthesised via a divergent approach with an Ireland-Claisen rearrangement, a stereodivergent reductive 1,6-enyne cyclisation and a tandem 1,2-addition/oxa-Michael/methylenation reaction sequence as key steps.474 Cytosporin D, an epoxyquinone metabolite of Eutypella scoparia,475 has been prepared from the Diels–Alder adduct of cyclopentadiene and 2-prenyl-p-benzoquinone,476 while helicascolide B, a lactone originally obtained from the fungus Helicascus kanaloanus,477 has been synthesised in seven steps from commercially available tiglic aldehyde.478 Leptosin D, originally obtained from a Leptosphaeria sp. associated with a brown alga479 has been synthesised via a strategy which first prepared the known terrestrial480 and marine481 fungal metabolite gliocladine C, which was then manipulated to access various tryptophan-derived epidithiodioxopiperazine NPs.482 Enantioselective total synthesis of (−)-penicipyrone, a polycyclic 4-hydroxy-2-pyrone metabolite of a Penicillium species associated with a Thai sea fan Annella sp.,483 was achieved in twelve steps by a biomimetic bimolecular cascade cyclisation featuring an intermolecular Michael addition/cyclo-(spiro-) ketalisation sequence484 and total syntheses of plectosphaeroic acids A–C (indoleamine 2,3-dioxygenase inhibitors from the fungus Plectosphaerella cucumerina485) have been accomplished.486,487 The quinoline alkaloid, 4,8-dimethyl-6-O-(2′,4′-di-O-methyl-β-D-xylopyranosyl)hydroxyquinoline, originally obtained from a Caribbean collection of Lyngbya majuscula,488 has been synthesised by a method which utilises unusual silyl group migrations489 and synthesis of nhatrangin A, an aplysiatoxin-related metabolite isolated from Vietnamese Lyngbya majuscula,490 has been accomplished and confirmed the absolute configuration originally proposed.491 (+)-Serinolamide A, a cannabinomimetic lipid metabolite of Panamanian Lyngbya majuscula492 has been synthesised from L-serine in nine steps with 30% overall yield493 and total synthesis of viequeamide A, a cyclic depsipeptide metabolite of the Puerto Rican “button” cyanobacterium Rivularia sp.,494 was achieved in ten linear steps based on three retrosynthetic fragments.495 Amphidinolide C, a macrocyclic lactone metabolite of the dinoflagellate Amphidinium sp.,496 has been synthesised through the use of a common intermediate to access both the C-1–C-8 and the C-18–C-25 sections.497
3.9 Assorted bioactivities
The sesterterpenes ophiobolin K,498,499 6-epi-ophiobolin K498,499 and 6-epi-ophiobolin G,499 known metabolites of both terrestrial498 and marine499 fungi were isolated from Emericella variecolor (sediment, Gokasyo Gulf, Mie Prefecture, Japan) as inhibitors of biofilm formation of Mycobacterium smegmatis and of M. bovis BCG at concentrations below those required for antimicrobial activity.500 Toluquinol, a methylhydroquinone known as a metabolite of the soil fungus Nectria erubescens501 was isolated from a Penicillium sp. (Instituto Biomar, León, Spain) as an antiangiogenesis agent, inhibiting the growth of endothelial and tumour cells via apoptosis after a cell cycle block and caspase activation.502 Several known fungal metabolites were isolated as selective inhibitors of PTP1B, a potential target for the treatment of type 2 diabetes and obesity.503Penicillium sp. (sediment, Wan Is., S. Korea) yielded fructigenine A504 and cyclopenol,505Eurotium sp. (sediment, Wan Is., Korea) yielded echinulin506 and flavoglaucin507 and a further Penicillium sp. (unidentified sponge, Jeju Is., S. Korea) was the source of viridicatol.505 Bis-N-norgliovictin, a known terrestrial508 and marine509,510 metabolite was isolated from a marine-derived endophytic fungus (no other details given), as an anti-inflammatory agent that inhibited LPS-induced TNF-α production in RAW264.7 cells.5113.10 Biosynthesis
The gene cluster responsible for the biosynthesis of the glycosylated diazofluorene polyketides lomaiviticins A–E,512,117 originally isolated from Salinispora pacifica (formerly Micromonospora lomaivitiensis), was identified in wild-type Salinispora tropica and several mutant strains through bioactivity-guided genome mining.513 An investigation of 163 strains of actinomycetes isolated from mangrove sediments via homologous screening of the biosynthetic genes and bioassay identified 16% of the strains as possessing the potential to produce halogenated NPs.514 The stephacidin and notoamide families of NPs occur in various Aspergillus species, both terrestrial515 and marine.516 In a further elaboration of the biosynthesis of these metabolites, notoamide T was identified as the likely precursor to stephacidin A and synthesised along with the C-6-epimer, 6-epi-notoamide T. Stephacidin A was chemically converted to notoamide T by reductive ring opening while notoamide T also underwent oxidative conversion to stephacidin A. [13C]2-Notoamide T was synthesised and fed to two Aspergillus strains resulting in significant incorporation into the advanced metabolite notoamide B.517 Analysis of transcriptome data of a number of saxitoxin (STX)-producing dinoflagellates, especially Alexandrium tamarense strains, identified 265 putative homologues of 13 cyanobacterial STX synthesis genes, including all of the genes directly involved in toxin synthesis. Putative homologues of four proteins group closely in phylogenies with cyanobacteria but the phylogenies do not support transfer of these genes directly between toxic cyanobacteria and dinoflagellates, suggesting that the STX synthesis pathway was likely to have been assembled independently in cyanobacteria and dinoflagellates, but using some evolutionarily related proteins.5184 Green algae
Interest in green alga chemistry continued at a low ebb in 2013. Further work on Caulerpa racemosa (Zhanjiang coastline, China), previously the source of caulerpin and two related caulerpin derivatives,519 led to the discovery of two prenylated para-xylenes caulerprenylol A 509 and B 510 that were each weakly antifungal.520Interesting results were uncovered from the screening and careful bioassay-guided analysis of a collection of Floridian marine eukaryotic algae using an ARE-luciferase reporter gene assay that led to the detection and isolation of three mono-unsaturated fatty acids 511–513 from Ulva lactuca as activators of the ARE response. Each contained the identical Δ7,9-keto motif.521
A stereoselective synthesis of the C-8′–O–C-6′′ ether of the antimitotic agent nigricanoside A522 was successfully applied in model systems.523 Included in the green algal literature for 2013 were reports on the cytotoxic effects of clerosterol from Codium fragile524 on HTCLs525 and the spasmolytic effects of caulerpine526 on guinea pig ileum.527
5 Brown algae
The number of new compounds characterised in 2013 from the Ochrophyta was again relatively low and was dominated by terpenoid chemistry. Based on the in vitro cytotoxicity of a crude Dictyota dichotoma (Abu-Bakr, Red Sea, Egypt) extract an investigation was mounted and three new diterpenoids (Z)-pachydictyol B 514, (E)-pachydictyol B 515 and pachydictyol C 516 were characterised along with the known pachydictyol A528 and several other well-known brown algal metabolites.529Re-investigation of Dilophus spiralis (Elafonissos Is., Greece) resulted in the isolation of three new dolastanes 517–519 and five previously reported perhydroazulenes. The relative configurations were established for all three dolastanes and the absolute configuration of 518 established by conversion to a compound of known absolute configuration. The absolute configurations of 517 and 519 were assumed on the basis of biogenetic considerations.530
The cytotoxic meronorsesquiterpenoids cystoazorone A 520 and B 521 and meroditerpenoids cystoazorol A 522 and B 523 were isolated from Cystoseira abies-marina (Mosteiros, Sao Miguel Is., Azores)531 while a series of meroditerpenoids cystodione A–F 524–529, all with strong antioxidant properties in the ABTS assay, were isolated from Cystoseira usneoides (Gibraltar Strait).532
The mildly antiproliferative meroditerpenoid zonaquinone acetate 530 was obtained from a Jamaican Stypopodium zonale.533 Other known brown algal metabolites were co-isolated and these included flabellinone,534 not previously identified in S. zonale, stypoldione,535 and sargaol.536 The absolute configuration of 530 was determined by vibrational circular dichroism (VCD) calculations at several levels of theory.533
The synthesis of the core framework of the proposed structure of sargafuran537 was achieved but the 1H and 13C NMR spectral data of the synthetic analogue did not match suggesting that the originally proposed structure of sargafuran is incorrect.538 The data matched better with the known sargachromenol.539 Another total synthesis of (−)-ecklonialactone B,540 as well as the non-natural (+)-9,10-dihydro-ecklonialactone B, was reported.541 In papers covering biological properties of brown algal metabolites, four papers were published on the eckol group of phlorotannins describing anti-inflammatory properties,542,543 induction of apoptosis in carcinoma cells544 and potential as SARS inhibitors.545 Two surveys were published on the antioxidant potential of brown algal extracts which included an excellent summary from species across the phylum as well as the properties of individual brown algal metabolites.546,547 The antiviral properties of sulfoquinovosyldiacylglycerols from Sargassum vulgare (Ilha de Itacuruçá, S.E. Brazil) were evaluated548 and the antiviral activity of Dictyota diterpenes assessed in docking studies against HIV-1 reverse transcriptases.549 A putative inhibitory mechanism for RANK-induced osteoclast formation by sargachromanol G from Sargassum siliquastrum550 has been proposed551 and the antiplatelet and antithrombotic effects of sargahydroquinoic and sargaquinoic acids determined.552 In other reports of biological testing, the strong antimelanogenic properties of an extract from Dictyota coriacea (Jeju Is., S. Korea) were attributed to 1,9-dihydroxycrenulide553 and epiloliolide,554 known compounds.555 Seven known meroditerpenoids were isolated from Sargassum siliquastrum (Jeju Is., S. Korea) and evaluated for cytotoxicity against a range of HTCLs,556 while the cytotoxic sterol (24R)-hydroperoxy-24-vinylcholesterol557 was reported for the first time from Nizamuddinia zanardinii (Oman Sea).558 In a comprehensive study the anticancer effects of fucoxanthin were examined from a mechanistic perspective.559 In another wide-ranging study, 20 green and brown algal extracts from the French coast were evaluated against Trypanosoma brucei rhodesiense (T. b. rhodesiense).560 The Bifurcaria bifurcata extract showed the strongest trypanocidal activity which was tracked to eleganolone.561 The potential of the HPLC/NMR technique for chemical profiling and dereplication was illustrated with the characterisation of nine known compounds from Cystophora torulosa (Pt. Lonsdale, Victoria, Australia).562
6 Red algae
The nine new compounds reported from red algae in 2013 is a marked reduction in the number reported from the previous year (47). The relative configurations of the 30 stereogenic centres in the macrodiolide luminaolide 531 (Hydrolithon reinbodii)563 were assigned from NMR data, although the relationships of the two side chains to the macrolide ring are still to be established.564The structures of laurefurenynes A 532 and B 533 (Laurencia sp.)565 were reassigned following syntheses of 532566 and 533,567 respectively, and density functional theory (DFT) calculations of NMR chemical shift data.567 There is still doubt about the configuration of the closely related elatenyne (L. elata).568 Computational569 and synthetic570 efforts suggested a revised structure. However, recent more extensive NMR and chemical derivatisation studies proposed a further revision 534 but were unable to establish the absolute configuration.571
Various aspects of the configurations of armatols A–F (Chondria armata)572 have now been clarified through the total synthesis of armatol A 535 and hence by analogy to the structures for armatols B–F 536–540.573 This paper also reported the first total synthesis of dioxepandehydrothyrsiferol (Laurencia viridis)574 as the enantiomer.
The chamigrane sesquiterpenes yicterpene A 541 and B 542 were isolated from L. composita (Pingtan Is., China).575 Of the 7 compounds isolated from L. similis (Sepanggar Is., Kota Kinabalu, Sabah), ent-1(10)-aristolen-9β-ol 543 was claimed as an enantiomer of a known compound.576,577 Two bromophenols 544 and 545 with radical scavenging activity were obtained from Symphyocladia latiuscula (Qingdao, Shandong Province, China).578 This same collection of S. latiuscula also provided the weakly antifungal bromophenol sulfoxide 546.579
One new (547) and three known bromophenols isolated from Vertebrata lanosa (Oldervik, Ullsfjorden, Norway) had cellular antioxidant activities, the first time this activity has been reported for this class of compounds.580 The unprecedented polybrominated spiro-trisindole similisine A 548 and its enantiomer similisine B were obtained from Laurencia similis (S. China Sea).581
Synthesis of the two proposed diastereomers of prevezol C (L. obtusa)582 showed that neither is the structure of the NP.583 Parguerenes (L. filiformis)584 were identified as inhibitors of P-glycoprotein (ABCB1) in multidrug resistant human cancer cells.585 Five known bromophenols from a variety of red algae had inhibitory activity against glucose 6-phosphate dehydrogenase, this being the first report of such inhibitors from red algae.586 Analysis of the metabolite compositions of seasonal collections of Graciliaria gracilis (Lesina Lagoon, S. Adriatic Sea, Italy) led to the proposition for using G. gracilis as a multi products source for biotechnological, nutraceutical and pharmaceutical applications.587 Bioactive metabolites isolated from Asparagopsis taxiformis were found to have little potential for therapy services to fish infected with Streptococcus iniae.588
7 Sponges
Even with only 243 new compounds reported in 2013, a significant decrease in relation to previous years (19% and 33% down on 2011 and 2012, respectively),589,2 sponges remain the dominant phylum for the discovery of new marine-derived bioactives (see section 15 Conclusion). The modified sphingoid base halisphingosine B 549 was isolated from Haliclona tubifera (Santa Catarina, Brazil)590 while taurinated fatty acid 550 was isolated from Axinella sp. (Hainan Is., S. China Sea).591The asymmetric total synthesis of the “two-headed” sphingoid base rhizochalin C (Rhizochalina incrustata)592 has been completed.593 An Axinyssa djiferi found attached to mangrove tree roots (Djifer, Senegal) yielded axidjiferosides A–C 551–553, a mixture of which inhibited chloroquine-resistant P. falciparum.594
An acetylated nitrogenous glycolipid 554 was isolated from Plakinastrella clathrata (Gneerings Reef, Queensland, Australia), with the absolute configuration confirmed by synthesis of lipid-chain analogues. The compound was claimed to be a moderate anti-inflammatory by inhibition of PGE2 but no data was provided.595
Mycalol 555 is a glycerol ether isolated from Mycale acerata (Terra Nova Bay, Antarctica). A combination of chiroptical and Mosher's methods were used to assign the absolute configuration of this specific inhibitor of human anaplastic thyroid carcinomas, the most aggressive and currently untreatable thyroid gland malignancies, but inactive against other solid tumours.596 The absolute configuration of topsentolide C2556 (Topsentia sp.)597 was established by total synthesis of four possible diastereomers.598 The moderately antimicrobial fatty acid trimer manzamenone O 557 was isolated from Plakortis sp. (Manzamo, Okinawa).599
Sponges from the genus Petrosia continue to be a rich source of new polyacetylenes. The report of petrosiols A–E 558–562 from Petrosia strongylata (Ishigakijima Is., Okinawa) as inducers of nerve growth factor-like neuronal differentiation in PC12 cells was followed rapidly by reports of the total synthesis and absolute configuration of petrosiol D 560,600,601 and the discovery that 558 inhibits proliferation and migration of platelet derived growth factor-induced vascular smooth muscle cells and hence could be used as a lead for vascular disorders.602
The absolute configuration of the isolated methyl group of miyakosyne A 563 (Petrosia sp.)603 was established by chemical degradation and subsequent esterification with Ohrui's acid,604 thus correcting an earlier tentative assignment made from an analysis by X-ray crystallography of miyakosyne absorbed in a porous metal complex.71,605 A racemic mixture of C20 bisacetylenic alcohols 564 and 565 has been isolated from Callyspongia sp. (Iriomote Is., Okinawa), and separated by chiral HPLC. Total synthesis of both enantiomers and detailed biological evaluation showed 564 was more active than its enantiomer against HeLa and temperature sensitive rat lymphatic endothelial cells, thus defining the 1-yne-3-ol moiety as an essential pharmacophore.606 Petrosiacetylene E 566 (Petrosia sp. Dokdo Is., S. Korea) was a low μM inhibitor of multiple HTCLs.607
Petrosynic acids A–D 567–570 (Petrosia sp., Tutuila, American Samoa) all displayed similar activity versus various HTCLs and non-proliferative human fibroblasts and hence no therapeutic window is available.608
A New Caledonian Niphates sp. was the source of nepheliosyne B 571.609 Examination of Petrosia solida (Amami-Oshima, Japan) yielded petroacetylene 572 that inhibited starfish embryo blastulation.610
Bromoacetylene testafuran A 573 was isolated from Xestospongia testudinaria (Iwo Is., Kagoshima, Japan) along with four other polyacetylenes 574–577, all five of which induced apidogenesis (stimulation of the differentiation of preadipocytes to adipocytes), and hence may act as leads for treatment of cardiovascular disorders.611
An inhibitor of starfish egg maturation, bromotheoynic acid 578, was reported from Theonella swinhoei (Tanegashima, Kagoshima, Japan),612 while two further bromopolyacetylenes 579 and 580 were obtained from Haliclona sp. (Sharm Obhur, Jeddah, Saudi Arabia).613
Phosphoiodyns A 581 and B 582 are iodinated and phosphate containing alkynes from Placospongia sp. (Tong-Young City, S. Korea). Phosphoiodyn B was inactive, but 581 was a potent inhibitor of human peroxisome proliferator-activated receptor delta (hPPARδ) with 200-fold selectivity over other PPARs, and therefore a potent regulator of lipid and glucose metabolism, and potentially a lead for treating type 2 diabetes or metabolic disorders.614,615
Four mono- or di-iodinated polyacetylene acids were isolated from Suberites mammilaris (583 and 584) and S. japonicus (585 and 586) (Gageo Is., S. Korea). Anti-inflammatory bioactivity profiling of the methyl esters indicated that pre-treatment with the S. mammilaris metabolites inhibited nitrite production in LPS-stimulated RAW 267.4 macrophages while the S. japonicus metabolites inhibited NO production in BV2 microglial cells, with each pair being inactive in the other assay.616
A mixed extract from Smenospongia aurea, S. cerebriformis and Verongula rigida (Key Largo, Florida) yielded a linear phenyl alkene 587 with activity against HL-60 cells. Molecular modelling docking studies suggested that 587 had a pharmacophore similar to that of eribulin and hence potential to interfere with microtubule dynamics.617 Dysideolides A 588 and B 589 are methyl-branched lactones from Dysidea cinerea (Lang Co Beach, Vietnam),618 while 12-manadoperoxide B 590, manadoperoxidic acid B 591 and monoester 592 were reported from Plakortis lita (Bunaken Is., Manado, Indonesia). Both 591 and the likely oxidative breakdown product 592 showed potent antitrypanocidal activity against T. b. rhodesiense.619
Six new methylated peroxidic acids 593–598 were isolated from Plakortis simplex (Keomun Is., West Sea, S. Korea). All showed low moderate cytotoxic activity against RAW264.7 cells.620
A chemical ecological study of Discodermia dissoluta held in Santa Marta, Colombia has shown that <40 kg of sponge could be sufficient to sustainably produce 1 g of discodermolide621 over six months for clinical trials.622 A comprehensive study combining computation, chemical derivatisation and NMR studies was used to assign both the relative and absolute configurations of plakilactones G 599 and H 600 from a Fijian Plakinastrella mamillaris.623
Plakortoxides A 601 and B 602, simplextones C 603 and D 604 and plakorsin D 605 were all isolated from Plakortis simplex (Yongxing Is., S. China Sea) although only 603 showed activity.624
A two-sponge association between Plakortis communis and Agelas mauritiana (Mooloolaba, Queensland, Australia) yielded a new peroxy acid 606.625Plakinastrella mamillaris (Fiji Is.) produced plakortides R–U 607–610. Congener 610 was a potent antimalarial agent against chloroquine-resistant P. falciparum. The remaining compounds were less active and none of the compounds were cytotoxic against Vero cells at much higher concentrations.626
Gracilioether K 611 is a Pregnane X-Receptor (PXR) agonist with no activity against the Farnesoid X-Receptor isolated from Plakinastrella mamillaris (Fiji Is.). In silico docking studies suggested a similar binding motif to other gracilioether congeners.627 The sponge Hippospongia lachne (Xisha Is., S. China Sea) provided hippolachnin A 612, a compound with an unprecedented carbon skeleton, that was potently antifungal, but had no activity against three cancer cell lines. The absolute configuration of 612 was determined from comparison of calculated and experimental electronic circular dichroism (ECD) spectra.628
Manzamenones L–N 613–615 were isolated from Plakortis sp. (Manzamo, Okinawa). Manzamenones M and N showed some antimicrobial activity against E. coli, S. aureus and Cryptococcus neoformans (C. neoformans), while manzamenone L (isolated as a racemate) was inactive.629
Callylactam A 616 was isolated from Callyspongia sp. (Hainan Is., China),630 while allos-hemicalyculin 617 was reported from Discodermia calyx (Shikine-Jima Is., Japan). Photo-oxidative cleavage of the oxazole moiety of calyculin A was suggested as a route to the formation of 617.631 The lipopeptide ciliatamide D 618 was found from a dredged Stelletta sp. (170 m, Oshimashinsone seamount, Japan).632 This study also reaffirmed the absolute configuration of ciliatamide A (Aaptos ciliata) as that assigned during the original isolation,633 and subsequently incorrectly reassigned by synthesis.634
The sponge Lithoplocamia lithistoides (Madagascar) produced PM050489 619 and PM060184 620, polyketide amides that differ only in the presence of a chlorine atom. Both are active at sub-nanomolar levels against several cancer cell lines. The gram-scale total syntheses of each compound were also reported. PM060184 620 has undergone a remarkably rapid development from the source sponge collection in 2005 through isolation, characterisation and synthesis in 2006, to the commencement of phase I clinical trials in 2011.635
A detailed study of the terrestrial myxobacterial genera Sorangium and Jahnella has delineated the biosynthesis of the microsclerodermins, unusual peptides isolated from Microscleroderma and Theonella sponges,636,637 hence suggesting the likely microbial origin of these NPs.638 Gombamide A 621, a disulfide linked hexapeptide, was isolated from Clathria gombawuiensis (Gageo-Do, S. Korea).639 Stylissatin A 622, a cyclic heptapeptide from Stylissa massa (Loloata Is., Papua New Guinea), inhibited NO production in LPS-stimulated macrophages,640 while euryjanicins E–G 623–625 are phenylalanine- and proline-rich heptapeptides from Prosuberites laughlini (Puerto Rico).641
Although the structure of the NP is yet to be reported, the proline-rich octapeptide phakellistatin-19 626 has been synthesised. Interestingly, the bioactivity of the natural (GI50 = 440–515 nM vs. three cell lines) and synthetic (not active) versions differ significantly, a puzzling discrepancy that has been noted previously.642,643
The antifungal activity of the theonellamides (Theonella sp.)644 has been linked to their ability to bind to the 3β-OH of sterols in lipid bilayers. This was established using solid state 2H-NMR and surface plasmon resonance spectroscopies.645 Sulfinyltheonellapeptolide 627 and theonellapeptolide If 628 were isolated from Theonella swinhoei (N. Sulawesi, Indonesia), both with similar activities against HepG2 hepatic carcinoma cells.646
The total synthesis of yaku'amide A 629 (Ceratopsion sp.)647 established the configuration of the C-terminal methyl. Altering the configuration of the methyl had no significant effect on bioactivity.648 Asteropsin A 630 (Asteropus sp., Geoje Is., S. Korea) is a cysteine-knot peptide with an unusual N-terminal pyroglutamate residue that enhanced neuronal Ca2+ influx in murine cerebrocortical neuron cells and therefore may be useful for the treatment of topical pain or hypertension.649
The total syntheses of 18-epi-latrunculol A (Negombata magnifica)650 and haliclamide (Haliclona sp.)651 have been achieved, with the latter study determining the absolute configuration 631 of the NP.652,653 Two separate collections of Pachastrissa nux (Koh Tao, Surat Thani Province, and Chumphon Is. National Park, Thailand) yielded the antimalarial trioxazole macrolide kabiramide L 632.654
A comprehensive study using J-based conformational analysis, the universal NMR database and chemical derivatisations, established the absolute configurations of theonezolide A–C 633–635, originally isolated from a Theonella sponge.655–657
Theonella swinhoei (Bunaken Marine Park, Manado, Indonesia) provided isoswinholide B 636 and swinholide K 637. Interestingly 636 was completely inactive while 637 showed significant potency against HepG2 cells consistent with other swinholide congeners.658
The absolute configuration of (−)-dysibetaine CPa 638 (Dysidea herbacea)659 was established by total synthesis, although the current study incorrectly mentions Lendenfeldia chondrodes as the original source.660
The synthesis of nakinadine C (Amphimedon sp.)661 confirmed the absolute structure.662 Synthesis also confirmed the structures of batzellasides A and C (Batzella sp.).663,664 Manzamine A (Haliclona sp.)665 inhibited autophagy, and hence could prevent pancreatic cancer, by uncoupling vacuolar ATPases,666 as well as suppressing hyperlipidaemia and hence atherosclerosis in apoE-deficient mice.667 Zamamiphidin A 639 is a moderately antibacterial (S. aureus) manzamine-type alkaloid isolated along with ircinic acid 2 640 from Amphimedon sp. (Zamami, Okinawa).668
The synthesis of two unstable stereoisomers of 'upenamide (Echinochalina sp.)669 has shown that the putative structure was incorrect, although the constitution of the NP could not be established and given the paucity of remaining compound, structural revision will be difficult.670 The sponge Haliclona sp. (d'Urville Is., New Zealand) yielded dehydrohaliclocyclins C 641 and F 642 but lack of material prevented bioactivity profiling.671Plakortis simplex (Keomun Is., West Sea, S. Korea) provided two regioisomeric alkylpyridinium carboxylates 643 and 644.620 The pyridinium diamine callyimine A 645 was obtained from Callyspongia sp. (Hainan Is., China).630
Synthesis confirmed the structures of amphimedosides A–C (Amphimedon sp.).672,673 Pyrinodemins G–I 646–648 are bis-3-alkylpyridinium alkaloids from Amphimedon sp. (Okinawa), although the exact positioning of the alkyne functionalities is uncertain and hence the compounds are likely a mixture of related congeners.674
High-level DFT calculations helped confirm the unusually deshielded 13C chemical shifts found in trikentramides A–D 649–652, isolated using an NMR-guided approach from Trikentrion flabelliforme (East Point Bommies, Northern Territory, Australia).675 The synthesis of igzamide (Plocamissa igzo)676 was achieved.677 Three 5-hydroxyindole compounds 653–655 were reported from Scalarispongia sp. (Dokdo, S. Korea).678
Hyrtioreticulin F 656 was obtained from Hyrtios reticulatus (N. Sulawesi, Indonesia) and is the likely product of a Pictet–Spengler reaction between tryptophan, alanine and glycine.679 The bis-indole 6′'-debromohamacanthin A (Spongosorites sp.)680 inhibited angiogenesis by suppressing vascular endothelial growth factor VEGFR2-mediated PI3K/ALT/mTOR signalling in human umbilical vascular endothelial cells and mouse embryonic stem cells.681
Hyrtioerectines D–F 657–659 are indolo-β-carboline alkaloids from a Red Sea Hyrtios species, with all three showing antimicrobial and radical scavenging activity.682 Two brominated indolo-carbazoles 660 and 661 were isolated from a deep water Asteropus sp. (offshore from Bimini, Ocean Cay, Bahamas). While catechol 660 showed antimicrobial activity (C. albicans and MRSA), sulfonate 661 was completely inactive.683
Hyrtimomines A–C 662–664 are 5-hydroxyindole alkaloids from Hyrtios sp. (Kerama Is., Okinawa), although only 662 showed activity against tumour cells.684 Hyrtimomines D 665 and E 666 from the same collection are bisindole dimers with some activity against C. albicans, C. neoformans, S. aureus and Trichophyton mentagrophytes.685
The Australian sponge Plakortis lita (Tydeman Reef, Queensland) yielded thiaplakortones A–D 667–670 following HTS of a library of 202![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 983 fractions from 18
983 fractions from 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 453 extracts. All were potent inhibitors of P. falciparum with 667 showing greater than 60-fold selectivity for Plasmodium over human embryonic kidney cells.686 The total syntheses of zyzzyanones A–D (Zyzzya fuliginosa)687,688 have been achieved.689 Atkamine A 671 is a new pyrroloiminoquinone isolated from a deep water Latrunculia sp. (Aleutian Is., Alaska). Olefin metathesis was used to identify the location of the side-chain alkene of this surprisingly inactive metabolite.690
453 extracts. All were potent inhibitors of P. falciparum with 667 showing greater than 60-fold selectivity for Plasmodium over human embryonic kidney cells.686 The total syntheses of zyzzyanones A–D (Zyzzya fuliginosa)687,688 have been achieved.689 Atkamine A 671 is a new pyrroloiminoquinone isolated from a deep water Latrunculia sp. (Aleutian Is., Alaska). Olefin metathesis was used to identify the location of the side-chain alkene of this surprisingly inactive metabolite.690
Of four new aaptamine-derivatives 672–675 (Aaptos suberitoides, Ambon, Indonesia) only 674 showed any activity against murine lymphoma.691 The total synthesis of 2-deoxy-2-aminokealiiquinone (Leucetta chagosensis)692 confirmed the structure of the NP.693
Pulchranins A–C 676–678, isolated from two dredged Monachora pulchra samples (Kuril Is. Chain, Russia), were moderately active inhibitors of the transient receptor potential cationic channel subfamily V (capsaicin receptor), and hence are pain and thermal reception modulators.694,695 The same sponge that yielded pulchranins B and C also yielded monanchomycalin C 679, a modest inhibitor of MDA-MB-231 breast cancer cells.696
Spongiacidin C (Stylissa massa, Indonesia)697 is the first selective inhibitor of USP-7 over other ubiquitin-specific-processing proteases to be isolated from a natural source, and hence is a new lead as an oncological therapeutic.698 Nagelamides U–Z 680–685 are bromopyrrole alkaloids from Agelas sp. (Kerama Is., Okinawa) with a variety of biological activities, especially the inhibition of the growth of C. albicans. Congeners 683 and 684 were isolated as racemates.699,700
Three new bromotyrosine compounds 686–688 were isolated from Aplysina sp. (Ladda Reef, S. China Sea),701 while the structures of ma'edamines A and B (Suberea sp.)702 have been confirmed by synthesis.703
Pseudoceratina verrucosa (Hook Reef Lagoon, Queensland, Australia) yielded pseudoceralidinone A 689 and aplysamine 7 690, with the absolute configurations established by Mosher's method and by total synthesis, respectively. The latter compound inhibited the growth of PC3 prostate adenocarcinoma cells while the former was inactive.704
Eight new bromotyrosine derivatives of the psammaplysin 691–693, ceratinamide 694 and subereamide 695–698 classes were isolated from Suberea sp. (Chuuk, Federated States of Micronesia). Only psammaplysin X 691 and the 19-hydroxy derivative 692 showed activity against six HTCLs.705
Sesquibastadin 699 (Ianthella basta, Ambon, Indonesia) is a trimer of hemibastadin that inhibited a variety of protein kinases from a panel of 24 enzymes, but had no effect on the proliferation of murine lymphoma cells (L5178Y).706
The Verongid sponges Ianthella basta and Aplysina cavernicola were examined for the presence of brominated skeletal components within their organic and siliceous matrices. The conclusions drawn from this work were that the bastadin and aerothionin compounds found are likely of microbial origin and that the known secondary metabolites are not associated with the sponge skeletons. However, a considerable quantity of brominated mass was found within the skeleton and it is possible that this represents tightly bound sponge-derived secondary metabolites with a defensive role.707 Reticulatins A 700 and B 701 are dimethylimidazolium cations isolated from Hyrtios reticulatus (N. Sulawesi, Indonesia). Surprisingly, they differ in absolute configuration of the side chain carbinol.679
Bis-uracil 702 was isolated from Agelas clathrodes (Yongxing Is., S. China Sea).708 A Fasciospongia sp. (Weizhou Is., Guangxi, China) gave the sesquiterpene alkaloid fasciospyrinadine 703,709 while Dysidea avara (Fethiye, Turkey) yielded the merosesquiterpenoid N-methylmelemeleone-A 704.710
Samples of Dactylospongia elegans collected in both Malaysia and Palau contained the related 5,8-di-epi-ilimaquinone 705, 4,5-di-epi-dactylospongiaquinone 706, 8-epi-dactyloquinone 707, 10,17-O-cyano,4,5-di-epi-dactylospongiaquinone 708 and cyclospongiacatechol 709. All five compounds showed antiproliferative effects at high concentrations while 706 and 707 also activated Hypoxia Inducible Factor-1 (HIF-1), with the 1,4-benzoquinone moiety demonstrated as essential for activity.711
The asymmetric total synthesis of strongylin A (Strongylophora hartmani)712 confirmed the absolute configuration,713 while synthesis of dysideavarone A (Dysidea avara)714 confirmed the structure and also provided material to demonstrate the compound's potent antimicrobial activity, especially against Gram-positive bacteria, in particular various Staphylococci spp.715 The bisabolane sesquiterpenoids 3-oxobolene 710 and 1-oxocurcuphenol 711 were isolated from Myrmekioderma sp. (Phi-Phi Is., Thailand) and were potent inhibitors of HT-29 cancer cells.716
Ianthellalactams A 712 and B 713 (Ianthella flabelliformis, Port Philip Heads, Victoria, Australia) did not inhibit Glycine-gated chloride channel receptors (GlyR) like other related glycinal lactams.717 Euryspongins A–C 714–716 (Euryspongia sp., Iriomote Is., Okinawa) have rare six- or eight-membered skeletons with either fused furan or γ-lactone rings. The presence of the C-4 hydroxyl group in all three compounds was thought to totally abrogate activity compared with other active analogues.718
Phorbasin H, originally isolated from Phorbas gukulensis719 but differing from another structure with the same name from Phorbas sp.,720 inhibits the hypha-specific HWP1 and ALS3 mRNAs of C. albicans, preventing the yeast-to-hyphae transition and therefore inhibits virulence of the pathogen.721 Axistatins 1–3 717–719 are pyrimidine diterpenoids from Agelas axifera (Koror, Republic of Palau). All three were low μM inhibitors of various human and murine cancer cell lines, as well as being potent broad-spectrum antibiotics against several Gram-positive and negative bacteria.722
Phenotypic screening using zebra fish as a genome-wide eukaryote assay identified kalihinol F (Acanthella sp.)723 as a copper chelator, resulting in abnormal development as indicated by an undulating notochord, and both pigmentation and neural defects. This study exemplifies the use of zebra fish as a viable chemical genetic tool for assessing bioactives in a complex eukaryotic organism.724 The total syntheses of cyanthiwigins A, C (Epipolasis reiswigi)725 and H (Myrmekioderma styx)726 have been achieved, confirming the absolute structures.727Hamigera tarangaensis (Cape Karikari, North Is., New Zealand) provided hamigerans F–K 720–725, 10-epi-hamigeran K 726, 4-bromohamigeran K 727, hamigeran L 728 and the methyl ester 729, hamigeran A ethyl ester 730 and an unrelated congener of epi-verrucosane 731. All but the latter compound inhibited the growth of HL-60 cells while chemical genetic screening using yeast as a model organism showed that the mode of action of the antifungal hamigeran G 721 was via influence of Golgi function and Golgi vesicle formation.728
Two new isoneoamphilectane diterpenes 732 and 733 were isolated from Svenzea flava (previously described as Pseudoaxinella flava) (Great Inagua Is., Bahamas). The absolute configurations of these compounds were secured by comparison of experimental and calculated VCD data. Both compounds inhibited the growth of Mycobacterium, but had no effect on mammalian cell lines.729 Petronigrione 734 is a cembranoid dimer from Petrosia nigricans (Haivan, Danang, Vietnam) with moderate activity against HTCLs,730 while Phorbas gukhulensis (Gagu-Do Is., S. Korea) yielded the diterpene pseudo-dimers gukulenin C–F 735–738. All four were cytotoxic against K562 and A549 cancer cell lines but none showed any activity against various microbes.731
The norsesterterpene cyclic peroxides 13,14-epoxymuqublin A 739 and the 9,10-epoxy isomer 740 were isolated from Diacarnus erythraeanus (Elfanadir, Hurghada coast, Egypt). Both were low micromolar inhibitors of various HTCLs.732
Collections of the Homoscleromorpha sponge Oscarella balibaloi at two sites near Marseilles (Mediterranean Sea) yielded the glucosidated sesterterpenes balibaloside 741, 6′′-O-acetylbalibaloside 742, 6′′′-O-acetylbalibaloside 743 and 6′′,6′′′-O-diacetylbalibaloside 744. These metabolites are the first glycosidated sesterterpenes reported and although tested in a wide variety of assays, proved to be inactive.733
Hyrtios communis (Northern Reef region, Palau) yielded thorectidaeolide A 745, the 4-acetoxy congener 746, and thorectidaeolides B–E 747–750. Compounds 745–747 inhibited HIF-1 yet did not show any antiproliferative effects against the parent T47D or NDA-MB-231 breast cancer cell lines.734
A Canadian Phorbas sp. (Ansell Point, Howe Sound, British Columbia), provided four distinct sesterterpenes ansellone B 751, phorbadione 752, secoepoxyansellone A 753 and alotaketal C 754. The latter compound showed similar levels of activation of cAMP signalling to the standard probe, forskolin.735
Five new sesterterpenes were reported from three different Psammocinia sp. (various locations in New South Wales and Victoria, Australia). Ircinianin lactam A 755, the sulfate derivative 756, oxoircinianin 757, oxoircinianin lactam A 758 and ircinianin lactone A 759 were all assessed for GlyR modulating activity with 755 and 758 being selective and potent potentiators of α3-GlyR and α1-GlyR, respectively, having potential as leads for treatment of inflammatory pain, epilepsy and both breathing or movement disorders.736
Phorbaketals D–K 760–767 and phorbin A 768 were reported from Monachora sp. (Gageo Is., Korea), with 764 and 765 weakly active against A498 cancer cells. The absolute configurations of all the new compounds were established by Mosher's method and comparison of CD-curves with known congeners.737
The scalarane sesterterpenoid hippospongide C 769 (Hippospongia sp., Tai-Tung, Taiwan) had moderate activity against four HTCLs,738 while 12-deacetoxy-23-hydroxyscalardial 770, 12-deacetoxy-23-hydroxyhyrtiolide 771 and 12-O-acetyl-16-deacetoxy-23-acetoxyscalarafuran 772 from Psammocinia sp. (S. Korea) were inactive in all assays used.739
Four new scalaranes 773–776 were reported from Carteriospongia sp. (Nosy Be, Madagascar) with 773 and 774 being significantly more active than the other two congeners, indicating the importance of the aldehyde pharmacophore.740
Cholic acid-3,7-diacetate 777 was isolated as an MNP for the first time from Siphonochalina fortis (Bahia Bustamante, Chubat, Argentina),741 while the 5α,8α-epidioxy sterol 3-acetylaxinysterol 778 was isolated from Axinyssa sp. (Pingtung, Taiwan).742Haliclona crassiloba (Dongshan Is., Guangdong, China) yielded halicrasterols A–D 779–782 with moderate activity against various microbial pathogens.743
Cyclopropanated sterols aragusterol I 783, 21-O-octadecanoyl-xestokerol A 784 and 7β-hydroxypetrosterol 785 were isolated from Xestospongia testudinaria (Truong Sa Archipelago, Khanh Hoa, Vietnam). Both 783 and 784 had antifouling potential (growth inhibition of Pseudoalteromonas and Polaribacter bacterial species) at similar levels of activity to the now-banned antifoulant marine pollutant tributyltin oxide.744
The structure of the unusual autophagy-modulating aminosterol clionamine B (Cliona celata)745 was confirmed by synthesis, which also confirmed the assumed absolute configuration.746 A Corticium sp. (New Britain, Papua New Guinea) yielded the steroidal alkaloid plakinamine M 786, which displayed antitubercular activity.747 Stellettin N 787 is an isomalabaricane triterpene acid from Stelletta sp. (Lingshui Bay, Hainan, China), which along with the other isolated congeners presented a chemotaxonomic link between three genera within the sponge order Astrophorida.748
A dredged Penares sp. (Vietnam) provided six new lanosterol congeners 788–793. Only 793 showed any significant activity against HL-60 cells. A combination of CD and X-ray data allowed the assignment of absolute configuration for 789 and also permitted reassignment of the aglycone of eryloside U 794 from 7α,8α to 7β,8β.749,750
Finally, ulososide F 795, urabosides A 796 and B 797 are triterpene saponins from Ectyoplasia ferox (Caribbean Sea, Colombia), with 797 being the first reported compound with both C-4 methyls elevated to the carboxylic acid oxidation state.751
8 Cnidarians
The number of new compounds reported from cnidarians in 2013 (281) has increased by 38% over the average for each of the previous 10 years. In addition to an epidioxysterol (see later), three ceramides 798–800 were isolated from Sinularia candidula (Safaga, Egyptian Red Sea).752 Of the three ceramides, 798 was the most potent anti-H5N1 virus agent.Pyrimidinedione 801 was reported from Verrucella umbraculum (Hainan Is., S. China Sea),753 while Mediterranean specimens of the scleractinian coral Astroides calycularis afforded the new aplysinopsin analogue 802.754 The highly strained cyclo-1,3-carbazole structure originally proposed for antipathine A (Antipathes dichotoma)755,756 has been corrected to the 2,3-carbazole 803 by total synthesis.757 Polyacetylenic montiporic acid D 804 (Montipora digitata, Sesoko Is., Okinawa, Japan) exhibited only mild antibacterial and antioxidant properties.758
New clovane-type sesquiterpenes rumphellclovane C–E 805–807 and four unnamed variants 808–811 were reported from the same collection of Rumphella antipathies (Southern Taiwan).759,760 The latter four compounds are reported as NPs for the first time. Clovane 808 inhibited superoxide generation and elastase release by stimulated human neutrophils.
