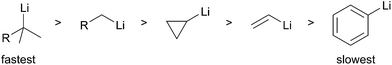Generation, structure and reactivity of tertiary organolithium reagents
Matthew A.
Perry
*a and
Scott D.
Rychnovsky
b
aAnacor Pharmaceuticals, Inc., 1020 E. Meadow Circle, Palo Alto, CA, USA. E-mail: mperry@anacor.com
bDepartment of Chemistry, University of California, 1102 Natural Sciences 2, Irvine, CA, USA. E-mail: srychnov@uci.edu
First published on 5th December 2014
Abstract
Covering: up to 2014
Tertiary alkyllithium reagents are very useful intermediates in synthesis. Alkyllithium reagents with adjacent heteroatoms may be formed stereoselectively or may react stereoselectively, and have been used in the synthesis of alkaloids, C-glycosides and spirocycles. An overview of the generation, reactivity and stereochemistry of tertiary alkyllithium reagents will be presented, as well as examples of their use in organic synthesis. The discussion will be focused on a conceptual understanding of the generation and reactivity of these intermediates. The reactions described herein generate fully substituted carbon atoms, and the forces driving stereoselectivity will be discussed in detail. Where appropriate, computational results will be introduced to provide a better understanding for the structure and reactivity of tertiary alkyllithium reagents.
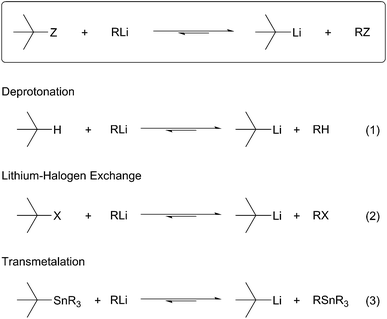
Preparation of organolithium reagents
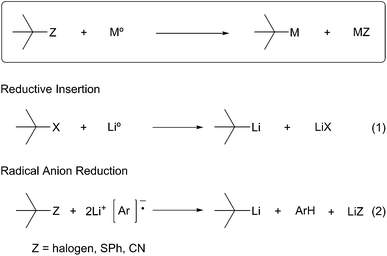
All organolithium species are prepared ultimately from the obvious precursor, lithium–metal. The limited compatibility of lithium–metal with organic substrates has resulted in the development and use of more general methods for the selective introduction of C–Li bonds. Schlosser describes two main methods to generate organolithium species.1 The first method, termed permutational interconversion, involves the displacement of a substituent Z with subsequent transfer of the electrofugal group to the original metal carrier (Scheme 1). This method may be further divided into three classes based on the mechanism of the transformation; (1) deprotonation, (2) transmetalation, and (3) lithium–halogen exchange. Deprotonation is the most direct method, but is limited by the low acidities of C–H bonds, poor regioselectivity, and limited substrate scope (Scheme 1, eqn (1)). Lithium–halogen exchange may be the most common method to selectively introduce a C–Li bond (Scheme 1, eqn (2)). Early work performed by Gilman determined lithium–halogen exchange to be an equilibrium process favoring the formation of the less basic, more stable organolithium.2 Transmetalation occurs through formation of a lithium–metal ate complex followed by lithium–metal exchange:3 the most applicable method involves Sn–Li exchange (Scheme 1, eqn (3)). Organolithiums tend to react rapidly and reversibly with stannanes under thermodynamic control to produce the more stable organolithium. To a large extent the current knowledge regarding reactivity and configurational stability of organolithiums was elucidated by their preparation from organostannanes. In general, permutational interconversion is the most mild and substrate compatible method for the generation of functionalized organolithium species.The second general method for preparing organolithium species is reductive insertion. This process involves insertion of lithium–metal into an activated C–Z bond (Z = I, Br, Cl, CN, etc.) with concomitant release of Z as a nucleofugal group (Scheme 2). The common commercially available unfunctionalized organolithium reagents (n-, sec-, tert-butyllithium) are prepared by reductive lithiation of alkyl chlorides and lithium–metal.4 In alkyl halides, an electron from a lithium atom enters the C–X σ* orbital with concomitant C–X sigma bond cleavage to the alkyl radical and halide anion in a concerted process.5 The radical intermediate is then reduced by a second lithium atom to provide the organolithium (Scheme 2, eqn (1)). Since the reduction is a radical process, the relative rate of formation is contingent upon the stability of the radical intermediate. Complementary to deprotonation, reductive lithiation is fastest for more substituted alkyllithiums and slowest for aryllithiums (Fig. 2).
Arene radical anion reducing reagents
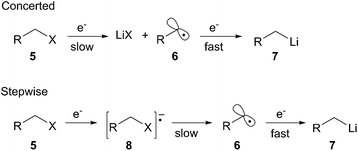
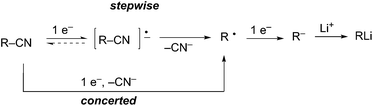
More recently, arene radical anion reductions have been developed to circumvent the variability and undesired reactivity of lithium–metal reductions (Scheme 2, eqn (2)). Polynuclear aromatics accept electrons from lithium–metal to form radical anion intermediates that act as single electron transfer (SET) reagents between the metal and the reducible functionality (Fig. 1). The “dissolving metal” conditions provided by arene radical anions are homogeneous and therefore provide efficient and rapid electron transfer under a wide range of temperatures. In addition, the stoichiometry between the arene radical species and the compound to be reduced is precisely controlled. One limitation to dissolving metal conditions involves the nucleophilic addition of the intermediate arene radical/organolithium to an electrophile on the substrate being reduced. Another drawback is the radical anion solution must be freshly prepared before use; their highly reducing nature results in a short shelf life. This method is complementary to traditional dissolving metal conditions (i.e. Li/NH3 or Na/NH3) in which the anion generated upon reductive insertion of lithium or sodium is rapidly protonated by the solvent ammonia.Reductive lithiation proceeds by the same general mechanism regardless of the radical anion carrier used. The first step involves a SET to the LUMO of the nucleofugal substituent of the substrate being reduced. The loss of the nucleofuge to form the intermediate alkyl radical may proceed through either a stepwise or concerted process (Scheme 3). A stepwise process is possible when the nucleofuge is able to accept an electron into a low-lying empty d-orbital or π*-orbital. A concerted process occurs when the nucleofuge is unable to accommodate an additional electron into a low energy molecular orbital prior to bond cleavage. The first SET to form the alkyl radical intermediate is the rate-limiting step in the reduction to an organolithium. The second SET from a second equivalent of radical anion carrier forms the organolithium intermediate.
The rate of reduction for the first SET is dictated by the relative stability of the resultant radical intermediate. Therefore, the reduction is fastest for tertiary alkyllithiums and slowest for aryllithiums (Fig. 2).
Reductive decyanation is mechanistically related to reductive desulfurization6 and dehalogenation.7 Initial single electron transfer (SET) to the alkyl nitrile, a potentially reversible process, leads to a transient radical anion that fragments to generate an alkyl radical and cyanide (Scheme 4). Alternately, a concerted single electron transfer/cleavage sequence can also lead directly to the alkyl radical. A second single electron transfer to the alkyl radical produces a carbanion that is protonated in protic media (e.g. NH3) or trapped by an electrophile in aprotic media (e.g. THF, ether).
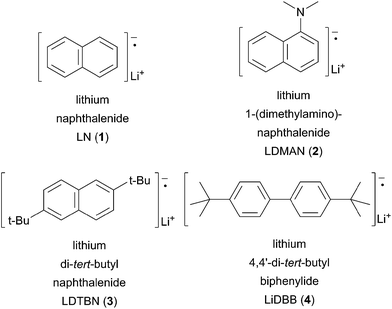
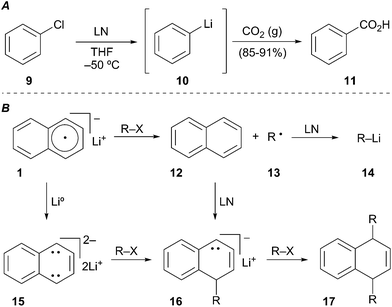
Screttas was the first to describe the use of a polynuclear aromatic radical carrier for reductive lithiation.8 In this case, naphthalene was used with lithium–metal to mediate the reduction of chlorobenzene (9) to phenyllithium (10). Electrophilic trapping of the organolithium with carbon dioxide gas efficiently provided benzoic acid (11, Scheme 5A). The limited utility of LN (1) arises from the propensity of naphthalene to undergo deleterious alkylation or dimerization (Scheme 5B). In the desired pathway, reduction of naphthalene by lithium–metal generates radical anion 1 that mediates two sequential SET processes with RX to provide the alkyllithium 14. Unproductive pathways stem from the Birch reduction of LN to the aromatic dianion 15. Coupling of the dianion with either RX or the alkyl radical 13 afford alkylated anion 16. The resulting anion 16 may undergo a second alkylation reaction in the presence of primary or secondary alkyl halides (RX) to generate 17. To avoid the undesired by-product reactivity associated with lithium naphthalenide (LN) and chromatographic separation of naphthalene, other arene radical anion carriers were developed.