Sesquiterpenes capillosanane A–N 812–825 and seco-variants capillosanane O–R 826–829 were isolated from Sinularia capillosa (Sanya Bay, Hainan Province, China).761 Absolute configurations were established by combinations of chemical conversions, Mosher's method, CD analysis and biogenetic reasoning. Capillosanane A exhibited antifouling activity against B. amphitrite.
Further examples of tricyclic sesquiterpenes were reported from Lemnalia philippinensis (philippinlins A 830 and B 831) collected at Lanyu, Taiwan762 and Paralemnalia thyrsoides (parathyrsoidins A–D 832–835) collected at Sansiantai, Taitong County also in Taiwan.763
Spiro-butenolides sinularianins C 836 and D 837 and potential biosynthetically-related precursors sinularianins E 838 and F 839 were isolated as mild inhibitors of NF-κB activation from Sinularia sp. (Dongluo Is., Hainan Province, China).764
Perezoperezone 840 and curcuperezone 841 (Pseudopterogorgia rigida, Caribbean Sea) are envisaged to arise, in the case of 840, from non-symmetrical dimerisation of known co-metabolite perezone765 and in the case of 841, through coupling of perezone and α-curcumene.766 Flexibilisquinone 842 (cultured specimen of Sinularia flexibilis)767 was claimed to be the enantiomer of sarcophytonone (Sarcophyton crassocaule)768 based upon optical rotation data (842 [α]D −19.6, sarcophytonone [α]D +5.8).
Of two new C19-norditerpenes 12-hydroxy-scabrolide A 843 and 13-epi-scabrolide C 844 (Sinularia maxima, Nha Trang Bay, Vietnam) the latter was identified as an inhibitor of the production of IL-6 and IL-12 by LPS-stimulated bone marrow-derived dendritic cells.769 In addition to five sterols (see later), δ-lactone 845 was isolated from Scleronephthya gracillimum (Green Is., Taiwan) as a modest inhibitor of expression of iNOS and COX-2 in stimulated macrophages.770 The weakly cytotoxic spatane diterpene leptoclalin A 846 was reported from cultured specimens of Sinularia leptoclados.771
As with most years, a diverse array of cembranoid diterpenes were reported from soft corals in 2013. Arbolides A 847 and B 848, epoxy-alcohols with the former also containing a hydroperoxide functional group, were obtained from Sinularia arborea (southern Taiwan).772 Similarly functionalised cembranes flexibilins A 849 and B 850 in addition to ε-lactone-containing flexibilin C 851 were reported from S. flexibilis also collected from southern Taiwan.773
The absolute configuration of co-metabolite (−)-sandensolide (Dendronephthya sp.)774 was confirmed by X-ray crystal analysis. Of the δ-lactones 11-acetylsinuflexolide 852 and dihydro analogue 853 (S. flexibilis, Pingtung county, Taiwan), only the former exhibited cytotoxicity as anticipated for an exomethylene-conjugated lactone.775Sinularia flexibilis (southern Taiwan) was also the source of flexibilin D 854 and of known congener 5-dehydrosinulariolide776 the absolute configuration of which was determined by X-ray crystal analysis.777 The same publication and a second one778 also described sinulanorcembranolide A 855 and the 1-epi-diastereomer 856 from the same collection of S. gaweli (Sansiantai, Taitung county, Taiwan).
While cembranoid cugibberosene A 857 (S. gibberosa, Pingtung, Taiwan)779 was found to be devoid of cytotoxic or antibacterial properties, one of sinulariols T–Z5858–869 (S. rigida, Sanya Bay, Hainan Is., S. China Sea), specifically 864, exhibited effects against the model fouling organisms B. amphitrite and B. neritina.780
The casbane family of cembranoid diterpenes is characterised by the presence of a fused dimethyl-cyclopropyl ring. Of new examples sinularcasbanes A–F 870–875 (Sinularia sp., Ximao Is., Hainan, S. China Sea), 871 and 874 exhibited modest ability to inhibit NO production by stimulated macrophages.781
Two separate collections of Lobophytum sp. yielded epoxycembranes 876–880 (Ximao Is., Sanya Bay, Hainan, China)782 and α-methylene-γ-lactones 881–883 (Sanya Bay, Hainan, China).783 This is the first report 880 as an NP. Epoxycembrane 878 was a modest inhibitor of NO production by stimulated macrophages, while 881–883 were each found to be moderately cytotoxic towards a range of human and murine tumour cell lines.
Hydroxycembrane sarcophytol W 884 was isolated from Sarcophyton sp. (Xuwen coral reef area, Guangdong Province, China) – absolute configuration was assigned based upon that determined for a previously reported (Sinularia ovispiculata)784 co-metabolite.785Sarcophyton ehrenbergi (San-hsian-tai, Taitong county, Taiwan) was the source of diterpenes ehrenbergol C 885 and acetylehrenberoxide B 886 which both exhibited mild cytotoxicity (P388) but 886 was more potent as an anti-human cytomegalovirus agent.786
Of the sarcophyolides B–E 887–890 isolated from S. elegans (Xidao Is., Hainan, China), the structures and absolute configurations of 887 and 888 were established by X-ray crystal studies.787
Sarcophyolide B exhibited modest cytotoxicity. Red Sea collections of S. glaucum afforded 891–893 with 893 being reported as an NP for the first time.788 While 891 and 892 were equally cytotoxic towards a melanoma and a mouse kidney cell line, 892 exhibited selectivity towards the tumour cell line. Due to the presence of the conjugated triene functionality in sarglaucol 894 (S. glaucum, Sanya Bay, Hainan, China), it can be considered a diene-precursor to biscembranoids typically isolated from soft corals of the genus Sarcophyton.789
The same collection of S. elegans that afforded sarcophyolides B–E (see earlier) also yielded new examples of an isobiscembranoid and biscembranoids, the sarcophytolides G–L 895–900.790 These structures represent minor modifications to previously reported biscembranoids, being a dihydroxylated analogue of lobophytone F,791 a positional isomer of lobophytone S,792 a methoxylated analogue of lobophytone H,793 a dehydrated analogue of methyl tortuoate A,794 an oxidised analogue of lobophytone U795 and a 31-epimer also of lobophytone U, respectively.
Further investigation of S. latum (Sanya, Hainan Province), that had previously afforded, amongst other metabolites, biscembranoids bislatumlides A and B,796 has now yielded four more congeners bislatumlides C–F 901–904.797 Detailed examination of the absolute configuration of 901 and 903 by time dependent DFT (TDDFT) calculations of ECD data necessitated reassignment of the C-21 configuration of bislatumlides A 905 and B 906.
Re-isolation of methyl tortuoate D798 (Sarcophyton tortuosum, Yalong Bay, Hainan, China) has led to its structural revision to 907, with absolute configuration assigned by comparison of ECD data with that of co-metabolite ximaolide A.799 The study also concluded that the structure previously attributed to lobophytone K (Lobophytum pauciflorum)800 should also be corrected to 907.
A Red Sea (Hurghada) collection of S. trocheliophorum provided trochelioids A 908 and B 909 and 16-oxosarcophytonin E 910, the latter reported for the first time as an NP.801
In three separate accounts, sixteen new cembranoids were reported from S. trocheliophorum (Yalong Bay, Hainan, China). Of methyl sarcotroates A 911 and B 912, and sarcophytonolides M–R 913–918, only hydroperoxide-containing 912 and sarcophytonolide N 914 were found to inhibit human PTP1B.802,803
Also isolated were ε-lactone-containing cembranolides sartrolide A–G 919–925 and dimer bissartrolide 926.804 The unusual (1E,3Z)-diene configuration of 925 was supported by X-ray crystal analysis of isomeric co-metabolites sarcrassin D805 and emblide.806 Bissartrolide represents a dimer of sartrolide A and a free carboxylic acid analogue of sarcophytonolide B.807
A total of 34 new briarane diterpenes were reported from two collections of Dichotella gemmacea: gemmacolides AA–AR 927–944 (S. China Sea)808 and dichotellides F–U 945–960 (Meishan Is., Hainan, China).809 The absolute configurations of 927–944 were assigned by comparison of ECD data with those of dichotellide T 941, the absolute configuration of which was established by X-ray crystal analysis. Modest to moderate levels of cytotoxicity were observed for the gemmacolides while the dichotellides were all poorly cytotoxic with some examples exhibiting strong antifouling activity.
Junceella fragilis (Tai-Tong county, Taiwan) was the source of four more briaranes, frajunolide P–S 961–964.810 Hirsutalins I–M 965–969 are eunicellin diterpenes isolated from Cladiella hirsuta (Sianglu Islet, Penghu Is., Taiwan).811 Moderate inhibition of NO production by stimulated macrophages for 967 was shown.
In addition to a number of related metabolites, C. krempfi (Weizhou Is., S. China Sea) yielded oxylitophynol 970, litophynol A acetate 971, litophynol C 972 and krempfenin 973.812 Reduction of 970 gave a product identical to known co-metabolite litophynol A,813 subsequent acetylation of which afforded a product identical to 971.
Also isolated from C. krempfi (Penghu Is., Taiwan) were krempfielins E–M 974–982.814,815 Although 974–977 were inactive in antitumour and anti-inflammatory assays, structurally related co-metabolites did exhibit activity.
Of four new eunicellins reported from Cladiella sp. (Penghu Is., Taiwan), cladieunicellins I 983, K 984, and L 985 and litophynin I diacetate 986, the latter has been previously reported816 as a semi-synthetic derivative.817,818 Cladieunicellins I and L exhibited moderate activity towards an HTCL.
An unusual member of the klymollins I–S 987–997 (Klyxum molle, Penghu Is., Taiwan) is the phenylacetate-bearing klymollin M 991.819 This same metabolite was the most potent of the set, exhibiting cytotoxicity and the ability to inhibit superoxide generation and elastase release from stimulated human neutrophils.
In addition to two eunicellin diterpenes sibogin A 998 and B 999, investigation of the NPs from Muricella sibogae (Weizhou Is., China) also afforded three new seco-sterols sibogol A–C 1000–1002.820 A further two seco-sterols 1003 and 1004 were reported from Sinularia nanolobata (Xiao-Liuqiu Is., Pingtung county, Taiwan)821 and eleven, subergorgol A–J 1005–1014 and 1015, from Subergorgia suberosa (Meishan coast, S. China Sea).822 The latter unnamed seco-sterol is reported as an NP for the first time. Subergorgols C 1007 and D 1008, and F 1010 and G 1011 were isolated as their respective epimeric pairs but with unassigned configuration. The latter two were considered artefacts of isolation. While 1013 was found to be the most cytotoxic (moderate), 1015 was devoid of activity.
As well as a modestly bioactive δ-lactone noted earlier, an extract of Scleronephthya gracillimum (Green Is., Taiwan) also afforded pregnanes sclerosteroid J–N 1016–1020.770 Sclerosteroids K and M were more active than the other metabolites at inhibiting expression of iNOS and COX-2 in stimulated macrophages. Pregnane 1021 (Carijoa sp., Weizou Is., S. China Sea) exhibited potent antimicrobial properties.823
In addition to a number of known congeners, three new mildly cytotoxic polyhydroxylated steroids 1022–1024 were isolated from Sarcophyton sp. (Weizhou Is., S. China Sea).824 The possible artefactual origin of methylether 1022 was noted.
A collection of Anthogorgia caerulea from the same general location afforded caerulsteroid A 1025.825 Three studies of Red Sea (Hurghada) cnidarians afforded steroids – hurgadacin 1026 was isolated from Sinularia polydactyla,826 gorgostane 1027 (= 11-acetyl-sarcoaldosterol A827) from Heteroxenia ghardaqensis828 and zahramycins A 1028 and B 1029 from Sarcophyton trocheliophorum.829 Zahramycin B exhibited modest antibacterial activity.
Fourteen new sterols 1030–1043, plus one 1044 reported as an NP for the first time, were isolated as mildly cytotoxic constituents of Menella kanisa (coast of Beihai, Guangxi province, China).830
In addition to a number of co-metabolites, muriflasteroids A–C 1045–1047 were identified as weak to moderate cytotoxins (Muriceopsis flavida, Beihai, Guangxi province, China).831 Sterols containing 24(28)-unsaturation were reported from Sinularia depressa (1048 and 1049, Lingshui Bay, Hainan, S. China Sea)832 and Nephthea chabrolii (nebrosteroids Q–S, 1050–1052, San-Hsian-Tai coast, Taitong county, Taiwan).833 The latter three sterols exhibited mild cytotoxicity.
While sterols 1053–1059 (Sarcophyton sp., Weizhou Is., S. China Sea)834 exhibited variable levels of antimicrobial activity, dissesterol 1060 (Sinularia dissecta, Hai Van-Son Cha, Hue, Vietnam) was a strong inhibitor of IL-12 p40 cytokine production by stimulated bone marrow-derived dendritic cells.835 One new 18-acetoxy sterol 1061 was isolated from a South China Sea (Xuwen coral reef area) collection of Sarcophyton sp.836
Two 5α,8α-epidioxysterols were reported: mildly cytotoxic 1062 from Sinularia gaweli (Sansiantai, Taitung county, Taiwan)837 and antiviral (H5N1) 1063 from S. candidula (Safaga, Egyptian Red Sea).752
A range of ring-A cross-conjugated steroids, including lactone side-chained withanolides, were reported from cnidarians. All five cholestadienones 1064–1068 (Nepthea sp., Naozhou Is., S. China Sea)838 exhibited cytotoxicity towards a panel of HTCLs, while of three carboxylic acid-containing examples, paraminabic acid A–C 1069–1071 (Paraminabea acronocephala, Pingtung county, Taiwan), 1071 exhibited the most potent cytotoxicity.839
Sinubrasolides A–G 1072–1078 are withanolide-type steroids isolated from cultured specimens of Sinularia brassica (Taiwan) – the structure of 1075 is notable for containing an unusual spiroketal moiety.840 Mild cytotoxicity was observed for 1072, 1073 and 1076.
Acetylation of hemiacetal-containing nephthoacetal 1079 (Nephthea sp., Naozhou Is., S. China Sea) yielded two acetates.841 The NP inhibited the settlement of B. neritina larvae, an activity not observed for the acetate derivatives, while all three compounds were mildly cytotoxic to HeLa cells.
Finally, new steroidal glycosides junceelloside E–G 1080–1082 were reported from Dichotella gemmacea (Beihai, China).842 Detailed analysis of the nature of the arabinopyranose subunits (thiocarbamoyl-thiazolidine derivative) identified junceelloside E to contain the β-L anomer while junceellosides F and G contained the more standard β-D anomer. The arabinopyranose unit present in co-metabolite junceelloside C (Junceella juncea)843 was corrected from β-D to β-L (1083).
The structure of (−)-sinularianin B (Sinularia sp.)844 has been confirmed and absolute configuration established via synthesis which made use of sulfone-mediated tandem intramolecular-intermolecular alkylation.845 Comparison of NMR and chiroptical data for two diastereomers of (+)-sarcophytonolide C (Sarcophyton sp.)807 synthesised via a route including macrolactonisation and transannular RCM steps has confirmed the structure and established the absolute configuration of the NP.846 A general strategy for the synthesis of cladiellin diterpenoid NPs has been exemplified with the synthesis of ten examples.847 Efforts to mimic the putative carbon-centred radical reactions proposed for the biosynthesis of selected norcembranoids in Sinularia sp. were unsuccessful – a new model pathway was proposed, acting via a (3 + 2) transannular cyclisation reaction.848 In a related study, the 5,5,6- and 5,5,7-tricyclic ring systems found in the cnidarian metabolites plumarellide and rameswaralide were constructed from linear furanbutenolide precursors under acidic conditions, suggesting a potential biosynthetic mechanism involving two-step carbocation cyclisation sequences.849 Further investigation of the previously reported anti-inflammatory activity of the sesquiterpene lemnalol (Lemnalia sp.)850 has revealed that intramuscular injection leads to attenuation of inflammation in a monosodium urate model of human gouty arthritis, and that the NP also suppressed neutrophil infiltration and expression of related pro-inflammatory cytokines.851 While the mechanisms of cytotoxicity of the cembranoid 5-episinuleptolide acetate852 appear to include inhibition of levels of Hsp90 and induction of apoptosis,853 11-episinulariolide acetate854 targets EGF-mediated cytoplasmic calcium levels and inhibits COX-2 and IL-8 expression.855 Of a range of exo-methylene lactone-containing cembranoids tested for immunomodulatory effects, lobocrassin B (Lobophytum crassum)856 was the most effective at blocking TNF-α production and attenuating LPS-stimulated dendritic cell maturation and endocytosis.857 A hydroxypropyl-β-cyclodextrin formulation of pseudopterosin A (Pseudopterogorgia elisabethae)858 was more effective at inducing HUVEC cell proliferation than a DMSO solution of the NP – the change in formulation allowed observation of the decoupling of proliferative and cytotoxic effects.859 The previously reported ability of hippuristanol (Isis hippuris)860 to inhibit RNA helicase and eukaryotic initiation factor 4A, has prompted further investigation of the biological properties of the spiroacetal-containing steroid, identifying it as an inhibitor of primary effusion lymphoma cells, inducing G1 phase arrest, caspase activation and apoptosis.861 Activity was also observed in an in vivo model. Further investigation of anti-inflammatory (15R)-prostaglandins from Plexaura homomalla862 has identified the metabolites (15R)-PGE2 and (15R)-OAc-PGA2 as topically active inhibitors of oedema formation, leucocyte degranulation and elastase enzyme activity.863 As noted in the previous review in this series, simplexin Q (Klyxum simplex)864 is a duplicate of klysimplexin C865 and simplexin S864 is identical to cladieunicellin G (Cladiella sp.).866,867
9 Bryozoans
Only one new metabolite was reported from bryozoans in the last year, continuing the trend of minimal NP research efforts on this phylum. A new alkaloid, 7-bromo-1-ethyl-β-carboline 1084 was isolated from Pterocella vesiculosa (Aldermen Islands, New Zealand).868Wilsoniamines A and B, tribrominated alkaloids originally obtained from an Australian collection of Amathia wilsoni,869 have been synthesised in two steps featuring a condensation reaction between (2,4,6-tribromo-3-methoxyphenyl)acetaldehyde and (S)-N-methylpyrrolidine-2-carboxamide as a key step.870 Convolutamydine A, a dibrominated oxindole originally obtained from Amathia convoluta,871 (along with two synthetic analogues) has/have been shown to possess antinociceptive effects comparable to those of morphine.872
10 Molluscs
The number of new metabolites reported from molluscs (15) is just over half the yearly average number reported over the past decade. The previously noted ability of molluscs to acylate dinoflagellate-produced toxin okadaic acid has been confirmed with acylating activity located in the digestive gland of various molluscs.873 A range of unusual Δ8 unsaturated 4-methyl and 4,4-dimethyl sterols was identified in extracts of the gonads of the Japanese limpet Cellana grata and C. toreuma.874 Matrix solid-phase dispersion combined with GC-MS was demonstrated as a useful technique to detect the presence of brominated diphenyl ethers and newer halogenated flame retardants in mussel, cockle and clam extracts.875 New onchidione analogues 1085–1088 and ilikonapyrone esters 1089–1094 were reported from different Onchidium sp. molluscs.876 Acylation of 1086 gave 1087 and 1088, while reduction of co-metabolite onchidione afforded two diastereomers, one of which was identical to onchidionol 1086. The configurational relationships between 1089–1094 were identified by methanolysis of each, affording a product identical to co-metabolite ilikonapyrone.877 Mild cytotoxicity was observed for some of the compounds.Two formamide-containing pupukeanane sesquiterpenoid congeners 1095 and 1096, the latter previously known as a synthetic derivative, were reported from the tubercle nudibranch Phyllidia coelestis (Koh-Ha Islet, Krabi province, Thailand).878 Moderate to strong cytotoxicity towards tumour cell lines was observed.
The absolute configuration of the mildly cytotoxic cyclic dodecapeptide cycloforskamide 1097 (Pleurobranchus forskalii, Ishigaki Is., Okinawa) was established by combinations of ozonolysis and acid hydrolysis.879 In addition to this peptide, the ergot alkaloid ergosinine880 was also isolated, an unusual finding as ergot alkaloids are usually only isolated from terrestrial higher plants and fungi.881
A potentially artefactual hydroperoxide, phototridachiapyrone J 1098 was isolated from the sacoglossan mollusc Elysia patagonica (San Jorge Gulf, Patagonia, Argentina).882 The search for new leads for the treatment of leishmaniasis has identified the known 5α,8α-epidioxycholest-6-en-3β-ol (Dolabrifera dolabrifera) as mildly active against the amastigote form with nearly sixty-fold selectivity versus Vero cells.883 The structure of furan 1099 (Hypselodoris jacksoni, S. E. Queensland) was confirmed and absolute configuration established by a thorough study using combinations of synthesis, chiral HPLC and MPA derivatisation.884
A new enantioselective route to oxazinin alkaloids (Mytilus galloprovincialis)885,886 has helped confirm the absolute configuration of amongst others, oxazinin-1 and -2.887 Synthesis of a library of analogues of cytotoxic depsipeptide kulokekahilide-2 (Philinopsis speciosa)888,889 has revealed requirements of conformation, ring formation and ring size for biological potency.890 Aplysiatoxin (Stylocheilus longicauda)891 is a potent PKC binding tumour promoter – synthesis and evaluation of simplified debromo analogues suggest that activation of PKCδ might play a role in the observed antiproliferative activity.892 Following the synthesis of sanguinamide B (Hexabranchus sanguineus),893 the same group has reported that the use of biotinylated analogues of two cytotoxic D-Phe analogues in combination with pull-down assays have identified cellular targets that include eukaryotic ribosomal subunits.894 Close investigation of the mechanisms of cell death induced by the compounds indicates that the exact mechanism depends on the position of the D-Phe group. The results of a dose-escalating phase I study of kahalalide F (originally mollusc Elysia rufescens and green alga Bryopsis pennata)895 have been reported,896 while evaluation of a kahalalide F analogue, elisidepsin, against a panel of tumour cell lines suggests that cell lines that exhibit high E-cadherin, ErbB3 and Muc1 gene expression can be regarded as being sensitive to the clinical candidate.897 Drug resistance was associated with the presence of KRAS activating mutations. Using constrained NOESY NMR data, a conformational search has helped assign the configuration (3S) in the 9-methyl-3-decanol subunit of kahalalide Y (Elysia rufescens):898 unfortunately the study made use of the enantiomer of the NP and so the configuration should in fact be (3R).899,900 Investigation of the mechanism of cytotoxic action of aplyronine A (Aplysia kurodai)901 using photoaffinity biotinylated derivatives has identified aplyronine A to synergistically bind to tubulin in association with actin in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, leading to inhibition of tubulin polymerisation, and ultimately prevention of spindle formation and mitosis.902 Similar experiments using aplyronine C902 (lacks the trimethylserine sidechain of aplyronine A;903 three orders of magnitude less cytotoxic) showed it to bind to actin, as previously reported, but it did not bind to tubulin in this present study. Model compounds of the N-methylformamide sidechain of aplyronine A exhibit cytotoxicity towards tumour cell lines which is strongly correlated with their ability to induce the disruption of actin filaments.904
1 ratio, leading to inhibition of tubulin polymerisation, and ultimately prevention of spindle formation and mitosis.902 Similar experiments using aplyronine C902 (lacks the trimethylserine sidechain of aplyronine A;903 three orders of magnitude less cytotoxic) showed it to bind to actin, as previously reported, but it did not bind to tubulin in this present study. Model compounds of the N-methylformamide sidechain of aplyronine A exhibit cytotoxicity towards tumour cell lines which is strongly correlated with their ability to induce the disruption of actin filaments.904
11 Tunicates (ascidians)
The 35 new tunicate-derived NPs presented in this review is average for the number reported per annum over the last decade. The sulfonated serinol lipids siladenoserinol A–L 1100–1111 (Didemnidae, North Sulawesi, Indonesia) inhibited the interaction of tumour suppressor p53 with Hdm2, potentially leading to reactivation of p53 and induction of apoptosis in cancer cells.905 The absolute configuration of 1100 was established by a combination of degradation, modified Mosher's analysis and comparison with similar fragments of defined configuration.Two new examples of the rare 1,2,4-thiadiazole ring system, polycarpathiamine A 1112 and B 1113, were isolated from Polycarpa aurata (Ambon, Indonesia). While 1112 exhibited sub-micromolar cytotoxicity (L5178Y), 1113 was inactive.906 The regiochemistry of the 1,2,4-thiadiazole ring was established by analysis of 1H–15N HMBC data and by synthesis of a model compound.
A diverse range of pteridine (duramidines A–D 1114–1117), thymidine (leptoclinidines A 1118 and B 1119), choline (durabetaines A 1120 and B 1121) and imidazole (leptoclinidamines D–F 1122–1124) analogues was isolated from Leptoclinides durus (Swains Reef, Great Barrier Reef).907
Four methylsulfinyladenosine derivatives, momusine A–D 1125–1128, isolated as pairs of interconverting isomers, were reported from extracts of Herdmania momus (Jeju Is., S. Korea).908
The structures of the modestly cytotoxic dioxothiazinomeroterpenes conthiaquinone A 1129 and B 1130 (Aplidium conicum, Porto Cesareo, Lecce, Italy) were established by interpretation of NMR data in combination with DP4 calculated chemical shifts.909 The absolute configuration of 1129 was proposed from TDDFT calculated ECD data.
Four new examples of pyridoacridine alkaloids, shermilamine F 1131, dehydrokuanoniamine F 1132 arnoamine C 1133 and D 1134 (Cystodytes violatinctus, Solomon Is.) exhibited modest cytotoxicity towards a panel of HTCLs.910 A variant biosynthetic pathway to address the formation of arnoamines C and D was proposed. New analogues of the structurally related styelsamines911 were prepared and assessed for DNA binding ability and cytotoxicity.912
A sperm activation and attractant 1135 was isolated from egg seawater of Ascidia sydneiensis; structure elucidation by NMR and MS was performed on 2.6 μg (4 nmol) of material.913 The proposed planar and stereo structure of 1135 was supported by the synthesis of model compounds. The structure of this sperm attractant is very similar to that previously reported from Ciona intestinalis and C. savignyi.914
Synthesis and cytotoxic evaluation of aminol lipids clavaminol G915 (Clavelina phlegrea)916 and crucigasterins A, B and D917,918 (Pseudodistoma crucigaster)919 have been reported; mild activity was observed. First syntheses of kottamide E920 (Pycnoclavella kottae),921 lukianol B922 (unidentified tunicate)923 and eudistomin Y7924–926 (Eudistoma sp.)927 have been published. Syntheses of an isomer of didemnaketal A928 and the proposed structure of didemnaketal B929 (Didemnum sp.)930 provide further evidence that the NPs require revision of configurational assignments. Synthesis and structure–activity relationship studies on orthidine F931 (antimalarial),932 ascidiathiazone933 (antimalarial),934 meridianin G935 (antimalarial),936 perspicamide A937 (antileishmanial),938 rigidin939 (antitumour),940 and ningalin B941 (P-glycoprotein modulator)942 have been reported. Further investigation of recently reported ascidian metabolites of the cadiolide and synoilide families of furanones (Synoicum sp.)943 has identified cadiolides E, H and I as being potent inhibitors of C. albicans isocitrate lyase, an enzyme associated with microorganism virulence.944 Semi-synthetic N-acyl derivatives of ecteinascidin 770 (Ecteinascidia thurstoni)945 has identified quinoline- and fluorocinnamoyl-containing examples that exhibit 50–70 fold increased cytotoxicity towards the HCT-116 cell line versus the parent NP.946 Minor corrections to manuscripts describing the mandelalides (Lissoclinum sp.)947 and herdmanine K (Herdmania momus)948 have been noted.
12 Echinoderms
The 33 new metabolites reported from echinoderms in this review is lower than the average number reported per annum over the last decade. A commercially available specimen of the starfish Asterias rollestoni (Xiamen food market, China) afforded the tetraosides 1136 and 1137 (ref. 949) while Astropecten polyacanthus (Cat Ba, Haiphong, Vietnam) contained the inactive or mildly cytotoxic sterols astropectenol A–D 1138–1141.950 The latter set of compounds was also reported to inhibit the expression of pro-inflammatory cytokines in bone marrow-derived dendritic cells.951Aphelasteroside E 1142, which contains the rare sulfation at C-26, was isolated from Aphelasterias japonica (Poset Bay, Sea of Japan)952 and the C-24-arabinosides pectinioside H–J 1143–1145 were identified in extracts of Asterina pectinifera (Dalian coast, Yellow Sea, China).953
Tetraosides typicoside A11146 (the 24E isomer of previously reported intercedenside A (Mensamaria intercedens)954), A2, B1, C1 and C21147–1150 are minor metabolites isolated from the sea cucumber Actinocucumis typica (Vizhinjam coast, Arabian Sea, India).955 Antifungal, haemolytic and cytotoxic evaluations of the five NPs identified widespread activity, with typicoside C1 being markedly less active in all assays. The presence of disulfated tetraoside turquetoside A 1151, which contains the rare 3-O-methyl-D-quinovose sugar unit, in both Staurocucumis turqueti and S. liouvillei suggests the sugar is a taxonomic character of this particular genus of Antarctic sea cucumber.956
Of the disulfated pentaosides cucumarioside I1, I3 and I41152–1154 (Eupentacta fraudatrix, Peter the Great Gulf, Sea of Japan), only 1152 exhibited biological activity including cytotoxicity (weak) and haemolytic activity (strong).957 Pentaosides cladoloside B11155 and B21156 and hexaosides cladoloside C, C1, C2 and D 1157–1160 (Cladolabes schmeltzii, Nha Trang Gulf, S. China Sea) all exhibited similar levels of strong cytotoxicity and haemolytic activity.958
Extracts of the starfish Astropecten monacanthus (Cat Ba, Haiphong, Vietnam) afforded the hexaosides astrosterioside A–C 1161–1163 and pentaoside astrosterioside D 1164.959 While 1161 and 1163 exhibited mild inhibition of IL-6 production by stimulated bone marrow-derived dendritic cells, diketo-containing 1164 exhibited potent inhibition of production of IL-6, IL-12 p40 and TNF-α.
The pyrrole and furan oligoglycosides astebatherioside A–D 1165–1168 were reported from the starfish Asterina batheri (Catba, Haiphong, Vietnam).960 While 1165 was either inactive or weakly active, 1166–1168 demonstrated inhibition of IL-12 p40 production, and to a lesser extent of IL-6 production, in LPS-stimulated bone marrow-derived dendritic cells.
The synthesis of goniopectenoside B (starfish Goniopecten demonstrans)961 has been reported.962 Purified polar steroids previously reported from the starfish Patiria pectinifera and Distolasterias nipon are potent enhancers of neurite outgrowth and acted as neuroprotectors against damage caused by oxygen-glucose deprivation.963 Crude preparations of cerebrosides from the sea cucumber Acaudina molpadioides and the starfish Asterias amurensis were found to protect PC12 cells from oxidative damage due to exposure to H2O2 or tert-butyl hydroperoxide.964 In both cases the neuroprotection appeared to be conferred by upregulation of superoxide dismutase activity and modulation of components of the mitochondrial apoptotic pathway. High-energy CID tandem mass analysis has been used to determine the structures of ceramides and cerebrosides isolated from Distolasterias nipon.965 Stable isotope biosynthesis feeding experiments have determined that dietary cholesterol and cholesterol 3-sulfate are elaborated into polyhydroxylated sterols in the starfish Patiria (= Asterina) pectinifera.966
13 Mangroves
Aerial parts of the mangrove plant Kandelia obovata (Ximen Is., Zhejiang Province, China) afforded two new furofuran lignans kandelisesquilignan A 1169 and B 1170.967 Similar levels of antioxidant activity (DPPH assay) were observed for 1169 and 1170versus ascorbic acid.Ethanolic extracts of the bark of Ceriops decandra (Godavari estuary, Andhra Pradesh, India) afforded diterpenes decandrin A–K 1171–1181, the structures of which encompass abietane and podocarpane skeletons,968 while the wood of Excoecaria agallocha (Corangi forest, Godavari estuary) afforded ent-isopimarane diterpenoids agallochaexcoerin D–F 1182–1184.969
Two triterpenes tiliacol A 1185 and B 1186 were isolated from the semi-mangrove plant Hibiscus tiliaceus (Hainan Is., China).970
A diverse range of liminoids were reported from extracts of Xylocarpus granatum: granatumins H–K 1187–1190 (seeds, Krishna estuary, Andhra Pradesh),971 xylomexicanins C 1191 and D 1192 (seeds, Hainan Province, China),972 and xylogranins A 1193 and B 1194 (leaves, Sundarbans Mangrove Forest, Bangladesh).973 Xylomexicanin C 1191 exhibited modest cytotoxicity towards a breast tumour cell line, while xylogranin B 1194 and co-metabolite swietephragmin were potent inhibitors of the Wnt signalling pathway and exhibited sub-micromolar cytotoxicity towards two human colorectal tumour cell lines.
Extracts of the seeds of X. moluccensis (Trang province, Thailand) afforded thaixylomolins A–F 1195–1200.974,975 The structure of 1195 was secured by X-ray diffraction analysis and TDDFT calculations were used to establish the absolute configurations of 1196 and 1197, while 1198 was assigned by comparison of ECD data. The 4-hydro-dithiosulfonate, bruguiesulfurol (Bruguiera gymnorhiza)976 was detected as a modest inhibitor of PTP1B, prompting its synthesis and preparation of a library of analogues, some of which exhibited more potent activity.977
14 Miscellaneous
Investigation of water conditioned with sea lamprey (Petromyzon marinus) larvae afforded the hexahydrophenanthrene sulfate petromyzonin 1201.978 Absolute configuration was assigned by ECD analysis. Petromyzonin elicited potent (10−11 M) response in electro-olfactogram recordings using olfactory epithelia of adult male sea lamprey, indicating a likely ecological role as an odorant.The detection of 5,11-dideoxytetrodotoxin,979 isolated as an NP for the first time, in the pufferfish Takifugu poecilonotus and the flatworm Planocerid sp. 1 (Guam) prompted speculation on the putative biosynthetic pathways for the biosynthesis and metabolism of tetrodotoxins.980 A new method involving sonication, SPE and LC-MS/MS has been reported to allow simultaneous quantification of Pacific ciguatoxins-1, -2 and -3 in the whole blood of fish.981
15 Conclusion
Fifty years ago in 1963 just four papers were published on MNPs with only one paper containing new compounds. At that time MNPs was becoming established as a field of interest. In this Conclusion the phylum-preferences of the MNP community across the subsequent 50 years period are examined. These preferences are presented (Fig. 1) as the annual number of publications reporting the isolation of new compounds for each phylum that has been sampled over this period. The most aggressively selected phylum has been the Porifera, but the popularity of this target has diminished somewhat since the mid 1990s coinciding with the very rapid rise in popularity of the Ascomycota, Actinobacteria and the Cyanobacteria. The Cnidaria have steadily increased in popularity across the years and while the phyla Rhodophyta, Ochrophyta, Echinodermata and Mollusca were as popular as the Porifera in the early years, interest waned in later years. The popularity of these phyla in earlier years may have been a reflection of the relative ease of collection by snorkeling and shore-wading as in the 1960s and 1970s SCUBA diving was more of a specialist technique. The numeric totals for the 50 years of collection are given in Table 1 along with the percentage contribution of each phylum to the marine literature. For the 50 years period from 1963 9220 papers have reported the isolation of 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 662 new compounds. These 9220 papers constitute 37% of the total papers in MarinLit.77 The other 17
662 new compounds. These 9220 papers constitute 37% of the total papers in MarinLit.77 The other 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 284 papers are associated with topics such as reviews, syntheses, stereochemistry, corrections of structure or stereochemistry, bioactivities, and ecological surveys. Other data shown in Table 1 include the numbers of compounds reported/phylum over the 50 years period as well as the % contributions each phylum has made to the number of papers reporting new compounds or the number of compounds. These relative proportions are comparable as the number of isolated compounds reported/paper is 2–3 across most of the phyla. Also included are the recognised totals of species/phylum from the World Register of Marine Species (WORMS),982 allowing comparison of the numbers of samples of each phylum collected with the actual number of recognised species. This comparison should be used carefully as multiple collections of the same species have been made, or the sample may have only been identified to the genus level. However the comparison does offer insights into the coverage of each phylum. This point is emphasised by considering the various contributions the most studied genus for each phylum has made. For example, for the Rhodophyta the most studied genus has been Laurencia. Even though Laurencia is only one of 84 genera studied, this one genus contributed 369 out of the total 672 papers from the Rhodophyta describing new MNPs and was the source of 824 of the 1668 new compounds from this phylum. The important consideration is that even though multiple collections of the same genus/species may have been made, the same genus or species at different locations is giving rise to a different suite of metabolites. Apart from Porifera and Cyanobacteria, the coverage of most phyla is very limited, although the credibility of the numbers of species recognised by WORMS982 for the Ascomycota and Actinobacteria is probably suspect as much of the micro-world has yet to be fully recognised. Over the past 50 years MNP chemists have studied samples collected from 1300 genera across 28 phyla. These 28 phyla represent an estimated (WORMS) 210
284 papers are associated with topics such as reviews, syntheses, stereochemistry, corrections of structure or stereochemistry, bioactivities, and ecological surveys. Other data shown in Table 1 include the numbers of compounds reported/phylum over the 50 years period as well as the % contributions each phylum has made to the number of papers reporting new compounds or the number of compounds. These relative proportions are comparable as the number of isolated compounds reported/paper is 2–3 across most of the phyla. Also included are the recognised totals of species/phylum from the World Register of Marine Species (WORMS),982 allowing comparison of the numbers of samples of each phylum collected with the actual number of recognised species. This comparison should be used carefully as multiple collections of the same species have been made, or the sample may have only been identified to the genus level. However the comparison does offer insights into the coverage of each phylum. This point is emphasised by considering the various contributions the most studied genus for each phylum has made. For example, for the Rhodophyta the most studied genus has been Laurencia. Even though Laurencia is only one of 84 genera studied, this one genus contributed 369 out of the total 672 papers from the Rhodophyta describing new MNPs and was the source of 824 of the 1668 new compounds from this phylum. The important consideration is that even though multiple collections of the same genus/species may have been made, the same genus or species at different locations is giving rise to a different suite of metabolites. Apart from Porifera and Cyanobacteria, the coverage of most phyla is very limited, although the credibility of the numbers of species recognised by WORMS982 for the Ascomycota and Actinobacteria is probably suspect as much of the micro-world has yet to be fully recognised. Over the past 50 years MNP chemists have studied samples collected from 1300 genera across 28 phyla. These 28 phyla represent an estimated (WORMS) 210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 marine species out of an estimated total of 224
892 marine species out of an estimated total of 224![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 070 for all marine phyla.982 In other words MNP chemists have collected widely, but perhaps thinly across the Kingdoms. The Kingdom Virus (119 representatives) has not been sampled at all by MNP chemists and so has been excluded from consideration. As chemists we have published 9220 papers over the past 50 years describing new compounds from these 28 phyla. But, in fact, the 9220 papers describe compounds elicited from just 2657 named species with another 2485 occurrences across 484 genera that are only described as sp. So, the number of distinct species studied is a long way short of 9220. This nicely emphasises the point that there is still an enormous MNP resource waiting to be explored; probably in excess of 200
070 for all marine phyla.982 In other words MNP chemists have collected widely, but perhaps thinly across the Kingdoms. The Kingdom Virus (119 representatives) has not been sampled at all by MNP chemists and so has been excluded from consideration. As chemists we have published 9220 papers over the past 50 years describing new compounds from these 28 phyla. But, in fact, the 9220 papers describe compounds elicited from just 2657 named species with another 2485 occurrences across 484 genera that are only described as sp. So, the number of distinct species studied is a long way short of 9220. This nicely emphasises the point that there is still an enormous MNP resource waiting to be explored; probably in excess of 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 species still to be evaluated.
000 species still to be evaluated.
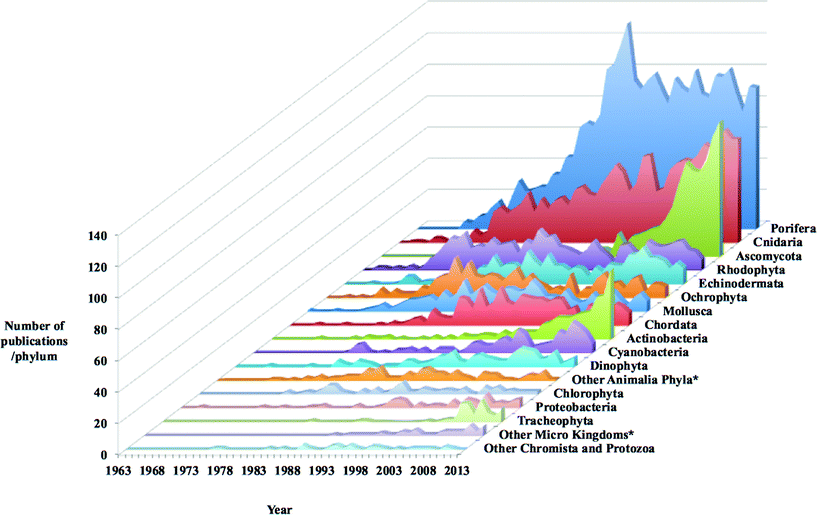 | ||
| Fig. 1 The phylum-preferences of the marine natural product research community across a 50-year period from 1963. | ||
| Kingdom | Number of genera | Most studied genus | Number of papers and compounds from the most studied genus | Papers/phylum | % MLit papers with new compounds | Compounds/phylum | % of MLit | Compounds | Compounds/paper | Number of recognised species | |
|---|---|---|---|---|---|---|---|---|---|---|---|
a In the 28 phyla sampled by MNP chemists WORMS lists 210![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 892 species. Across all Kingdoms WORMS lists 226 892 species. Across all Kingdoms WORMS lists 226![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 070 marine species. 070 marine species.
|
|||||||||||
| Animalia | Annelida | 13 | Odontosyllis | 5 | 12 | 24 | 0.3% | 46 | 0.2% | 1.9 | 2640 |
| Arthropoda | 8 | Megabalanus | 2 | 2 | 9 | 0.1% | 10 | 0.0% | 1.1 | 6216 | |
| Bryozoa | 24 | Bugula | 19 | 33 | 86 | 0.9% | 199 | 0.8% | 2.3 | 6036 | |
| Chordata | 66 | Didemnum | 54 | 144 | 434 | 4.7% | 1102 | 4.5% | 2.5 | 1898 | |
| Cnidaria | 161 | Sinularia | 234 | 678 | 1589 | 17.2% | 4949 | 20.1% | 3.1 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 839 839 |
|
| Echinodermata | 136 | Asterias | 35 | 69 | 493 | 5.3% | 1335 | 5.4% | 2.7 | 295 | |
| Hemichordata | 3 | Cephalodiscus | 9 | 19 | 11 | 0.1% | 27 | 0.1% | 2.5 | 30 | |
| Mollusca | 116 | Aplysia | 87 | 184 | 468 | 5.1% | 1095 | 4.4% | 2.3 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 233 233 |
|
| Nematoda | 2 | Amphiporus | 1 | 1 | 2 | 0.0% | 2 | 0.0% | 1.0 | 6997 | |
| Platyhelminthes | 3 | Amphiscolops | 2 | 2 | 3 | 0.0% | 6 | 0.0% | 2.0 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 836 836 |
|
| Porifera | 285 | Dysidea | 163 | 418 | 2991 | 32.4% | 8152 | 33.1% | 2.7 | 8320 | |
| Archaea | Euryarchaeota | 1 | Thermococcus | 2 | 24 | 2 | 0.0% | 24 | 0.1% | 12.0 | 98 |
| Bacteria | Actinobacteria | 28 | Streptomyces | 201 | 465 | 322 | 3.5% | 778 | 3.2% | 2.4 | 72 |
| Bacteroidetes | 9 | Rapidithrix | 3 | 4 | 12 | 0.1% | 24 | 0.1% | 2.0 | 235 | |
| Cyanobacteria | 28 | Lyngbya | 140 | 283 | 234 | 2.5% | 484 | 2.0% | 2.1 | 436 | |
| Firmicutes | 5 | Bacillus | 43 | 94 | 47 | 0.5% | 101 | 0.4% | 2.1 | 96 | |
| Proteobacteria | 38 | Pseudomonas | 16 | 33 | 110 | 1.2% | 225 | 0.9% | 2.0 | 772 | |
| Chromista | Bacillariophyta | 7 | Rhizosolenia | 2 | 7 | 10 | 0.1% | 24 | 0.1% | 2.4 | 2675 |
| Ciliophora | 4 | Euplotes | 8 | 24 | 12 | 0.1% | 37 | 0.2% | 3.1 | 2676 | |
| Cryptophyta | 1 | Chrysophaeum | 2 | 9 | 2 | 0.0% | 9 | 0.0% | 4.5 | 91 | |
| Dinophyta | 26 | Prorocentrum | 20 | 27 | 194 | 2.1% | 297 | 1.2% | 1.5 | 2194 | |
| Haptophyta | 6 | Coccolithus | 2 | 3 | 8 | 0.1% | 11 | 0.0% | 1.4 | 260 | |
| Ochrophyta | 57 | Dictyota | 94 | 249 | 460 | 5.0% | 1247 | 5.1% | 2.7 | 5009 | |
| Fungi | Ascomycota | 129 | Aspergillus | 187 | 545 | 783 | 8.5% | 2165 | 8.8% | 2.8 | 966 |
| Plantae | Chlorophyta | 29 | Caulerpa | 30 | 90 | 116 | 1.3% | 272 | 1.1% | 2.3 | 1810 |
| Rhodophyta | 84 | Laurencia | 369 | 824 | 672 | 7.3% | 1668 | 6.8% | 2.5 | 6399 | |
| Tracheophyta | 23 | Xylocarpus | 27 | 119 | 75 | 0.8% | 253 | 1.0% | 3.4 | 432 | |
| Protozoa | Euglenozoa | 1 | Euglena | 1 | 3 | 1 | 0.0% | 3 | 0.0% | 3.0 | 231 |
| Totals/Averages | 1300 | 1758 | 4361 | 9220 | 100% | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 662 662 |
100% | 2.7 | |||
16 Acknowledgements
We thank Dr Anthea Lees for assistance with the collection of data for this review.17 References
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2014, 31, 160–258 RSC
.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2013, 30, 237–323 RSC
.
- R. A. Hill, Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2013, 109, 146–166 RSC
.
- A. M. S. Mayer, A. D. Rodríguez, O. Taglialatela-Scafati and N. Fusetani, Mar. Drugs, 2013, 11, 2510–2573 CrossRef PubMed
.
- G. M. Cragg and D. J. Newman, Biochim. Biophys. Acta, 2013, 1830, 3670–3695 CrossRef CAS PubMed
.
- J. Khazir, B. A. Mir, S. A. Mir and D. Cowan, J. Asian Nat. Prod. Res., 2013, 15, 764–788 CrossRef CAS
.
- S. Vinothkumar and P. S. Parameswaran, Biotechnol. Adv., 2013, 31, 1826–1845 CrossRef CAS PubMed
.
- P. M. Murray, S. Moane, C. Collins, T. Beletskaya, O. P. Thomas, A. W. F. Duarte, F. S. Nobre, I. O. Owoyemi, F. C. Pagnocca, L. D. Sette, E. McHugh, E. Causse, P. Pérez-López, G. Feijoo, M. T. Moreira, J. Rubiolo, M. Leirós, L. M. Botana, S. Pinteus, C. Alves, A. Horta, R. Pedrosa, C. Jeffryes, S. N. Agathos, C. Allewaert, A. Verween, W. Vyverman, I. Laptev, S. Sineoky, A. Bisio, R. Manconi, F. Ledda, M. Marchi, R. Pronzato and D. J. Walsh, New Biotechnol., 2013, 30, 839–850 CrossRef CAS PubMed
.
- Y.-X. Li, S. W. A. Himaya and S.-K. Kim, Molecules, 2013, 18, 7886–7909 CrossRef CAS PubMed
.
- C. Gao, X. Yi, R. Huang, F. Yan, B. He and B. Chen, Chem. Biodiversity, 2013, 10, 1435–1447 CAS
.
- M. Menna, C. Imperatore, F. D'Aniello and A. Aiello, Mar. Drugs, 2013, 11, 1602–1643 CrossRef CAS PubMed
.
- M. Pelay-Gimeno, J. Tulla-Puche and F. Albericio, Mar. Drugs, 2013, 11, 1693–1717 CrossRef CAS PubMed
.
- N. E. Golantsov, A. A. Festa, A. V. Karchava and M. A. Yurovskaya, Chem. Heterocycl. Compd., 2013, 49, 203–225 CrossRef CAS
.
- A. Lorente, J. Lamariano-Merketegi, F. Albericio and M. Álvarez, Chem. Rev., 2013, 113, 4567–4610 CrossRef CAS PubMed
.
- Y. Li, L. Liang, W. Xiao, J. Liang and Y. Guo, Chin. J. Org. Chem., 2013, 33, 1157–1166 CrossRef CAS
.
- L.-F. Liang and Y.-W. Guo, Chem. Biodiversity, 2013, 10, 2161–2195 CAS
.
- F. Desriac, C. Jégou, E. Balnois, B. Brillet, P. Le Chevalier and Y. Fleury, Mar. Drugs, 2013, 11, 3632–3660 CrossRef PubMed
.
- V. L. Trainer, L. Moore, B. D. Bill, N. G. Adams, N. Harrington, J. Borchert, D. A. M. da Silva and B.-T. L. Eberhart, Mar. Drugs, 2013, 11, 1815–1835 CrossRef CAS PubMed
.
- V. Gouveia, A. M. L. Seca, M. C. Barreto and D. C. G. A. Pinto, Mini-Rev. Med. Chem., 2013, 13, 1150–1159 CrossRef CAS PubMed
.
- B.-G. Wang, J. B. Gloer, N.-Y. Ji and J.-C. Zhao, Chem. Rev., 2013, 113, 3632–3685 CrossRef CAS PubMed
.
- J. A. R. Salvador, J. F. S. Carvalho, M. A. C. Neves, S. M. Silvestre, A. J. Leitão, M. M. C. Silva and M. L. Sá e Melo, Nat. Prod. Rep., 2013, 30, 324–374 RSC
.
- L. Wang, B. Yang, X.-P. Lin, X.-F. Zhou and Y. Liu, Nat. Prod. Rep., 2013, 30, 455–473 RSC
.
- L. M. Blair and J. Sperry, J. Nat. Prod., 2013, 76, 794–812 CrossRef CAS PubMed
.
- Z. Jin, Nat. Prod. Rep., 2013, 30, 869–915 RSC
.
- A. Wang, Z. Zhao, X. Zheng and H. Cao, Chin. J. Org. Chem., 2013, 33, 483–491 CrossRef CAS
.
- K. D. Cusick and G. S. Sayler, Mar. Drugs, 2013, 11, 991–1018 CrossRef CAS PubMed
.
- A. ElMarrouni, A. Kolleth, R. Lebeuf, J. Gebauer, S. Prevost, M. Heras, S. Arseniyadis and J. Cossy, Nat. Prod. Commun., 2013, 8, 965–972 CAS
.
- Z. Qin, S. Huang, Y. Yu and H. Deng, Mar. Drugs, 2013, 11, 3970–3997 CrossRef CAS PubMed
.
- V. Valdiglesias, M. V. Prego-Faraldo, E. Pásaro, J. Méndez and B. Laffon, Mar. Drugs, 2013, 11, 4328–4349 CrossRef PubMed
.
- D. L. J. Clive and P. Cheng, Tetrahedron, 2013, 69, 5067–5078 CrossRef CAS
.
- H. Abida, S. Ruchaud, L. Rios, A. Humeau, I. Probert, C. De Vargas, S. Bach and C. Bowler, Mar. Drugs, 4594, 11, 4594–4611 CrossRef PubMed
.
- R. Subramani and W. Aalbersberg, Appl. Microbiol. Biotechnol., 2013, 97, 9291–9321 CrossRef CAS PubMed
.
- J. Vicente, A. Stewart, B. Song, R. T. Hill and J. L. Wright, Mar. Biotechnol., 2013, 15, 413–424 CrossRef CAS PubMed
.
- J.-R. Wang, W.-F. He and Y.-W. Guo, J. Asian Nat. Prod. Res., 2013, 15, 185–197 CrossRef PubMed
.
- P. Manivasagan, J. Venkatesan, K. Sivakumar and S.-K. Kim, Microbiol. Res., 2013, 168, 311–332 CrossRef CAS PubMed
.
- Y. K. Ng, A. K. Hewavitharana, R. Webb, P. N. Shaw and J. A. Fuerst, Appl. Microbiol. Biotechnol., 2013, 97, 3097–3108 CrossRef CAS PubMed
.
- L. T. Tan, Drug Discovery Today, 2013, 18, 863–871 CrossRef CAS PubMed
.
- C. Zhao, T. Zhu and W. Zhu, Chin. J. Org. Chem., 2013, 33, 1195–1234 CrossRef CAS
.
- Z.-Q. Xiong, J.-F. Wang, Y.-Y. Hao and Y. Wang, Mar. Drugs, 2013, 11, 700–717 CrossRef CAS PubMed
.
- W. H. Gerwick and A. M. Fenner, Microb. Ecol., 2013, 65, 800–806 CrossRef CAS PubMed
.
- K. Benkendorff, Mar. Drugs, 2013, 11, 1370–1398 CrossRef CAS PubMed
.
- K. Osako and V. L. Teixeira, Nat. Prod. Commun., 2013, 8, 533–538 CAS
.
- Y. M. Lee, M. J. Kim, H. Li, P. Zhang, B. Bao, K. J. Lee and J. H. Jung, Mar. Biotechnol., 2013, 15, 499–519 CrossRef CAS PubMed
.
- M. A. M. Mondol, H. J. Shin and M. T. Islam, Mar. Drugs, 2013, 11, 2846–2872 CrossRef PubMed
.
- W.-C. Wei, P.-J. Sung, C.-Y. Duh, B.-W. Chen, J.-H. Sheu and N.-S. Yang, Mar. Drugs, 2013, 11, 4083–4126 CrossRef PubMed
.
- J.-A. Kim and S.-K. Kim, Curr. Protein Pept. Sci., 2013, 14, 177–182 CrossRef CAS PubMed
.
- A. J. Jones, T. Grkovic, M. L. Sykes and V. M. Avery, Mar. Drugs, 2013, 11, 4058–4082 CrossRef PubMed
.
- R. Pangestuti and S.-K. Kim, Food Sci. Biotechnol., 2013, 22, 1175–1186 Search PubMed
.
- S. Indumathy and C. R. Dass, J. Pharm. Pharmacol., 2013, 65, 1280–1301 CrossRef CAS PubMed
.
- K. Petit and J. F. Biard, Anti-Cancer Agents Med. Chem., 2013, 13, 603–631 CrossRef CAS PubMed
.
- W. R. Sawadogo, M. Schumacher, M. H. Teiten, C. Cerella, M. Dicato and M. Diederich, Molecules, 2013, 18, 3641–3673 CrossRef CAS PubMed
.
- B. Pejin, K. K. Jovanovic, M. Mojovic and A. G. Savic, Curr. Top. Med. Chem., 2013, 13, 2745–2766 CrossRef CAS PubMed
.
- S. B. Bharate, S. D. Sawant, P. P. Singh and R. A. Vishwakarma, Chem. Rev., 2013, 113, 6761–6815 CrossRef CAS PubMed
.
- M.-G. Zhong, Y.-F. Xiang, X.-X. Qiu, Z. Liu, K. Kitazato and Y.-F. Wang, RSC Adv., 2013, 3, 313–328 RSC
.
- X. Zhou, J. Liu, B. Yang, X. Lin, X.-W. Yang and Y. Liu, Curr. Med. Chem., 2013, 20, 953–973 CAS
.
- Y.-Q. Wang and Z.-H. Miao, Mar. Drugs, 2013, 11, 903–933 CrossRef CAS PubMed
.
- P. A. Harnedy and R. J. FitzGerald, Curr. Protein Pept. Sci., 2013, 14, 162–172 CrossRef CAS PubMed
.
- R. Nasri and M. Nasri, Curr. Protein Pept. Sci., 2013, 14, 199–204 CrossRef CAS PubMed
.
- V. L. T. Hoang and S.-K. Kim, Curr. Protein Pept. Sci., 2013, 14, 205–211 CrossRef CAS PubMed
.
- T. Solov'eva, V. Davydova, I. Krasikova and I. Yermak, Mar. Drugs, 2013, 11, 2216–2229 CrossRef PubMed
.
- J.-T. Liu, X.-L. Lu, X.-Y. Liu, Y. Gao, B. Hu, B.-H. Jiao and H. Zheng, Mini-Rev. Med. Chem., 2013, 13, 617–626 CrossRef CAS PubMed
.
- J. S. Roy, K. L. Poulson-Ellestad, R. D. Sieg, R. X. Poulin and J. Kubanek, Nat. Prod. Rep., 2013, 30, 1364–1379 RSC
.
- A. Holland and S. Kinnear, Mar. Drugs, 2013, 11, 2239–2258 CrossRef PubMed
.
- J. C. Morris, Nat. Prod. Rep., 2013, 30, 783–805 RSC
.
- M. Gordaliza and P. G. Baraldi, Curr. Med. Chem., 2013, 20, 2798–2811 CrossRef CAS PubMed
.
- T. Nishikawa and M. Isobe, Chem. Rec., 2013, 13, 286–302 CrossRef CAS PubMed
.
- I. Paterson, P. Maltas and E. A. Anderson, Pure Appl. Chem., 2013, 85, 1133–1147 CrossRef CAS
.
- W. P. Unsworth and R. J. K. Taylor, Org. Biomol. Chem., 2013, 11, 7250–7261 CAS
.
- S. U. Kadam, B. K. Tiwari and C. P. O'Donnell, J. Agric. Food Chem., 2013, 61, 4667–4675 CrossRef CAS PubMed
.
- D. Forner, F. Berrué, H. Correa, K. Duncan and R. G. Kerr, Anal. Chim. Acta, 2013, 805, 70–79 CrossRef CAS PubMed
.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 495, 461–466 CrossRef CAS PubMed
.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 501, 262 CrossRef CAS
.
- Y. Zhang, S. Xiao, L. Sun, Z. Ge, F. Fang, W. Zhang, Y. Wang and Y. Cheng, Anal. Chim. Acta, 2013, 777, 49–56 CrossRef CAS PubMed
.
- J. Y. Yang, L. M. Sanchez, C. M. Rath, X. Liu, P. D. Boudreau, N. Bruns, E. Glukhov, A. Wodtke, R. de Felicio, A. Fenner, W. R. Wong, R. G. Linington, L. Zhang, H. M. Debonsi, W. H. Gerwick and P. C. Dorrestein, J. Nat. Prod., 2013, 76, 1686–1699 CrossRef CAS PubMed
.
- V. Gupta, R. S. Thakur, C. R. K. Reddy and B. Jha, RSC Adv., 2013, 3, 7037–7047 RSC
.
- M. C. Leal, M. H. G. Munro, J. W. Blunt, J. Puga, B. Jesus, R. Calado, R. Rosa and C. Madeira, Nat. Prod. Rep., 2013, 30, 1380–1390 RSC
.
- M. C. Leal, R. Calado, C. Sheridan, A. Alimonti and R. Osinga, Trends Biotechnol., 2013, 31, 555–561 CrossRef CAS PubMed
.
- http://pubs.rsc.org/marinlit .
- T. P. Wyche, M. Standiford, Y. Hou, D. Braun, D. A. Johnson, J. A. Johnson and T. S. Bugni, Mar. Drugs, 2013, 11, 5089–5099 CrossRef CAS PubMed
.
- Y. Hu, M. B. Potts, D. Colosimo, M. L. Herrera-Herrera, A. G. Legako, M. Yousufuddin, M. A. White and J. B. MacMillan, J. Am. Chem. Soc., 2013, 135, 13387–13392 CrossRef CAS PubMed
.
- H. J. Shin, F. S. Tareq, J. H. Kim, M. A. Lee, H.-S. Lee, Y.-J. Lee and J.-S. Lee, Heterocycles, 2013, 87, 307–318 CrossRef CAS
.
- R. W. Phelan, M. Barret, P. D. Cotter, P. M. O'Connor, R. Chen, J. P. Morrissey, A. D. W. Dobson, F. O'Gara and T. M. Barbosa, Mar. Drugs, 2013, 11, 1878–1898 CrossRef CAS PubMed
.
- M. A. M. Mondol, F. S. Tareq, J. H. Kim, M. A. Lee, H.-S. Lee, Y.-J. Lee, J. S. Lee and H. J. Shin, J. Nat. Prod., 2011, 74, 2582–2587 CrossRef CAS PubMed
.
- M. A. M. Mondol, F. S. Tareq, J. H. Kim, M. A. Lee, H.-S. Lee, J. S. Lee, Y.-J. Lee and H. J. Shin, J. Antibiot., 2013, 66, 89–95 CrossRef PubMed
.
- T. A. Mansoor, J. Hong, C.-O. Lee, S.-J. Bae, K. S. Im and J. H. Jung, J. Nat. Prod., 2005, 68, 331–336 CrossRef CAS PubMed
.
- X. Yang, Y. Shimizu, J. R. Steiner and J. Clardy, Tetrahedron Lett., 1993, 34, 761–764 CrossRef CAS
.
- S. Felder, S. Kehraus, E. Neu, G. Bierbaum, T. F. Schäberle and G. M. König, ChemBioChem, 2013, 14, 1363–1371 CrossRef CAS PubMed
.
- S. Felder, S. Dreisigacker, S. Kehraus, E. Neu, G. Bierbaum, P. R. Wright, D. Menche, T. F. Schäberle and G. M. König, Chem.–Eur. J., 2013, 19, 9319–9324 CrossRef CAS PubMed
.
- N. F. Montalvo, N. M. Mohamed, J. J. Enticknap and R. T. Hill, Antonie van Leeuwenhoek, 2005, 87, 29–36 CrossRef CAS PubMed
.
- J. Martín, T. d. S. Sousa, G. Crespo, S. Palomo, I. González, J. R. Tormo, M. de la Cruz, M. Anderson, R. T. Hill, F. Vicente, O. Genilloud and F. Reyes, Mar. Drugs, 2013, 11, 387–398 CrossRef PubMed
.
-
C. Hernandez, M. Librada, F. Romero Millan, A. Fernandez Medarde, R. I. Fernandez Chimeno and J. C. Hidalgo Villar, PCT Int. Appl., WO 2012062906 A1 20120518, 2012
.
-
G. B. Mahajan, S. D. George, P. V. Ranadive, P. D. S. Mishra, S. S. Eyyammadichiyil, R. M. Panshikar, S. N. Sawant, S. Krishna, M. Sivakumar, K. Pari, B. M. Thomas, Z. E. Patel, R. Vishwakarma, C. G. Naik, L. D'Souza and P. Devi, PCT Int. Appl., WO 2007119201, A2 20071025, 2007
.
- S. Palomo, I. Gonzalez, M. de la Cruz, J. Martín, J. R. Tormo, M. Anderson, R. T. Hill, F. Vicente, F. Reyes and O. Genilloud, Mar. Drugs, 2013, 11, 1071–1086 CrossRef CAS PubMed
.
- X. Just-Baringo, P. Bruno, L. K. Ottesen, L. M. Cañedo, F. Albericio and M. Álvarez, Angew. Chem., Int. Ed., 2013, 52, 7818–7821 CrossRef CAS PubMed
.
- S. Carlson, L. Marler, S.-J. Nam, B. D. Santarsiero, J. M. Pezzuto and B. T. Murphy, Mar. Drugs, 2013, 11, 1152–1161 CrossRef CAS PubMed
.
- F. Peng, C. Wang, Y. Xie, H. Jiang, L. Chen, P. Uribe, A. T. Bull, M. Goodfellow, H. Jiang and Y. Lian, Nat. Prod. Res., 2013, 27, 1366–1371 CrossRef PubMed
.
- D. W. Udwary, L. Zeigler, R. N. Asolkar, V. Singan, A. Lapidus, W. Fenical, P. R. Jensen and B. S. Moore, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 10376–10381 CrossRef CAS PubMed
.
- E. J. Skellam, A. K. Stewart, W. K. Strangman and J. L. C. Wright, J. Antibiot., 2013, 66, 431–441 CrossRef CAS PubMed
.
- K. W. Blake and P. G. Sammes, J. Chem. Soc. C, 1970, 980–984 RSC
.
- Y. Macherla, poster, 3rd Europ. Conf. Marine Nat. Prod., Elmau Castle, Germany, 2002 Search PubMed.
- J. Bryans, P. Charlton, I. Chicarelli-Robinson, M. Collins, R. Faint, C. Latham, I. Shaw and S. Trew, J. Antibiot., 1996, 49, 1014–1021 CrossRef CAS PubMed
.
- Q. Zhang, S. Li, Y. Chen, X. Tian, H. Zhang, G. Zhang, Y. Zhu, S. Zhang, W. Zhang and C. Zhang, J. Antibiot., 2013, 66, 31–36 CrossRef CAS PubMed
.
- I. Schneemann, B. Ohlendorf, H. Zinecker, K. Nagel, J. Wiese and J. F. Imhoff, J. Nat. Prod., 2010, 73, 1444–1447 CrossRef CAS PubMed
.
- Z. Lin, J. P. Torres, M. A. Ammon, L. Marett, R. W. Teichert, C. A. Reilly, J. C. Kwan, R. W. Hughen, M. Flores, M. D. Tianero, O. Peraud, J. E. Cox, A. R. Light, A. J. L. Villaraza, M. G. Haygood, G. P. Concepcion, B. M. Olivera and E. W. Schmidt, Chem. Biol., 2013, 20, 73–81 CrossRef CAS PubMed
.
- M. C. Kim, O.-W. Kwon, J.-S. Park, S. Y. Kim and H. C. Kwon, Chem. Pharm. Bull., 2013, 61, 511–515 CrossRef CAS PubMed
.
- P. Fu, P. Liu, Q. Gong, Y. Wang, P. Wang and W. Zhu, RSC Adv., 2013, 3, 20726–20731 RSC
.
- R. Raju, A. M. Piggott, X.-C. Huang and R. J. Capon, Org. Lett., 2011, 13, 2770–2773 CrossRef CAS PubMed
.