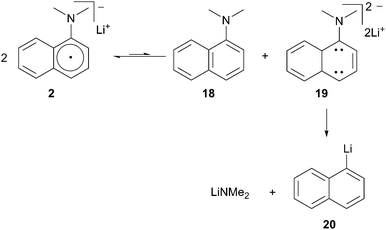
Cohen described the use of aqueous acid soluble 1-dimethylaminonaphthalene (DMAN) as an alternative solution to the issue of purification.9 The generation and use of lithium 1-(dimethylamino)-naphthalenide (2) requires that the reaction take place at −45 °C or below to avoid degradation of the radical anion to 1-lithionaphthalene.10 Cohen postulated a decomposition pathway involving an unfavorable equilibrium between two equivalents of LDMAN (2) to afford DMAN and dianion 19 (Scheme 6). The dianion further decomposes to the more stable lithium dimethyl amide and 1-lithionaphthalene 20.
In an effort to prevent the undesired alkylation problems, Freeman reported on the use of lithium 4,4′-di-tert-butylbiphenylide (LiDBB, 4) as an arene radical carrier.11 Freeman's reagent has the advantage of minimizing deleterious alkylation side reactions based on steric hindrance. The steric bulk of the tert-butyl substituents inhibit bond formation, which require distances of <2.0 Å. Fortunately, electron transfers can occur between 7–9 Å allowing for single electron reduction to occur.12 In addition to providing beneficial steric interactions, the tert-butyl groups also infer a higher reduction potential (Table 1).
| Arene | E 1/2 , V |
|---|---|
| a vs. Hg pool. | |
| Naphthalene | −1.98 |
| 2,6-Di-tert-butylnaphthalene | −2.07 |
| 2,7-Di-tert-butylnaphthalene | −2.09 |
| Biphenyl | −2.05 |
| 4,4′-Di-tert-butylbiphenyl | −2.14 |
| Anthracene | −1.46 |
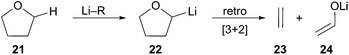
Solvent effects play a key role in the generation of organolithiums. The solubility and stability of an arene radical species dictates which solvents are appropriate. The extremely basic nature of organolithiums can result in deprotonation of ethereal solvents such as THF. Both LN and LiDBB have limited solubility in solvents other than THF as described by Cohen.13 At times, THF has been detrimental to the optimum utilization of reductive lithiation. The ability of organolithiums to remove a proton from the 2-position of THF can be a major problem chiefly for the more basic organolithiums or at temperatures above 0 °C.14 Deprotonation of THF produces lithiated intermediate 22 that subsequently undergoes a facile retro-[3 + 2] rearrangement to afford acetaldehyde enolate 24 and ethene gas (Scheme 7).15 The instability of highly basic tertiary organolithiums is typified by the relatively short 5.6 hour half-life of tert-BuLi in THF at −40 °C and 0.7 hour half-life at −20 °C.16 A decrease in the rate of electrophilic trapping of the tertiary organolithium–may result in greater competitive deprotonation of solvent. This decomposition pathway has become a fundamental limitation for reductive lithiation. The restrictions imposed by the solubility of the radical anion reagent and the high basicity of the organolithiums produced leave few alternative solvents that are compatible with the reaction conditions.
Tertiary α-alkoxyorganolithiums
Generation, structure and reactivity
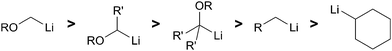
McGarvey et al. demonstrated that α-alkoxy substituted lithium species were stabilized compared to the alkyllithium counterparts.17 The relative stability of such α-alkoxyorganolithiums and alkyllithiums was determined through competitive exchange experiments (Fig. 3). Both primary and secondary α-alkoxyorganolithiums are more stable than methyllithium while the tertiary α-alkoxyorganolithium are more stable than primary alkyllithiums. In most cases, the stereospecific transformations of transmetalation from tin and subsequent electrophilic trapping allow for stereoselective C–C bond formation.Generation by permutational interconversion
Generation by reductive insertion
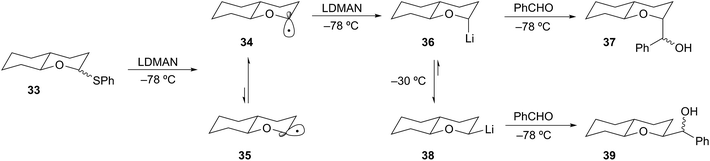
Complementary to the permutational interconversion approach is the reductive insertion method described earlier. Cohen demonstrated that formation of α-alkoxyorganolithium intermediates by reductive lithiation of thiophenyl ethers is a general and reliable method.24 These studies also show that stereoelectronic effects strongly influence the stereochemical disposition of the lithium species. For example, reductive lithiation (LDMAN) of thioether 33 gave a mixture of radicals 34 and 35. Rapid equilibration of 34 and 35 through a small energy barrier (<0.5 kcal mol−1)25 affords the thermodynamically preferred axial lithium 36 (Scheme 10).26 A favorable HOMO–SOMO anomeric stabilization involving overlap of the oxygen lone pair with the SOMO of the radical can rationalize the preferential formation of the axial radical 34.27 In this way, the configuration of thioether 33 is inconsequential to the stereochemical outcome of the reduction. Facile reduction of the radical 34 by a second equivalent of LDMAN (kred = 1 × 109 M s−1) provided the axial organolithium. Consequently, this organolithium is destabilized by an anti-anomeric (HOMO–HOMO) interaction.28 Organolithium 36 is configurationally stable at −78 °C and can be efficiently trapped to provide 37 in good yield and high diastereoselectivity (ax/eqn (95)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5). Epimerization of 36 can be accomplished by raising the temperature of the reaction to −30 °C to provide the lower energy isomer 38. Subsequent cooling to −78 °C and trapping with benzaldehyde provided 39 in 87
5). Epimerization of 36 can be accomplished by raising the temperature of the reaction to −30 °C to provide the lower energy isomer 38. Subsequent cooling to −78 °C and trapping with benzaldehyde provided 39 in 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 dr. While this example does not involve a tertiary α-alkoxyorganolithium, the stereochemical stability and lability of the secondary case described is an important feature of α-alkoxyorganolithium chemistry.
13 dr. While this example does not involve a tertiary α-alkoxyorganolithium, the stereochemical stability and lability of the secondary case described is an important feature of α-alkoxyorganolithium chemistry.
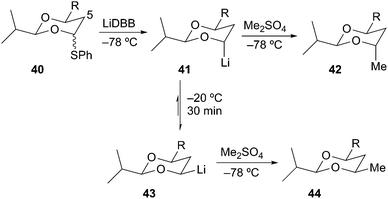
Rychnovsky and Skalitzky later demonstrated high levels of selectivity in the reductive lithiation of 4-phenythio-1,3-dioxanes to provide stereochemically defined 1,3 diols. Reductive lithiation of 40 with LiDBB at −78 °C gave the axial alkyllithium 41, which upon alkylation with dimethyl sulfate afforded the protected anti-1,3-diol 42 in 79% yield and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 selectivity (Scheme 11).29 The equatorial alkyllithium 43 was prepared by equilibration of axial organolithium 41 at −20 °C for 30 min followed by alkylation at −78 °C to give the protected syn-1,3-diol 44 in 79% yield and >99
1 selectivity (Scheme 11).29 The equatorial alkyllithium 43 was prepared by equilibration of axial organolithium 41 at −20 °C for 30 min followed by alkylation at −78 °C to give the protected syn-1,3-diol 44 in 79% yield and >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Beak has described these epimerizing alkyllithium systems as Dynamic Thermodynamic Resolutions in which the reactive intermediate is equilibrated prior to reaction with the next reagent.30
1 dr. Beak has described these epimerizing alkyllithium systems as Dynamic Thermodynamic Resolutions in which the reactive intermediate is equilibrated prior to reaction with the next reagent.30
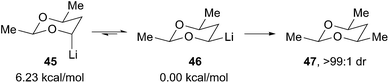
The equatorial alkyllithium 43 is more stable than the axial isomer 41 by at least 2.4 kcal mol−1 based on the equilibration experiment in Scheme 11. Rychnovsky and Skalitzky reported computational studies to evaluate the thermodynamic preference for equatorial over axial disposition.31 However, the studies were carried out many years ago using only modest levels of theory (B3LYP/6-31G(d), but larger systems were evaluated at the HF/3-21G minima) without explicit solvation. The calculations showed a strong preference for the equatorial isomer, varying from 5.3 to 6.2 kcal mol−1 depending upon the substitution pattern around the 4-lithio-1,3-dioxane ring (Scheme 12). Experimentally, the equilibration of systems with C-5 substitution equilibrated more slowly, but the calculated preference for the equatorial isomer was essentially unchanged. This conclusion is consistent with experiments where the equilibrium was approached from both directions. Although all of the 4-lithio-1,3-dioxane systems studied showed a large thermodynamic preference for the equatorial isomer, the equilibration strategy was only practical for the less substituted systems as shown in Scheme 11.
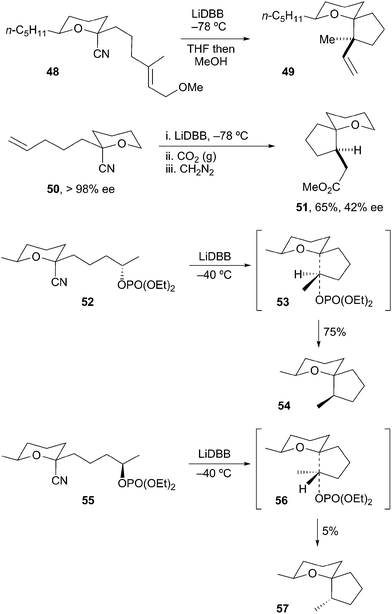
The generation and spiroannulation of tertiary α-alkoxyorganolithiums has also been described. Rychnovsky and Takaoka reported on the annulation of lithiated THP 48 onto tethered alkenes to afford spirocycle 49 (Scheme 13).32 In a similar fashion, Rychnovsky et al. cyclized lithiated THP 50 by a carbolithiation/carboxylation strategy to provide spiro-THP 51 in good yield and high diastereoselectivity. The optical purity of the reductive cyclization product 51 (42% ee) indicated a lifetime for the radical intermediate (ca. 2.4 × 10−7 s) that was too brief for a radical cyclization to take place. Thus, the reduction of 50 to 51 proceeds via cyclization of the alkyllithium. Rychnovsky and Morin later described the stereoselective spiroannulation of lithiated THP 52 onto resolved secondary tethered electrophiles to provide spirocycles such as 54.33 As might be expected based on transition states 53 and 56, the rate of cyclization is strongly dependent on the configuration of the electrophilic carbon. In all instances, the tertiary α-alkoxyorganolithium intermediate was configurationally stable at −78 °C and reacted with retention of configuration.