- R. Raju, A. M. Piggott, M. Conte, Z. Tnimov, K. Alexandrov and R. J. Capon, Chem.–Eur. J., 2010, 16, 3194–3200 CrossRef CAS PubMed
.
- R. Raju, A. M. Piggott, M. Quezada and R. J. Capon, Tetrahedron, 2013, 69, 692–698 CrossRef CAS
.
- Z. Wang, P. Fu, P. Liu, P. Wang, J. Hou, W. Li and W. Zhu, Chem. Biodiversity, 2013, 10, 281–287 CAS
.
- Z.-C. Wu, S. Li, S.-J. Nam, Z. Liu and C. Zhang, J. Nat. Prod., 2013, 76, 694–701 CrossRef CAS PubMed
.
- N. I. Kalinovskaya, L. A. Romanenko, A. I. Kalinovsky, P. S. Dmitrenok and S. A. Dyshlovoy, Nat. Prod. Commun., 2013, 8, 381–384 CAS
.
- L. Kjaerulff, A. Nielsen, M. Mansson, L. Gram, T. O. Larsen, H. Ingmer and C. H. Gotfredsen, Mar. Drugs, 2013, 11, 5051–5062 CrossRef CAS PubMed
.
- H. K. Zane and A. Butler, J. Nat. Prod., 2013, 76, 648–654 CrossRef CAS PubMed
.
- D.-C. Oh, P. G. Williams, C. A. Kauffman, P. R. Jensen and W. Fenical, Org. Lett., 2006, 8, 1021–1024 CrossRef CAS PubMed
.
- A. L. Lane, S.-J. Nam, T. Fukuda, K. Yamanaka, C. A. Kauffman, P. R. Jensen, W. Fenical and B. S. Moore, J. Am. Chem. Soc., 2013, 135, 4171–4174 CrossRef CAS PubMed
.
- C. M. Woo, N. E. Beizer, J. E. Janso and S. B. Herzon, J. Am. Chem. Soc., 2012, 134, 15285–15288 CrossRef CAS PubMed
.
- C. M. Woo, S. L. Gholap and S. B. Herzon, J. Nat. Prod., 2013, 76, 1238–1241 CrossRef CAS PubMed
.
- X.-W. Yang, G.-Y. Zhang, J.-X. Ying, B. Yang, X.-F. Zhou, A. Steinmetz, Y.-H. Liu and N. Wang, Mar. Drugs, 2013, 11, 33–39 CrossRef CAS PubMed
.
- A. I. M. Khedr, I. Kouno, T. Tanaka and K. Yamada, Heterocycles, 2013, 87, 1029–1037 CrossRef CAS
.
- X.-Y. Lian and Z. Zhang, Nat. Prod. Res., 2013, 27, 2161–2167 CrossRef CAS PubMed
.
- A. Yang, L. Si, Z. Shi, L. Tian, D. Liu, D. Zhou, P. Proksch and W. Lin, Org. Lett., 2013, 15, 5366–5369 CrossRef CAS PubMed
.
- Y. Song, H. Huang, Y. Chen, J. Ding, Y. Zhang, A. Sun, W. Zhang and J. Ju, J. Nat. Prod., 2013, 76, 2263–2268 CrossRef CAS PubMed
.
- N. Liu, F. Shang, L. Xi and Y. Huang, Mar. Drugs, 2013, 11, 1524–1533 CrossRef CAS PubMed
.
-
A. D. Batcho and W. Leimgruber, U.S. Pat., US 3524849 A 19700818, 1970
.
- M. R. Pena and J. K. Stille, J. Am. Chem. Soc., 1989, 111, 5417–5424 CrossRef CAS
.
- R. H. Jiao, H. Xu, J. T. Cui, H. M. Ge and R. X. Tan, J. Appl. Microbiol., 2013, 114, 1046–1053 CrossRef CAS PubMed
.
- I. Djinni, A. Defant, M. Kecha and I. Mancini, Mar. Drugs, 2013, 11, 124–135 CrossRef CAS PubMed
.
- S. T. Khan, H. Komaki, K. Motohashi, I. Kozone, A. Mukai, M. Takagi and K. Shin-ya, Environ. Microbiol., 2011, 13, 391–403 CrossRef CAS PubMed
.
- M. Izumikawa, T. Kawahara, J.-H. Hwang, M. Takagi and K. Shin-Ya, Biosci., Biotechnol., Biochem., 2013, 77, 663–665 CrossRef CAS PubMed
.
- S. Imai, A. Shimazu, K. Furihata, K. Furihata, Y. Hayakawa and H. Seto, J. Antibiot., 1990, 43, 1606–1607 CrossRef CAS PubMed
.
- P. B. Gomes, M. Nett, H.-M. Dahse and C. Hertweck, J. Nat. Prod., 2010, 73, 1461–1464 CrossRef CAS PubMed
.
-
A. Zeeck, S. Breiding-Mack, S. Grabley, H. Voelskow and G. Seibert, Eur. Pat. Appl., EP 260486 A1 19880323, 1998
.
- J. Ren, D. Liu, L. Tian, Y. Wei, P. Proksch, J. Zeng and W. Lin, Bioorg. Med. Chem. Lett., 2013, 23, 301–304 CrossRef CAS PubMed
.
- Z.-Y. You, Y.-H. Wang, Z.-G. Zhang, M.-J. Xu, S.-J. Xie, T.-S. Han, L. Feng, X.-G. Li and J. Xu, Mar. Drugs, 2013, 11, 4035–4049 CrossRef PubMed
.
- P. Fu, F. Kong, Y. Wang, Y. Wang, P. Liu, G. Zuo and W. Zhu, Chin. J. Chem., 2013, 31, 100–104 CrossRef CAS
.
- X. Alvarez–Mico, P. R. Jensen, W. Fenical and C. C. Hughes, Org. Lett., 2013, 15, 988–991 CrossRef PubMed
.
- S. M. Mantovani and B. S. Moore, J. Am. Chem. Soc., 2013, 135, 18032–18035 CrossRef CAS PubMed
.
- H. Kanzaki, S. Yanagisawa and T. Nitoda, J. Antibiot., 2000, 53, 1257–1264 CrossRef CAS PubMed
.
- R. Brown, C. Kelley and S. E. Wiberley, J. Org. Chem., 1965, 30, 277–280 CrossRef CAS
.
- P. Wang, L. Xi, P. Liu, Y. Wang, W. Wang, Y. Huang and W. Zhu, Mar. Drugs, 2013, 11, 1035–1049 CrossRef CAS PubMed
.
- C. Chen, J. Wang, H. Guo, W. Hou, N. Yang, B. Ren, M. Liu, H. Dai, X. Liu, F. Song and L. Zhang, Appl. Microbiol. Biotechnol., 2013, 97, 3885–3892 CrossRef CAS PubMed
.
- T. A. Knappe, U. Linne, S. Zirah, S. Rebuffat, X. Xie and M. A. Marahiel, J. Am. Chem. Soc., 2008, 130, 11446–11454 CrossRef CAS PubMed
.
- S. Um, Y.-J. Kim, H. Kwon, H. wen, S.-H. Kim, H. C. Kwon, S. Park, J. Shin and D.-C. Oh, J. Nat. Prod., 2013, 76, 873–879 CrossRef CAS PubMed
.
- K. Shiomi, H. Iinuma, M. Hamada, H. Naganawa, M. Manabe, C. Matsuki, T. Takeuchi and H. Umezawa, J. Antibiot., 1986, 39, 487–493 CrossRef CAS PubMed
.
- K. Shiomi, H. Nakamura, H. Iinuma, H. Naganawa, T. Takeuchi, H. Umezawa and Y. Iitaka, J. Antibiot., 1987, 40, 1213–1219 CrossRef CAS PubMed
.
- Y.-B. Cheng, P. R. Jensen and W. Fenical, Eur. J. Org. Chem., 2013, 18, 3751–3757 CrossRef PubMed
.
- Z. Wu, S. Li, J. Li, Y. Chen, K. Saurav, Q. Zhang, H. Zhang, W. Zhang, W. Zhang, S. Zhang and C. Zhang, Mar. Drugs, 2013, 11, 2113–2125 CrossRef PubMed
.
-
Y. Igarashi, T. Iida, K. Miyanochi and Y. Sudo, Jpn. Kokai Tokkyo Koho, JP 2011010586 A 20110120, 2011
.
- K. H. Jang, S.-J. Nam, J. B. Locke, C. A. Kauffman, D. S. Beatty, L. A. Paul and W. Fenical, Angew. Chem., Int. Ed., 2013, 52, 7822–7824 CrossRef CAS PubMed
.
- K. Takada, A. Ninomiya, M. Naruse, Y. Sun, M. Miyazaki, Y. Nogi, S. Okada and S. Matsunaga, J. Org. Chem., 2013, 78, 6746–6750 CrossRef CAS PubMed
.
- A. Pesic, H. I. Baumann, K. Kleinschmidt, P. Ensle, J. Wiese, R. D. Süssmuth and J. F. Imhoff, Mar. Drugs, 2013, 11, 4834–4857 CrossRef CAS PubMed
.
- Y. Zhang, X. Zhou, H. Huang, X. Tian, Y. Song, S. Zhang and J. Ju, J. Antibiot., 2013, 66, 327–331 CrossRef CAS PubMed
.
- D. E. Williams, D. S. Dalisay, F. Li, J. Amphlett, W. Maneerat, M. A. G. Chavez, Y. A. Wang, T. Matainaho, W. Yu, P. J. Brown, C. H. Arrowsmith, M. Vedadi and R. J. Andersen, Org. Lett., 2013, 15, 414–417 CrossRef CAS PubMed
.
- S.-J. Nam, C. A. Kauffman, P. R. Jensen and W. Fenical, Tetrahedron, 2011, 67, 6707–6712 CrossRef CAS PubMed
.
- S.-J. Nam, C. A. Kauffman, L. A. Paul, P. R. Jensen and W. Fenical, Org. Lett., 2013, 15, 5400–5403 CrossRef CAS PubMed
.
- M. S. Abdelfattah, Nat. Prod. Res., 2013, 27, 2126–2131 CrossRef CAS PubMed
.
- E. Z. Ilan, M. R. Torres, J. Prudhomme, K. Le Roch, P. R. Jensen and W. Fenical, J. Nat. Prod., 2013, 76, 1815–1818 CrossRef PubMed
.
- C. Wu, Y. Tan, M. Gan, Y. Wang, Y. Guan, X. Hu, H. Zhou, X. Shang, X. You, Z. Yang and C. Xiao, J. Nat. Prod., 2013, 76, 2153–2157 CrossRef CAS PubMed
.
- S. Um, T. J. Choi, H. Kim, B. Y. Kim, S.-H. Kim, S. K. Lee, K.-B. Oh, J. Shin and D.-C. Oh, J. Org. Chem., 2013, 78, 12321–12329 CrossRef CAS PubMed
.
- M. Bae, H. Kim, Y. Shin, B. Y. Kim, S. K. Lee, K.-B. Oh, J. Shin and D.-C. Oh, Mar. Drugs, 2013, 11, 2882–2893 CrossRef PubMed
.
- H.-Q. Pan, S.-Y. Zhang, N. Wang, Z.-L. Li, H.-M. Hua, J.-C. Hu and S.-J. Wang, Mar. Drugs, 2013, 11, 3891–3901 CrossRef CAS PubMed
.
- Y. Igarashi, T. Zhou, S. Sato, T. Matsumoto, L. Yu and N. Oku, Org. Lett., 2013, 15, 5678–5681 CrossRef CAS PubMed
.
- X.-W. Yang, K. Peng, Z. Liu, G.-Y. Zhang, J. Li, N. Wang, A. Steinmetz and Y. Liu, J. Nat. Prod., 2013, 76, 2360–2363 CrossRef CAS PubMed
.
- X. Xu, L. Yin, S. Wang, H. Liu, J. Gao and S. Zhao, Rec. Nat. Prod., 2013, 7, 292–295 CAS
.
- D.-C. Oh, W. K. Strangman, C. A. Kauffman, P. R. Jensen and W. Fenical, Org. Lett., 2007, 9, 1525–1528 CrossRef CAS PubMed
.
- Y. Xu, R. D. Kersten, S.-J. Nam, L. Lu, A. M. Al-Suwailem, H. Zheng, W. Fenical, P. C. Dorrestein, B. S. Moore and P.-Y. Qian, J. Am. Chem. Soc., 2012, 134, 8625–8632 CrossRef CAS PubMed
.
-
I. Kaneko, H. Minekura, Y. Takeuchi, K. Kodama, T. Nakamura, H. Haruyama and Y. Sakaida, Japanese Patent, JP 06298796 A 1994
.
- A. C. Ross, Y. Xu, L. Lu, R. D. Kersten, Z. Shao, A. M. Al-Suwailem, P. C. Dorrestein, P.-Y. Qian and B. S. Moore, J. Am. Chem. Soc., 2013, 135, 1155–1162 CrossRef CAS PubMed
.
- S. Um, Y. Pyee, E.-H. Kim, S. K. Lee, J. Shin and D.-C. Oh, Mar. Drugs, 2013, 11, 611–622 CrossRef CAS PubMed
.
- X. Li, S. Vanner, W. Wang, Y. Li, V. A. Gallardo and N. A. Magarvey, J. Antibiot., 2013, 66, 443–446 CrossRef CAS PubMed
.
- J. Riedlinger, A. Reicke, H. Zaehner, B. Krismer, A. T. Bull, L. A. Maldonado, A. C. Ward, M. Goodfellow, B. Bister, D. Bischoff, R. D. Süssmuth and H.-P. Fiedler, J. Antibiot., 2004, 57, 271–279 CrossRef CAS PubMed
.
- S. Keller, G. Nicholson, C. Drahl, E. Sorensen, H.-P. Fiedler and R. D. Süssmuth, J. Antibiot., 2007, 60, 391–394 CrossRef CAS PubMed
.
- Q. Wang, F. Song, X. Xiao, P. Huang, L. Li, A. Monte, W. M. Abdel-Mageed, J. Wang, H. Guo, W. He, F. Xie, H. Dai, M. Liu, C. Chen, H. Xu, M. Liu, A. M. Piggott, X. Liu, R. J. Capon and L. Zhang, Angew. Chem., Int. Ed., 2013, 52, 1231–1234 CrossRef CAS PubMed
.
- R. Raju, A. M. Piggott, M. M. Conte and R. J. Capon, Org. Biomol. Chem., 2010, 8, 4682–4689 CAS
.
- R. Sugiyama, S. Nishimura and H. Kakeya, Tetrahedron Lett., 2013, 54, 1531–1533 CrossRef CAS
.
- K. Motohashi, M. Takagi and K. Shin-ya, J. Nat. Prod., 2010, 73, 226–228 CrossRef CAS PubMed
.
- T. Hosoya, T. Hirokawa, M. Takagi and K. Shin-ya, J. Nat. Prod., 2012, 75, 285–289 CrossRef CAS PubMed
.
- K. Motohashi, M. Takagi and K. Shin-ya, J. Nat. Prod., 2013, 76, 1230 CrossRef CAS
.
- T. Hosoya, T. Hirokawa, M. Takagi and K. Shin-ya, J. Nat. Prod., 2013, 76, 1231 CrossRef CAS
.
- T. Kameyama, A. Takahashi, S. Kurasawa, M. Ishizuka, Y. Okami, T. Takeuchi and H. Umezawa, J. Antibiot., 1987, 40, 1664–1670 CrossRef CAS PubMed
.
- A. Takahashi, H. Nakamura, T. Kameyama, S. Kurasawa, H. Naganawa, Y. Okami, T. Takeuchi, H. Umezawa and Y. Iitaka, J. Antibiot., 1987, 40, 1671–1676 CrossRef CAS PubMed
.
- G. Winkelmann, B. Busch, A. Hartmann, G. Kirchhof, R. Sussmuth and G. Jung, BioMetals, 1999, 12, 255–264 CrossRef CAS PubMed
.
- M. J. Fujita, K. Nakano and R. Sakai, Molecules, 2013, 18, 3917–3926 CrossRef CAS PubMed
.
- Y. Hu, K. Wang and J. B. MacMillan, Org. Lett., 2013, 15, 390–393 CrossRef CAS PubMed
.
- R. D. Shingare, R. Velayudham, J. R. Gawade and D. S. Reddy, Org. Lett., 2013, 15, 4556–4559 CrossRef CAS PubMed
.
- G. Deltour, F. Binon, F. Henaux and R. Charlier, Arch. Int. Pharmacodyn. Ther., 1961, 131, 84–106 CAS
.
- H. Koshima, K. Ding, Y. Chisaka, T. Matsuura, I. Miyahara and K. Hirotsu, J. Am. Chem. Soc., 1997, 119, 10317–10324 CrossRef CAS
.
- C. Gang, M.-X. Shen, W. Xin, X.-M. Fan, H.-M. Ma, H.-H. Wu and Y.-H. Pei, Chem. Nat. Compd., 2013, 49, 291–293 CrossRef CAS
.
-
K. Hong, G. Yuan, H. Lin, Q. Xie, C. Wang, X. Huang and Y. Tang, Faming Zhuanli Shenqing, CN 101792474 A 20100804, 2010
.
- G. Yuan, K. Hong, H. Lin, Z. She and J. Li, Mar. Drugs, 2013, 11, 817–829 CrossRef CAS PubMed
.
- W.-J. Ding, S.-Q. Zhang, J.-H. Wang, Y.-X. Lin, Q.-X. Liang, W.-J. Zhao and C.-Y. Li, J. Asian Nat. Prod. Res., 2013, 15, 209–214 CrossRef CAS PubMed
.
- G. Assante, S. Dallavalle, L. Malpezzi, G. Nasini, S. Burruano and L. Torta, Tetrahedron, 2005, 61, 7686–7692 CrossRef CAS
.
- Suciati, J. A. Fraser, L. K. Lambert, G. K. Pierens, P. V. Bernhardt and M. J. Garson, J. Nat. Prod., 2013, 76, 1432–1440 CrossRef CAS PubMed
.
-
D. Li, Q. Gu, G. Zhang and T. Zhu, Faming Zhuanli Shenqing, CN 102001921 A 20110406, 2011
.
- G. Zhang, G. Wu, T. Zhu, T. Kurtán, A. Mándi, J. Jiao, J. Li, X. Qi, Q. Gu and D. Li, J. Nat. Prod., 2013, 76, 1946–1957 CrossRef CAS PubMed
.
- L.-R. Xie, D.-Y. Li, Z.-L. Li, H.-M. Hua, P.-L. Wang and X. Wu, Nat. Prod. Res., 2013, 27, 847–850 CrossRef CAS PubMed
.
- L. Chen, W.-W. Zhang, Q.-H. Zheng, Q.-Y. Liu, P. Zhong, X. Hu, Z.-X. Fang and Q.-Q. Zhang, Heterocycles, 2013, 87, 861–868 CrossRef CAS
.
- O. I. Zhuravleva, S. S. Afiyatullov, E. A. Yurchenko, V. A. Denisenko, N. N. Kirichuk and P. S. Dmitrenok, Nat. Prod. Commun., 2013, 8, 1071–1074 CAS
.
- W. Jiang, P. Ye, C.-T. A. Chen, K. Wang, P. Liu, S. He, X. Wu, L. Gan, Y. Ye and B. Wu, Mar. Drugs, 2013, 11, 4761–4772 CrossRef CAS PubMed
.
- G.-X. Zhou, E. M. K. Wijeratne, D. Bigelow, L. S. Pierson III, H. D. VanEtten and A. A. L. Gunatilaka, J. Nat. Prod., 2004, 67, 328–332 CrossRef CAS PubMed
.
- W. Keller-Schierlein and E. Kupfer, Helv. Chim. Acta, 1979, 62, 1501–1524 CrossRef CAS
.
- Z. Lin, T. Zhu, H. Wei, G. Zhang, H. Wang and Q. Gu, Eur. J. Org. Chem., 2009, 3045–3051 CrossRef CAS
.
- T. Tomikawa, K. Shin-Ya, H. Seto, N. Okusa, T. Kajiura and Y. Hayakawa, J. Antibiot., 2002, 55, 666–668 CrossRef CAS PubMed
.
- C.-J. Zheng, C.-L. Shao, L.-Y. Wu, M. Chen, K.-L. Wang, D.-L. Zhao, X.-P. Sun, G.-Y. Chen and C.-Y. Wang, Mar. Drugs, 2013, 11, 2054–2068 CrossRef PubMed
.
- X. Liu, F. Song, L. Ma, C. Chen, X. Xiao, B. Ren, X. Liu, H. Dai, A. M. Piggott, Y. Av-Gay, L. Zhang and R. J. Capon, Tetrahedron Lett., 2013, 54, 6081–6083 CrossRef CAS
.
- G.-Y. Li, B.-G. Li, T. Yang, J.-H. Yin, H.-Y. Qi, G.-Y. Liu and G.-L. Zhang, J. Nat. Prod., 2005, 68, 1243–1246 CrossRef CAS PubMed
.
- K. Arai, T. Yoshimura, Y. Itatani and Y. Yamamoto, Chem. Pharm. Bull., 1983, 31, 925–933 CrossRef CAS
.
- M. H. Haroon, S. R. Premaratne, M. I. Choudhry and H. R. W. Dharmaratne, Nat. Prod. Res., 2013, 27, 1060–1066 CrossRef CAS PubMed
.
- F. He, J. Bao, X.-Y. Zhang, Z.-C. Tu, Y.-M. Shi and S.-H. Qi, J. Nat. Prod., 2013, 76, 1182–1186 CrossRef CAS PubMed
.
- S. Sureram, C. Kesornpun, C. Mahidol, S. Ruchirawat and P. Kittakoop, RSC Adv., 2013, 3, 1781–1788 RSC
.
- S. Nozoe, M. Morisaki, K. Fukushima and S. Okuda, Tetrahedron Lett., 1968, 42, 4457–4458 CrossRef
.
- H.-B. Liu, R. Edrada-Ebel, R. Ebel, Y. Wang, B. Schulz, S. Draeger, W. E. G. Mueller, V. Wray, W.-H. Lin and P. Proksch, Helv. Chim. Acta, 2011, 94, 623–631 CrossRef CAS
.
- X.-H. Liu, F.-P. Miao, M.-F. Qiao, R. H. Cichewicz and N.-Y. Ji, RSC Adv., 2013, 3, 588–595 RSC
.
- K. Yamada, M. Doi, T. Yamada, K. Minoura and A. Numata, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 2000, 42, 397–402 Search PubMed
.
- K. Nakanishi, M. Doi, Y. Usami, T. Amagata, K. Minoura, R. Tanaka, A. Numata and T. Yamada, Tetrahedron, 2013, 69, 4617–4623 CrossRef CAS
.
- N.-Y. Ji, X.-H. Liu, F.-P. Miao and M.-F. Qiao, Org. Lett., 2013, 15, 2327–2329 CrossRef CAS PubMed
.
- L.-N. Zhou, H.-Q. Gao, S.-X. Cai, T.-J. Zhu, Q.-Q. Gu and D.-H. Li, Helv. Chim. Acta, 2011, 94, 1065–1070 CrossRef CAS
.
- H. Gao, L. Zhou, S. Cai, G. Zhang, T. Zhu, Q. Gu and D. Li, J. Antibiot., 2013, 66, 539–542 CrossRef CAS PubMed
.
- M. N. Oliveira, L. S. Santos, G. M. S. P. Guilhon, A. S. Santos, I. C. S. Ferreira, M. L. Lopes-Junior, M. S. P. Arruda, A. M. R. Marinho, M. N. da Silva, E. Rodrigues-Filho and M. C. F. Oliveira, J. Braz. Chem. Soc., 2011, 22, 993–996 CrossRef CAS
.
- H.-F. Sun, X.-M. Li, L.-H. Meng, C.-M. Cui, S.-S. Gao, C.-S. Li and B.-G. Wang, Helv. Chim. Acta, 2013, 96, 458–462 CrossRef CAS
.
- R.-R. Sun, F.-P. Miao, J. Zhang, G. Wang, X.-L. Yin and N.-Y. Ji, Magn. Reson. Chem., 2013, 51, 65–68 CrossRef CAS PubMed
.
- J. Peng, X.-Y. Zhang, Z.-C. Tu, X.-Y. Xu and S.-H. Qi, J. Nat. Prod., 2013, 76, 983–987 CrossRef CAS PubMed
.
- S. Tsukamoto, H. Hirota, M. Imachi, M. Fujimuro, H. Onuki, T. Ohta and H. Yokosawa, Bioorg. Med. Chem. Lett., 2005, 15, 191–194 CrossRef CAS PubMed
.
- T. Kuwana, M. Miyazaki, H. Kato and S. Tsukamoto, Chem. Pharm. Bull., 2013, 61, 105–107 CrossRef CAS PubMed
.
- J. Bao, X.-Y. Zhang, X.-Y. Xu, F. He, X.-H. Nong and S.-H. Qi, Tetrahedron, 2013, 69, 2113–2117 CrossRef CAS
.
- Y. Zhou, A. Debbab, A. Mándi, V. Wray, B. Schulz, W. E. G. Müller, M. Kassack, W. Lin, T. Kurtán, P. Proksch and A. H. Aly, Eur. J. Org. Chem., 2013, 5, 894–906 CrossRef
.
- J. Wang, Z. Lu, P. Liu, Y. Wang, J. Li, K. Hong and W. Zhu, Planta Med., 2012, 78, 1861–1866 CrossRef CAS PubMed
.
- K. A. Miller, S. Tsukamoto and R. M. Williams, Nat. Chem., 2009, 1, 63–68 CrossRef CAS PubMed
.
- H. Kato, T. Yoshida, T. Tokue, Y. Nojiri, H. Hirota, T. Ohta, R. M. Williams and S. Tsukamoto, Angew. Chem., Int. Ed., 2007, 46, 2254–2256 CrossRef CAS PubMed
.
- S. Li, J. M. Finefield, J. D. Sunderhaus, T. J. McAfoos, R. M. Williams and D. H. Sherman, J. Am. Chem. Soc., 2012, 134, 788–791 CrossRef CAS PubMed
.
- M. Chen, C.-L. Shao, X.-M. Fu, R.-F. Xu, J.-J. Zheng, D.-L. Zhao, Z.-G. She and C.-Y. Wang, J. Nat. Prod., 2013, 76, 547–553 CrossRef CAS PubMed
.
- S. Tsukamoto, H. Kato, M. Samizo, Y. Nojiri, H. Onuki, H. Hirota and T. Ohta, J. Nat. Prod., 2008, 71, 2064–2067 CrossRef CAS PubMed
.
- S. Tsukamoto, H. Umaoka, K. Yoshikawa, T. Ikeda and H. Hirota, J. Nat. Prod., 2010, 73, 1438–1440 CrossRef CAS PubMed
.
- J. M. Finefield and R. M. Williams, J. Org. Chem., 2010, 75, 2785–2789 CrossRef CAS PubMed
.
- J. M. Finefield and R. M. Williams, J. Org. Chem., 2013, 78, 8214 CrossRef CAS
.
- S. Tsukamoto, H. Kato, M. Samizo, Y. Nojiri, H. Ohnuki, H. Hirota and T. Ohta, J. Nat. Prod., 2013, 76, 1233 CrossRef CAS
.
- S. Tsukamoto, H. Umaoka, K. Yoshikawa, T. Ikeda and H. Hirota, J. Nat. Prod., 2013, 76, 1232 CrossRef CAS
.
- J. Bao, X.-Y. Xu, X.-Y. Zhang and S.-H. Qi, Nat. Prod. Commun., 2013, 8, 1127–1128 CAS
.
- G. N. Belofsky, P. R. Jensen, M. K. Renner and W. Fenical, Tetrahedron, 1998, 54, 1715–1724 CrossRef CAS
.
- G. Assante, L. Carmada, R. Locci, L. Merlini, G. Nasini and E. Papadopoulos, J. Agric. Food Chem., 1981, 29, 785–787 CrossRef CAS
.
- X. Xu, F. He, X. Zhang, J. Bao and S. Qi, Food Chem. Toxicol., 2013, 53, 46–51 CrossRef CAS PubMed
.
- X.-Y. Xu, X.-Y. Zhang, F. He, J. Peng, X.-H. Nong and S.-H. Qi, Nat. Prod. Commun., 2013, 8, 1069–1070 CAS
.
- M. F. Elsebai, V. Rempel, G. Schnakenburg, S. Kehraus, C. E. Müller and G. M. König, ACS Med. Chem. Lett., 2011, 2, 866–869 CrossRef CAS PubMed
.
- F. Zhu, G. Y. Chen, J. Wu and J. Pan, Nat. Prod. Res., 2013, 27, 1960–1964 CrossRef CAS PubMed
.
- L.-Y. Zang, W. Wei, T. Wang, Y. Guo, R.-X. Tan and H.-M. Ge, Nat. Prod. Bioprospect., 2012, 2, 117–120 CrossRef CAS
.
- E. Yoshida, H. Fujimoto, M. Baba and M. Yamazaki, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1993, 35, 290–297 Search PubMed
.
- X.-L. Sun, H. Takayanagi, K. Matsuzaki, H. Tanaka, K. Furuhata and S. Omura, J. Antibiot., 1996, 49, 689–692 CrossRef CAS PubMed
.
- N. Jansen, B. Ohlendorf, A. Erhard, T. Bruhn, G. Bringmann and J. F. Imhoff, Mar. Drugs, 2013, 11, 800–816 CrossRef CAS PubMed
.
- G. Schlingmann, L. Milne and G. T. Carter, Tetrahedron, 2002, 58, 6825–6835 CrossRef CAS
.
- J. Silber, B. Ohlendorf, A. Labes, A. Erhard and J. F. Imhoff, Mar. Drugs, 2013, 11, 3309–3323 CrossRef PubMed
.
-
H. Matsumoto, K. Yoshikawa, S. Arihara and T. Miyataka, Jpn. Kokai Tokkyo Koho, JP 2005213144 A 20050811, 2005
.
- Y. Zhao, S.-Q. Li, H.-J. Li and W.-J. Lan, Chem. Nat. Compd., 2013, 49, 653–656 CrossRef CAS
.
- Y.-M. Ying, W.-G. Shan, L.-W. Zhang, Y. Chen and Z.-J. Zhan, Helv. Chim. Acta, 2013, 96, 2092–2097 CrossRef CAS
.
- H.-J. Li, Y.-L. Xie, Z.-L. Xie, Y. Chen, C.-K. Lam and W.-J. Lan, Mar. Drugs, 2012, 10, 627–638 CrossRef CAS PubMed
.
- H. Takazawa and S. Kashino, Chem. Pharm. Bull., 1991, 39, 555–557 CrossRef CAS PubMed
.
- E. Amouzou, W. A. Ayer and L. M. Browne, J. Nat. Prod., 1989, 52, 1042–1054 CrossRef CAS
.
- F. Bohlmann, E. Inhoffen and P. Herbst, Chem. Ber., 1957, 90, 1661–1667 CrossRef CAS
.
- H.-J. Li, T. Chen, Y.-L. Xie, W.-D. Chen, X.-F. Zhu and W.-J. Lan, Mar. Drugs, 2013, 11, 551–558 CrossRef CAS PubMed
.
- J. Jeon, E. Julianti, H. Oh, W. Park, D.-C. Oh, K.-B. Oh and J. Shin, Tetrahedron Lett., 2013, 54, 3111–3115 CrossRef CAS
.
- P. Sun, D.-X. Xu, A. Mándi, T. Kurtán, T.-J. Li, B. Schulz and W. Zhang, J. Org. Chem., 2013, 78, 7030–7047 CrossRef CAS PubMed
.
- L. Sun, D. Li, M. Tao, Y. Chen, Q. Zhang, F. Dan and W. Zhang, Nat. Prod. Res., 2013, 27, 1298–1304 CrossRef CAS PubMed
.
- T. Amagata, M. Tanaka, T. Yamada, K. Minoura and A. Numata, J. Nat. Prod., 2008, 71, 340–345 CrossRef CAS PubMed
.
- T. Amagata, M. Tanaka, T. Yamada, Y.-P. Chen, K. Minoura and A. Numata, Tetrahedron Lett., 2013, 54, 5960–5962 CrossRef CAS
.
- J. Sanz, A. C. Soria and M. C. Garcia-Vallejo, J. Chromatogr. A, 2004, 1024, 139–146 CrossRef CAS PubMed
.
- K. Mori, Agric. Biol. Chem., 1976, 40, 1617–1619 CrossRef CAS
.
- H. Zhu, X. Hua, T. Gong, J. Pang, Q. Hou and P. Zhu, Phytochem. Lett., 2013, 6, 392–396 CrossRef CAS
.
- A. N. Yurchenko, O. F. Smetanina, Y. V. Khudyakova, N. N. Kirichuk, E. L. Chaikina, M. M. Anisimov and S. S. Afiyatullov, Chem. Nat. Compd., 2013, 49, 857–860 CrossRef CAS
.
- S. B. Singh, D. L. Zink, G. F. Bills, A. Teran, K. C. Silverman, R. B. Lingham, P. Felock and D. J. Hazuda, Bioorg. Med. Chem. Lett., 2003, 13, 713–717 CrossRef CAS PubMed
.
- K. Koyama, K. Kinoshita, N. Hamada, S. Natori and Y. Iitaka, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1987, 29, 713–720 CAS
.
- X. Kong, X. Ma, Y. Xie, S. Cai, T. Zhu, Q. Gu and D. Li, Arch. Pharmacal Res., 2013, 36, 739–744 CrossRef CAS PubMed
.
- A. Eamvijarn, N. M. Gomes, T. Dethoup, J. Buaruang, L. Manoch, A. Silva, M. Pedro, I. Marini, V. Roussis and A. Kijjoa, Tetrahedron, 2013, 49, 8583–8591 CrossRef
.
- N. Xu, Y. Cao, L. Wang, G. Chen and Y.-H. Pei, J. Asian Nat. Prod. Res., 2013, 15, 731–736 CrossRef CAS PubMed
.
- F.-T. Sun, G. Chen, J. Bai, W. Li and Y.-H. Pei, J. Asian Nat. Prod. Res., 2012, 14, 1109–1115 CrossRef CAS PubMed
.
- Z. Mosadeghzad, Z. Zuriati, A. Asmat, U. Gires, R. Wickneswari, P. Pittayakhajonwut and G. H. N. Farahani, Chem. Nat. Compd., 2013, 49, 621–625 CrossRef CAS
.
- C.-Y. An, X.-M. Li, C.-S. Li, S.-S. Gao, Z. Shang and B.-G. Wang, Helv. Chim. Acta, 2013, 96, 682–687 CrossRef CAS
.
- P. Wang, D. Li, L. Xie, X. Wu, H. Hua and Z. Li, Nat. Prod. Commun., 2013, 8, 1397–1398 Search PubMed
.
- S. Shen, W. Li and J. Wang, Nat. Prod. Res., 2013, 27, 2286–2291 CrossRef CAS PubMed
.
- Y.-L. Sun, J. Bao, K.-S. Liu, X.-Y. Zhang, F. He, Y.-F. Wang, X.-H. Nong and S.-H. Qi, Planta Med., 2013, 79, 1474–1479 CrossRef CAS PubMed
.
- T. Sassa, H. Kachi and M. Nukina, J. Antibiot., 1985, 38, 439–441 CrossRef CAS PubMed
.
- M. Imran, L. Mitu, S. Latif, Z. Mahmood, I. Naimat, S. S. Zaman and S. Fatima, J. Serb. Chem. Soc., 2010, 75, 1075–1084 CrossRef CAS
.
- C.-S. Li, X.-M. Li, S.-S. Gao, Y.-H. Lu and B.-G. Wang, Mar. Drugs, 2013, 11, 3068–3076 CrossRef PubMed
.
- F. Sóti, M. Incze, M. Kajtár-Peredy, E. Baitz-Gács, L. Imre and L. Farkas, Chem. Ber., 1977, 110, 979–984 CrossRef
.
- N. Tan, Y. Tao, J. Pan, S. Wang, F. Xu, Z. She, Y. Lin and E. B. G. Jones, Chem. Nat. Compd., 2008, 44, 296–300 CrossRef CAS
.
- K. Drauz, A. Kleemann, J. Martens, P. Scherberich and F. Effenberger, J. Org. Chem., 1986, 51, 3494–3498 CrossRef CAS
.
- S. Jin, P. Wessig and J. Liebscher, Eur. J. Org. Chem., 2000, 1993–1999 CrossRef CAS
.