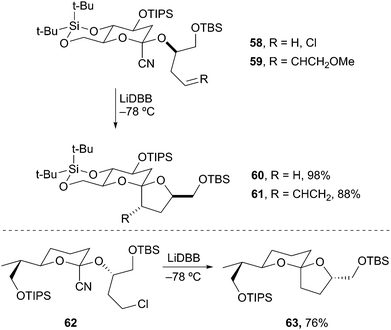
Spiroacetal motifs are common in natural products and several methods have been developed for their synthesis.34 Most methods rely on acid-catalyzed cyclization, which lead to a thermodynamic mixture of spiroacetals. The most stable configuration usually positions both oxygen atoms in a spatial arrangement favoring double anomeric stabilization.35 A number of natural products contain contra-thermodynamic spiroacetals (only a single anomeric stabilization) and have been more difficult to access. Rychnovsky and coworkers accomplished the construction of contra-thermodynamic spiroacetals by reductive lithiation and intramolecular trapping of 2-cyanotetrahydropyranyl acetals. Specifically, reductive lithiation of 2-cyano-THP 58 produced an axially disposed 2-lithio-THP that cyclized with retention to give spiroacetal 60 in 98% yield as a single diastereomer (Scheme 14). The cyclization also proceeds via an SN2′-type mechanism to provide 4-alkenyl tetrahydrofuran spiroacetal 61. Rychnovsky and Vellucci subsequently applied this approach to the synthesis of the A/B non-anomeric spiroacetal of pectenotoxin 2.36 The reductive cyclization of cyano acetal 62 provided the non-anomeric spiroacetal 63 in 76% yield as a single diastereomer. This approach constitutes the first highly diastereoselective approach to the less stable spiroacetal of the pectenotoxin natural products.
Tertiary α-aminoorganolithiums
Generation, structure and reactivity
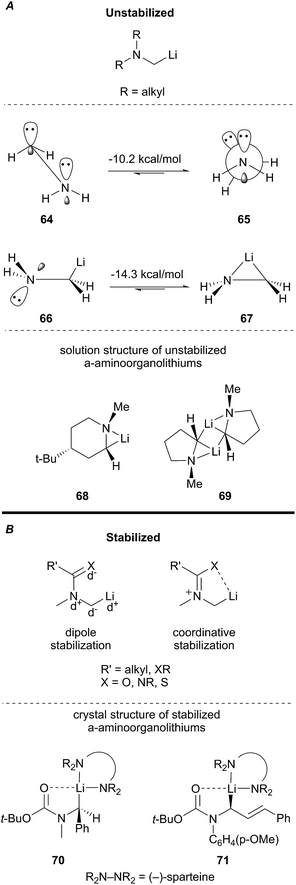
The reactivity of a α-aminoorganolithium depends on the nature of substitution at nitrogen. Broadly, there are two classes of α-aminoorganolithiums: unstabilized and stabilized α-aminoorganolithiums (Fig. 4).37 Unstabilized α-aminoorganolithiums possess simple alkyl substituents that infer little to no stereoelectronic or coordinative stabilization of the C–Li bond. Computational studies indicate that N–Li bridging favors the syn conformation (67) in α-aminoorganolithiums. The solution state structure of 68 and 69 support that hypothesis (Fig. 4A).38 The analogous syn conformation of the α-aminocarbanion (64) is 10.2 kcal mol−1 higher in energy and prefers to adopt a staggered geometry (65). However, nitrogen–lithium bridging plays a negligible role in the case of stabilized α-aminoorganolithiums (Fig. 4B). Stabilized α-aminoorganolithiums benefit from a combination of a favorable dipole arrangement and heteroatom–Li coordination to form a carbon centered anion.39 This effect has been unequivocally demonstrated in the crystal structures of N-acyl α-aminoorganolithiums 70 and 71.40Generation by permutational interconversion
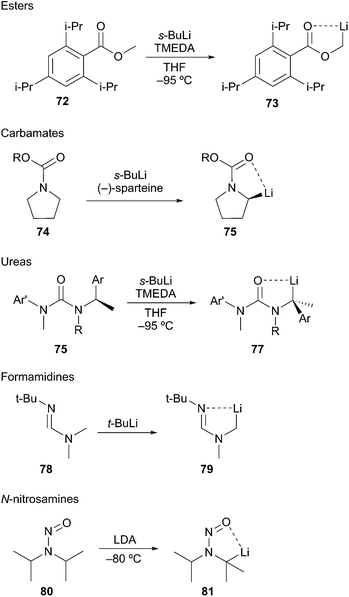
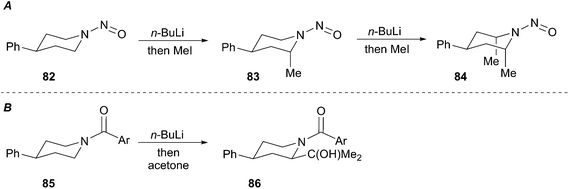
Seebach developed efficient low-temperature lithiation procedures of N-nitrosamines using lithium diisopropylamide (LDA).47 The resulting lithiated species react in good yields with a wide range of electrophiles. A noteworthy application of this method involves the lithiation and alkylation of tertiary α-amino positions as illustrated in Scheme 15 (i.e.81). Further developments revealed a highly diastereoselective alkylation of cyclohexyl nitrosamine 82 to give exclusively axially alkylated products 83 and 84 (Scheme 16A). The major drawback of N-nitrosamines is attributed to their mutagenic and carcinogenic properties.48 Extreme care to minimize exposure is necessary when using any N-nitrosamine reagents. Based on work conducted by Fraser,49 Seebach showed that 4-phenylpiperidine-derived amide 85 undergoes lithiation and alkylation at the equatorial position to give 2,4-cis-piperidine 86 (Scheme 16B).50 The stereochemical disparity in the alkylation of the N-nitroso and N-acyl examples suggested the organolithium intermediates were configurationally stable. Few examples of discrete tertiary organolithiums generated by deprotonation have been reported. As such, the description of configurationally stable secondary α-aminoorganolithiums will be discussed as a means of exemplifying the stereoelectronic and steric factors that govern stereoselectivity and reactivity.
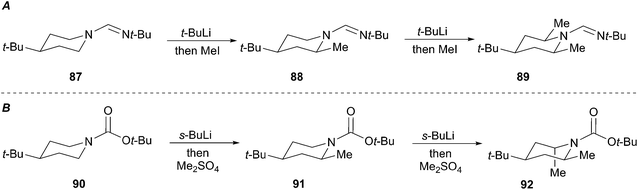
Both Meyers and Beak investigated the use of activating groups of greater synthetic utility. Meyers focused on N-formamidinyl activating groups and Beak employed the N-Boc group. Meyers devised a sequence involving successive equatorial lithiation/alkylation to give exclusively the 2,4,6-cis derivative 89 (Scheme 17A).51 Beak's lithiation/alkylation sequence proceeds first with equatorial alkylation then with axial lithiation/alkylation to provide the 2,4-cis-6-trans piperidine 92 as a single diastereomer (Scheme 17B).52
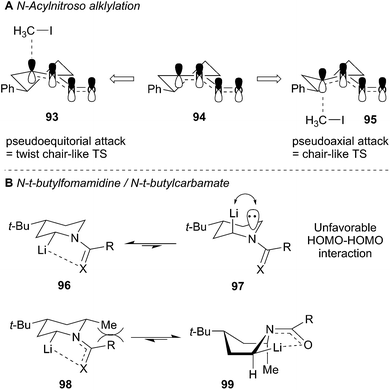
The proposed rationalization for axial-selective N-nitroso alkylation is based on the strong electron-withdrawing capability of the nitroso group. The anion is largely delocalized in a coplanar C–N–N–O π-system, which favors axial alkylation over equatorial alkylation for stereoelectronic purposes. Specifically, a lower energy chair-like transition state (95) that leads to axial alkylation is preferred over a higher energy twist-boat-like transition state (93) that leads to equatorial alkylation (Fig. 5A). For N-tert-butylformamidinyl and N-Boc lithiation and alkylations the delocalization of the nitrogen lone pair into the carbonyl π-bond is less significant than the N-nitroso systems. As a result, the C–Li bond prefers to adopt an orthogonal orientation to the axial nitrogen lone pair (96) to avoid the high energy (∼17 kcal mol−1) HOMO–HOMO interaction (97) (Fig. 5B). Efficient O–Li coordination also stabilizes this geometry. Subsequent alkylation with retention of configuration provides the equatorially substituted product 91. For the N-t-butylformamidinyl case, the second alkylation sequence occurs as above to provide the 2,4,6-cis product 89. In contrast, the second alkylation of N-Boc piperidines gives rise to a 2,4-cis-6-trans product 92. Resonance induced planarization of the N–C–O bonds creates A1,3-strain between the Boc group and the equatorial substituent (98). Conformational adjustment to the lower energy twist-boat-like conformation (99) allows lithiation to occur at a pseudo-equatorial position to give a 2,6-trans relationship.