- Y. Kimura, T. Yoshinari, H. Koshino, S. Fujioka, K. Okada and A. Shimada, Biosci., Biotechnol., Biochem., 2007, 71, 1896–1901 CrossRef CAS PubMed
.
- M.-H. Wang, X.-M. Li, C.-S. Li, N.-Y. Ji and B.-G. Wang, Mar. Drugs, 2013, 11, 2230–2238 CrossRef PubMed
.
- M. Vansteelandt, E. Blanchet, M. Egorov, F. Petit, L. Toupet, A. Bondon, F. Monteau, B. Le Bizec, O. P. Thomas, Y. F. Pouchus, R. Le Bot and O. Grovel, J. Nat. Prod., 2013, 76, 297–301 CrossRef CAS PubMed
.
- H. Gao, L. Zhou, D. Li, Q. Gu and T.-J. Zhu, Helv. Chim. Acta, 2013, 96, 514–519 CrossRef CAS
.
- E. Julianti, J.-H. Lee, L. Liao, W. Park, S. Park, D.-C. Oh, K.-B. Oh and J. Shin, Org. Lett., 2013, 15, 1286–1289 CrossRef CAS PubMed
.
- A. Numata, C. Takahashi, T. Matsushita, T. Miyamoto, K. Kawai, Y. Usami, E. Matsumura, M. Inoue, H. Ohishi and T. Shingu, Tetrahedron Lett., 1992, 33, 1621–1624 CrossRef CAS
.
- C.-B. Cui, H. Kakeya and H. Osada, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 1996, 38, 49–54 Search PubMed
.
- C. Takahashi, T. Matsushita, M. Doi, K. Minoura, T. Shingu, Y. Kumeda and A. Numata, J. Chem. Soc., Perkin Trans. 1, 1995, 2345–2353 RSC
.
- P. M. Scott, J. Polonsky and M. A. Merrien, J. Agric. Food Chem., 1979, 27, 201–202 CrossRef CAS
.
- K. Nozawa and S. Nakajima, J. Nat. Prod., 1979, 42, 374–377 CrossRef CAS
.
- S. Ohmomo, T. Sato, T. Utagawa and M. Abe, Agric. Biol. Chem., 1975, 3, 1333–1334 CrossRef
.
- K. F. Nielsen, M. W. Sumarah, J. C. Frisvad and J. D. Miller, J. Agric. Food Chem., 2006, 54, 3756–3763 CrossRef CAS PubMed
.
- F. He, Z. Han, J. Peng, P.-Y. Qian and S.-H. Qi, Nat. Prod. Commun., 2013, 8, 329–332 CAS
.
- R. J. Capon, M. Stewart, R. Ratnayake, E. Lacey and J. H. Gill, J. Nat. Prod., 2007, 70, 1746–1752 CrossRef CAS PubMed
.
- D.-S. Lee, J.-H. Jang, W. Ko, K.-S. Kim, J. H. Sohn, M.-S. Kang, J. S. Ahn, Y.-C. Kim and H. Oh, Mar. Drugs, 2013, 11, 1409–1426 CrossRef CAS PubMed
.
- H. Ren, L. Tian, Q. Gu and W. Zhu, Arch. Pharmacal Res., 2006, 29, 59–63 CrossRef CAS
.
- V. H. Powell and M. D. Sutherland, Aust. J. Chem., 1967, 20, 541–553 CrossRef CAS
.
- T. Jiang, L. Tian, A. Guo, H. Fu, Y. Pei and W. Lin, Yaoxue Xuebao, 2002, 37, 271–274 CAS
.
- J. Bao, Y.-L. Sun, X.-Y. Zhang, Z. Han, H.-C. Gao, F. He, P.-Y. Qian and S.-H. Qi, J. Antibiot., 2013, 66, 219–223 CrossRef CAS PubMed
.
- J. Qi, C.-L. Shao, Z.-Y. Li, L.-S. Gan, X.-M. Fu, w.-T. Bian, H.-Y. Zhao and C.-Y. Wang, J. Nat. Prod., 2013, 76, 571–579 CrossRef CAS PubMed
.
- M. H. Kossuga, A. G. Ferreira, L. D. Sette and R. G. S. Berlinck, Rev. Bras. Farmacogn., 2012, 22, 257–267 CrossRef CAS
.
- M. H. Kossuga, A. G. Ferreira, L. D. Sette and R. G. S. Berlinck, Nat. Prod. Commun., 2013, 8, 721–724 CAS
.
- G. Wu, A. Lin, Q. Gu, T. Zhu and D. Li, Mar. Drugs, 2013, 11, 1399–1408 CrossRef CAS PubMed
.
- P. S. Baran and E. J. Corey, J. Am. Chem. Soc., 2002, 124, 7904–7905 CrossRef CAS PubMed
.
- T. H. Quang, D.-S. Lee, J. H. Sohn, Y.-C. Kim and H. Oh, Bull. Korean Chem. Soc., 2013, 34, 3109–3112 CrossRef CAS
.
- E. Li, L. Jiang, L. Guo, H. Zhang and Y. Che, Bioorg. Med. Chem., 2008, 16, 7894–7899 CrossRef CAS PubMed
.
- M.-Y. Wei, D. Li, C.-L. Shao, D.-S. Deng and C.-Y. Wang, Mar. Drugs, 2013, 11, 1050–1060 CrossRef CAS PubMed
.
- B. Wu, X. Wu, M. Sun and M. Li, Mar. Drugs, 2013, 11, 2713–2721 CrossRef PubMed
.
- M. F. Elsebai, S. Kehraus and G. M. König, Steroids, 2013, 78, 880–883 CrossRef CAS PubMed
.
- V. Rukachaisirikul, S. Kannai, S. Klaiklay, S. Phongpaichit and J. Sakayaroj, Tetrahedron, 2013, 69, 6981–6986 CrossRef CAS
.
- C.-L. Shao, R.-F. Xu, M.-Y. Wei, Z.-G. She and C.-Y. Wang, J. Nat. Prod., 2013, 76, 779–782 CrossRef CAS PubMed
.
- M. J. Vazquez, A. Vega, A. Rivera-Sagredo, M. D. Jimenez-Alfaro, E. Diez and J. A. Hueso-Rodriguez, Tetrahedron, 2004, 60, 2379–2385 CrossRef CAS
.
- X. Ma, L. Li, T. Zhu, M. Ba, G. Li, Q. Gu, Y. Guo and D. Li, J. Nat. Prod., 2013, 76, 2298–2306 CrossRef CAS PubMed
.
- M. Muto, H. Kohri, H. Ishitani and K. Higaki, Nagoya Kogyo Daigaku Gakuho, 1970, 22, 159–164 Search PubMed
.
- C. Almeida, E. Eguereva, S. Kehraus and G. M. König, J. Nat. Prod., 2013, 76, 322–326 CrossRef CAS PubMed
.
- A. Haga, H. Tamoto, M. Ishino, E. Kimura, T. Sugita, K. Kinoshita, K. Takahashi, M. Shiro and K. Koyama, J. Nat. Prod., 2013, 76, 750–754 CrossRef CAS PubMed
.
- L. Chen, P. Zhong, J.-R. Pan, K.-J. Zhou, K. Huang, Z.-X. Fang and Q.-Q. Zhang, Heterocycles, 2013, 87, 645–655 CrossRef CAS
.
- J. Ren, Y. Yang, D. Liu, W. Chen, P. Proksch, B. Shao and W. Lin, J. Chromatogr. A, 2013, 1309, 90–95 CrossRef CAS PubMed
.
- A. Carroux, A.-I. van Bohemen, C. Roullier, T. R. du Pont, M. Vansteelandt, A. Bondon, A. Zalouk-Vergnoux, Y. F. Pouchus and N. Ruiz, Chem. Biodiversity, 2013, 10, 772–786 CAS
.
- I. Panizel, O. Yarden, M. Ilan and S. Carmeli, Mar. Drugs, 2013, 11, 4937–4960 CrossRef CAS PubMed
.
- Z.-L. Xie, H.-J. Li, L.-Y. Wang, W.-L. Liang, W. Liu and W.-J. Lan, Nat. Prod. Commun., 2013, 8, 67–68 CAS
.
- B. R. Clark, R. J. Capon, E. Lacey, S. Tennant and J. H. Gill, Org. Biomol. Chem., 2006, 4, 1520–1528 CAS
.
- M. M. Chien, P. L. Schiff Jr, D. J. Slatkin and J. E. Knapp, Lloydia, 1977, 40, 301–302 CAS
.
- S. Iwasaki, H. Muro, S. Nozoe, S. Okuda and Z. Sato, Tetrahedron Lett., 1972, 13–16 CrossRef CAS
.
- X.-H. Nong, Z.-H. Zheng, X.-Y. Zhang, X.-H. Lu and S.-H. Qi, Mar. Drugs, 2013, 11, 1718–1727 CrossRef CAS PubMed
.
- L. Chen, W. Liu, K. Huang, X. Hu, Z.-X. Fang, J.-L. Wu and Q.-Q. Zhang, Heterocycles, 2011, 83, 1853–1858 CrossRef CAS
.
- L. Xu, J. Xue, H. Xu, X. Liu, W. Ma and X. Wei, Heterocycles, 2006, 68, 1955–1959 CrossRef CAS
.
- L. Chen, K. Huang, P. Zhong, X. Hu, Z.-X. Fang, J. Wu and Q.-Q. Zhang, Heterocycles, 2012, 85, 413–419 CrossRef CAS
.
- N. Khamthong, V. Rukachaisirikul, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Tetrahedron, 2012, 68, 8245–8250 CrossRef CAS
.
- C.-L. Shao, H.-X. Wu, C.-Y. Wang, Q.-A. Liu, Y. Xu, M.-Y. Wei, P.-Y. Qian, Y.-C. Gu, C.-J. Zheng, Z.-G. She and Y.-C. Lin, J. Nat. Prod., 2011, 74, 629–633 CrossRef CAS PubMed
.
- C.-L. Shao, H.-X. Wu, C.-Y. Wang, Q.-A. Liu, Y. Xu, M.-Y. Wei, P.-Y. Qian, Y.-C. Gu, C.-J. Zheng, Z.-G. She and Y.-C. Lin, J. Nat. Prod., 2013, 76, 302 CrossRef CAS
.
-
A. McClay, H. van Den Berg, P. Johnston, W. Watters, K. McGarell, D. Waugh, P. Armstrong, Z. Delbederi, C. Higgins and T. Mills, PCT Int. Appl., WO 2006046071 A1 20060504, 2006
.
- C. D. Donner and M. Gill, J. Chem. Soc., Perkin Trans. 1, 2002, 938–948 RSC
.
- K. Yun, Z. Feng, H. D. Choi, J. S. Kang and B. W. Son, Chem. Nat. Compd., 2013, 49, 24–26 CrossRef CAS
.
- S. Tamura and A. Sakurai, Agric. Biol. Chem., 1964, 28, 331–336 CrossRef CAS
.
- A. Abdel-Lateff, T. Okino, W. M. Alarif and S. S. Al-Lihaibi, J. Saudi Chem. Soc., 2013, 17, 161–165 CrossRef CAS
.
- H. Nagasawa, A. Suzuki and S. Tamura, Agric. Biol. Chem., 1978, 42, 1303–1304 CrossRef CAS
.
- A. J. Flewelling, J. A. Johnson and C. A. Gray, Nat. Prod. Commun., 2013, 8, 373–374 CAS
.
- G. Gatti, R. Cardillo, C. Fuganti and D. Ghiringhelli, J. Chem. Soc., Chem. Commun., 1976, 435–436 RSC
.
- D.-L. Li, X.-M. Li, T.-G. Li, H.-Y. Dang and B.-G. Wang, Helv. Chim. Acta, 2008, 91, 1888–1893 CrossRef CAS
.
- H. Gao, T. Zhu, D. Li, Q. Gu and W. Liu, Arch. Pharmacal Res., 2013, 36, 952–956 CrossRef CAS PubMed
.
- S.-W. Sun, C.-Z. Ji, Q.-Q. Gu, D.-H. Li and T.-J. Zhu, J. Asian Nat. Prod. Res., 2013, 15, 956–961 CrossRef CAS PubMed
.
- S. A. Neff, S. U. Lee, Y. Asami, J. S. Ahn, H. Oh, J. Baltrusaitis, J. B. Gloer and D. T. Wicklow, J. Nat. Prod., 2012, 75, 464–472 CrossRef CAS PubMed
.
- C.-Y. An, X.-M. Li, H. Luo, C.-S. Li, M.-H. Wang, G.-M. Xu and B.-G. Wang, J. Nat. Prod., 2013, 76, 1896–1901 CrossRef CAS PubMed
.
- C.-Y. An, X.-M. Li, C.-S. Li, M.-H. Wang, G.-M. Xu and B.-G. Wang, Mar. Drugs, 2013, 11, 2682–2694 CrossRef PubMed
.
- D. Liu, X.-M. Li, C.-S. Li and B.-G. Wang, Helv. Chim. Acta, 2013, 96, 1055–1061 CrossRef CAS
.
- N. Ojima, S. Takenaka and S. Seto, Phytochemistry, 1973, 12, 2527–2529 CrossRef CAS
.
- P. Karrer, K. A. Gehrckens and W. Heuss, Helv. Chim. Acta, 1926, 9, 446–457 CrossRef CAS
.
- H. Gao, W. Guo, Q. Wang, L. Zhang, M. Zhu, T. Zhu, Q. Gu, W. Wang and D. Li, Bioorg. Med. Chem. Lett., 2013, 23, 1776–1778 CrossRef CAS PubMed
.
- S. Cai, S. Sun, H. Zhou, X. Kong, T. Zhu, D. Li and Q. Gu, J. Nat. Prod., 2011, 74, 1106–1110 CrossRef CAS PubMed
.
- T. J. Greshock, A. W. Grubbs, P. Jiao, D. T. Wicklow, J. B. Gloer and R. M. Williams, Angew. Chem., Int. Ed., 2008, 47, 3573–3577 CrossRef CAS PubMed
.
- S. Cai, Y. Luan, X. Kong, T. Zhu, Q. Gu and D. Li, Org. Lett., 2013, 15, 2168–2171 CrossRef CAS PubMed
.
- C. Deng, C. Huang, Q. Wu, J. Pang and Y. Lin, Nat. Prod. Res., 2013, 27, 1882–1887 CrossRef CAS PubMed
.
- G. A. Ellestad, R. H. Evans Jr and M. P. Kunstmann, Tetrahedron Lett., 1971, 497–500 CrossRef CAS
.
- H.-F. Sun, X.-M. Li, L. Meng, C.-M. Cui, S.-S. Gao, C.-S. Li, C.-G. Huang and B.-G. Wang, J. Nat. Prod., 2012, 75, 148–152 CrossRef CAS PubMed
.
- H. M. T. Bandara Herath, W. H. M. W. Herath, P. Carvalho, S. I. Khan, B. L. Tekwani, S. O. Duke, M. Tomaso-Peterson and N. P. D. Nanayakkara, J. Nat. Prod., 2009, 72, 2091–2097 CrossRef CAS PubMed
.
- B. Caron and P. Brassard, Tetrahedron, 1991, 47, 4287–4298 CrossRef CAS
.
- C.-M. Deng, S.-X. Liu, C.-H. Huang, J.-Y. Pang and Y.-C. Lin, Mar. Drugs, 2013, 11, 2616–2624 CrossRef PubMed
.
- X. Huang, H. Huang, H. Li, X. Sun, H. Huang, Y. Lu, Y. Lin, Y. Long and Z. She, Org. Lett., 2013, 15, 721–723 CrossRef CAS PubMed
.
- Z. Xiao, H. Huang, C. Shao, X. Xia, L. Ma, X. Huang, Y. Lu, Y. Lin, Y. Long and Z. She, Org. Lett., 2013, 15, 2522–2525 CrossRef CAS PubMed
.
- G. Buechi, K. C. Luk, B. Kobbe and J. M. Townsend, J. Org. Chem., 1977, 42, 244–246 CrossRef CAS
.
- J. Clardy, J. P. Springer, G. Buechi, K. Matsuo and R. Wightman, J. Am. Chem. Soc., 1975, 97, 663–665 CrossRef CAS PubMed
.
- N. Koyama, Y. Inoue, M. Sekine, Y. Hayakawa, H. Homma, S. Oinmura and H. Tomoda, Org. Lett., 2008, 10, 5273–5276 CrossRef CAS PubMed
.
- J. Peng, T. Lin, W. Wang, Z. Xin, T. Zhu, Q. Gu and D. Li, J. Nat. Prod., 2013, 76, 1133–1140 CrossRef CAS PubMed
.
- W. Ebrahim, A. H. Aly, A. Mandi, F. Totzke, M. H. G. Kubbutat, V. Wray, W.-H. Lin, H. Dai, P. Proksch, T. Kurtán and A. Debbab, Eur. J. Org. Chem., 2012, 3476–3484 CrossRef CAS
.
- W. Ebrahim, A. H. Aly, V. Wray, P. Proksch and A. Debbab, Tetrahedron Lett., 2013, 54, 6611–6614 CrossRef CAS
.
- L. Calcul, C. Waterman, W. S. Ma, M. D. Lebar, C. Harter, T. Mutka, L. Morton, P. Maignan, A. van Olphen, D. E. Kyle, L. Vrijmoed, K.-L. Pang, C. Pearce and B. J. Baker, Mar. Drugs, 2013, 11, 5036–5050 CrossRef CAS PubMed
.
- D. Liu, X.-M. Li, C.-S. Li and B.-G. Wang, Helv. Chim. Acta, 2013, 96, 437–444 CrossRef CAS
.
- Z. Huang, C. Shao, Y. Chen, Z. She, Y. Lin and S. Zhou, Chem. Nat. Compd., 2007, 43, 655–658 CrossRef CAS
.
- G. N. Belofsky, J. B. Gloer, D. T. Wicklow and P. F. Dowd, Tetrahedron, 1995, 51, 3959–3968 CrossRef CAS
.
- T. Fehr and W. Acklin, Helv. Chim. Acta, 1966, 49, 1907–1910 CrossRef CAS
.
- H. Sun, S. Gao, X. Li, C. Li and B. Wang, Chin. J. Oceanol. Limnol., 2013, 31, 464–470 CrossRef CAS
.
- H. Seya, K. Nozawa, S. Udagawa, S. Nakajima and K. Kawai, Chem. Pharm. Bull., 1986, 34, 2411–2416 CrossRef CAS PubMed
.
- P. G. Mantle and C. M. Weedon, Phytochemistry, 1994, 36, 1209–1217 CrossRef CAS
.
- Y. Fan, Y. Wang, P. Liu, P. Fu, T. Zhu, W. Wang and W. Zhu, J. Nat. Prod., 2013, 76, 1328–1336 CrossRef CAS PubMed
.
- L.-H. Meng, X.-M. Li, C.-T. Lv, C.-S. Li, G.-M. Xu, C.-G. Huang and B.-G. Wang, J. Nat. Prod., 2013, 76, 2145–2149 CrossRef CAS PubMed
.
- CAS Registry Number: 1235379-39-1, Ambinter Stock Screening Collection, Ambinter, Amb15769954, 21 Feb 2014.
- CAS Registry Number: 1235379-39-1, Analyticon Discovery MEGx Catalog, AnalytiCon Discovery GmbH, NP-002355, NP-003261, 29 Jan 2014.
- CAS Registry Number: 1235379-39-1, K13.052.753, Aurora Screening Library, Aurora Fine Chemicals LLC, 3 Jul 2013.
- M. L. Wang, C. H. Lu, Q. Y. Xu, S. Y. Song, Z. Y. Hu and Z. H. Zheng, Molecules, 2013, 18, 5723–5735 CrossRef CAS PubMed
.
- X. Huang, X. Sun, B. Ding, M. Lin, L. Liu, H. Huang and Z. She, Planta Med., 2013, 79, 1572–1575 CrossRef CAS PubMed
.
- K. Otoguro, K. Shiomi, Y. Yamaguchi, N. Arai, T. Sunazuka, R. Masuma, Y. Iwai and S. Omura, J. Antibiot., 2000, 53, 50–57 CrossRef CAS PubMed
.
- J. Yang, R. Huang, S. Qiu, Z. She and Y. Lin, Nat. Prod. Res., 2013, 27, 1902–1905 CrossRef CAS PubMed
.
- Y. Hemberger, J. Xu, V. Wray, P. Proksch, J. Wu and G. Bringmann, Chem.–Eur. J., 2013, 19, 15556–15564 CrossRef CAS PubMed
.
- D. Rönsberg, A. Debbab, A. Mándi, V. Wray, H. Dai, T. Kurtán, P. Proksch and A. H. Aly, Tetrahedron Lett., 2013, 54, 3256–3259 CrossRef
.
- W. J. McGahren, G. A. Ellestad, G. O. Morton, M. P. Kunstmann and P. Mullen, J. Org. Chem., 1973, 38, 3542–3544 CrossRef CAS PubMed
.
- Z. Huang, J. Yang, F. Lei, Z. She and Y. Lin, Chem. Nat. Compd., 2013, 49, 27–30 CrossRef CAS
.
- J. X. Yang, S. Qiu, Z. She and Y. Lin, Chem. Nat. Compd., 2013, 49, 31–33 CrossRef CAS
.
- J. X. Yang, S. Qiu, Z. She and Y. Lin, Chem. Nat. Compd., 2013, 49, 246–248 CrossRef CAS
.
- M. M. Wagenaar and J. Clardy, J. Nat. Prod., 2001, 64, 1006–1009 CrossRef CAS
.
- V. Rukachaisirikul, U. Sommart, S. Phongpaichit, J. Sakayaroj and K. Kirtikara, Phytochemistry, 2008, 69, 783–787 CrossRef CAS PubMed
.
- S. Cao, D. W. McMillin, G. Tamayo, J. Delmore, C. S. Mitsiades and J. Clardy, J. Nat. Prod., 2012, 75, 793–797 CrossRef CAS PubMed
.
- B. Ding, J. Yuan, X. Huang, W. wWn, X. Zhu, Y. Liu, H. Li, Y. Lu, L. He, H. Tan and Z. She, Mar. Drugs, 2013, 11, 4961–4972 CrossRef CAS PubMed
.
- Y. Shiono, T. Sasaki, F. Shibuya, Y. Yasuda, T. Koseki and U. Supratman, Nat. Prod. Commun., 2013, 8, 1735–1737 CAS
.
- J. Wang, W. Ding, C. Li, S. Huang, Z. She and Y. Lin, Chem. Nat. Compd., 2013, 49, 799–802 CrossRef CAS
.
- Z. Feng and L. Yongcheng, Chin. Sci. Bull., 2006, 51, 1426–1430 CrossRef
.
- C.-L. Shao, C.-Y. Wang, Y.-C. Gu, M.-Y. Wei, J.-H. Pan, D.-S. Deng, Z.-G. She and Y.-C. Lin, Bioorg. Med. Chem. Lett., 2010, 20, 3284–3286 CrossRef CAS PubMed
.
- L. A. Shaala, D. T. A. Youssef, K. L. McPhail and M. Elbandy, Phytochem. Lett., 2013, 6, 183–188 CrossRef CAS
.
- S. P. Gunasekera, R. Ritson-Williams and V. J. Paul, J. Nat. Prod., 2008, 71, 2060–2063 CrossRef CAS PubMed
.
- J. I. Jiménez, T. Vansach, W. Y. Yoshida, B. Sakamoto, P. Pörzgen and F. D. Horgen, J. Nat. Prod., 2009, 72, 1573–1578 CrossRef PubMed
.
- E. Mevers, T. Byrum and W. H. Gerwick, J. Nat. Prod., 2013, 76, 1810–1814 CrossRef CAS PubMed
.
- C. C. Thornburg, E. S. Cowley, J. Sikorska, L. A. Shaala, J. E. Ishmael, D. T. A. Youssef and K. L. McPhail, J. Nat. Prod., 2013, 76, 1781–1788 CrossRef CAS PubMed
.
- L. T. Tan, T. Okino and W. H. Gerwick, Mar. Drugs, 2013, 11, 3015–3024 CrossRef PubMed
.
- R. Montaser, V. J. Paul and H. Luesch, Org. Lett., 2013, 15, 4050–4053 CrossRef CAS PubMed
.
- C. M. Pavlik, C. Y. B. Wong, S. Ononye, D. D. Lopez, N. Engene, K. L. McPhail, W. H. Gerwick and M. J. Balunas, J. Nat. Prod., 2013, 76, 2026–2033 CrossRef CAS PubMed
.
- K. Kumagai, M. Tsuda, E. Fukushi and J. Kawabata, Heterocycles, 2013, 87, 2615–2623 CrossRef CAS
.
- R. Watanabe, H. Uchida, T. Suzuki, R. Matsushima, M. Nagae, Y. Toyohara, M. Satake, Y. Oshima, A. Inoue and T. Yasumoto, Tetrahedron, 2013, 69, 10299–10303 CrossRef CAS
.
- C. Tsukano and M. Sasaki, Tetrahedron Lett., 2006, 47, 6803–6807 CrossRef CAS
.
- Y. Tanaka, M. Satake, M. Yotsu-Yamashita and Y. Oshima, Heterocycles, 2013, 87, 2037–2046 CrossRef CAS
.
- B. S. Hwang, E. Y. Yoon, H. S. Kim, W. Yih, J. Y. Park, H. J. Jeong and J.-R. Rho, Bioorg. Med. Chem. Lett., 2013, 23, 3023–3027 CrossRef CAS PubMed
.
- H. Uchida, Y. Taira and T. Yasumoto, Rapid Commun. Mass Spectrom., 2013, 27, 1999–2008 CrossRef CAS PubMed
.
- J. L. Dahmen and J. D. Leblond, Protist, 2013, 164, 183–194 CrossRef CAS PubMed
.
- A. I. Selwood, A. L. Wilkins, R. Munday, F. Shi, L. L. Rhodes and P. T. Holland, Tetrahedron Lett., 2013, 54, 4705–4707 CrossRef CAS
.
- M. Ishibashi, N. Yamaguchi, T. Sasaki and J. Kobayashi, J. Chem. Soc., Chem. Commun., 1994, 1455–1456 RSC
.
- Y. Takahashi, T. Kubota, M. Imachi, M. R. Wälchli and J. Kobayashi, J. Antibiot., 2013, 66, 277–279 CrossRef CAS PubMed
.
- J. D. Leblond, H. I. Timofte, S. A. Roche and N. M. Porter, Phycol. Res., 2010, 58, 222–229 CrossRef CAS
.
- A. Nagatsu, M. Watanabe, K. Ikemoto, M. Hashimoto, N. Murakami, J. Sakakibara, H. Tokuda, H. Nishino, A. Iwashima and K. Yazawa, Bioorg. Med. Chem. Lett., 1994, 4, 1619–1622 CrossRef CAS
.
- A. H. Banskota, R. Stefanova, P. Gallant and P. J. McGinn, J. Appl. Phycol., 2013, 25, 349–357 CrossRef CAS
.
- A. Arakaki, D. Iwama, Y. Liang, N. Murakami, M. Ishikura, T. Tanaka and T. Matsunaga, Phytochemistry, 2013, 85, 107–114 CrossRef CAS PubMed
.
- E. Julianti, H. Oh, H.-S. Lee, D.-C. Oh, K.-B. Oh and J. Shin, Tetrahedron Lett., 2012, 53, 2885–2886 CrossRef CAS
.
- K. Banert, Tetrahedron Lett., 2012, 53, 6443–6445 CrossRef CAS
.
- L. A. Januar and T. F. Molinski, Org. Lett., 2013, 15, 2370–2373 CrossRef CAS PubMed
.
- Q.-X. Wu, M. S. Crews, M. Draskovic, J. Sohn, T. A. Johnson, K. Tenney, F. A. Valeriote, X.-J. Yao, L. F. Bjeldanes and P. Crews, Org. Lett., 2010, 12, 4458–4461 CrossRef CAS PubMed
.
- J.-C. Zhao, S.-M. Yu, Y. Liu and Z.-J. Yao, Org. Lett., 2013, 15, 4300–4303 CrossRef CAS PubMed
.
- Y. Zhuang, X. Teng, Y. Wang, P. Liu, H. Wang, J. Li, G. Li and W. Zhu, Tetrahedron, 2011, 67, 7085–7089 CrossRef CAS
.
- L. Wang and W. Zhu, Tetrahedron Lett., 2013, 54, 6729–6731 CrossRef CAS
.
- J. Zhang, L. He, H. Xue and R. Feng, Chin. Chem. Lett., 1990, 1, 223–224 CAS
.
- J. Zhang and L. He, Yaoxue Xuebao, 1986, 21, 273–278 CAS
.
- M. Tsuda, Y. Kasai, K. Komatsu, T. Sone, M. Tanaka, Y. Mikami and J. Kobayashi, Org. Lett., 2004, 6, 3087–3089 CrossRef CAS PubMed
.
- T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao and J. Kobayashi, J. Org. Chem., 2005, 70, 9430–9435 CrossRef CAS PubMed
.
- Z. Bian, C. C. Marvin and S. F. Martin, J. Am. Chem. Soc., 2013, 135, 10886–10889 CrossRef CAS PubMed
.
- K. Kong, J. A. Enquist Jr, M. E. McCallum, G. M. Smith, T. Matsumaru, E. Menhaji-Klotz and J. L. Wood, J. Am. Chem. Soc., 2013, 135, 10890–10893 CrossRef CAS PubMed
.
- Y. Sun, L. Tian, J. Huang, H.-Y. Ma, Z. Zheng, A.-L. Lv, K. Yasukawa and Y.-H. Pei, Org. Lett., 2008, 10, 393–396 CrossRef CAS PubMed
.
- H. Shigehisa, Y. Suwa, N. Furiya, Y. Nakaya, M. Fukushima, Y. Ichihashi and K. Hiroya, Angew. Chem., Int. Ed., 2013, 52, 3646–3649 CrossRef CAS PubMed
.
- Q. Li, Y.-S. Xu, G. A. Ellis, T. S. Bugni, Y. Tang and R. P. Hsung, Tetrahedron Lett., 2013, 54, 5567–5572 CrossRef CAS PubMed
.
- Z.-J. Lin, Z.-Y. Lu, T.-J. Zhu, Y.-C. Fang, Q.-Q. Gu and W.-M. Zhu, Chem. Pharm. Bull., 2008, 56, 217–221 CrossRef CAS PubMed
.
- K. Kempf, A. Raja, F. Sasse and R. Schobert, J. Org. Chem., 2013, 78, 2455–2461 CrossRef CAS PubMed
.
- J. Xu, J. Kjer, J. Sendker, V. Wray, H. Guan, R. Edrada, W. Lin, J. Wu and P. Proksch, J. Nat. Prod., 2009, 72, 662–665 CrossRef CAS PubMed
.
- A. M. Beekman and R. A. Barrow, J. Nat. Prod., 2013, 76, 2054–2059 CrossRef CAS PubMed
.
- F. Xu, J. Pang, B. Lu, J. Wang, Y. Zhang, Z. She, L. L. P. Vrijmoed, E. B. Gareth Jones and Y. Lin, Chin. J. Chem., 2009, 27, 365–368 CrossRef CAS
.
- R.-A. F. Rarig, M. N. Tran and D. M. Chenoweth, J. Am. Chem. Soc., 2013, 135, 9213–9219 CrossRef CAS PubMed
.
- W. P. Frankmolle, G. Knubel, R. E. Moore and G. M. L. Patterson, J. Antibiot., 1992, 45, 1458–1466 CrossRef CAS PubMed
.
- I. Bonnard, M. Rolland, C. Francisco and B. Banaigs, Lett. Pept. Sci., 1997, 4, 289–292 CAS
.
- N. Maru, O. Ohno and D. Uemura, Tetrahedron Lett., 2010, 51, 6384–6387 CrossRef CAS
.
- F. Boyaud, Z. Mahiout, C. Lenoir, S. Tang, J. Wdzieczak–Bakala, A. Witczak, I. Bonnard, B. Banaigs, T. Ye and N. Inguimbert, Org. Lett., 2013, 15, 3898–3901 CrossRef CAS PubMed
.
- B. Adams, P. Poerzgen, E. Pittman, W. Y. Yoshida, H. E. Westenburg and F. D. Horgen, J. Nat. Prod., 2008, 71, 750–754 CrossRef CAS PubMed
.
- P. K. Gajula, S. Sharma, R. S. Ampapathi and T. K. Chakraborty, Org. Biomol. Chem., 2013, 11, 257–260 CAS
.
- M. Murata, S. Matsuoka, N. Matsumori, G. K. Paul and K. Tachibana, J. Am. Chem. Soc., 1999, 121, 870–871 CrossRef CAS
.
- M. Ebine, M. Kanemoto, Y. Manabe, Y. Konno, K. Sakai, N. Matsumori, M. Murata and T. Oishi, Org. Lett., 2013, 15, 2846–2849 CrossRef CAS PubMed
.
- M. A. M. Mondol, J. H. Kim, M. A. Lee, F. S. Tareq, H.-S. Lee, Y.-J. Lee and H. J. Shin, J. Nat. Prod., 2011, 74, 1606–1612 CrossRef CAS PubMed
.
- V. T. Salunkhe, S. Bhosale, P. Punde, D. Bhuniya and S. Koul, Tetrahedron Lett., 2013, 54, 2489–2491 CrossRef CAS
.
- E. N. Reddy, A. Krishnaiah and T. P. Rao, Tetrahedron: Asymmetry, 2013, 24, 724–728 CrossRef CAS
.
- S. Das and R. K. Goswami, J. Org. Chem., 2013, 78, 7274–7280 CrossRef CAS PubMed
.
- N. N. Rao and H. M. Meshram, Tetrahedron Lett., 2013, 54, 4544–4546 CrossRef
.
- F. S. Tareq, J. H. Kim, M. A. Lee, H.-S. Lee, Y.-J. Lee, J. S. Lee and H. J. Shin, Org. Lett., 2012, 14, 1464–1467 CrossRef CAS PubMed
.
- C. R. Reddy, E. Jithender and K. R. Prasad, J. Org. Chem., 2013, 78, 4251–4260 CrossRef CAS PubMed
.
- F. S. Tareq, J. H. Kim, M. A. Lee, H.-S. Lee, Y.-J. Lee, J. S. Lee and H. J. Shin, Org. Lett., 2013, 15, 2071 CrossRef CAS
.
- H. Huang, Y. Yao, Z. He, T. Yang, J. Ma, X. Tian, Y. Li, C. Huang, X. Chen, W. Li, S. Zhang, C. Zhang and J. Ju, J. Nat. Prod., 2011, 74, 2122–2127 CrossRef CAS PubMed
.
- S. Tagawa, T. Choshi, A. Okamoto, T. Nishiyama, S. Watanabe, N. Hatae and S. Hibino, Heterocycles, 2013, 87, 357–367 CrossRef CAS
.
- Y. Matsuo, K. Kanoh, T. Yamori, H. Kasai, A. Katsuta, K. Adachi, K. Shin-ya and Y. Shizuri, J. Antibiot., 2007, 60, 251–255 CrossRef CAS PubMed
.
- Y. Matsuo, K. Kanoh, H. Imagawa, K. Adachi, M. Nishizawa and Y. Shizuri, J. Antibiot., 2007, 60, 256–260 CrossRef CAS PubMed
.
- C.-C. Lin, W. Tantisantisom and S. R. McAlpine, Org. Lett., 2013, 15, 3574–3577 CrossRef CAS PubMed
.
- T. Tamaoki, K. Shirahata, T. Iida and F. Tomita, J. Antibiot., 1981, 34, 1525–1530 CrossRef CAS PubMed
.
- R. P. Maskey, E. Helmke, O. Kayser, H. H. Fiebig, A. Maier, A. Busche and H. Laatsch, J. Antibiot., 2004, 57, 771–779 CrossRef CAS PubMed
.
- T. Magauer, D. J. Smaltz and A. G. Myers, Nat. Chem. Biol., 2013, 5, 886–893 CrossRef CAS PubMed
.
- S. Sato, F. Iwata, T. Mukai, S. Yamada, J. Takeo, A. Abe and H. Kawahara, J. Org. Chem., 2009, 74, 5502–5509 CrossRef CAS PubMed
.
- O. F. Jeker and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 3474–3477 CrossRef CAS PubMed
.
- C. He, C. Zhu, Z. Dai, C.-C. Tseng and H. Ding, Angew. Chem., Int. Ed., 2013, 52, 13256–13260 CrossRef CAS PubMed
.
- M. L. Ciavatta, M. P. Lopez-Gresa, M. Gavagnin, R. Nicoletti, E. Manzo, E. Mollo, Y.-W. Guo and G. Cimino, Tetrahedron, 2008, 64, 5365–5369 CrossRef CAS
.
- J. Vannada, L. Niehues, B. König and G. Mehta, Tetrahedron, 2013, 69, 6034–6040 CrossRef CAS
.
- G. K. Poch and J. B. Gloer, J. Nat. Prod., 1989, 52, 257–260 CrossRef CAS
.
- J. S. Yadav, A. B. Reddy and K. S. Shankar, Synthesis, 2013, 45, 1034–1038 CrossRef CAS
.
- C. Takahashi, A. Numata, Y. Ito, E. Matsumura, H. Araki, H. Iwaki and K. Kushida, J. Chem. Soc., Perkin Trans. 1, 1994, 1859–1864 RSC
.
- J.-Y. Dong, H.-P. He, Y.-M. Shen and K.-Q. Zhang, J. Nat. Prod., 2005, 68, 1510–1513 CrossRef CAS PubMed
.
- F.-Z. Wang, Z. Huang, X.-F. Shi, Y.-C. Chen, W.-M. Zhang, X.-P. Tian, J. Li and S. Zhang, Bioorg. Med. Chem. Lett., 2012, 22, 7265–7267 CrossRef CAS PubMed
.
- J. E. DeLorbe, D. Horne, R. Jove, S. M. Mennen, S. Nam, F.-L. Zhanag and L. E. Overman, J. Am. Chem. Soc., 2013, 135, 4117–4128 CrossRef CAS PubMed
.
- K. Trisuwan, V. Rukachaisirikul, Y. Sukpondma, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Chem. Pharm. Bull., 2009, 57, 1100–1102 CrossRef CAS PubMed
.
- L. Song, H. Yao, L. Zhu and R. Tong, Org. Lett., 2013, 15, 6–9 CrossRef CAS PubMed
.
- G. Carr, W. Tay, H. Bottriell, S. K. Andersen, A. G. Mauk and R. J. Andersen, Org. Lett., 2009, 11, 2996–2999 CrossRef CAS PubMed
.
- S. Y. Jabri and L. E. Overman, J. Am. Chem. Soc., 2013, 135, 4231–4234 CrossRef CAS PubMed
.
- S. Y. Jabri and L. E. Overman, J. Org. Chem., 2013, 78, 8766–8788 CrossRef CAS PubMed
.
- J. Orjala and W. H. Gerwick, Phytochemistry, 1997, 45, 1087–1090 CrossRef CAS PubMed
.
- A. Phanumartwiwath, T. W. Hornsby, J. Jamalis, C. D. Bailey and C. L. Willis, Org. Lett., 2013, 15, 5734–5737 CrossRef CAS PubMed
.
- G. E. Chlipala, P. H. Tri, N. van Hung, A. Krunic, S. H. Shim, D. D. Soejarto and J. Orjala, J. Nat. Prod., 2010, 73, 784–787 CrossRef CAS PubMed
.
- A. Kamal and S. R. Vangala, Org. Biomol. Chem., 2013, 11, 4442–4448 CAS
.
- M. Gutierrez, A. R. Pereira, H. M. Debonsi, A. Ligresti, V. Di Marzo and W. H. Gerwick, J. Nat. Prod., 2011, 74, 2313–2317 CrossRef CAS PubMed
.
- Y.-R. Gao, S.-H. Guo, Z.-X. Zhang, S. Mao, Y.-L. Zhang and Y.-Q. Wang, Tetrahedron Lett., 2013, 54, 6511–6513 CrossRef CAS
.
- P. D. Boudreau, T. Byrum, W.-T. Liu, P. C. Dorrestein and W. H. Gerwick, J. Nat. Prod., 2012, 75, 1560–1570 CrossRef CAS PubMed
.
- D. Wang, S. Song, Y. Tian, Y. Xu, Z. Miao and A. Zhang, J. Nat. Prod., 2013, 76, 974–978 CrossRef CAS PubMed
.
- J. Kobayashi, M. Ishibashi, M. R. Walchli, H. Nakamura, Y. Hirata, T. Sasaki and Y. Ohizumi, J. Am. Chem. Soc., 1988, 110, 490–494 CrossRef CAS
.
- S. Mahapatra and R. G. Carter, J. Am. Chem. Soc., 2013, 135, 10792–10803 CrossRef CAS PubMed
.
- S. B. Singh, J. L. Smith, G. S. Sabnis, A. W. Dombrowski, J. M. Schaeffer, M. A. Goetz and G. F. Bills, Tetrahedron, 1991, 47, 6931–6938 CrossRef CAS
.
- H. Wei, T. Itoh, M. Kinoshita, Y. Nakai, M. Kurotaki and M. Kobayashi, Tetrahedron, 2004, 60, 6015–6019 CrossRef CAS
.
- M. Arai, H. Niikawa and M. Kobayashi, J. Nat. Med., 2013, 67, 271–275 CrossRef CAS PubMed
.
- S. T. Carey and M. S. R. Nair, J. Nat. Prod., 1979, 42, 231 CrossRef CAS
.
- M. García-Caballero, M. Marí-Beffa, L. Cañedo, M. A. Medina and A. R. Quesada, Biochem. Pharmacol., 2013, 85, 1727–1740 CrossRef PubMed
.
- J. H. Sohn, Y.-R. Lee, D.-S. Lee, Y.-C. Kim and H. Oh, J. Microbiol. Biotechnol., 2013, 23, 1206–1211 CrossRef CAS PubMed
.
- K. Arai, K. Kimura, T. Mushiroda and Y. Yamamoto, Chem. Pharm. Bull., 1989, 37, 2937–2939 CrossRef CAS
.
- Y. S. Mohammed and M. Luckner, Tetrahedron Lett., 1963, 1953–1958 CrossRef CAS
.
- A. Quilico, Gazz. Chim. Ital., 1948, 78, 111–135 CAS
.
- B. S. Gould and H. Raistrick, Biochem. J., 1934, 28, 1640–1656 CrossRef CAS PubMed
.
- M. Isaka, S. Palasarn, P. Rachtawee, S. Vimuttipong and P. Kongsaeree, Org. Lett., 2005, 7, 2257–2260 CrossRef CAS PubMed
.
- W. Zhao, Q. Gu and W. Zhu, Huaxue Yanjiu, 2007, 18, 10–13 CAS
.
- X. Han, X. Xu, C. Cui and Q. Gu, Zhongguo Yaowu Huaxue Zazhi, 2007, 17, 155–159 CAS
.
- Y. Song, H. Dou, W. Gong, X. Liu, Z. Yu, E. Li, R. Tan and Y. Hou, Eur. J. Pharmacol., 2013, 705, 49–60 CrossRef CAS PubMed
.
- H. He, W.-D. Ding, V. S. Bernan, A. D. Richardson, C. M. Ireland, M. Greenstein, G. A. Ellestad and G. T. Carter, J. Am. Chem. Soc., 2001, 123, 5362–5363 CrossRef CAS PubMed
.
- R. D. Kersten, A. L. Lane, M. Nett, T. K. S. Richter, B. M. Duggan, P. C. Dorrestein and B. S. Moore, ChemBioChem, 2013, 14, 955–962 CrossRef CAS PubMed
.
- X.-G. Li, X.-M. Tang, J. Xiao, G.-H. Ma, L. Xu, S.-J. Xie, M.-J. Xu, X. Xiao and J. Xu, Mar. Drugs, 2013, 11, 3875–3890 CrossRef PubMed
.
- J. Qian-Cutrone, S. Huang, Y.-Z. Shu, D. Vyas, C. Fairchild, A. Menendez, K. Krampitz, R. Dalterio, S. E. Klohr and Q. Gao, J. Am. Chem. Soc., 2002, 124, 14556–14557 CrossRef CAS PubMed
.
- Y. Ding, J. R. de Wet, J. Cavalcoli, S. Li, T. J. Greshock, K. A. Miller, J. M. Finefield, J. D. Sunderhaus, T. J. McAfoos, S. Tsukamoto, R. M. Williams and D. H. Sherman, J. Am. Chem. Soc., 2010, 132, 12733–12740 CrossRef CAS PubMed
.
- J. D. Sunderhaus, T. J. McAfoos, J. M. Finefield, H. Kato, S. Li, S. Tsukamoto, D. H. Sherman and R. M. Williams, Org. Lett., 2013, 15, 22–25 CrossRef CAS PubMed
.
- J. D. Hackett, J. H. Wisecaver, M. L. Brosnahan, D. M. Kulis, D. M. Anderson, D. Bhattacharya, F. G. Plumley and D. L. Erdner, Mol. Biol. Evol., 2013, 30, 70–78 CrossRef CAS PubMed
.
- D.-Q. Liu, S.-C. Mao, X.-Q. Yu, L.-H. Feng and X.-P. Lai, Heterocycles, 2012, 85, 661–666 CrossRef CAS
.
- A.-H. Liu, D.-Q. Liu, T.-J. Liang, X.-Q. Yu, M.-T. Feng, L.-G. Yao, Y. Fang, B. Wang, L.-H. Feng, M.-X. Zhang and S.-C. Mao, Bioorg. Med. Chem. Lett., 2013, 23, 2491–2494 CrossRef CAS PubMed
.
- R. Wang, V. J. Paul and H. Luesch, Free Radical Biol. Med., 2013, 57, 141–153 CrossRef CAS PubMed
.
- D. E. Williams, C. M. Sturgeon, M. Roberge and R. J. Andersen, J. Am. Chem. Soc., 2007, 129, 5822–5823 CrossRef CAS PubMed
.
- N. Kinashi, K. Fujiwara, T. Tsunoda, R. Katoono, H. Kawai and T. Suzuki, Tetrahedron Lett., 2013, 54, 4564–4567 CrossRef CAS
.
- I. Rubinstein and L. J. Goad, Phytochemistry, 1974, 13, 481–484 CrossRef CAS
.
- A. D. Kim, Y. Lee, S.-H. Kang, G. Y. Kim, H. S. Kim and J. W. Hyun, Mar. Drugs, 2013, 11, 418–430 CrossRef CAS PubMed
.
-
G. Aguilar-Santos and M. S. Doty, Drugs Sea, Trans. Symp., ed. H. D. Freudenthal, 1968, pp. 173–176 Search PubMed
.
- L. H. A. Cavalcante-Silva, A. C. D. Correia, J. M. Barbosa, B. A. da Silva, B. V. D. Santos, D. P. de Lira, J. C. F. Sousa, G. E. C. de Miranda, F. D. Cavalcante and M. S. Alexandre-Moreira, Mar. Drugs, 2013, 11, 1553–1564 CrossRef CAS PubMed
.
- D. R. Hirschfeld, W. Fenical, G. H. Y. Lin, R. M. Wing, P. Radlick and J. J. Sims, J. Am. Chem. Soc., 1973, 95, 4049–4050 CrossRef CAS
.
- G. S. E. Abou-El-Wafa, M. Shaaban, K. A. Shaaban, M. E. E. El-Naggar, A. Maier, H. H. Fiebig and H. Laatsch, Mar. Drugs, 2013, 11, 3109–3123 CrossRef PubMed
.
- E. Ioannou, C. Vagias and V. Roussis, Mar. Drugs, 2013, 11, 1104–1112 CrossRef CAS PubMed
.
- V. L. M. Gouveia, A. M. L. Seca, M. C. Barreto, A. I. Neto, A. Kijjoa and A. M. S. Silva, Phytochem. Lett., 2013, 6, 593–597 CrossRef CAS
.
- C. de los Reyes, H. Zbakh, V. Motilva and E. Zubía, J. Nat. Prod., 2013, 76, 621–629 CrossRef CAS PubMed
.
- N. Penicooke, K. Walford, S. Badal, R. Delgoda, L. A. D. Williams, P. Joseph-Nathan, B. Gordillo-Roman and W. Gallimore, Phytochemistry, 2013, 87, 96–101 CrossRef CAS PubMed
.
- O. M. M. Sabry, S. Andrews, K. L. McPhail, D. E. Goeger, A. Yokochi, K. T. LePage, T. F. Murray and W. H. Gerwick, J. Nat. Prod., 2005, 68, 1022–1030 CrossRef CAS PubMed
.
- W. H. Gerwick, W. Fenical, N. Fritsch and J. Clardy, Tetrahedron Lett., 1979, 20, 145–148 CrossRef
.
- A. Numata, S. Kanbara, C. Takahashi, R. Fujiki, M. Yoneda, Y. Usami and E. Fujita, Phytochemistry, 1992, 31, 1209–1213 CrossRef CAS
.
- Y. Kamei, M. Sueyoshi, K.-i. Hayashi, R. Terada and H. Nozaki, J. Antibiot., 2009, 62, 259–263 CrossRef CAS PubMed
.
- R. Katsuta, K. Aoki, A. Yajima and T. Nukada, Tetrahedron Lett., 2013, 54, 347–350 CrossRef CAS
.
- Y. Seo, K. E. Park and T. J. Nam, Bull. Korean Chem. Soc., 2007, 28, 1831–1833 CrossRef CAS
.
- K. Kurata, K. Taniguchi, K. Shiraishi, N. Hayama, I. Tanaka and M. Suzuki, Chem. Lett., 1989, 267–270 CrossRef CAS
.
- J. Becker, L. Butt, V. von Kiedrowski, E. Mischler, F. Quentin and M. Hiersemann, Org. Lett., 2013, 15, 5982–5985 CrossRef CAS PubMed
.
- H. A. Jung, S. E. Jin, B. R. Ahn, C. M. Lee and J. S. Choi, Food Chem. Toxicol., 2013, 59, 199–206 CrossRef CAS PubMed
.
- T.-H. Kwon, H.-J. Suh, I.-K. Lee, B.-S. Yun, T.-W. Kim, D.-I. Hwang, Y.-J. Kim, M.-J. Kim, O.-O. Kwon, C.-G. Kim and N.-H. Park, Eur. Food Res. Technol., 2013, 237, 501–508 CrossRef CAS
.
- J.-S. Yoon, A. K. Yadunandam, S.-J. Kim, H.-C. Woo, H.-R. Kim and G.-D. Kim, J. Nat. Med., 2013, 67, 519–527 CrossRef CAS PubMed
.
- J.-Y. Park, J. H. Kim, J. M. Kwon, H.-J. Kwon, H. J. Jeong, Y. M. Kim, D. Kim, W. S. Lee and Y. B. Ryu, Bioorg. Med. Chem., 2013, 21, 3730–3737 CrossRef CAS PubMed
.
- E. M. Balboa, E. Conde, A. Moure, E. Falqué and H. Domínguez, Food Chem., 2013, 138, 1764–1785 CrossRef CAS PubMed
.
- K. H. S. Farvin and C. Jacobsen, Food Chem., 2013, 138, 1670–1681 CrossRef PubMed
.
- E. Plouguerné, L. M. de Souza, G. L. Sassaki, J. F. Cavalcanti, M. T. V. Romanos, B. A. P. da Gama, R. C. Pereira and E. Barreto-Bergter, Mar. Drugs, 2013, 11, 4628–4640 CrossRef PubMed
.
- L. A. Miceli, V. L. Teixeira, H. C. Castro, C. R. Rodrigues, J. F. R. Mello, M. G. Albuquerque, L. M. Cabral, M. A. de Brito and A. M. T. de Souza, Mar. Drugs, 2013, 11, 4127–4143 CrossRef PubMed
.
- W.-J. Yoon, S.-J. Heo, S.-C. Han, H.-J. Lee, G.-J. Kang, E.-J. Yang, S.-S. Park, H.-K. Kang and E.-S. Yoo, Food Chem. Toxicol., 2012, 50, 3273–3279 CrossRef CAS PubMed
.
- W.-J. Yoon, K.-N. Kim, S.-J. Heo, S.-C. Han, J. Kim, Y.-J. Ko, H.-K. Kang and E.-S. Yoo, Biochem. Biophys. Res. Commun., 2013, 434, 892–897 CrossRef CAS PubMed
.
- B.-G. Park, S. Oh, D. Kwon, Y. Cui, J. Ham, W.-S. Shin and S. Lee, Bull. Korean Chem. Soc., 2013, 34, 3121–3124 CrossRef CAS
.
- S. L. Midland, R. M. Wing and J. J. Sims, J. Org. Chem., 1983, 48, 1906–1909 CrossRef CAS
.
- K. E. Park, Y. A. Kim, H. A. Jung, H. J. Lee, J.-W. Ahn, B.-J. Lee and Y. Seo, J. Korean Chem. Soc., 2004, 48, 394–398 CrossRef CAS
.
- R. K. Ko, M.-C. Kang, S. S. Kim, T. H. Oh, G.-O. Kim, C.-G. Hyun, J. W. Hyun and N. H. Lee, Nat. Prod. Commun., 2013, 8, 427–428 CAS
.
- J. I. Lee, M. K. Kwak, H. Y. Park and Y. Seo, Nat. Prod. Commun., 2013, 8, 431–432 CAS
.
- M. Guyot, M. Morel and C. Belaud, J. Chem. Res., Synop., 1983, 188–189 CAS
.
- M. H. Moghadam, J. Firouzi, S. Saeidnia, H. Hajimehdipoor, S. Jamili, A. Rustaiyan and A. R. Gohari, Daru, J. Pharm. Sci., 2013, 21–24 Search PubMed
.
- S. R. Kumar, M. Hosokawa and K. Miyashita, Mar. Drugs, 2013, 11, 5130–5147 CrossRef CAS PubMed
.
- J. B. Gallé, B. Attioua, M. Kaiser, A. M. Rusig, A. Lobstein and C. Vonthron-Sénécheau, Mar. Drugs, 2013, 11, 599–610 CrossRef PubMed
.
- C. Francisco, G. Combaut, J. Teste and M. Prost, Phytochemistry, 1978, 17, 1003–1005 CrossRef CAS
.
- S. Urban and M. Timmers, Nat. Prod. Commun., 2013, 8, 715–719 CAS
.
- M. Kitamura, P. J. Schupp, Y. Nakano and D. Uemura, Tetrahedron Lett., 2009, 50, 6606–6609 CrossRef CAS PubMed
.
- N. Maru, T. Inuzuka, K. Yamamoto, M. Kitamura, P. J. Schupp, K. Yamada and D. Uemura, Tetrahedron Lett., 2013, 54, 4385–4387 CrossRef CAS
.
- W. M. Abdel-Mageed, R. Ebel, F. A. Valeriote and M. Jaspars, Tetrahedron, 2010, 66, 2855–2862 CrossRef CAS
.
- M. T. Holmes and R. Britton, Chem.–Eur. J., 2013, 19, 12649–12652 CrossRef CAS PubMed
.
- D. J. Shepherd, P. A. Broadwith, B. S. Dyson, R. S. Paton and J. W. Burton, Chem.–Eur. J., 2013, 19, 12644–12648 CrossRef CAS PubMed
.
- J. G. Hall and J. A. Reiss, Aust. J. Chem., 1986, 39, 1401–1409 CrossRef CAS
.
- S. G. Smith, R. S. Paton, J. W. Burton and J. M. Goodman, J. Org. Chem., 2008, 73, 4053–4062 CrossRef CAS PubMed
.
- B. S. Dyson, J. W. Burton, T. I. Sohn, B. Kim, H. Bae and D. Kim, J. Am. Chem. Soc., 2012, 134, 11781–11790 CrossRef CAS PubMed
.
- R. Brkljača and S. Urban, Nat. Prod. Commun., 2013, 8, 729–732 Search PubMed
.
- M. L. Ciavatta, S. Wahidulla, L. D'Souza, G. Scognamiglio and G. Cimino, Tetrahedron, 2001, 57, 617–623 CrossRef CAS
.
- B. S. Underwood, J. Tanuwidjaja, S.-S. Ng and T. F. Jamison, Tetrahedron, 2013, 69, 5205–5220 CrossRef CAS PubMed
.
- C. P. Manríquez, M. L. Souto, J. A. Gavin, M. Norte and J. J. Fernández, Tetrahedron, 2001, 57, 3117–3123 CrossRef
.
- X. D. Li, F. P. Miao, X. R. Liang, B. G. Wang and N. Y. Ji, RSC Adv., 2013, 3, 1953–1956 RSC
.
- T. Kamada and C. S. Vairappan, Nat. Prod. Commun., 2013, 8, 287–288 CAS
.
- L. Shide, A. Olbrich, R. Mayer and G. Rücker, Planta Med., 1987, 53, 556–558 CrossRef CAS PubMed
.
- X. Xu, L. Yin, L. Gao, J. Gao, J. Chen, J. Li and F. Song, Mar. Drugs, 2013, 11, 842–847 CrossRef CAS PubMed
.
- X. Xu, L. Yin, Y. Wang, S. Wang and F. Song, Nat. Prod. Res., 2013, 27, 723–726 CrossRef CAS PubMed
.
- E. K. Olsen, E. Hansen, J. Isaksson and J. H. Andersen, Mar. Drugs, 2013, 11, 2769–2784 CrossRef PubMed
.
- W.-S. Sun, S. Su, R.-X. Zhu, G.-Z. Tu, W. Cheng, H. Liang, X.-Y. Guo, Y.-Y. Zhao and Q.-Y. Zhang, Tetrahedron Lett., 2013, 54, 3617–3620 CrossRef CAS
.
- D. Iliopoulou, N. Mihopoulos, C. Vagias, P. Papazafiri and V. Roussis, J. Org. Chem., 2003, 68, 7667–7674 CrossRef CAS PubMed
.
- A. E. Leung, M. Blair, C. M. Forsyth and K. L. Tuck, Org. Lett., 2013, 15, 2198–2201 CrossRef CAS PubMed
.
- S. J. Rochfort and R. J. Capon, Aust. J. Chem., 1996, 49, 19–26 CAS
.
- X.-c. Huang, Y.-L. Sun, A. A. Salim, Z.-S. Chen and R. J. Capon, Biochem. Pharmacol., 2013, 85, 1257–1268 CrossRef CAS PubMed
.
- D. Mikami, H. Kurihara, S. M. Kim and K. Takahashi, Mar. Drugs, 2013, 11, 4050–4057 CrossRef PubMed
.
- M. Francavilla, M. Franchi, M. Monteleone and C. Caroppo, Mar. Drugs, 2013, 11, 3754–3776 CrossRef PubMed
.
- L. Mata, E. Wright, L. Owens, N. Paul and R. de Nys, J. Appl. Phycol., 2013, 25, 1963–1973 CrossRef CAS
.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2012, 29, 144–222 RSC
.
- T. F. Molinski, R. Biegelmeyer, E. P. Stout, X. Wang, M. L. C. Frota and A. T. Henriques, J. Nat. Prod., 2013, 76, 374–381 CrossRef CAS PubMed
.
- R. Huang, Y. Peng, X. Zhou, X. Yang and Y. Liu, Nat. Prod. Res., 2013, 27, 1537–1541 CrossRef CAS PubMed
.
- T. N. Makarieva, P. S. Dmitrenok, A. M. Zakharenko, V. A. Denisenko, A. G. Guzii, R. Li, C. K. Skepper, T. F. Molinksi and V. A. Stonik, J. Nat. Prod., 2007, 70, 1991–1998 CrossRef CAS PubMed
.
- J. Ko and T. F. Molinski, J. Org. Chem., 2013, 78, 498–505 CrossRef CAS PubMed
.
- F. Farokhi, P. Grellier, M. Clément, C. Roussakis, P. M. Loiseau, E. Genin-Seward, J. M. Kornprobst, G. Barnathan and G. Wielgosz-Collin, Mar. Drugs, 2013, 11, 1304–1315 CrossRef CAS PubMed
.
- P. L. Katavic, K. W. L. Yong, J. N. Herring, M. A. Deseo, J. T. Blanchfield, V. Ferro and M. J. Garson, Tetrahedron, 2013, 69, 8074–8079 CrossRef CAS
.
- A. Cutignano, G. Nuzzo, D. D'Angelo, E. Borbone, A. Fusco and A. Fontana, Angew. Chem., Int. Ed., 2013, 52, 9256–9260 CrossRef CAS PubMed
.
- X. Luo, F. Li, J. Hong, C.-O. Lee, C. J. Sim, K. S. Im and J. H. Jung, J. Nat. Prod., 2006, 69, 567–571 CrossRef CAS PubMed
.
- R. Towada, Y. Kurashina and S. Kuwahara, Tetrahedron Lett., 2013, 54, 6878–6881 CrossRef CAS
.
- N. Tanaka, M. Asai, A. Takahashi-Nakaguchi, T. Gonoi, J. Fromont and J. Kobayashi, Org. Lett., 2013, 15, 2518–2521 CrossRef CAS PubMed
.
- K. Horikawa, T. Yagyu, Y. Yoshioka, T. Fujiwara, A. Kanamoto, T. Okamoto and M. Ojika, Tetrahedron, 2013, 69, 101–106 CrossRef CAS
.
- A. S. Reddy and P. Srihari, Tetrahedron Lett., 2013, 54, 6370–6372 CrossRef
.
- B.-K. Choi, B.-Y. Cha, T. Yagyu, J.-T. Woo and M. Ojika, Bioorg. Med. Chem., 2013, 21, 1804–1810 CrossRef CAS PubMed
.
- Y. Hitora, K. Takada, S. Okada and S. Matsunaga, Tetrahedron, 2011, 67, 4530–4534 CrossRef CAS
.
- Y. Hitora, K. Takada and S. Matsunaga, Tetrahedron, 2013, 69, 11070–11073 CrossRef CAS
.
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen and M. Fujita, Nature, 2013, 501, 262 CrossRef CAS
.
- T. Shirouzu, K. Watari, M. Ono, K. Koizumi, I. Saiki, C. Tanaka, R. W. M. van Soest and T. Miyamoto, J. Nat. Prod., 2013, 76, 1337–1342 CrossRef CAS PubMed
.
- Y.-J. Lee, S.-J. Yoo, J. S. Kang, J. Yun, H. J. Shin, J. S. Lee and H.-S. Lee, Lipids, 2013, 48, 87–91 CrossRef CAS PubMed
.
- E. J. Mejia, L. B. Magranet, N. J. De Voogd, K. TenDyke, D. Qiu, Y. Y. Shen, Z. Zhou and P. Crews, J. Nat. Prod., 2013, 76, 425–432 CrossRef CAS PubMed
.
- N. Legrave, S. Hamrouni-Buonomo, M. Dufies, V. Guérineau, J. Vacelet, P. Auberger, P. Amade and M. Mehiri, Mar. Drugs, 2013, 11, 2282–2292 CrossRef PubMed
.
- S. Ohta, T. Ogawa, E. Ohta, T. Ikeuchi, K. Kamemura and S. Ikegami, Nat. Prod. Res., 2013, 27, 1842–1847 CrossRef CAS PubMed
.
- T. Akiyama, K. Takada, T. Oikawa, N. Matsuura, Y. Ise, S. Okada and S. Matsunaga, Tetrahedron, 2013, 69, 6560–6564 CrossRef CAS
.
- N. Aoki, K. Yamamoto, T. Ogawa, E. Ohta, T. Ikeuchi, K. Kamemura, S. Ikegami and S. Ohta, Nat. Prod. Res., 2013, 27, 117–122 CrossRef CAS PubMed
.
- W. M. Alarif, A. Abdel-Lateff, S. S. Al-Lihaibi, S.-E. N. Ayyad and F. A. Badria, Z. Naturforsch., C: J. Biosci., 2013, 68, 70–75 CrossRef CAS
.
- H. Kim, J. Chin, H. Choi, K. Baek, T.-G. Lee, S. E. Park, W. Wang, D. Hahn, I. Yang, J. Lee, B. Mun, M. Ekins, S.-J. Nam and H. Kang, Org. Lett., 2013, 15, 100–103 CrossRef CAS PubMed
.
- H. Kim, J. Chin, H. Choi, K. Baek, T.-G. Lee, S. E. Park, W. Wang, D. Hahn, I. Yang, J. Lee, B. Mun, M. Ekins, S.-J. Nam and H. Kang, Org. Lett., 2013, 15, 5614 CrossRef CAS
.
- B. S. Hwang, K. Lee, C. Yang, E. J. Jeong and J.-R. Rho, J. Nat. Prod., 2013, 76, 2355–2359 CrossRef CAS PubMed
.
- I. H. Hwang, J. Oh, A. Kochanowska-Karamyan, R. J. Doerksen, M. Na and M. T. Hamann, Tetrahedron Lett., 2013, 54, 3872–3876 CrossRef CAS
.
- P. V. Kiem, N. X. Nhiem, N. V. Quang, C. V. Minh, N. H. Nam, N. T. Cuc, H. L. T. Anh, B. H. Tai, P. H. Yen, N. X. Cuong, N. P. Thao, N. T. Hoai, N. Y. Kim, S. J. Park and K. S. Hyun, Nat. Prod. Commun., 2013, 8, 1751–1752 Search PubMed
.
- G. Chianese, F. Scala, B. Calcinai, C. Cerrano, H. A. Dien, M. Kaiser, D. Tasdemir and O. Taglialatela-Scafati, Mar. Drugs, 2013, 11, 3297–3308 CrossRef PubMed
.
- J. S. Oh, B. S. Hwang, O.-H. Kang, D.-Y. Kwon and J.-R. Rho, Mar. Drugs, 2013, 11, 4407–4418 CrossRef PubMed
.
- S. P. Gunasekera, M. Gunasekera, R. E. Longley and G. K. Schulte, J. Org. Chem., 1990, 55, 4912–4915 CrossRef CAS
.
- C. Ruiz, K. Valderrama, S. Zea and L. Castellanos, Mar. Biotechnol., 2013, 15, 571–583 CrossRef CAS PubMed
.
- S. Di Micco, A. Zampella, M. V. D'Auria, C. Festa, S. De Marino, R. Riccio, C. P. Butts and G. Bifulco, Beilstein J. Org. Chem., 2013, 9, 2940–2949 CrossRef CAS PubMed
.
- J. Zhang, X. Tang, J. Li, P. Li, N. J. de Voogd, X. Ni, X. Jin, X. Yao, P. Li and G. Li, J. Nat. Prod., 2013, 76, 600–606 CrossRef CAS PubMed
.
- P. Jumaryatno, L. K. Lambert, J. N. A. Hooper, J. T. Blanchfield and M. J. Garson, Nat. Prod. Commun., 2013, 8, 725–728 CAS
.
- C. Festa, S. De Marino, M. V. D'Auria, O. Taglialatela-Scafati, E. Deharo, S. Petek and A. Zampella, Tetrahedron, 2013, 69, 3706–3713 CrossRef CAS
.
- C. Festa, C. D'Amore, B. Renga, G. Lauro, S. De Marino, M. V. D'Auria, G. Bifulco, A. Zampella and S. Fiorucci, Mar. Drugs, 2013, 11, 2314–2327 CrossRef PubMed
.
- S.-J. Piao, Y.-L. Song, W.-H. Jiao, F. Yang, X.-F. Liu, W.-S. Chen, B.-N. Han and H.-W. Lin, Org. Lett., 2013, 15, 3526–3529 CrossRef CAS PubMed
.
- T. Kubota, Y. Ishiguro, A. Takahashi-Nakaguchi, J. Fromont, T. Gonoi and J. Kobayashi, Bioorg. Med. Chem. Lett., 2013, 23, 244–247 CrossRef CAS PubMed
.
- B. Yang, H. Tao, X. Zhou, X.-P. Lin and Y. Liu, Nat. Prod. Res., 2013, 27, 433–437 CrossRef CAS PubMed
.
- M. Kimura, T. Wakimoto and I. Abe, Tetrahedron Lett., 2013, 54, 114–116 CrossRef CAS
.
- Y. Imae, K. Takada, S. Okada, Y. Ise, H. Yoshimura, Y. Morii and S. Matsunaga, J. Nat. Prod., 2013, 76, 755–758 CrossRef CAS PubMed
.
- Y. Nakao, S. Kawatsu, C. Okamoto, M. Okamoto, Y. Matsumoto, S. Matsunaga, R. van Soest and N. Fusetani, J. Nat. Prod., 2008, 71, 469–472 CrossRef CAS PubMed
.
- J. A. Lewis, R. N. Daniels and C. W. Lindsley, Org. Lett., 2008, 10, 4545–4548 CrossRef CAS PubMed
.
- M. J. Martin, L. Coello, R. Fernández, F. Reyes, A. Rodríguez, C. Murcia, M. Garranzo, C. Mateo, F. Sánchez–Sancho, S. Bueno, C. de Eguilior, A. Francesch, S. Munt and C. Cuevas, J. Am. Chem. Soc., 2013, 135, 10164–10171 CrossRef CAS PubMed
.
- C. A. Bewley, C. Debitus and D. J. Faulkner, J. Am. Chem. Soc., 1994, 116, 7631–7636 CrossRef CAS
.
- E. W. Schmidt and D. J. Faulkner, Tetrahedron, 1998, 54, 3043–3056 CrossRef CAS
.
- T. Hoffmann, S. Müller, S. Nadmid, R. Garcia and R. Müller, J. Org. Chem., 2013, 135, 16904–16911 CAS
.
- J.-K. Woo, J.-E. Jeon, C.-K. Kim, C.-J. Sim, D.-C. Oh, K.-B. Oh and J. Shin, J. Nat. Prod., 2013, 76, 1380–1383 CrossRef CAS PubMed
.
- M. Kita, B. Gise, A. Kawamura and H. Kigoshi, Tetrahedron Lett., 2013, 54, 6826–6828 CrossRef CAS
.
- E. Avilés and A. D. Rodríguez, Tetrahedron, 2013, 69, 10797–10804 CrossRef
.
- A. Napolitano, M. Rodriquez, I. Bruno, S. Marzocco, G. Autore, R. Riccio and L. Gomez-Paloma, Tetrahedron Lett., 2003, 59, 10203–10211 CrossRef CAS
.
- M. Pelay-Gimeno, A. Meli, J. Tulla-Puche and F. Albericio, J. Med. Chem., 2013, 56, 9780–9788 CrossRef CAS PubMed
.
- S. Matsunaga and N. Fusetani, J. Org. Chem., 1995, 60, 1177–1181 CrossRef CAS
.
- R. A. Espiritu, N. Matsumori, M. Murata, S. Nishimura, H. Kakeya, S. Matsunaga and M. Yoshida, Biochemistry, 2013, 52, 2410–2418 CrossRef CAS PubMed
.
- A. Sinisi, B. Calcinai, C. Cerrano, H. A. Dien, A. Zampella, C. D'Amore, B. Renga, S. Fiorucci and O. Taglialatela-Scafati, Beilstein J. Org. Chem., 2013, 9, 1643–1651 CrossRef PubMed
.
- R. Ueoka, Y. Ise, S. Ohtsuka, S. Okada, T. Yamori and S. Matsunaga, J. Am. Chem. Soc., 2010, 132, 17692–17694 CrossRef CAS PubMed
.
- T. Kuranaga, Y. Sesoko, K. Sakata, N. Maeda, A. Hayata and M. Inoue, J. Am. Chem. Soc., 2013, 135, 5467–5474 CrossRef CAS PubMed
.
- H. Li, J. J. Bowling, F. R. Fronczek, J. Hong, S. V. Jabba, T. F. Murray, N.-C. Ha, M. T. Hamann and J. H. Jung, Biochim. Biophys. Acta, 2013, 1830, 2591–2599 CrossRef CAS PubMed
.
- T. Amagata, T. A. Johnson, R. H. Cichewicz, K. Tenney, S. L. Mooberry, J. Media, M. Edelstein, F. A. Valeriote and P. Crews, J. Med. Chem., 2008, 51, 7234–7242 CrossRef CAS PubMed
.
- A. Randazzo, C. Debitus and L. Gomez-Paloma, Tetrahedron, 2001, 57, 4443–4446 CrossRef CAS
.
- B. D. Williams and A. B. Smith III, Org. Lett., 2013, 15, 4584–4587 CrossRef CAS PubMed
.