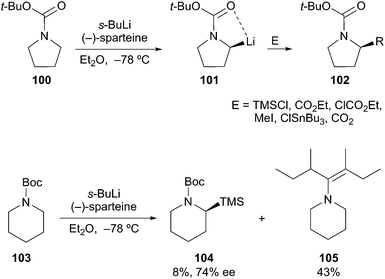
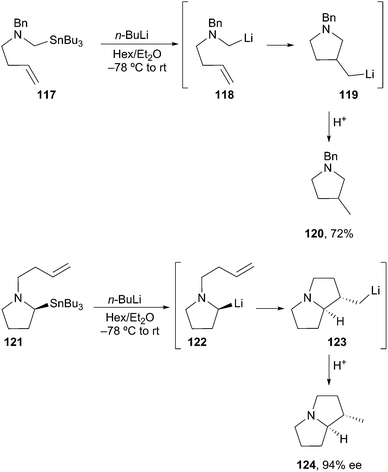
Following the work of Nozaki53 and Hoppe,54 Beak described the asymmetric deprotonation of N-Boc-directed pyrrolidine 100 by a (−)-sparteine·s-BuLi complex (Scheme 18). Subsequent electrophilic alkylation provided the substituted pyrrolidine 102 in good yield and high enantioselectivity. Since the first report in 1991,55 considerable attention has been focused on the mechanistic and synthetic utility of this method.56 In simple cases, sparteine and related amines are effective chiral ligands. One notable limitation is the use of Beak's protocol in the deprotonation of N-Boc piperidine 103. Lithiation followed by silylation provided adduct 104 in low yield and with moderate enantioselectivity. Unfortunately, the favored course of the reaction involved sec-BuLi addition into the Boc carbonyl to give 105.57 Interestingly, O'Brien and co-workers reported on the asymmetric deprotonation and electrophilic trapping of piperidine 103 with s-BuLi and a (+)-sparteine surrogate in moderate to high yield and moderate enantioselectivity (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 er, 76% ee).58 Gawley and Beng have also reported on the catalytic dynamic resolution of rac-2-lithio-N-Boc-piperidine using a chiral ligand in the presence of TMEDA to afford various 2-substituted piperidines with high enantioselectivity (96
12 er, 76% ee).58 Gawley and Beng have also reported on the catalytic dynamic resolution of rac-2-lithio-N-Boc-piperidine using a chiral ligand in the presence of TMEDA to afford various 2-substituted piperidines with high enantioselectivity (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to >99
4 to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er).59
1 er).59
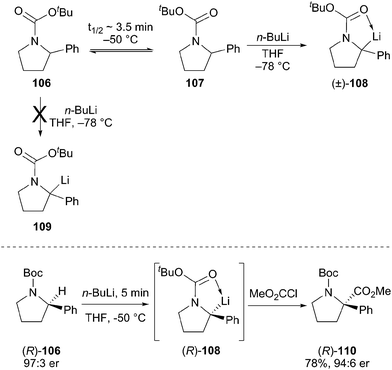
Recent work has shown that configurationally stable alkyllithium reagents can be generated by direct deprotonation of N-Boc amides with an activating group such as a phenyl ring. O'Brien and Coldham studied the deprotonation reaction using React-IR and found that the two rotamers of the Boc carbamate show very different reactivity (Scheme 19).60 The example of N-Boc-2-phenylpyrrolidine is particularly demanding. Deprotonation of rotamer 107 at −78 °C takes place rapidly, but the other rotamer 106 does not react at low temperatures. The interconversion of rotamers 106 and 107 has a measured barrier of ca. 15.4 kcal mol−1, which leads to a half-life of rotation of ∼10 h at −78 °C and ∼3.5 min at −50 °C. Rotamer interconversion is thus the slow step in the deprotonation reaction at −78 °C, but warming to 0 °C leads to complete and efficient deprotonation. The corresponding N-Boc-2-phenylpiperidine rotamers interconvert much more rapidly and can be efficiently deprotonated at low temperature.
Optically pure N-Boc-2-phenylpyrrolidine (R)-106 can be deprotonated and alkylated with a high degree of retention (Scheme 19).54 Under carefully optimized conditions, the deprotonation of pyrrolidine (R)-106 at −50 °C for 5 min generates the tertiary alkyllithium (R)-108, which slowly epimerizes at this temperature. By keeping the reaction times short and using reactive electrophiles, this alkyllithium reagent can be trapped with overall retention of configuration in good yield. The method works well with the analogous piperidine, and provides a very nice strategy to prepare optically pure, fully substituted centers adjacent to nitrogen in these important ring systems. In 2014, Coldham et al. reported on the kinetic resolution of rac-N-Boc-2-phenylpiperidines with n-BuLi/(−)-sparteine or n-BuLi/(+)-sparteine in moderate yield and high enantioselectivity to afford 2,2-disubstituted piperidines.61
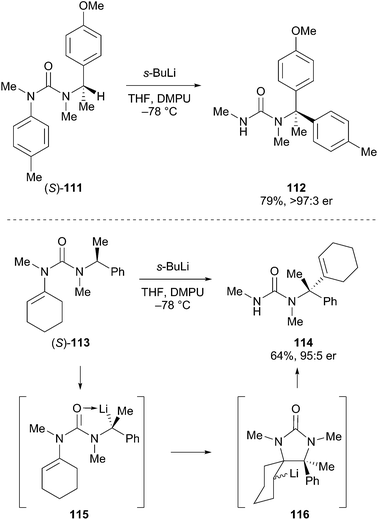
An interesting method to prepare optically enriched fully substituted centers adjacent to nitrogen atoms was developed by Clayden.62 For example, the reaction is initiated by deprotonation of optically pure benzylic urea (S)-111 (Scheme 20). Intramolecular migration of the aryl group took place with retention of configuration to give urea 112 in good overall yield. Similarly, alkene migration was demonstrated in the transformation of 113 to 114 with good levels of selectivity. The proposed mechanism involved deprotonation with retention of configuration to produce the alkyllithium 115, which is configurationally stable under the reaction conditions. The migration proceeds by cyclization to 116 and ring opening to produce the fully substituted carbon atom adjacent to nitrogen. These transformations are effective in the absence of any obvious anion-stabilizing group on the aryl ring or alkene, and led to optically active amines that would be difficult to access through other methods.
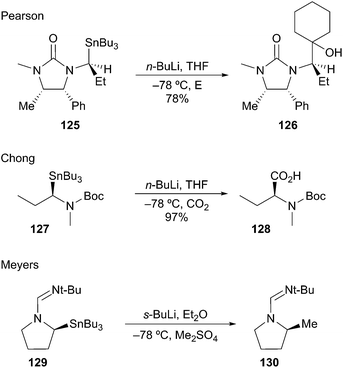
Pearson was the first to demonstrate Sn–Li exchange to generate stabilized α-aminoorganolithiums.65 Transmetalation of 125 at −78 °C afforded the organolithium with retention, which was trapped with cyclohexanone to afford 126 in good yield (Scheme 22). Chong and co-workers also demonstrated the generation and electrophilic trapping of an α-aminoorganolithium from enantioenriched stannane 127 to afford acid 128 with high stereofidelity.66 Meyers later demonstrated the configurational stability of formamide-stabilized α-aminoorganolithium at −78 °C from stannane 129; subsequent methylation provided 130.67
Generation by reductive insertion
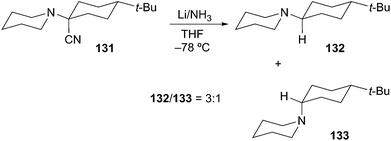
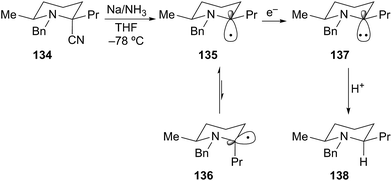
The last, and perhaps, most efficient method for the generation of α-aminoorganometallics is reductive decyanation/metalation of suitable functional groups by low-valent metals (e.g. zero-valent Group 1 metals: Li, Na, and K). In 1970, Welvart described the reduction of a tertiary α-aminonitrile precursor under dissolving metal conditions (Li/NH3 or Na/NH3) to effect replacement of the nitrile group by a hydrogen atom (Scheme 23).68Husson later described the stereoselective reductive decyanation of 2-cyanopiperidines under dissolving metal conditions.69 Reductive decyanation (Na/NH3) of 134 to afforded piperidine 138 as a single diastereomer with 2,6-cis relative stereochemistry (Scheme 24). The selectivity in this process is rationalized by an anomeric-type effect as described earlier for the case of α-alkoxyorganolithium generation. Initial SET produces a rapidly equilibrating mixture of anomeric radicals 135 and 136 where equilibrium lies toward the pseudo-axial radical 135 due to a stabilizing HOMO–SOMO interaction. A second SET produces the axial carbanion 137. Rapid protonation of the highly basic anion proceeds with retention of configuration to generate the cis product 138. As noted above, this process proceeds first through the thermodynamically preferred axial radical configuration and then through the thermodynamically disfavored axial anion configuration (HOMO–HOMO interaction). On this basis, the equatorial anion should be preferred as in the N-acyl/formamidinyl piperidine lithiations shown in Schemes 16 and 17.