- B. Pfeiffer, S. Speck–Gisler, L. Barandun, U. Senft, C. de Groot, I. Lehmann, W. Ganci, J. Gertsch and K. H. Altmann, J. Org. Chem., 2013, 78, 2553–2563 CrossRef CAS PubMed
.
- T. Sirirak, L. Brecker and A. Plubrukarn, Nat. Prod. Res., 2013, 27, 1213–1219 CrossRef CAS PubMed
.
- J. Kobayashi, K. Kondo, M. Ishibashi, M. R. Walchli and T. Nakamura, J. Am. Chem. Soc., 1993, 115, 6661–6665 CrossRef CAS
.
- K. Kondo, M. Ishibashi and J. Kobayashi, Tetrahedron, 1994, 50, 8355–8362 CrossRef CAS
.
- K. Nozawa, M. Tsuda, N. Tanaka, T. Kubota, E. Fukushi, J. Kawabata and J. Kobayashi, Tetrahedron Lett., 2013, 54, 783–787 CrossRef CAS
.
- A. Sinisi, B. Calcinai, C. Cerrano, H. A. Dien, A. Zampella, C. D'Amore, B. Renga, S. Fiorucci and O. Taglialatela–Scafati, Bioorg. Med. Chem., 2013, 21, 5332–5338 CrossRef CAS PubMed
.
- R. Sakai, K. Suzuki, K. Shimamoto and H. Kamiya, J. Org. Chem., 2004, 69, 1180–1185 CrossRef CAS PubMed
.
- M. Sakai, Y. Ishikawa, S. Takamizawa and M. Oikawa, Tetrahedron Lett., 2013, 54, 5911–5912 CrossRef CAS
.
- T. Nishi, T. Kubota, J. Fromont, T. Sasaki and J. Kobayashi, Tetrahedron, 2008, 64, 3127–3132 CrossRef CAS
.
- S. G. Davies, P. M. Roberts, R. S. Shah and J. E. Thomson, Tetrahedron Lett., 2013, 54, 6423–6426 CrossRef CAS
.
- N. L. Seagraves and P. Crews, J. Nat. Prod., 2005, 68, 118–121 CrossRef PubMed
.
- T. Okaki, R. Fujimura, M. Sekiguchi, D. Zhou, K. Sugimoto, D. Minato, Y. Matsuya, A. Kato, I. Adachi, Y. Tezuka, R. A. Saporito and N. Toyooka, Eur. J. Org. Chem., 2013, 14, 2841–2848 CrossRef
.
- R. Sakai, T. Higa, C. W. Jefford and G. Bernardinelli, J. Am. Chem. Soc., 1986, 108, 6404–6405 CrossRef CAS
.
- G. Kallifatidis, D. Hoepfner, T. Jaeg, E. A. Guzmán and A. E. Wright, Mar. Drugs, 2013, 11, 3500–3516 CrossRef CAS PubMed
.
- K. Eguchi, Y. Fujiwara, A. Hayashida, H. Horlad, H. Kato, H. Rotinsulu, F. Losung, R. E. P. Mangindaan, N. J. de Voogd, M. Takeya and S. Tsukamoto, Bioorg. Med. Chem., 2013, 21, 3831–3838 CrossRef CAS PubMed
.
- T. Kubota, Y. Kamijyo, A. Takahashi-Nakaguchi, J. Fromont, T. Gonoi and J. Kobayashi, Org. Lett., 2013, 15, 610–612 CrossRef CAS PubMed
.
- J. I. Jiménez, G. Goetz, C. M. S. Mau, W. Y. Yoshida, P. J. Scheuer, R. T. Williamson and M. Kelly, J. Org. Chem., 2000, 65, 8465–8469 CrossRef
.
- W. P. Unsworth, K. A. Gallagher, M. Jean, J. P. Schmidt, L. J. Diorazio and R. J. K. Taylor, Org. Lett., 2013, 15, 262–265 CrossRef CAS PubMed
.
- V. Damodaran, J. L. Ryan and R. A. Keyzers, J. Nat. Prod., 2013, 76, 1997–2001 CrossRef CAS PubMed
.
- Y. Takekawa, S. Matsunaga, R. W. M. van Soest and N. Fusetani, J. Nat. Prod., 2006, 69, 1503–1505 CrossRef CAS PubMed
.
- J. M. Langenhan, E. Mullarky, D. K. Rogalsky, J. R. Rohlfing, A. E. Tjaden, H. M. Werner, L. M. Rozal and S. A. Loskot, J. Org. Chem., 2013, 78, 1670–1676 CrossRef CAS PubMed
.
- T. Kubota, K. Kura, J. Fromont and J. Kobayashi, Tetrahedron, 2013, 69, 96–100 CrossRef CAS
.
- S. Khokhar, Y. Feng, M. R. Campitelli, R. J. Quinn, J. N. A. Hooper, M. G. Ekins and R. A. Davis, J. Nat. Prod., 2013, 76, 2100–2105 CrossRef CAS PubMed
.
- E. Dumdei and R. J. Andersen, J. Nat. Prod., 1993, 56, 792–794 CrossRef CAS
.
- E. Dickson, B. R. Copp and D. Barker, Tetrahedron Lett., 2013, 54, 5239–5242 CrossRef CAS
.
- Y.-J. Lee, D.-G. Lee, H. S. Rho, V. B. Krasokhin, H. J. Shin, J. S. Lee and H.-S. Lee, J. Heterocycl. Chem., 2013, 50, 1400–1404 CrossRef CAS
.
- K. Imada, E. Sakai, H. Kato, T. Kawabata, S. Yoshinaga, T. Nehira, H. Terasawa and S. Tsukamoto, Tetrahedron, 2013, 69, 7051–7055 CrossRef CAS
.
- B. Bao, Q. Sun, X. Yao, J. Hong, C.-O. Lee, C. J. Sim, K. S. Im and J. H. Jung, J. Nat. Prod., 2005, 68, 711–715 CrossRef CAS PubMed
.
- G. D. Kim, O. J. Cheong, S. Y. Bae, J. Shin and S. K. Lee, Mar. Drugs, 2013, 11, 1087–1103 CrossRef CAS PubMed
.
- D. T. A. Youssef, L. A. Shaala and H. Z. Asfour, Mar. Drugs, 2013, 11, 1061–1070 CrossRef CAS PubMed
.
- F. Russell, D. Harmody, P. J. McCarthy, S. A. Pomponi and A. E. Wright, J. Nat. Prod., 2013, 76, 1989–1992 CrossRef CAS PubMed
.
- R. Momose, N. Tanaka, J. Fromont and J. Kobayashi, Org. Lett., 2013, 15, 2010–2013 CrossRef CAS PubMed
.
- N. Tanaka, R. Momose, Y. Takahashi, T. Kubota, A. Takahashi-Nakaguchi, T. Gonoi, J. Fromont and J. Kobayashi, Tetrahedron Lett., 2013, 54, 4038–4040 CrossRef CAS
.
- R. A. Davis, S. Duffy, S. Fletcher, V. M. Avery and R. J. Quinn, J. Org. Chem., 2013, 78, 9608–9613 CrossRef CAS PubMed
.
- N. K. Utkina, A. E. Makarchenko, V. A. Denisenko and P. S. Dmitrenok, Tetrahedron Lett., 2004, 45, 7491–7494 CrossRef CAS
.
- N. K. Utkina, A. E. Makarchenko and V. A. Denisenko, J. Nat. Prod., 2005, 68, 1424–1427 CrossRef CAS PubMed
.
- D. H. Nadkarni, S. Murugesan and S. E. Velu, Tetrahedron, 2013, 69, 4105–4113 CrossRef CAS PubMed
.
- Y. Zou and M. T. Hamann, Org. Lett., 2013, 15, 1516–1519 CrossRef CAS PubMed
.
- C.-D. Pham, R. Hartmann, W. E. G. Müller, N. de Voogd, D. Lai and P. Proksch, J. Nat. Prod., 2013, 76, 103–106 CrossRef CAS PubMed
.
- X. Fu, J. R. Barnes, T. Do and F. J. Schmitz, J. Nat. Prod., 1997, 60, 497–498 CrossRef CAS
.
- J. Das, A. Bhan, S. S. Mandal and C. J. Lovely, Bioorg. Med. Chem. Lett., 2013, 23, 6183–6187 CrossRef CAS PubMed
.
- A. G. Guzii, T. N. Makarieva, Y. V. Korolkova, Y. A. Andreev, I. V. Mosharova, K. M. Tabakmaher, V. A. Denisenko, P. S. Dmitrenok, E. K. Ogurtsova, A. S. Antonov, H.-S. Lee and E. V. Grishin, Tetrahedron Lett., 2013, 54, 1247–1250 CrossRef CAS
.
- T. N. Makarieva, E. K. Ogurtsova, Y. V. Korolkova, Y. A. Andreev, I. V. Mosharova, K. M. Tabakmakher, A. G. Guzii, V. A. Denisenko, P. S. Dmitrenok, H.-S. Lee, E. V. Grishin and V. Stonik, Nat. Prod. Commun., 2013, 8, 1229–1232 CAS
.
- K. M. Tabakmakher, V. A. Denisenko, A. G. Guzii, P. S. Dmitrenok, S. A. Dyshlovoy, H. S. Lee and T. N. Makarieva, Nat. Prod. Commun., 2013, 8, 1399–1402 CAS
.
- K. Inaba, H. Sato, M. Tsuda and J. Kobayashi, J. Nat. Prod., 1998, 61, 693–695 CrossRef CAS PubMed
.
- M. Yamaguchi, M. Miyazaki, M. P. Kodrasov, H. Rotinsulu, F. Losung, R. E. P. Mangindaan, N. J. de Voogd, H. Yokosawa, B. Nicholson and S. Tsukamoto, Bioorg. Med. Chem. Lett., 2013, 23, 3884–3886 CrossRef CAS PubMed
.
- N. Tanaka, T. Kusama, A. Takahashi-Nakaguchi, T. Gonoi, J. Fromont and J. Kobayashi, Tetrahedron Lett., 2013, 54, 3794–3796 CrossRef CAS
.
- N. Tanaka, T. Kusama, A. Takahashi-Nakaguchi, T. Gonoi, J. Fromont and J. Kobayashi, Org. Lett., 2013, 15, 3262–3265 CrossRef CAS PubMed
.
- E. A. Santalova, V. A. Denisenko and P. S. Dmitrenok, Chem. Nat. Compd., 2013, 49, 75–78 CrossRef CAS
.
- K. Hirano, T. Kubota, M. Tsuda, K. Watanabe, J. Fromont and J. Kobayashi, Tetrahedron, 2000, 56, 8107–8110 CrossRef CAS
.
- S. Saha, C. V. R. Reddy, T. Chiranjeevi, U. Addepally, T. S. C. Rao and B. Patro, Bioorg. Med. Chem. Lett., 2013, 23, 1013–1016 CrossRef CAS PubMed
.
- T. D. Tran, N. B. Pham, G. Fechner, J. N. A. Hooper and R. J. Quinn, J. Nat. Prod., 2013, 76, 516–523 CrossRef CAS PubMed
.
- Y.-J. Lee, S. Han, H.-S. Lee, J. S. Kang, J. Yun, C. J. Sim, H. J. Shin and J. S. Lee, J. Nat. Prod., 2013, 76, 1731–1736 CrossRef CAS PubMed
.
- H. Niemann, W. Lin, W. E. G. Müller, M. Kubbutat, D. Lai and P. Proksch, J. Nat. Prod., 2013, 76, 121–125 CrossRef CAS PubMed
.
- K. Kunze, H. Niemann, S. Ueberlein, R. Schulze, H. Ehrlich, E. Brunner, P. Proksch and K.-H. van Pée, Mar. Drugs, 2013, 11, 1271–1287 CrossRef PubMed
.
- F. Yang, R.-H. Ji, J. Li, J.-H. Gan and H.-W. Lin, Nat. Prod. Commun., 2013, 8, 1713–1714 CAS
.
- Z.-B. Zhao, J.-Z. Sun, S.-C. Mao and Y.-W. Guo, J. Asian Nat. Prod. Res., 2013, 15, 198–202 CrossRef CAS PubMed
.
- A. N. E.-S. Hamed, W. Wätjen, R. Schmitz, Y. Chovolou, R. Edrada-Ebel, D. T. A. Youssef, M. S. Kamel and P. Proksch, Nat. Prod. Commun., 2013, 8, 289–292 Search PubMed
.
- L. Du, Y.-D. Zhou and D. G. Nagle, J. Nat. Prod., 2013, 76, 1175–1181 CrossRef CAS PubMed
.
- A. E. Wright, S. A. Rueth and S. S. Cross, J. Nat. Prod., 1991, 54, 1108–1111 CrossRef CAS
.
- T. Kamishima, T. Kikuchi and T. Katoh, Eur. J. Org. Chem., 2013, 21, 4558–4563 CrossRef
.
- W.-H. Jiao, X.-J. Huang, J.-S. Yang, F. Yang, S.-J. Piao, H. Gao, J. Li, W.-C. Ye, X.-S. Yao, W.-S. Chen and H.-W. Lin, Org. Lett., 2012, 14, 202–205 CrossRef CAS PubMed
.
- B. Schmalzbauer, J. Herrmann, R. Muller and D. Menche, Org. Lett., 2013, 15, 964–967 CrossRef CAS PubMed
.
- A. Yegdaneh, S. Putchakarn, S. Yuenyongsawad, A. Ghannadi and A. Plubrukarn, Nat. Prod. Commun., 2013, 8, 1355–1357 CAS
.
- W. Balansa, R. Islam, D. F. Gilbert, F. Fontaine, X. Xiao, H. Zhang, A. M. Piggott, J. W. Lynch and R. J. Capon, Bioorg. Med. Chem., 2013, 21, 4420–4425 CrossRef CAS PubMed
.
- H. Yamazaki, T. Nakazawa, D. A. Sumilat, O. Takahashi, K. Ukai, S. Takahashi and M. Namikoshi, Bioorg. Med. Chem. Lett., 2013, 23, 2151–2154 CrossRef CAS PubMed
.
- H.-S. Park, S. Y. Park, C. J. Sim and J.-R. Rho, Chem. Pharm. Bull., 2008, 56, 1198–1200 CrossRef
.
- H. Zhang, J. M. Major, R. J. Lewis and R. J. Capon, Org. Biomol. Chem., 2008, 6, 3811–3815 CAS
.
- S.-H. Lee, J.-E. Jeon, C.-H. Ahn, S.-C. Chung, J. Shin and K.-B. Oh, Appl. Microbiol. Biotechnol., 2013, 97, 3141–3148 CrossRef CAS PubMed
.
- G. R. Pettit, Y. Tang, Q. Zhang, G. T. Bourne, C. A. Arm, J. E. Leet, J. C. Knight, R. K. Pettit, J.-C. Chapuis, D. L. Doubek, F. J. Ward, C. Weber and J. N. A. Hooper, J. Nat. Prod., 2013, 76, 420–424 CrossRef CAS PubMed
.
- A. Patra, C. W. J. Chang, P. J. Scheuer, G. D. van Duyne, G. K. Matsumoto and J. Clardy, J. Am. Chem. Soc., 1984, 106, 7981–7983 CrossRef CAS
.
- I. T. Sandoval, E. J. Manos, R. M. Van Wagoner, R. G. C. Delacruz, K. Edes, D. R. Winge, C. M. Ireland and D. A. Jones, Chem. Biol., 2013, 20, 753–763 CrossRef CAS PubMed
.
- D. Green, I. Goldberg, Z. Stein, M. Ilan and Y. Kashman, Nat. Prod. Lett., 1992, 1, 193–199 CrossRef CAS
.
- J. Peng, K. Walsh, V. Weedman, J. D. Bergthold, J. Lynch, K. L. Lieu, I. A. Braude, M. Kelly and M. T. Hamann, Tetrahedron, 2002, 58, 7809–7819 CrossRef CAS
.
- C. Wang, D. Wang and S. Gao, Org. Lett., 2013, 15, 4402–4405 CrossRef CAS PubMed
.
- A. J. Singh, J. D. Dattelbaum, J. J. Field, Z. Smart, E. F. Woolly, J. M. Barber, R. Heathcott, J. H. Miller and P. T. Northcote, Org. Biomol. Chem., 2013, 11, 8041–8051 CAS
.
- E. Avilés, A. D. Rodríguez and J. Vicente, J. Org. Chem., 2013, 78, 11294–11301 CrossRef PubMed
.
- N. X. Nhiem, N. V. Quang, C. V. Minh, D. T. T. Hang, H. L. T. Anh, B. H. Tai, P. H. Yen, N. T. Hoai, D. C. Thung and P. V. Kiem, Nat. Prod. Commun., 2013, 8, 1209–1212 Search PubMed
.
- J.-E. Jeon, L. Liao, H. Kim, C. J. Sim, D.-C. Oh, K.-B. Oh and J. Shin, J. Nat. Prod., 2013, 76, 1679–1685 CrossRef CAS PubMed
.
- F. Lefranc, G. Nuzzo, N. A. Hamdy, I. Fakhr, L. M. Y. Banuls, G. Van Goietsenoven, G. Villani, V. Mathieu, R. van Soest, R. Kiss and M. L. Ciavatta, J. Nat. Prod., 2013, 76, 1541–1547 CrossRef CAS PubMed
.
- C. Audoin, D. Bonhomme, J. Ivanisevic, M. de la Cruz, B. Cautain, M. C. Monteiro, F. Reyes, L. Rios, T. Perez and O. P. Thomas, Mar. Drugs, 2013, 11, 1477–1489 CrossRef CAS PubMed
.
- J. Li, L. Du, M. Kelly, Y.-D. Zhou and D. G. Nagle, J. Nat. Prod., 2013, 76, 1492–1497 CrossRef CAS PubMed
.
- J. Daoust, M. Chen, M. Wang, D. E. Williams, M. A. G. Chavez, Y. A. Wang, C. E. Merchant, A. Fontana, T. J. Kieffer and R. J. Andersen, J. Org. Chem., 2013, 78, 8267–8273 CrossRef CAS PubMed
.
- W. Balansa, R. Islam, F. Fontaine, A. M. Piggott, H. Zhang, X. Xiao, T. L. Webb, D. F. Gilbert, J. W. Lynch and R. J. Capon, Org. Biomol. Chem., 2013, 11, 4695–4701 CAS
.
- W. Wang, B. Mun, Y. Lee, M. V. Reddy, Y. Park, J. Lee, H. Kim, D. Hahn, J. Chin, M. Ekins, S.-J. Nam and H. Kang, J. Nat. Prod., 2013, 76, 170–177 CrossRef CAS PubMed
.
- Y.-M. Fuh, M.-C. Lu, C.-H. Lee and J.-H. Su, Nat. Prod. Commun., 2013, 8, 571–572 CAS
.
- D. Hahn, D. H. Won, B. Mun, H. Kim, C. Han, W. Wang, T. Chun, S. Park, D. Yoon, H. Choi, S.-J. Nam, M. Ekins, J. Chin and H. Kang, Bioorg. Med. Chem. Lett., 2013, 23, 2336–2339 CrossRef CAS PubMed
.
- L. Harinantenaina, P. J. Brodie, J. Maharavo, G. Bakary, K. TenDyke, Y. Shen and D. G. I. Kingston, Bioorg. Med. Chem., 2013, 21, 2912–2917 CrossRef CAS PubMed
.
- L. P. P. Cano, S. A. Bartolotta, N. A. Casanova, G. E. Siless, E. Portmann, L. Schejter, J. A. Palermo and M. A. Carballo, Steroids, 2013, 78, 982–986 CrossRef PubMed
.
- T.-R. Su, K.-J. Liang, M.-Y. Chiang, M.-C. Lu, Y.-J. Wu and J.-H. Su, Nat. Prod. Commun., 2013, 8, 1535–1536 CAS
.
- Z.-B. Cheng, H. Xiao, C.-Q. Fan, Y.-N. Lu, G. Zhang and S. Yin, Steroids, 2013, 78, 1353–1358 CrossRef CAS PubMed
.
- X. C. Nguyen, A. Longeon, V. C. Pham, F. Urvois, C. Bressy, T. T. V. Trinh, H. N. Nguyen, V. K. Phan, V. M. Chau, J.-F. Briand and M.-L. Bourguet–Kondracki, J. Nat. Prod., 2013, 76, 1313–1318 CrossRef CAS PubMed
.
- R. A. Keyzers, J. Daoust, M. T. Davies-Coleman, R. van Soest, A. Balgi, E. Donohue, M. Roberge and R. J. Andersen, Org. Lett., 2008, 10, 2959–2962 CrossRef CAS PubMed
.
- R. Forestieri, E. Donohue, A. Balgi, M. Roberge and R. J. Andersen, Org. Lett., 2013, 15, 3918–3921 CrossRef CAS PubMed
.
- Z. Lu, M. Koch, M. K. Harper, T. K. Matainaho, L. R. Barrows, R. M. Van Wagoner and C. M. Ireland, J. Nat. Prod., 2013, 76, 2150–2152 CrossRef CAS PubMed
.
- D.-Q. Xue, S.-C. Mao, X.-Q. Yu and Y.-W. Guo, Biochem. Syst. Ecol., 2013, 49, 101–106 CrossRef CAS
.
- S. S. Afiyatullov, A. I. Kalinovsky, A. S. Antonov, L. P. Ponomarenko, P. S. Dmitrenok, D. L. Aminin, V. B. Krasokhin, V. M. Nosova and A. V. Kisin, J. Nat. Prod., 2007, 70, 1871–1877 CrossRef CAS PubMed
.
- S. A. Kolesnikova, E. G. Lyakhova, A. I. Kalinovsky, M. A. Pushilin, S. S. Afiyatullov, E. A. Yurchenko, S. A. Dyshlovoy, C. V. Minh and V. A. Stonik, J. Nat. Prod., 2013, 76, 1746–1752 CrossRef CAS PubMed
.
- J. Colorado, D. Muñoz, D. Marquez, M. E. Marquez, J. Lopez, O. P. Thomas and A. Martinez, Molecules, 2013, 18, 2598–2610 CrossRef CAS PubMed
.
- S. Ahmed, A. Ibrahim and A. S. Arafa, Tetrahedron Lett., 2013, 54, 2377–2381 CrossRef CAS
.
- R. Huang, Y. Peng, X. Zhou, M. Fu, S. Tian and Y. Liu, Nat. Prod. Res., 2013, 27, 319–322 CrossRef CAS PubMed
.
- N. Cachet, L. Loffredo, O. O. Vicente and O. P. Thomas, Phytochem. Lett., 2013, 6, 205–208 CrossRef CAS
.
- S.-H. Qi, G.-C. Su, Y.-F. Wang, Q.-Y. Liu and C.-H. Gao, Chem. Pharm. Bull., 2009, 57, 87–88 CrossRef CAS PubMed
.
- S.-H. Qi, G.-C. Su, Y.-F. Wang, Q.-Y. Liu and C.-H. Gao, Chem. Pharm. Bull., 2013, 61, 887 CrossRef CAS
.
- A. Berndt, M. Gruner, A. W. Schmidt and H.-J. Knölker, Synlett, 2013, 24, 2102–2106 CrossRef CAS
.
- S. Kodani, K. Sato, T. Higuchi, B. E. Casareto and Y. Suzuki, Nat. Prod. Res., 2013, 27, 1859–1862 CrossRef CAS PubMed
.
- H.-M. Chung, J.-H. Su, T.-L. Hwang, J.-J. Li, J.-J. Chen, Y.-H. Chen, Y.-C. Chang, Y.-D. Su, Y.-H. Chen, L.-S. Fang, J.-H. Sheu, W.-H. Wang and P.-J. Sung, Tetrahedron, 2013, 69, 2740–2744 CrossRef CAS
.
- H.-M. Chung, W.-H. Wang, T.-L. Hwang, Y.-C. Wu and P.-J. Sung, Nat. Prod. Commun., 2013, 8, 1037–1040 CAS
.
- D. Chen, W. Chen, D. Liu, L. van Ofwegen, P. Proksch and W. Lin, J. Nat. Prod., 2013, 76, 1753–1763 CrossRef CAS PubMed
.
- Y.-J. Xio, J.-H. Su, B.-W. Chen, Y.-J. Tseng, Y.-C. Wu and J.-H. Sheu, Mar. Drugs, 2013, 11, 3735–3741 CrossRef PubMed
.
- Y.-J. Tseng, Y.-S. Lee, S.-K. Wang, J.-H. Sheu and C.-Y. Duh, Mar. Drugs, 2013, 11, 2501–2509 CrossRef PubMed
.
- B. Yang, S. Liao, X. Lin, J. Wang, J. Liu, X. Zhou, X. Yang and Y. Liu, Mar. Drugs, 2013, 11, 4741–4750 CrossRef CAS PubMed
.
- E. R. Wagner, R. D. Moss, R. M. Brooker, J. P. Heeschen, W. J. Potts and M. L. Dilling, Tetrahedron Lett., 1965, 4233–4239 CrossRef CAS
.
- P. Georgantea, E. Ioannou, C. Vagias and V. Roussis, Tetrahedron Lett., 2013, 54, 6920–6922 CrossRef CAS
.
- Y.-F. Lin, C.-Y. Kuo, Z.-H. Wen, Y.-Y. Lin, W.-H. Wang, J.-H. Su, J.-H. Sheu and P.-J. Sung, Molecules, 2013, 18, 8160–8167 CrossRef CAS PubMed
.
- L. Li, C.-Y. Wang, C.-L. Shao, L. Han, X.-P. Sun, J. Zhao, Y.-W. Guo, H. Huang and H.-S. Guan, J. Asian Nat. Prod. Res., 2009, 11, 851–855 CrossRef PubMed
.
- N. P. Thao, N. H. Nam, N. X. Cuong, T. H. Quang, P. T. Tung, L. D. Dat, D. Chae, S. Kim, Y.-S. Koh, P. V. Kiem, C. V. Minh and Y. H. Kim, Bioorg. Med. Chem. Lett., 2013, 23, 228–231 CrossRef PubMed
.
- H.-Y. Fang, C.-H. Hsu, C.-H. Chao, Z.-H. Wen, Y.-C. Wu, C.-F. Dai and J.-H. Sheu, Mar. Drugs, 2013, 11, 1853–1865 CrossRef CAS PubMed
.
- T.-C. Tsai, Y.-J. Wu, J.-H. Su, W.-T. Lin and Y.-S. Lin, Mar. Drugs, 2013, 11, 114–123 CrossRef CAS PubMed
.
- K.-H. Chen, C.-F. Dai, M.-C. Lu, J.-J. Li, J.-J. Chen, Y.-C. Chang, Y.-D. Su, W.-H. Wang and P.-J. Sung, Mar. Drugs, 2013, 11, 3372–3380 CrossRef PubMed
.
- L.-C. Hu, J.-H. Su, M. Y.-N. Chiang, M.-C. Lu, T.-L. Hwang, Y.-H. Chen, W.-P. Hu, N.-C. Lin, W.-H. Wang, L.-S. Fang, Y.-H. Kuo and P.-J. Sung, Mar. Drugs, 2013, 11, 1999–2012 CrossRef PubMed
.
- A. Ma, Z. Deng, L. van Ofwegen, M. Bayer, P. Proksch and W. Lin, J. Nat. Prod., 2008, 71, 1152–1160 CrossRef CAS PubMed
.
- C.-C. Su, B.-S. Wong, C. Chin, Y.-J. Wu and J.-H. Su, Int. J. Mol. Sci., 2013, 14, 4317–4325 CrossRef CAS PubMed
.
- Y.-S. Lin, C.-H. Chen, C.-C. Liaw, Y.-C. Chen, Y.-H. Kuo and Y.-C. Shen, Tetrahedron, 2009, 65, 9157–9164 CrossRef CAS
.
- L.-C. Hu, W.-H. Yen, J.-H. Su, M. Y.-N. Chiang, Z.-H. Wen, W.-F. Chen, T.-J. Lu, Y.-W. Chang, Y.-H. Chen, W.-H. Wang, Y.-C. Wu and P.-J. Sung, Mar. Drugs, 2013, 11, 2154–2167 CrossRef PubMed
.
- W.-H. Yen, Y.-D. Su, Y.-C. Chang, Y.-H. Chen, Y.-H. Chen, C.-F. Dai, Z.-H. Wen, J.-H. Su and P.-J. Sung, Tetrahedron Lett., 2013, 54, 2267–2270 CrossRef CAS
.
- H.-F. Lin, H.-J. Su, N.-L. Lee and J.-H. Su, Nat. Prod. Commun., 2013, 8, 1363–1364 CAS
.
- D. Lai, Z. Geng, Z. Deng, L. van Ofwegen, P. Proksch and W. Lin, J. Agric. Food Chem., 2013, 61, 4585–4592 CrossRef CAS PubMed
.
- J. Yin, M. Zhao, M. Ma, Y. Xu, Z. Xiang, Y. Cai, J. Dong, X. Lei, K. Huang and P. Yan, Mar. Drugs, 2013, 11, 455–465 CrossRef CAS PubMed
.
- M. Zhao, X. Li, F. Zhao, S. Cheng, Z. Xiang, J. Dong, K. Huang and P. Yan, Chem. Pharm. Bull., 2013, 61, 1323–1328 CrossRef CAS PubMed
.
- M. Zhao, J. Yin, W. Jiang, M. Ma, X. Lei, Z. Xiang, J. Dong, K. Huang and P. Yan, Mar. Drugs, 2013, 11, 1162–1172 CrossRef CAS PubMed
.
- C. B. Rao, C. Satyanarayana, D. S. Rao, D. V. Rao, E. Fahy and D. J. Faulkner, J. Nat. Prod., 1993, 56, 2003–2007 CrossRef CAS
.
- F. Cao, J. Zhou, K.-X. Xu, M.-Q. Zhang and C.-Y. Wang, Nat. Prod. Commun., 2013, 8, 1675–1678 CAS
.
- S.-K. Wang, M.-K. Hsieh and C.-Y. Duh, Mar. Drugs, 2013, 11, 4318–4327 CrossRef PubMed
.
- Z. Xi, W. Bie, W. Chen, D. Liu, L. van Ofwegen, P. Proksch and W. Lin, Mar. Drugs, 2013, 11, 3186–3196 CrossRef PubMed
.
- R. F. Abou El-Ezz, S. A. Ahmed, M. M. Radwan, N. A. Ayoub, M. S. Afifi, S. A. Ross, P. T. Szymanski, H. Fahmy and S. I. Khalifa, Tetrahedron Lett., 2013, 54, 989–992 CrossRef CAS
.
- C.-X. Zhang, X.-X. He, J. Zhang, Q. Guo, L.-F. Lei, J.-Y. Su and L.-M. Zeng, Nat. Prod. Res., 2013, 27, 782–786 CrossRef CAS PubMed
.
- Z. Xi, W. Bie, W. Chen, D. Liu, L. van Ofwegen, P. Proksch and W. Lin, Helv. Chim. Acta, 2013, 96, 2218–2227 CrossRef CAS
.
- P. C. Yan, Y. Lv, L. van Ofwegen, P. Proksch and W. Lin, Org. Lett., 2010, 12, 2484–2487 CrossRef CAS PubMed
.
- P. Yan, Z. Deng, L. van Ofwegen, P. Proksch and W. Lin, Mar. Drugs, 2010, 8, 2837–2848 CrossRef CAS PubMed
.
- P. Yan, Z. Deng, L. van Ofwegen, P. Proksch and W. Lin, Chem. Pharm. Bull., 2010, 58, 1591–1595 CrossRef CAS PubMed
.
- L.-M. Zeng, W.-J. Lan, J.-Y. Su, G.-W. Zhang, X.-L. Feng, Y.-J. Liang and X.-P. Yang, J. Nat. Prod., 2004, 67, 1915–1918 CrossRef CAS PubMed
.
- P. Yan, Z. Deng, L. van Ofwegen, P. Proksch and W. Lin, Chem. Biodiversity, 2011, 8, 1724–1734 CAS
.
- X.-H. Yan, M. Gavagnin, G. Cimino and Y.-W. Guo, Tetrahedron Lett., 2007, 48, 5313–5316 CrossRef CAS
.
- R. Jia, T. Kurtan, A. Mandi, X.-H. Yan, W. Zhang and Y.-W. Guo, J. Org. Chem., 2013, 78, 3113–3119 CrossRef CAS PubMed
.
- W.-J. Lan, S.-L. Wang and H.-J. Li, Nat. Prod. Commun., 2009, 4, 1193–1196 CAS
.
- Y.-F. Li, L.-L. He, H.-L. Liu, L.-F. Liang, H.-B. Zhang and Y.-W. Guo, J. Asian Nat. Prod. Res., 2013, 15, 566–573 CrossRef CAS PubMed
.
- P. Yan, Z. Deng, L. van Ofwegen, P. Proksch and W. Lin, Chem. Pharm. Bull., 2010, 58, 1591–1595 CrossRef CAS PubMed
.
- M.-E. F. Hegazy, T. A. Mohamed, F. F. Abdel-Latif, M. S. Alsaid, A. A. Shahat and P. W. Pare, Phytochem. Lett., 2013, 6, 383–386 CrossRef CAS
.
- L.-F. Liang, T. Kurtan, A. Mandi, L.-G. Yao, J. Li, W. Zhang and Y.-W. Guo, Org. Lett., 2013, 15, 274–277 CrossRef CAS PubMed
.
- L.-F. Liang, L.-X. Gao, J. Li, O. Taglialatela-Scafati and Y.-W. Guo, Bioorg. Med. Chem., 2013, 21, 5076–5080 CrossRef CAS PubMed
.
- L.-F. Liang, L.-F. Lan, O. Taglialatela-Scafati and Y.-W. Guo, Tetrahedron, 2013, 69, 7381–7386 CrossRef CAS
.
- C. Zhang, J. Li, J. Su, Y. Liang, X. Yang, K. Zheng and L. Zeng, J. Nat. Prod., 2006, 69, 1476–1480 CrossRef CAS PubMed
.
- J. A. Toth, B. J. Burreson, P. J. Scheuer, J. Finer-Moore and J. Clardy, Tetrahedron, 1980, 36, 1307–1309 CrossRef CAS
.
- R. Jia, Y.-W. Guo, E. Mollo and G. Cimino, Helv. Chim. Acta, 2005, 88, 1028–1033 CrossRef CAS
.
- C. Li, M. Jiang, M.-P. La, T.-J. Li, H. Tang, P. Sun, B.-S. Liu, Y.-H. Yi, Z. Liu and W. Zhang, Mar. Drugs, 2013, 11, 1565–1582 CrossRef CAS PubMed
.
- J.-F. Sun, Z. Han, X.-F. Zhou, B. Yang, X. Lin, J. Liu, Y. Peng, X.-W. Yang and Y. Liu, Tetrahedron, 2013, 69, 871–880 CrossRef CAS
.
- C.-C. Liaw, Y.-C. Lin, Y.-S. Lin, C.-H. Chen, T.-L. Hwang and Y.-C. Shen, Mar. Drugs, 2013, 11, 2042–2053 CrossRef PubMed
.
- B.-W. Chen, S.-Y. Wang, C.-Y. Huang, S.-L. Chen, Y.-C. Wu and J.-H. Sheu, Tetrahedron, 2013, 69, 2296–2301 CrossRef CAS
.
- Y.-S. Cai, L.-G. Yao, A. Di Pascale, C. Irace, E. Mollo, O. Taglialatela-Scafati and Y.-W. Guo, Tetrahedron, 2013, 69, 2214–2219 CrossRef CAS
.
- T. Miyamoto, K. Yamada, N. Ikeda, T. Komori and R. Higuchi, J. Nat. Prod., 1994, 57, 1212–1219 CrossRef CAS
.
- C.-J. Tai, J.-H. Su, C.-Y. Huang, M.-S. Huang, Z.-H. Wen, C.-F. Dai and J.-H. Sheu, Mar. Drugs, 2013, 11, 788–799 CrossRef CAS PubMed
.
- Y.-N. Lee, C.-J. Tai, T.-L. Hwang and J.-H. Sheu, Mar. Drugs, 2013, 11, 2741–2750 CrossRef PubMed
.
- M. Ochi, K. Yamada, K. Kataoka, H. Kotsuki and K. Shibata, Chem. Lett., 1992, 155–158 CrossRef CAS
.