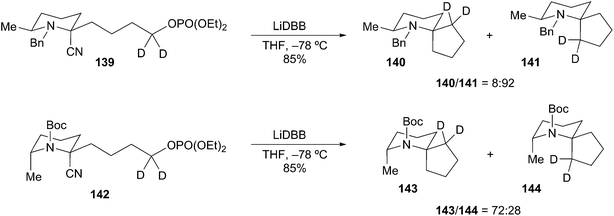
Rychnovsky and Wolckenhauer have also reported several examples for the generation of tertiary α-aminoorganolithiums by reductive decyanation.70 Their study examined the roles of protecting group and tether length with regards to cyclization of substituted 2-cyanopiperidines. As with examples described above, the use of unstabilized (N-Bn) versus stabilized (N-Boc) organolithiums had a noticeable influence on the course of the reductive cyclization. Reductive decyanation of N-Bn protected cyanopiperidine 139 (LiDBB, −78 °C) afforded cyclized diastereomers 141 and 140 in a 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 ratio (Scheme 25). The major cyclization product 141 arises from cyclization with retention from an axial organolithium. The configuration of the organolithium is assumed based on analogy to the deuterium-trapped alkyllithium of a similar piperidine (Scheme 27). In contrast, subjecting N-Boc cyanopiperidine 142 to the same reductive cyclization conditions produced diastereomeric products 143 and 144 in a 72
8 ratio (Scheme 25). The major cyclization product 141 arises from cyclization with retention from an axial organolithium. The configuration of the organolithium is assumed based on analogy to the deuterium-trapped alkyllithium of a similar piperidine (Scheme 27). In contrast, subjecting N-Boc cyanopiperidine 142 to the same reductive cyclization conditions produced diastereomeric products 143 and 144 in a 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 ratio. Trapping studies of a model system with trimethyl phosphate indicated that the equatorial organolithium was the major species in solution. These results exemplify the disparate yet complementary nature of seemingly similar reactions where only the protecting group dictates the course of the reaction.
28 ratio. Trapping studies of a model system with trimethyl phosphate indicated that the equatorial organolithium was the major species in solution. These results exemplify the disparate yet complementary nature of seemingly similar reactions where only the protecting group dictates the course of the reaction.
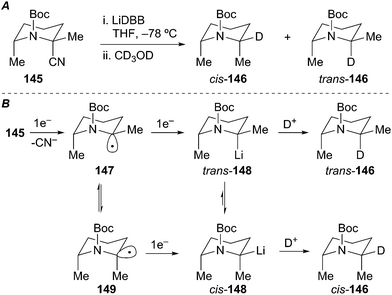
Wolckenhauer and Rychnovsky also explored the stereoselectivity in the reductive decyanation of simple N-benzyl 2-cyanopiperidine and N-Boc 2-cyanopiperidine. Rapid (<1 h) CD3OD quenches afforded low ratios of diastereomeric products cis/trans-146 with the cis-146 predominating (Scheme 26A). Slow equilibration of the intermediate diastereomeric organolithiums was observed at low temperature (−78 °C, t = 1 h gave 62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 38 cis/trans versus t = 12.5 h, 78
38 cis/trans versus t = 12.5 h, 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 cis/trans) over extended time periods. Conducting the reductive lithiation at −40 °C provided higher diastereomer ratios (94
22 cis/trans) over extended time periods. Conducting the reductive lithiation at −40 °C provided higher diastereomer ratios (94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 cis/trans) with lower yield and deuterium incorporation. The observed selectivity can be rationalized by considering the stability of the intermediate radical and organolithium intermediates. Single-electron transfer to produce an axially disposed anomerically stabilized radical (147) would be expected. In the N-Boc piperidine case, the anomeric stabilization is diminished due to delocalization of the nitrogen lone pair into the carbonyl moiety. Additionally, A1,3-strain between the Boc group and the equatorial methyl group results in isomerization to the equatorially disposed radical intermediate 149. Further single-electron reduction of the diastereomeric radical intermediates leads to α-aminoorganolithiums trans/cis-148. An anti-anomeric (HOMO–HOMO) effect of the C–Li bond and the nitrogen lone pair electrons, and A1,3-strain arising from interactions between the Boc group and the equatorial methyl group favor formation of the equatorial organolithium intermediate. Coordinative stabilization between the Boc group and the equatorial lithium may also favor equilibration to the equatorial organolithium cis-148. The combination of these effects overrides the 1,3-diaxial (Me–Me) interactions in the cis-isomer. Deuterium incorporation with retention of configuration produces a mixture of the 2,6-trans and 2,6-cis piperidines. Assuming a thermodynamic preference for formation of cis-148, the slow equilibration at −78 °C indicates a substantial barrier to inversion. Computational work by Gawley et al. suggests an inversion barrier of ∼16 kcal mol−1 for related N-Boc 2-lithiopyrrolidines.71
6 cis/trans) with lower yield and deuterium incorporation. The observed selectivity can be rationalized by considering the stability of the intermediate radical and organolithium intermediates. Single-electron transfer to produce an axially disposed anomerically stabilized radical (147) would be expected. In the N-Boc piperidine case, the anomeric stabilization is diminished due to delocalization of the nitrogen lone pair into the carbonyl moiety. Additionally, A1,3-strain between the Boc group and the equatorial methyl group results in isomerization to the equatorially disposed radical intermediate 149. Further single-electron reduction of the diastereomeric radical intermediates leads to α-aminoorganolithiums trans/cis-148. An anti-anomeric (HOMO–HOMO) effect of the C–Li bond and the nitrogen lone pair electrons, and A1,3-strain arising from interactions between the Boc group and the equatorial methyl group favor formation of the equatorial organolithium intermediate. Coordinative stabilization between the Boc group and the equatorial lithium may also favor equilibration to the equatorial organolithium cis-148. The combination of these effects overrides the 1,3-diaxial (Me–Me) interactions in the cis-isomer. Deuterium incorporation with retention of configuration produces a mixture of the 2,6-trans and 2,6-cis piperidines. Assuming a thermodynamic preference for formation of cis-148, the slow equilibration at −78 °C indicates a substantial barrier to inversion. Computational work by Gawley et al. suggests an inversion barrier of ∼16 kcal mol−1 for related N-Boc 2-lithiopyrrolidines.71
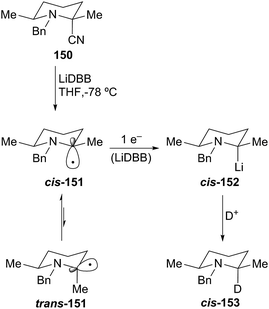
The analogous N-benzyl 2-cyanopiperidine gave complementary diastereoselectivity compared to the N-Boc 2-cyanopiperidine case. Reductive lithiation of 150 proceeded with high stereoselectivity (>95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 cis/trans) to produce the cis-lithiopiperidine 152, which appears to be configurationally stable at −78 °C for at least one hour (Scheme 27). The axial incorporation of the proton can be explained by carbon–CN bond cleavage by single-electron transfer to predominantly generate the anomerically-stabilized axial radical cis-151. The subsequent single-electron transfer occurs with retention of configuration to form the axial 2-lithiopiperidine cis-152, which reacts with retention of configuration to produce cis-N-benzyl piperidine 153.
5 cis/trans) to produce the cis-lithiopiperidine 152, which appears to be configurationally stable at −78 °C for at least one hour (Scheme 27). The axial incorporation of the proton can be explained by carbon–CN bond cleavage by single-electron transfer to predominantly generate the anomerically-stabilized axial radical cis-151. The subsequent single-electron transfer occurs with retention of configuration to form the axial 2-lithiopiperidine cis-152, which reacts with retention of configuration to produce cis-N-benzyl piperidine 153.
As shown above, the reductive lithiation of N-Boc 2-cyanopiperidines produces organolithium diastereomers with less than 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
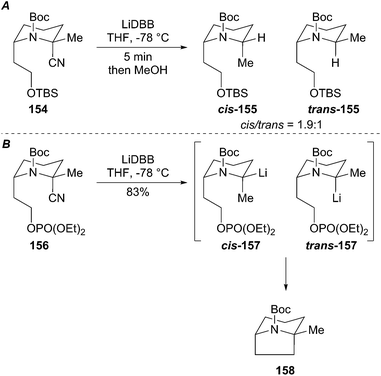
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at −78 °C, which slowly equilibrate over several hours. Reductive lithiation of analog 154 also produced a mixture of organolithium diastereomers cis/trans-155 (Scheme 28A). By proper positioning of the tethered leaving group on the N-Boc piperidine, one organolithium diastereomer is forced to react with retention of configuration and the other with inversion. Alternatively, the organolithium diastereomer may be non-reactive towards cyclization with inversion and may be protonated upon quenching the reaction. A reasonable mechanism based on the reductive decyanation product ratio of cis/trans-155 involves non-stereoselective reductive lithiation to generate a pair of diastereomeric organolithiums (cis/trans-157) where one organolithium diastereomer reacts via a SE2ret pathway while the other diastereomer reacts via SE2inv. The hypothesis was confirmed by reducing the 2-cyanopiperidine phosphate 156 to give the bridged bicycle 158 in 83% yield as a single diastereomer (Scheme 28B). This example demonstrates that a stereogenic organolithium can be forced to proceed through a non-preferred electrophilic substitution pathway by conformational constraint.
1 ratio at −78 °C, which slowly equilibrate over several hours. Reductive lithiation of analog 154 also produced a mixture of organolithium diastereomers cis/trans-155 (Scheme 28A). By proper positioning of the tethered leaving group on the N-Boc piperidine, one organolithium diastereomer is forced to react with retention of configuration and the other with inversion. Alternatively, the organolithium diastereomer may be non-reactive towards cyclization with inversion and may be protonated upon quenching the reaction. A reasonable mechanism based on the reductive decyanation product ratio of cis/trans-155 involves non-stereoselective reductive lithiation to generate a pair of diastereomeric organolithiums (cis/trans-157) where one organolithium diastereomer reacts via a SE2ret pathway while the other diastereomer reacts via SE2inv. The hypothesis was confirmed by reducing the 2-cyanopiperidine phosphate 156 to give the bridged bicycle 158 in 83% yield as a single diastereomer (Scheme 28B). This example demonstrates that a stereogenic organolithium can be forced to proceed through a non-preferred electrophilic substitution pathway by conformational constraint.
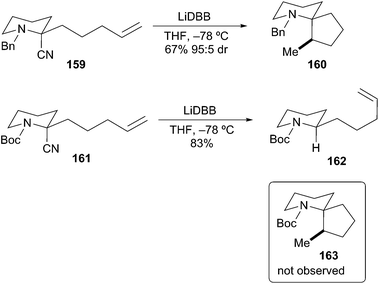
Bahde and Rychnovsky also described the different reactivity of N-Boc and N-Bn protected α-aminoorganolithiums.72 Reductive lithiation of N-Bn-2-cyanopiperidine 159 followed by carbolithiation cyclization produced spiropiperidine 160 in good yield with excellent diastereoselectivity (Scheme 29). In an analogous experiment, reductive lithiation of the N-Boc-2-cyanopiperidine 161 produced an α-aminoorganolithium species that did not undergo cyclization to 163; only reduced starting material 162 was isolated. Coldham et al. have reported a similar lack of cyclization for N-Boc alkenyl stannanes.73 These examples further demonstrate the reactivity differences between unstabilized and stabilized α-aminoorganolithium species.
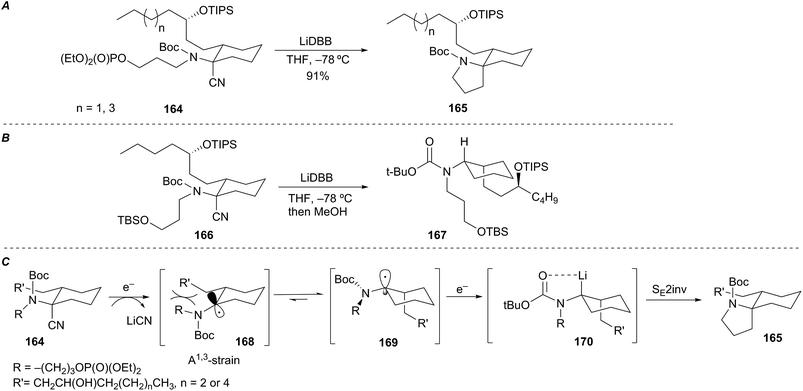
Rychnovsky et al. described an efficient and highly stereoselective reductive cyclization of an exocyclic α-aminoorganolithium to form the spiropyrrolidine ring system of the lepadiformine alkaloids (Scheme 30A).74 The mechanistic origin of selectivity was of interest and prompted an investigation aimed at identifying the configuration of the intermediate organolithium. Reductive lithiation of control substrate 166 followed by protonation of the resultant organolithium gave cis-disubstituted cyclohexane 167 (Scheme 30B). Assuming protonation proceeded with retention of configuration,75 the mechanism of the reductive cyclization may progress as shown in Scheme 30C. The radical intermediate 168, stabilized by interaction with the nitrogen electrons, creates a sterically encumbered environment between the equatorial R′ group and the nitrogen R group (or N-Boc group) resulting in a conformational isomerization to produce radical 169. Further reduction of the axially disposed radical 169 to alkyllithium 170 allows for cyclization via a SEinv pathway and leads to spiropyrrolidine 165. The overall stereochemical outcome of the event is cyclization with retention of configuration through a double inversion sequence.
Tertiary alkyl organolithiums
Generation, structure and reactivity
Reported concurrently by Wittig76 and Gilman,77 lithium halogen exchange has proven to be a useful method for the selective introduction of carbon–lithium bonds. This equilibrium process provides rapid and efficient preparation of primary and secondary alkyllithiums from alkyl bromides and iodides from less stable organolithiums (e.g. tert-butyllithium).78 Therefore, the generation of tertiary alkyllithiums by equilibration with another unstable tertiary organolithium is energetically and synthetically prohibitive. Similar to lithium–halogen exchange, tin–lithium exchange also relies on an equilibrium process that is limited by the unfavorable formation of less stable unfunctionalized tertiary alkyllithiums. Deprotonation is by far the least common method for their preparation. Lithiation by deprotonation to form unstabilized alkyllithiums is confounded by the very low acidity of the C–H bond, the lack of intramolecular coordination of the electron deficient lithium atom to a heteroatom, and/or the lack of an adjacent empty orbital or electron withdrawing group necessary for stabilization of the electron rich C–Li bond. The best method for generating tertiary unstabilized alkyllithiums is reductive insertion.Generation by reductive insertion
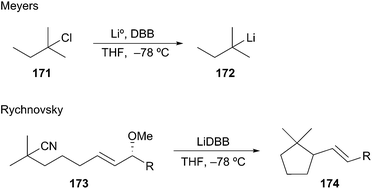
In contrast to deprotonation, the rate of reductive lithiation is fastest for substituted alkyllithiums (Fig. 2). This trend logically coincides with the relative stabilities of the intermediate radicals, which are generated in the rate-determining step of the reduction sequence. Meyers showed that the radical anion carrier DBB was very efficient at forming tert-amyl lithium 172 by reductive lithiation (Scheme 31).79 In 2004, Rychnovsky and La Cruz reported on the syn-SN2′ cyclization of a tertiary alkyllithium onto a tethered allylic methoxy ether to give cyclopentyl alkene 174 in 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
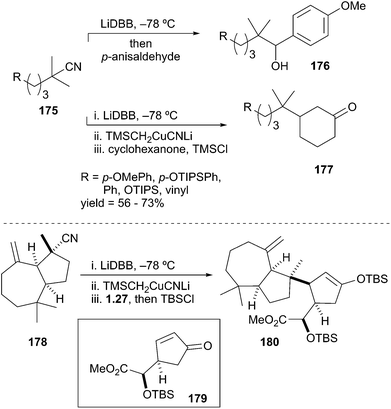
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er.80 More recently, Overman et al. demonstrated the formation and reactivity of unstabilized tertiary alkyllithium reagents and their derived cuprates in bimolecular reactions with carbon-centered electrophiles.81 The reductive lithiation and electrophilic trapping of tertiary nitriles 175 with p-anisaldehyde gave neopentyl alcohols 176 in synthetically useful yields (Scheme 32). The approach was then extended to the formation of tertiary organocuprates by transmetalation. Generation of the organolithium from nitriles 175 followed by transmetalation and subsequent conjugate addition to the cyclohexenone afforded β-substituted cyclohexanone derivatives 177. The utility of this transformation was highlighted by the union of hydrazulene nitrile 178 to cyclopentenone 179 to fashion the C8–C14 bond and the C8 quaternary stereocenter of the rearranged spongian diterpene aplyviolene precursor 180.82 The above examples highlight the facile reduction of alkyl nitriles and halogens to provide synthetically useful tertiary alkyllithium intermediates.83 However, this area of research remains underdeveloped in part due to the highly basic and reactive nature of the generated alkyllithium species.
5 er.80 More recently, Overman et al. demonstrated the formation and reactivity of unstabilized tertiary alkyllithium reagents and their derived cuprates in bimolecular reactions with carbon-centered electrophiles.81 The reductive lithiation and electrophilic trapping of tertiary nitriles 175 with p-anisaldehyde gave neopentyl alcohols 176 in synthetically useful yields (Scheme 32). The approach was then extended to the formation of tertiary organocuprates by transmetalation. Generation of the organolithium from nitriles 175 followed by transmetalation and subsequent conjugate addition to the cyclohexenone afforded β-substituted cyclohexanone derivatives 177. The utility of this transformation was highlighted by the union of hydrazulene nitrile 178 to cyclopentenone 179 to fashion the C8–C14 bond and the C8 quaternary stereocenter of the rearranged spongian diterpene aplyviolene precursor 180.82 The above examples highlight the facile reduction of alkyl nitriles and halogens to provide synthetically useful tertiary alkyllithium intermediates.83 However, this area of research remains underdeveloped in part due to the highly basic and reactive nature of the generated alkyllithium species.
Conclusion
Functionalized organometallic species are versatile reagents for carbon–carbon bond formation constituting a key step during the synthesis of many organic non-natural and natural products. Tertiary α-heteroatom organolithiums constitute a unique class due to their high reactivity and interesting modes of stereoselectivity. In general, the stereoselective generation of organolithiums by either permutational interconversion or direct insertion is difficult to predict without robust and abundant empirical data and analysis. The examples contained in this article highlight the unique nature of seemingly similar systems as it relates to changes in stereoselectivity and reactivity. The design and use of appropriately poised substrates as well as new investigations into the generation and selectivity of organolithium species will continue to provide the synthetic chemist a powerful tool in the construction of ever increasingly complex molecules.Notes and references
- M. Schlosser, in Organometallics in Synthesis: A Manual, ed. M. Schlosser, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2nd edn, ch. 1, pp. 1–352 Search PubMed.