- T.-H. Chen, M.-C. Lu, Y.-C. Chang, Y.-D. Su, Y.-H. Chen, N.-C. Lin, L.-S. Fang, Y.-C. Wu and P.-J. Sung, Mar. Drugs, 2013, 11, 4585–4593 CrossRef CAS PubMed
.
- F.-Y. Shih, T.-H. Chen, M.-C. Lu, W.-F. Chen, Z.-H. Wen, Y.-H. Kuo and P.-J. Sung, Int. J. Mol. Sci., 2013, 14, 21781–21789 CrossRef PubMed
.
- M.-C. Lin, B.-W. Chen, C.-Y. Huang, C.-F. Dai, T.-L. Hwang and J.-H. Sheu, J. Nat. Prod., 2013, 76, 1661–1667 CrossRef CAS PubMed
.
- T.-T. Li, X.-L. Tang, C.-L. Chen, X.-W. Zhang, R.-C. Wu, H.-Y. Zhu, P.-L. Li and G.-Q. Li, Helv. Chim. Acta, 2013, 96, 1188–1196 CrossRef CAS
.
- Y.-J. Tseng, S.-K. Wang and C.-Y. Duh, Mar. Drugs, 2013, 11, 3288–3296 CrossRef PubMed
.
- G. Zhang, X. Tang, C. Cheng, K. Gong, X. Zhang, H. Zhu, R. Wu, P. Li and G. Li, Steroids, 2013, 78, 845–850 CrossRef CAS PubMed
.
- H.-Y. Zhao, C.-L. Shao, Z.-Y. Li, L. Han, F. Cao and C.-Y. Wang, Molecules, 2013, 18, 3458–3466 CrossRef CAS PubMed
.
- K.-K. Gong, X.-L. Tang, G. Zhang, C.-L. Cheng, X.-W. Zhang, P.-L. Li and G.-Q. Li, Mar. Drugs, 2013, 11, 4788–4798 CrossRef CAS PubMed
.
- L.-L. Sun, C.-L. Shao, H. Hang, Z.-Y. Guo, Q. Xing and C.-Y. Wang, Nat. Prod. Res., 2013, 27, 1159–1166 CrossRef CAS PubMed
.
- M. Shaaban, K. A. Shaaban and M. A. Ghani, Steroids, 2013, 78, 866–873 CrossRef CAS PubMed
.
- A. Umeyama, N. Shoji, M. Ozeki and S. Arihara, J. Nat. Prod., 1996, 59, 894–895 CrossRef CAS
.
- A. I. Elshamy, A. F. Abdel-Razik, M. I. Nassar, T. K. Mohamed, M. A. Ibrahim and S. M. El-Kousy, Nat. Prod. Res., 2013, 27, 1250–1254 CrossRef CAS PubMed
.
- M. Shaaban, M. A. Ghani and K. A. Shaaban, Z. Naturforsch., B: J. Chem. Sci., 2013, 68, 939–945 CAS
.
- P. Wang, H. Tang, B.-S. Liu, T.-J. Li, P. Sun, W. Zhu, Y.-P. Luo and W. Zhang, Steroids, 2013, 78, 951–958 CrossRef CAS PubMed
.
- T.-F. Liu, X. Lu, H. Tang, M.-M. Zhang, P. Wang, P. Sun, Z.-Y. Liu, Z.-L. Wang, L. Li, Y.-C. Rui, T.-J. Li and W. Zhang, Steroids, 2013, 78, 108–114 CrossRef CAS PubMed
.
- L.-F. Liang, X.-J. Wang, H.-Y. Zhang, H.-L. Liu, J. Li, L.-F. Lan, W. Zhang and Y.-W. Guo, Bioorg. Med. Chem. Lett., 2013, 23, 1334–1337 CrossRef CAS PubMed
.
- S.-K. Wang, S.-Y. Puu and C.-Y. Duh, Mar. Drugs, 2013, 11, 571–580 CrossRef CAS PubMed
.
- Z. Wang, H. Tang, P. Wang, W. Gong, M. Xue, H. Zhang, T. Liu, B. Liu, Y. Yi and W. Zhang, Mar. Drugs, 2013, 11, 775–787 CrossRef CAS PubMed
.
- N. P. Thao, N. H. Nam, N. X. Cuong, B. H. Tai, T. H. Quang, N. T. T. Ngan, B. T. T. Luyen, S. Y. Yang, C. H. Choi, S. Kim, D. Chae, Y.-S. Koh, P. V. Kiem, C. V. Minh and Y. H. Kim, Bull. Korean Chem. Soc., 2013, 34, 949–952 CrossRef
.
- L.-L. Sun, X.-M. Fu, X.-B. Li, Q. Xing and C.-Y. Wang, Nat. Prod. Res., 2013, 27, 2006–2011 CrossRef CAS PubMed
.
- W.-H. Yen, W.-F. Chen, C.-H. Cheng, C.-F. Dai, M.-C. Lu, J.-H. Su, Y.-D. Su, Y.-H. Chen, Y.-C. Chang, Y.-H. Chen, J.-H. Sheu, C.-S. Lin, Z.-H. Wen and P.-J. Sung, Molecules, 2013, 18, 2895–2903 CrossRef CAS PubMed
.
- J. Zhang, X.-J. Liao, K.-L. Wang, Z. Deng and S.-H. Xu, Steroids, 2013, 78, 396–400 CrossRef CAS PubMed
.
- C.-H. Chao, Y.-C. Wu, Z.-H. Wen and J.-H. Sheu, Mar. Drugs, 2013, 11, 136–145 CrossRef CAS PubMed
.
- C.-Y. Huang, C.-C. Liaw, B.-W. Chen, P.-C. Chen, J.-H. Su, P.-J. Sung, C.-F. Dai, M. Y. Chiang and J.-H. Sheu, J. Nat. Prod., 2013, 76, 1902–1908 CrossRef CAS PubMed
.
- J. Zhang, L.-C. Li, K.-L. Wang, X.-J. Liao, Z. Deng and S.-H. Xu, Bioorg. Med. Chem. Lett., 2013, 23, 1079–1082 CrossRef CAS PubMed
.
- M. Jiang, P. Sun, H. Tang, B.-S. Liu, T.-J. Li, C. Li and W. Zhang, J. Nat. Prod., 2013, 76, 764–768 CrossRef CAS PubMed
.
- S. Qi, S. Zhang, J. Huang, Z. Xiao, J. Wu and Q. Li, Magn. Reson. Chem., 2005, 43, 266–268 CrossRef CAS PubMed
.
- C.-H. Chao, C.-H. Hsieh, S.-P. Chen, C.-K. Lu, C.-F. Dai and J.-H. Sheu, Tetrahedron Lett., 2006, 47, 5889–5891 CrossRef CAS
.
- K. Ota and H. Miyaoka, Chem. Commun., 2013, 49, 8148–8150 RSC
.
- H. Takamura, K. Iwamoto, E. Nakao and I. Kadota, Org. Lett., 2013, 15, 1108–1111 CrossRef CAS PubMed
.
- J. S. Clark, R. Berger, S. T. Hayes, H. M. Senn, L. J. Farrugia, L. H. Thomas, A. J. Morrison and L. Gobbi, J. Org. Chem., 2013, 78, 673–696 CrossRef CAS PubMed
.
- M. J. Palframan and G. Pattenden, Tetrahedron Lett., 2013, 54, 6822–6825 CrossRef CAS
.
- M. J. Palframan and G. Pattenden, Tetrahedron Lett., 2013, 54, 324–328 CrossRef CAS
.
- H. Kikuchi, Y. Tsukitani, Y. Yamada, K. Iguchi, S. A. Drexler and J. Clardy, Tetrahedron Lett., 1982, 23, 1063–1066 CrossRef CAS
.
- H.-P. Lee, S.-Y. Huang, Y.-Y. Lin, H.-M. Wang, Y.-H. Jean, S.-F. Wu, C.-Y. Duh and Z.-H. Wen, Mar. Drugs, 2013, 11, 99–113 CrossRef CAS PubMed
.
- W.-H. Yen, L.-C. Hu, J.-H. Su, M.-C. Lu, W.-H. Twan, S.-Y. Yang, Y.-C. Kuo, C.-F. Weng, C.-H. Lee, Y.-H. Kuo and P.-J. Sung, Molecules, 2012, 17, 14058–14066 CrossRef CAS PubMed
.
- K.-J. Huang, Y.-C. Chen, M. El-Shazly, Y.-C. Du, J.-H. Su, C.-W. Tsao, W.-H. Yen, W.-B. Chang, Y.-D. Su, Y.-T. Yeh and M.-C. Lu, Molecules, 2013, 18, 2924–2933 CrossRef CAS PubMed
.
- Y. Kashman, M. Bodner, Y. Loya and Y. Benayahu, Isr. J. Chem., 1977, 16, 1–3 CrossRef CAS
.
- W.-L. Hsu, S.-J. Chiu, Y.-T. Tsai, C.-M. Chang, J.-Y. Wang, E. T. Wang, M.-F. Hou, C.-Y. Huang, J.-H. Sheu and W.-C. Chang, Molecules, 2013, 18, 7023–7034 CrossRef CAS PubMed
.
- C.-Y. Kao, J.-H. Su, M.-C. Lu, T.-L. Hwang, W.-H. Wang, J.-J. Chen, J.-H. Sheu, Y.-H. Kuo, C.-F. Weng, L.-S. Fang, Z.-H. Wen and P.-J. Sung, Mar. Drugs, 2011, 9, 1319–1331 CrossRef CAS PubMed
.
- C.-Y. Lin, M.-C. Lu, J.-H. Su, C.-L. Chu, D. Shiuan, C.-F. Weng, P.-J. Sung and K.-J. Huang, Mar. Drugs, 2013, 11, 1336–1350 CrossRef CAS PubMed
.
- S. A. Look, W. Fenical, G. K. Matsumoto and J. Clardy, J. Org. Chem., 1986, 51, 5140–5145 CrossRef CAS
.
- D. R. Day, S. Jabaiah, R. S. Jacobs and R. D. Little, Mar. Drugs, 2013, 11, 3258–3271 CrossRef PubMed
.
- T. Higa, J. Tanaka, Y. Tsukitani and H. Kikuchi, Chem. Lett., 1981, 1647–1650 CrossRef CAS
.
- C. Ishikawa, J. Tanaka, H. Katano, M. Senba and N. Mori, Mar. Drugs, 2013, 11, 3410–3424 CrossRef CAS PubMed
.
- C. Schneider, M. L. Manier, D. L. Hachey and A. R. Brash, Lipids, 2002, 37, 217–221 CrossRef CAS PubMed
.
- E. Reina, F. A. Ramos, L. Castellanos, M. Aragon and L. F. Ospina, J. Pharm. Pharmacol., 2013, 65, 1643–1652 CrossRef CAS PubMed
.
- S.-L. Wu, J.-H. Su, C.-Y. Huang, C. J. Tai, P.-J. Sung, C.-C. Liaw and J.-H. Sheu, Mar. Drugs, 2012, 10, 1203–1211 CrossRef CAS PubMed
.
- B.-W. Chen, Y.-C. Wu, M. Y. Chiang, J.-H. Su, W.-H. Wang, T.-Y. Fan and J.-H. Sheu, Tetrahedron, 2009, 65, 7016–7022 CrossRef CAS
.
- Y.-H. Chen, T.-L. Hwang, Y.-D. Su, Y.-C. Chang, P.-H. Hong, L.-C. Hu, W.-H. Yen, H.-Y. Hsu, S.-J. Huang, Y.-H. Kuo and P.-J. Sung, Chem. Pharm. Bull., 2012, 60, 160–163 CrossRef CAS PubMed
.
- S.-L. Wu, J.-H. Su, C.-Y. Huang, C.-J. Tai, P.-J. Sung, C.-C. Liaw and J.-H. Sheu, Mar. Drugs, 2013, 11, 5087–5088 CrossRef CAS
.
- M. R. Prinsep and M. Dumté, Nat. Prod. Commun., 2013, 8, 693–694 CAS
.
- A. R. Carroll, S. Duffy, M. Sykes and V. M. Avery, Org. Biomol. Chem., 2011, 9, 604–609 CAS
.
- F. A. Khan and S. Ahmad, Tetrahedron Lett., 2013, 54, 2996–2998 CrossRef CAS
.
- Y. Kamano, H. Zhang, Y. Ichihara, H. Kizu, K. Komiyama and G. R. Pettit, Tetrahedron Lett., 1995, 36, 2783–2784 CrossRef CAS
.
- G. S. M. Figueiredo, R. S. Zardo, B. V. Silva, F. A. Violante, A. C. Pinto and P. D. Fernandes, Pharmacol., Biochem. Behav., 2013, 103, 431–439 CrossRef CAS PubMed
.
- K. Konoki, T. Onoda, R. Watanabe, Y. Cho, S. Kaga, T. Suzuki and M. Yotsu-Yamashita, Mar. Drugs, 2013, 11, 300–315 CrossRef CAS PubMed
.
- H. Kawashima, M. Ohnishi and S. Ogawa, J. Oleo Sci., 2013, 62, 465–470 CrossRef CAS PubMed
.
- E. Villaverde-de-Sáa, C. Valls-Cantenys, J. B. Quintana, R. Rodil and R. Cela, J. Chromatogr. A, 2013, 85–94 CrossRef PubMed
.
- M. Carbone, M. L. Ciavatta, J.-R. Wang, I. Cirillo, V. Mathieu, R. Kiss, E. Mollo, Y.-W. Guo and M. Gavagnin, J. Nat. Prod., 2013, 76, 2065–2073 CrossRef CAS PubMed
.
- C. M. Ireland, J. E. Biskupiak, G. J. Hite, M. Rapposch, P. J. Scheuer and J. R. Ruble, J. Org. Chem., 1984, 49, 559–561 CrossRef CAS
.
- S. Jaisamut, S. Prabpai, C. Tancharoen, S. Yuenyongsawad, S. Hannongbua, P. Kongsaeree and A. Plubrukarn, J. Nat. Prod., 2013, 76, 2158–2161 CrossRef CAS PubMed
.
- K. C. Tan, T. Wakimoto, K. Takada, T. Ohtsuki, N. Uchiyama, Y. Goda and I. Abe, J. Nat. Prod., 2013, 76, 1388–1391 CrossRef CAS PubMed
.
- S. Smith and G. M. Timms, J. Chem. Soc., 1937, 396–401 RSC
.
- T. Wakimoto, K. C. Tan and I. Abe, Toxicon, 2013, 72, 1–4 CrossRef CAS PubMed
.
- M. Carbone, C. Muniain, F. Castelluccio, O. Iannicelli and M. Gavagnin, Biochem. Syst. Ecol., 2013, 49, 172–175 CrossRef CAS
.
- K. E. Clark, A. Capper, G. Della Togna, V. J. Paul, L. I. Romero, T. Johns, L. Cubilla-Rios and T. L. Capson, Nat. Prod. Commun., 2013, 8, 1537–1540 CAS
.
- I. W. Mudianta, V. L. Challinor, A. E. Winters, K. L. Cheney, J. J. De Voss and M. J. Garson, Beilstein J. Org. Chem., 2013, 9, 2925–2933 CrossRef PubMed
.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino, S. Magno, S. Ianaro and M. Di Rosa, Eur. J. Org. Chem., 2001, 49–53 CrossRef CAS
.
- P. Ciminiello, C. Dell'Aversano, E. Fattorusso, M. Forino, S. Magno, F. U. Santelia, V. I. Moutsos, E. N. Pitsinos and E. A. Couladouros, Tetrahedron, 2006, 62, 7738–7743 CrossRef CAS
.
- D. H. Dethe and A. Ranjan, RSC Adv., 2013, 3, 23692–23703 RSC
.
- Y. Nakao, W. Y. Yoshida, Y. Takada, J. Kimura, L. Yang, S. L. Mooberry and P. L. Scheuer, J. Nat. Prod., 2004, 67, 1332–1340 CrossRef CAS PubMed
.
- Y. Takada, M. Umehara, Y. Nakao and J. Kimura, Tetrahedron Lett., 2008, 49, 1163–1165 CrossRef CAS
.
- M. Umehara, T. Negishi, Y. Maehara, Y. Nakao and J. Kimura, Tetrahedron, 2013, 69, 3045–3053 CrossRef CAS
.
- Y. Kato and P. J. Scheuer, J. Am. Chem. Soc., 1974, 96, 2245–2246 CrossRef CAS PubMed
.
- Y. Hanaki, M. Kikumori, S. Ueno, H. Tokuda, N. Suzuki and K. Irie, Tetrahedron, 2013, 69, 7636–7645 CrossRef CAS
.
- D. S. Dalisay, E. W. Rogers, A. S. Edison and T. F. Molinski, J. Nat. Prod., 2009, 72, 732–738 CrossRef CAS PubMed
.
- W. Tantisantisom, D. M. Ramsey and S. R. McAlpine, Org. Lett., 2013, 15, 4638–4641 CrossRef CAS PubMed
.
- M. T. Hamann and P. J. Scheuer, J. Am. Chem. Soc., 1993, 115, 5825–5826 CrossRef CAS
.
- R. Salazar, H. Cortés-Funes, E. Casado, B. Pardo, A. López-Martín, C. Cuadra, J. Tabernero, C. Coronado, M. Garciá, A. S. Matos-Pita, B. Miguel-Lillo, M. Cullell-Young, J. L. Iglesias Dios and L. Paz-Ares, Cancer Chemother. Pharmacol., 2013, 72, 75–83 CrossRef CAS PubMed
.
- M. Serova, A. de Gramont, I. Bieche, M. E. Riveiro, C. M. Galmarini, M. Aracil, J. Jimeno, S. Faivre and E. Raymond, Mar. Drugs, 2013, 11, 944–959 CrossRef CAS PubMed
.
- K. V. Rao, M.-K. Na, J. C. Cook, J. Peng, R. Matsumoto and M. T. Hamman, J. Nat. Prod., 2008, 71, 772–778 CrossRef CAS PubMed
.
- M. A. Albadry, K. M. Elokely, B. Wang, J. J. Bowling, M. F. Abdelwahab, M. H. Hossein, R. J. Doerksen and M. T. Hamann, J. Nat. Prod., 2013, 76, 178–185 CrossRef CAS PubMed
.
- The error has been noted by the lead author and a correction will be forthcoming (M. Hamann, pers. commun.).
- K. Yamada, M. Ojika, T. Ishigaki, Y. Yoshida, H. Ekimoto and M. Arakawa, J. Am. Chem. Soc., 1993, 115, 11020–11021 CrossRef CAS
.
- M. Kita, Y. Hirayama, K. Yoneda, K. Yamagishi, T. Chinen, T. Usui, E. Sumiya, M. Uesugi and H. Kigoshi, J. Am. Chem. Soc., 2013, 135, 18089–18095 CrossRef CAS PubMed
.
- M. Ojika, H. Kigoshi, Y. Yoshida, T. Ishigaki, M. Nisiwaki, I. Tsukada, M. Arakawa, H. Ekimoto and K. Yamada, Tetrahedron, 2007, 63, 3138–3167 CrossRef CAS
.
- O. Ohno, M. Morita, K. Kitamura, T. Teruya, K. Yoneda, M. Kita, H. Kigoshi and K. Suenaga, Bioorg. Med. Chem. Lett., 2013, 23, 1467–1471 CrossRef CAS PubMed
.
- Y. Nakamura, H. Kato, T. Nishikawa, N. Iwasaki, Y. Suwa, H. Rotinsulu, F. Losung, W. Maarisit, R. E. P. Mangindaan, H. Morioka, H. Yokosawa and S. Tsukamoto, Org. Lett., 2013, 15, 322–325 CrossRef CAS PubMed
.
- C.-D. Pham, H. Weber, R. Hartmann, V. Wray, W. Lin, D. Lai and P. Proksch, Org. Lett., 2013, 15, 2230–2233 CrossRef CAS PubMed
.
- K. E. Rudolph, M. S. Liberio, R. A. Davis and A. R. Carroll, Org. Biomol. Chem., 2013, 11, 261–270 CAS
.
- J. L. Li, E. La Kim, H. Wang, J. Hong, S. Shin, C.-K. Lee and J. H. Jung, Bioorg. Med. Chem. Lett., 2013, 23, 4701–4704 CrossRef CAS PubMed
.
- M. Menna, A. Aiello, F. D'Aniello, C. Imperatore, P. Luciano, R. Vitalone, C. Irace and R. Santamaria, Eur. J. Org. Chem., 2013, 3241–3246 CrossRef CAS
.
- N. Bontemps, F. Gattacceca, C. Long, O. P. Thomas and B. Banaigs, J. Nat. Prod., 2013, 76, 1801–1805 CrossRef CAS PubMed
.
- B. R. Copp, J. Tompa, A. Tahir and C. M. Ireland, J. Org. Chem., 1998, 63, 8024–8026 CrossRef CAS
.
- H. K. H. Fong and B. R. Copp, Mar. Drugs, 2013, 11, 274–299 CrossRef CAS PubMed
.
- N. Matsumori, Y. Hiradate, H. Shibata, T. Oishi, S. Shimma, M. Toyoda, F. Hayashi, M. Yoshida, M. Murata and M. Morisawa, Org. Lett., 2013, 15, 294–297 CrossRef CAS PubMed
.
- M. Yoshida, M. Murata, K. Inaba and M. Morisawa, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 14831–14836 CrossRef CAS PubMed
.
- T. V. K. Reddy, B. L. A. P. Devi, R. B. N. Prasad, P. Sujitha and C. G. Kumar, Eur. J. Med. Chem., 2013, 67, 384–389 CrossRef PubMed
.
- A. Aiello, E. Fattorusso, A. Giordano, M. Menna, C. Navarrete and E. Muñoz, Tetrahedron, 2009, 65, 4384–4388 CrossRef CAS
.
- J. N. Kumar, P. R. Reddy, B. Das, C. G. Kumar and P. Sujitha, Bioorg. Med. Chem. Lett., 2013, 23, 5192–5194 CrossRef CAS PubMed
.
- J. N. Kumar and B. Das, Tetrahedron Lett., 2013, 54, 3865–3867 CrossRef CAS
.
- M. L. Ciavatta, E. Manzo, G. Nuzzo, G. Villani, M. Varcamonti and M. Gavagnin, Tetrahedron, 2010, 66, 7533–7538 CrossRef CAS
.
- T. B. Parsons, N. Spencer, C. W. Tsang and R. S. Grainger, Chem. Commun., 2013, 49, 2296–2298 RSC
.
- D. R. Appleton and B. R. Copp, Tetrahedron Lett., 2003, 44, 8963–8965 CrossRef CAS
.
- K. Takamura, H. Matsuo, A. Tanaka, J. Tanaka, T. Fukuda, F. Ishibashi and M. Iwao, Tetrahedron, 2013, 69, 2782–2788 CrossRef CAS
.
- W. Y. Yoshida, K. K. Lee, A. R. Carroll and P. J. Scheuer, Helv. Chim. Acta, 1992, 75, 1721–1725 CrossRef CAS
.
- H. Jin, P. Zhang, K. Bijian, S. Ren, S. Wan, M. A. Alaoui-Jamali and T. Jiang, Mar. Drugs, 2013, 11, 1427–1439 CrossRef CAS PubMed
.
- T. H. Trieu, J. Dong, Q. Zhang, B. Zheng, T.-Z. Meng, X. Lu and X.-X. Shi, Eur. J. Org. Chem., 2013, 3271–3277 CrossRef CAS
.
- J. D. Panarese and S. P. Waters, Org. Biomol. Chem., 2013, 11, 3428–3431 CAS
.
- W. Wang, S.-J. Nam, B.-C. Lee and H. Kang, J. Nat. Prod., 2008, 71, 163–166 CrossRef CAS PubMed
.
- L. Peng, F.-M. Zhang, B.-M. Yang, X.-B. Zhang, W.-X. Liu, S.-Y. Zhang and Y.-Q. Tu, Tetrahedron Lett., 2013, 54, 6514–6516 CrossRef CAS
.
- H. Fuwa, K. Sekine and M. Sasaki, Org. Lett., 2013, 15, 3970–3973 CrossRef CAS PubMed
.
- B. C. M. Potts, D. J. Faulkner, J. A. Chan, G. C. Simolike, P. Offen, M. E. Hemling and T. A. Francis, J. Am. Chem. Soc., 1991, 113, 6321–6322 CrossRef CAS
.
- A. N. Pearce, E. W. Chia, M. V. Berridge, E. W. Maas, M. J. Page, J. L. Harper, V. L. Webb and B. R. Copp, Tetrahedron, 2008, 64, 5748–5755 CrossRef CAS
.
- L. P. P. Liew, M. Kaiser and B. R. Copp, Bioorg. Med. Chem. Lett., 2013, 23, 452–454 CrossRef CAS PubMed
.
- A. N. Pearce, E. W. Chia, M. V. Berridge, G. R. Clark, J. L. Harper, L. Larsen, E. W. Maas, M. J. Page, N. B. Perry, V. L. Webb and B. R. Copp, J. Nat. Prod., 2007, 70, 936–940 CrossRef CAS PubMed
.
- C. F. C. Lam, N. Pearce, S. H. Tan, M. Kaiser and B. R. Copp, Mar. Drugs, 2013, 11, 3472–3499 CrossRef CAS PubMed
.
- A. M. Seldes, M. F. Rodriguez Brasco, L. Hernandez Franco and J. A. Palermo, Nat. Prod. Res., 2007, 21, 555–563 CrossRef CAS PubMed
.
- S. B. Bharate, R. R. Yadav, S. I. Khan, B. L. Tekwani, M. R. Jacob, I. A. Khan and R. A. Vishwakarma, MedChemComm, 2013, 4, 1042–1048 RSC
.
- M. J. McKay, A. R. Carroll and R. J. Quinn, J. Nat. Prod., 2005, 68, 1776–1778 CrossRef CAS PubMed
.
- A. K. Pandey, R. Sharma, R. Shivahare, A. Arora, N. Rastogi, S. Gupta and P. M. S. Chauhan, J. Org. Chem., 2013, 78, 1534–1546 CrossRef CAS PubMed
.
- J. Kobayashi, J.-F. Cheng, Y. Kikuchi, M. Ishibashi, S. Yamamura, Y. Ohizumi, T. Ohta and S. Nozoe, Tetrahedron Lett., 1990, 31, 4617–4620 CrossRef CAS
.
- L. V. Frolova, I. V. Magedov, A. E. Romero, M. Karki, I. Otero, K. Hayden, N. M. Evdokimov, L. M. Y. Banuls, S. K. Rastogi, W. R. Smith, S.-L. Lu, R. Kiss, C. B. Shuster, E. Hamel, T. Betancourt, S. Rogelj and A. Kornienko, J. Med. Chem., 2013, 56, 6886–6900 CrossRef CAS PubMed
.
- H. Kang and W. Fenical, J. Org. Chem., 1997, 62, 3254–3262 CrossRef CAS PubMed
.
- J. W. Bin, I. L. K. Wong, X. Hu, Z. X. Yu, L. F. Xing, T. Jiang, L. M. C. Chow and W. S. Biao, J. Med. Chem., 2013, 56, 9057–9070 CrossRef CAS PubMed
.
- T. H. Won, J.-e. Jeon, S.-H. Kim, S.-H. Lee, B.-J. Rho, D.-C. Oh, K.-B. Oh and J. Shin, J. Nat. Prod., 2012, 75, 2055–2061 CrossRef CAS PubMed
.
- C.-H. Ahn, T. H. Won, H. Kim, J. Shin and K.-B. Oh, Bioorg. Med. Chem. Lett., 2013, 23, 4099–4101 CrossRef CAS PubMed
.
- K. Suwanborirux, K. Charupant, S. Amnuoypol, A. Kubo and N. Saito, J. Nat. Prod., 2002, 65, 935–937 CrossRef CAS
.
- M. Tsujimoto, W. Lowtangkitcharoen, N. Mori, W. Pangkruang, P. Putongking, K. Suwanborirux and N. Saito, Chem. Pharm. Bull., 2013, 61, 1052–1064 CrossRef CAS PubMed
.
- J. Sikorska, A. M. Hau, C. Anklin, S. Parker-Nance, M. T. Davies-Coleman, J. E. Ishmael and K. L. McPhail, J. Org. Chem., 2013, 78, 2812 CrossRef CAS
.
- J. L. Li, B. Xiao, M. Park, E. S. Yoo, S. Shin, J. Hong, H. Y. Chung, H. S. Kim and J. H. Jung, J. Nat. Prod., 2013, 76, 815 CrossRef CAS
.
- G.-Y. Zhang, H.-H. Ren, Y.-B. Zhang, L.-Q. Ma, Y.-L. Yang and S. Wang, Biochem. Syst. Ecol., 2013, 51, 203–206 CrossRef CAS
.
- N. P. Thao, N. X. Cuong, B. T. T. Luyen, N. H. Nam, P. V. Cuong, N. V. Thanh, N. X. Nhiem, T. T. H. Hanh, E.-J. Kim, H.-K. Kang, P. V. Kiem, C. V. Minh and Y. H. Kim, Chem. Pharm. Bull., 2013, 61, 1044–1051 CrossRef PubMed
.
- N. P. Thao, N. X. Cuong, B. T. T. Luyen, T. H. Quang, T. T. H. Hanh, S. Kim, Y.-S. Koh, N. H. Nam, P. V. Kiem, C. V. Minh and Y. H. Kim, Mar. Drugs, 2013, 11, 2917–2926 CrossRef PubMed
.
- R. S. Popov, N. V. Ivanchina, A. A. Kicha, T. B. Malyarenko, A. I. Kalinovskii and P. S. Dmitrenok, Chem. Nat. Compd., 2013, 49, 286–290 CrossRef CAS
.
- Z. Li, G. Chen, X. Lu, H. Wang, B. Feng and Y. Pei, Nat. Prod. Res., 2013, 27, 1816–1822 CrossRef CAS PubMed
.
- Z.-R. Zou, Y.-H. Yi, H.-M. Wu, J.-H. Wu, C.-C. Liaw and H.-K. Lee, J. Nat. Prod., 2003, 66, 1055–1060 CrossRef CAS PubMed
.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Martyyas, V. I. Kalinin, P. Jayasandhya, G. C. Rajan and K. P. Padmakumar, Nat. Prod. Commun., 2013, 8, 301–310 CAS
.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjashchenko, P. S. Dmitrenok, V. I. Kalinin, S. Taboada and C. Avila, Biochem. Syst. Ecol., 2013, 51, 45–49 CrossRef CAS
.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjashchenko, P. S. Dmitrenok, E. A. Martyyas and V. I. Kalinin, Nat. Prod. Commun., 2013, 8, 1053–1058 CAS
.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Yurchenko, I. Y. Dolmatov, V. I. Kalinin and V. A. Stonik, Nat. Prod. Commun., 2013, 8, 1527–1534 CAS
.
- N. P. Thao, N. X. Cuong, B. T. T. Luyen, N. V. Thanh, N. X. Nhiem, Y.-S. Koh, B. M. Ly, N. H. Nam, P. V. Kiem, C. V. Minh and Y. H. Kim, J. Nat. Prod., 2013, 76, 1764–1770 CrossRef PubMed
.
- N. P. Thao, L. D. Dat, N. T. Ngoc, V. A. Tu, T. T. H. Hanh, P. T. T. Huong, N. X. Nhiem, B. H. Tai, N. X. Cuong, N. H. Nam, P. V. Cuong, S. Y. Yang, S. Kim, D. Chae, Y.-S. Koh, P. V. Kiem, C. V. Minh and Y. H. Kim, Bioorg. Med. Chem. Lett., 2013, 23, 1823–1827 CrossRef CAS PubMed
.
- S. De Marino, M. Iorizzi, F. Zollo, C. D. Amsler, S. P. Greer and J. B. McClintock, Eur. J. Org. Chem., 2000, 4093–4098 CrossRef CAS
.
- G. Xiao and B. Yu, Chem.–Eur. J., 2013, 7708–7712 CrossRef CAS PubMed
.
- N. V. Palyanova, T. M. Pankova, M. V. Starostina, A. A. Kicha, N. V. Ivanchina and V. A. Stonik, Mar. Drugs, 2013, 11, 1440–1455 CrossRef CAS PubMed
.
- F.-J. Wu, Y. Xue, Q.-J. Tang, J. Xu, L. Du, C.-H. Xue, K. Takahashi and Y.-M. Wang, J. Oleo Sci., 2013, 62, 717–727 CrossRef CAS PubMed
.
- J. S. Yoo, T. Park, G. Bang, C. Lee, J.-R. Rho and Y. H. Kim, J. Mass Spectrom., 2013, 48, 164–171 CrossRef CAS PubMed
.
- N. V. Ivanchina, A. A. Kicha, T. V. Malyarenko, A. I. Kalinovsky, P. S. Dmitrenok and V. A. Stonik, Steroids, 2013, 78, 1183–1191 CrossRef CAS PubMed
.
- H. Nan, H. Lin, Z. Qian and H. Yin, Heterocycles, 2013, 87, 1093–1098 CrossRef CAS
.
- H. Wang, M.-Y. Li, T. Satyanandamurty and J. Wu, Planta Med., 2013, 79, 666–672 CrossRef CAS PubMed
.
- M. G. Ponnapalli, M. Ankireddy, S. C. V. A. R. Annam, S. Ravirala, S. Sukki and V. R. Tuniki, Tetrahedron Lett., 2013, 54, 2942–2945 CrossRef
.
- C.-L. Cheng, Z.-Z. Wang, P.-L. Li, X.-W. Zhang, R.-C. Wu, H.-Y. Zhu, X.-L. Tang and G.-Q. Li, Chin. Chem. Lett., 2013, 24, 1080–1082 CrossRef CAS
.
- H. Chen, J. Zhang, M.-Y. Li, T. Satyanandamurty and J. Wu, Chem. Biodiversity, 2013, 10, 612–620 CAS
.
- Y.-B. Wu, Z.-Y. Ni, C.-H. Huo, J. Su, M. Dong, F. Sauriol, Q.-W. Shi, Y.-C. Gu and H. Kiyota, Biosci., Biotechnol., Biochem., 2013, 77, 736–740 CrossRef CAS PubMed
.
- K. Toume, K. Kamiya, M. A. Arai, N. Mori, S. K. Sadhu, F. Ahmed and M. Ishibashi, Org. Lett., 2013, 15, 6106–6109 CrossRef CAS PubMed
.
- J. Li, M.-Y. Li, T. Bruhn, F. Z. Katele, Q. Xiao, P. Pedpradab, J. Wu and G. Bringmann, Org. Lett., 2013, 15, 3682–3685 CrossRef CAS PubMed
.
- J. Li, M.-Y. Li, Q. Xiao, P. Pedpradab and J. Wu, Phytochem. Lett., 2013, 6, 482–485 CrossRef CAS
.
- S. Homhual, H.-J. Zhang, N. Bunyapraphatsara, T. P. Kondratyuk, B. D. Santarsiero, A. D. Mesecar, A. Herunsalee, W. Chaukul, J. M. Pezzuto and H. H. S. Fong, Planta Med., 2006, 72, 255–260 CrossRef CAS PubMed
.
- J. Chen, C.-S. Jiang, W.-Q. Ma, L.-X. Gao, J.-X. Gong, J.-Y. Li, J. Li and Y.-W. Guo, Bioorg. Med. Chem. Lett., 2013, 23, 5061–5065 CrossRef CAS PubMed
.
- K. Li, C. O. Brant, M. Huertas, S. K. Hur and W. Li, Org. Lett., 2013, 15, 5924–5927 CrossRef CAS PubMed
.
- M. Adachi, T. Imazu, M. Isobe and T. Nishikawa, J. Org. Chem., 2013, 78, 1699–1705 CrossRef CAS PubMed
.
- M. Yotsu-Yamashita, Y. Abe, Y. Kudo, R. Ritson-Williams, V. J. Paul, K. Konoki, Y. Cho, M. Adachi, T. Imazu, T. Nishikawa and M. Isobe, Mar. Drugs, 2013, 11, 2799–2813 CrossRef PubMed
.
- Y. L. Mak, J. J. Wu, W. H. Chan, M. B. Murphy, J. C. W. Lam, L. L. Chan and P. K. S. Lam, Anal. Bioanal. Chem., 2013, 405, 3331–3340 CrossRef CAS PubMed
.
- http://www.marinespecies.org, accessed June 2014.
| This journal is © The Royal Society of Chemistry 2015 |