- H. Gilman and R. G. Jones, Org. React., 1951, 6, 339 Search PubMed; R. G. Jones and H. Gilman, Chem. Rev., 1954, 54, 835 CrossRef CAS.
- W. C. Still, J. Am. Chem. Soc., 1978, 100, 1481 CrossRef CAS; N. Meyer and D. Seebach, Chem. Ber., 1980, 113, 1290 CrossRef.
- B. J. Wakefield, Organolithium Methods, Academic Press, London, 1988 Search PubMed.
- J. F. Garst, Acc. Chem. Res., 1974, 4, 400 CrossRef.
- T. Cohen and M. Bhupathy, Acc. Chem. Res., 1989, 22, 152 CrossRef CAS.
- C. P. Andrieux, I. Gallardo, J.-M. Saveant and K.-B. Su, J. Am. Chem. Soc., 1986, 108, 638 CrossRef CAS.
- C. G. Screttas, J. Chem. Soc., Chem. Commun., 1972, 752 RSC.
- T. Cohen and J. R. Matz, Synth. Commun., 1980, 10, 371 CrossRef.
- T. Cohen, J. P. Sherbine, R. R. Hutchins and M.-T. Lin, Organomet. Synth., 1986, 3, 361 Search PubMed.
- (a) P. K. Freeman and L. L. Hutchinson, Tetrahedron Lett., 1976, 22, 1849 CrossRef; (b) P. K. Freeman and L. L. Hutchinson, J. Org. Chem., 1980, 45, 1924 CrossRef CAS.
- K. Shimada and M. Szwarc, J. Am. Chem. Soc., 1975, 97, 3313 CrossRef CAS.
- T. Cohen, T. Kreethadumrongdat, X. Liu and V. Kulkarni, J. Am. Chem. Soc., 2001, 123, 3478 CrossRef CAS PubMed.
- (a) S. D. Rychnovsky, J. Org. Chem., 1989, 54, 4982 CrossRef CAS; (b) M. E. Jung and R. B. Blum, Tetrahedron Lett., 1977, 3791 CrossRef CAS; (c) A. Krief and P. Barbeaux, Synlett, 1990, 511 CrossRef CAS; (d) F. Chen, B. Mudryk and T. Cohen, Tetrahedron, 1994, 50, 12793 CrossRef CAS; (e) D. Cheng, S. Zhu, X. Liu, S. H. Norton and T. Cohen, J. Am. Chem. Soc., 1999, 121, 10241 CrossRef CAS; (f) B. Mudryk and T. Cohen, J. Am. Chem. Soc., 1993, 115, 3855 CrossRef CAS.
- (a) R. B. Bates, L. M. Kroposki and D. E. Potter, J. Org. Chem., 1972, 37, 560 CrossRef CAS; (b) J. Clayden and S. S. Yasin, New J. Chem., 2002, 26, 191 RSC.
- P. Stanetty and M. D. Mihovilovic, J. Org. Chem., 1997, 62, 1514 CrossRef CAS.
- (a) J. S. Sawyer, T. L. Macdonald and G. J. McGarvey, J. Am. Chem. Soc., 1984, 106, 3376 CrossRef CAS; (b) J. S. Sawyer, A. Kucerovy, T. L. Macdonald and G. J. McGarvey, J. Am. Chem. Soc., 1988, 110, 842 CrossRef CAS.
- (a) D. Hoppe and T. Krämer, Angew. Chem., Int. Ed. Engl., 1986, 98, 171 CrossRef CAS; (b) D. Hoppe, F. Hintze and P. Tebben, Angew. Chem., Int. Ed., 1990, 29, 1422 CrossRef; (c) P. Sommerfeld and D. Hoppe, Synlett, 1992, 764 CrossRef CAS PubMed; (d) F. Hintze and D. Hoppe, Synthesis, 1992, 1216 CrossRef CAS.
- T. Kramer and D. Hoppe, Tetrahedron Lett., 1987, 28, 5149 CrossRef.
- R. Mansueto, F. M. Perna, A. Salomone, S. Florio and V. Capriati, Chem. Commun., 2013, 49, 4911 RSC.
- R. Mansueto, F. M. Perna, A. Salomone, S. Florio and V. Capriati, Chem. Commun., 2013, 49, 10160 RSC.
- (a) H. K. Scott and V. K. Aggarwal, Chem.–Eur. J., 2011, 17, 13124 CrossRef CAS PubMed; (b) A. P. Pulis and V. K. Aggarwal, J. Am. Chem. Soc., 2012, 134, 7570 CrossRef CAS PubMed; (c) A. P. Pulis, P. Fackler and V. K. Aggarwal, Angew. Chem., Int. Ed., 2014, 53, 4382 CrossRef CAS PubMed; (d) A. P. Pulis, D. J. Blair, E. Torres and V. K. Aggarwal, J. Am. Chem. Soc., 2013, 135, 16054 CrossRef CAS PubMed; (e) K. R. Fandrick, N. D. Patel, J. A. Mulder, J. Gao, M. Konrad, E. Archer, F. G. Buono, A. Duran, R. Schmid, J. Daeubler, D. R. Fandrick, S. Ma, N. Grinberg, H. Lee, C. A. Busacca, J. J. Song, N. K. Yee and C. H. Senanayake, Org. Lett., 2014, 16, 4360 CrossRef CAS PubMed.
- (a) W. C. Still and C. Sreekumar, J. Am. Chem. Soc., 1980, 102, 1201 CrossRef CAS; (b) W. C. Still, J. Am. Chem. Soc., 1978, 100, 1481 CrossRef CAS.
- (a) T. Cohen and M.-T. Lin, J. Am. Chem. Soc., 1984, 106, 1130 CrossRef CAS; (b) T. Cohen and M. Bhupathy, Acc. Chem. Res., 1989, 152 CrossRef CAS; (c) T. Cohen and J. R. Matz, J. Am. Chem. Soc., 1980, 102, 6902 CrossRef.
- D. Griller, K. U. Ingold, P. J. Krusic and H. Fischer, J. Am. Chem. Soc., 1978, 100, 6750 CrossRef CAS.
- T. Cohen and M.-T. Lin, J. Am. Chem. Soc., 1984, 106, 1130 CrossRef CAS.
- For computational studies of related 2-cyanotetrahydropyran reductive decyanations, see: S. D. Rychnovsky, J. P. Powers and T. J. LePage, J. Am. Chem. Soc., 1992, 114, 8375 CrossRef CAS.
- A. I. Meyers, P. D. Edwards, W. F. Rieker and T. R. Bailey, J. Am. Chem. Soc., 1984, 106, 3270 CrossRef CAS.
- S. D. Rychnovsky and D. J. Skalitzky, J. Org. Chem., 1992, 57, 4336 CrossRef CAS.
- (a) P. Beak, D. R. Anderson and M. D. Curtis, Acc. Chem. Res., 2000, 33, 715 CrossRef CAS PubMed; (b) W. K. Lee, Y. S. Park and P. Beak, Acc. Chem. Res., 2009, 42, 224 CrossRef CAS PubMed.
- S. D. Rychnovsky, A. J. Buckmelter, V. H. Dahanukar and D. J. Skalitzky, J. Org. Chem., 1999, 64, 6849 CrossRef CAS PubMed.
- (a) L. R. Takaoka and S. D. Rychnovsky, Angew. Chem., Int. Ed., 2003, 42, 818 CrossRef PubMed; (b) S. D. Rychnovsky, T. Hata, A. I. Kim and A. J. Buckmelter, Org. Lett., 2001, 3, 807 CrossRef CAS PubMed.
- M. D. Morin and S. D. Rychnovsky, Org. Lett., 2005, 7, 2051 CrossRef CAS PubMed.
- (a) K. T. Mead and B. N. Brewer, Curr. Org. Chem., 2003, 7, 227 CrossRef CAS; (b) F. Perron and K. F. Albizati, Chem. Rev., 1989, 89, 1617 CrossRef CAS; (c) V. Vaillancourt, N. E. Pratt, F. Perron and K. F. Albizati, in Total Synthesis of Natural Products, ed. J. Apsimon, Wiley, New York, 1992, vol. 8, pp. 533–691 Search PubMed; (d) T. L. B. Boivin, Tetrahedron, 1987, 43, 3309 CrossRef CAS.
- F. Perron and K. F. Albizati, Chem. Rev., 1989, 89, 1617 CrossRef CAS.
- D. Vellucci and S. D. Rychnovsky, Org. Lett., 2007, 9, 711 CrossRef CAS PubMed.
- P. Beak and D. B. Reitz, Chem. Rev., 1978, 78, 275 CrossRef CAS.
- P. V. R. Schleyer, T. Clark, A. J. Kos, G. W. Spitznagel, C. Rohde, D. Arad, K. Houk and N. G. Rondan, J. Am. Chem. Soc., 1984, 106, 6467 CrossRef CAS.
- P. Beak, W. J. Zajdel and D. B. Reitz, Chem. Rev., 1984, 84, 471 CrossRef CAS.
- (a) D. J. Pippel, G. A. Weisenburger, S. R. Wilson and P. Beak, Angew. Chem., Int. Ed., 1998, 37, 2522 CrossRef CAS; (b) G. Boche, M. Marsch, J. Harbach, K. Harms, B. Ledig, F. Schubert, J. C. W. Lohrenz and H. Ahlbrecht, Chem. Ber., 1993, 126, 1887 CrossRef CAS.
- R. E. Gawley and Q. Zhang, Tetrahedron, 1994, 50, 6077 CrossRef CAS.
- (a) R. R. Fraser, T. B. Grindley and S. Passannanti, Can. J. Chem., 1975, 53, 2473 CrossRef CAS PubMed; (b) D. Seebach and D. Enders, Angew. Chem., Int. Ed., 1972, 11, 1101 CrossRef CAS; (c) D. Seebach and D. Enders, Angew. Chem., Int. Ed., 1975, 14, 15 CrossRef.
- Y. S. Park, M. L. Boys and P. Beak, J. Am. Chem. Soc., 1996, 118, 3757 CrossRef CAS.
- A. I. Meyers, B. Du and M. A. Gonzalez, J. Org. Chem., 1990, 55, 4218 CrossRef CAS.
- J. Clayden, J. Dufour, D. M. Grainger and M. Helliwell, J. Am. Chem. Soc., 2007, 129, 7488 CrossRef CAS PubMed.
- (a) P. Beak, T. A. Johnson, D. D. Kim and S. H. Lim, Topics in Organometallic Chemistry, in Organolithiums in Enantioselective Synthesis, ed. D.M. Hodgson, Springer-Verlag, Berlin, 2003, vol. 5, pp. 139–176 Search PubMed; (b) M. C. Whisler, S. MacNeil, V. Snieckus and P. Beak, Angew. Chem., Int. Ed., 2004, 43, 2206 CrossRef CAS PubMed.
- D. Seebach and D. Enders, Angew. Chem., Int. Ed., 1975, 14, 15 CrossRef.
- National Toxicology Program, Reports on Carcinogens, National Institutes of Health, 2011, vol. 12, p. 302 Search PubMed.
- R. R. Fraser, T. B. Grindley and S. Passannanti, Can. J. Chem., 1975, 53, 2473 CrossRef CAS PubMed.
- D. Seebach, W. Wykpiel, W. Lubosch and H.-O. Kalinowski, Helv. Chim. Acta, 1978, 61, 3100 CrossRef CAS.
- (a) T. T. Shawe and A. I. Meyers, J. Org. Chem., 1991, 56, 2751 CrossRef CAS; (b) A. I. Meyers, P. D. Edwards, W. F. Reiker and T. R. Bailey, J. Am. Chem. Soc., 1984, 106, 3270 CrossRef CAS; (c) A. I. Meyers and G. Milot, J. Am. Chem. Soc., 1993, 115, 6652 CrossRef CAS.
- (a) P. Beak and W. K. Lee, J. Org. Chem., 1990, 55, 2578 CrossRef CAS; (b) P. Beak and W. K. Lee, J. Org. Chem., 1993, 58, 1109 CrossRef CAS.
- H. Nozaki, T. Aratani, T. Toraya and R. Noyori, Tetrahedron, 1971, 27, 905 CrossRef CAS.
- D. Hoppe, F. Hintze and P. Tebben, Angew. Chem., Int. Ed., 1990, 29, 1422 CrossRef.
- S. T. Kerrick and P. Beak, J. Am. Chem. Soc., 1991, 113, 9708 CrossRef CAS.
- P. O'Brien, K. B. Wiberg, W. F. Bailey, J.-P. R. Hermet and M. J. McGrath, J. Am. Chem. Soc., 2004, 126, 15480 CrossRef PubMed and references therein.
- W. F. Bailey, P. Beak, S. T. Kerrick, S. Ma and K. B. Wiberg, J. Am. Chem. Soc., 2002, 124, 1889 CrossRef CAS PubMed.
- D. Stead, G. Carbone, P. O'Brien, K. R. Campos, I. Coldham and A. Sanderson, J. Am. Chem. Soc., 2010, 132, 7260 CrossRef CAS PubMed.
- (a) T. K. Beng and R. E. Gawley, J. Am. Chem. Soc., 2010, 132, 12216 CrossRef CAS PubMed; (b) T. K. Beng, W. S. Tyree, T. Parker, C. Su, P. G. Williard and R. E. Gawley, J. Am. Chem. Soc., 2012, 134, 16845 CrossRef CAS PubMed.
- (a) N. S. Sheikh, D. Leonori, G. Barker, J. D. Firth, K. R. Campos, A. J. H. M. Meijer, P. O'Brien and I. Coldham, J. Am. Chem. Soc., 2012, 134, 5300 CrossRef CAS PubMed; (b) X. Li and I. Coldham, J. Am. Chem. Soc., 2014, 136, 5551 CrossRef CAS PubMed; (c) X. Li, D. Leonori, N. S. Sheikh and I. Coldham, Chem.–Eur. J., 2013, 19, 7724 CrossRef CAS PubMed; (d) T. K. Beng, J. S. Woo and R. E. Gawley, J. Am. Chem. Soc., 2012, 134, 14764 CrossRef CAS PubMed.
- E. J. Cochrane, D. Leonori, L. A. Hassall and I. Coldham, Chem. Commun., 2014, 50, 9910 RSC.
- (a) J. Lefranc, A. M. Fournier, G. Mingat, S. Herbert, T. Marcelli and J. Clayden, J. Am. Chem. Soc., 2012, 134, 7286 CrossRef CAS PubMed; (b) A. M. Fournier, C. J. Nichols, M. A. Vincent, I. H. Hillier and J. Clayden, Chem.–Eur. J., 2012, 18, 16478 CrossRef CAS PubMed; (c) M. B. Tait, P. A. Ottersbach, D. J. Tetlow and J. Clayden, Org. Process Res. Dev., 2014, 18, 1245 CrossRef CAS.
- I. Coldham, R. Hufton and D. J. Snowden, J. Am. Chem. Soc., 1996, 118, 5322 CrossRef CAS.
- N. J. Ashweek, P. Brandt, I. Coldham, S. Dufour, R. E. Gawley, F. Haeffner, R. Klein and G. Sanchez-Jimenez, J. Am. Chem. Soc., 2005, 127, 449 CrossRef CAS PubMed.
- (a) W. H. Pearson and A. C. Lindbeck, J. Am. Chem. Soc., 1991, 113, 8546 CrossRef CAS; (b) W. H. Pearson, A. C. Lindbeck and J. W. Kampf, J. Am. Chem. Soc., 1993, 115, 2622 CrossRef CAS.
- J. M. Chong and S. B. Park, J. Org. Chem., 1992, 57, 2220 CrossRef CAS.
- A. I. Meyers and T. R. Elworthy, Tetrahedron, 1994, 50, 6089 CrossRef.
- C. Fabre and Z. Welvart, C. R. Acad. Sci. Ser., 1970, 270, 1887 CAS.
- M. Bonin, J. R. Romero, D. S. Grierson and H.-P. Husson, Tetrahedron Lett., 1982, 23, 3369 CrossRef CAS.
- S. A. Wolckenhauer and S. D. Rychnovsky, Tetrahedron, 2005, 61, 3371 CrossRef CAS PubMed.
- F. Haeffner, P. Brandt and R. E. Gawley, Org. Lett., 2002, 4, 2101 CrossRef CAS PubMed.
- R. J. Bahde and S. D. Rychnovsky, Org. Lett., 2008, 10, 4017 CrossRef CAS PubMed.
- (a) I. Coldham and R. Hufton, Tetrahedron, 1996, 52, 12541 CrossRef CAS; (b) I. Coldham, J.-C. Fernàndez, K. N. Price and D. J. Snowden, J. Org. Chem., 2000, 65, 3788 CrossRef CAS PubMed.
- (a) M. A. Perry, M. D. Morin, B. W. Slafer, S. A. Wolckenhauer and S. D. Rychnovsky, J. Am. Chem. Soc., 2010, 132, 9591 CrossRef CAS PubMed; (b) M. A. Perry, M. D. Morin, B. W. Slafer and S. D. Rychnovsky, J. Org. Chem., 2012, 77, 3390 CrossRef CAS PubMed.
- N. C. Faibish, Y. S. Park, S. Lee and P. Beak, J. Am. Chem. Soc., 1997, 119, 11561 CrossRef CAS.
- G. Wittig, U. Pockels and H. Dröge, Ber. Dtsch. Chem. Ges. A, 1938, 71, 1903 CrossRef.
- H. Gilman, W. Langham and A. L. Jacoby, J. Am. Chem. Soc., 1939, 61, 106 CrossRef CAS.
- W. F. Bailey and E. R. Punzalan, J. Org. Chem., 1990, 55, 5404 CrossRef CAS.
- D. J. Rawson and A. I. Meyers, Tetrahedron Lett., 1991, 32, 2095 CrossRef CAS.
- T. E. La Cruz and S. D. Rychnovsky, Chem. Commun., 2004, 168 RSC.
- M. J. Schnermann, N. L. Untiedt, G. Jiménez-Osés, K. N. Houk and L. E. Overman, Angew. Chem., Int. Ed., 2012, 51, 9581 CrossRef CAS PubMed.
- M. J. Schnermann and L. E. Overman, Angew. Chem., Int. Ed., 2012, 51, 9576 CrossRef CAS PubMed.
- For additional examples of tertiary alkyllithiums see; (a) W. F. Bailey and M. W. Carson, J. Org. Chem., 1998, 63, 9960 CrossRef CAS; (b) A. Krief, B. Remacle and J. Mercier, Synlett, 2000, 2000, 1443 CrossRef PubMed; (c) R. W. Hoffmann, R. Koberstein and K. Harms, J. Chem. Soc., Perkin Trans. 2, 1999, 183 RSC; (d) M. Yus, R. Ortiz and F. F. Huerta, Tetrahedron, 2003, 59, 8525 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |