Hydroxyapatite nanoparticles (HAP NPs): a green and efficient heterogeneous catalyst for three-component one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives in aqueous media†
Nasrin
Razavi
and
Batool
Akhlaghinia
*
Department of Chemistry, Faculty of Sciences, Ferdowsi University of Mashhad, Mashhad 9177948974, Iran. E-mail: akhlaghinia@um.ac.ir; Fax: +98-51-3879-5457; Tel: +98-51-3880-5527
First published on 4th November 2015
Abstract
Hydroxyapatite nanoparticles (HAP NPs) are found to be an efficient catalyst for synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives in aqueous media via a three-component one-pot condensation of isatoic anhydride and aromatic aldehydes with primary amines or ammonium salts. The nanocatalyst (characterized by FT-IR, XRD, SEM-EDS and TEM techniques) is easily recyclable six times without the significant loss of catalytic activity. Other remarkable features include the wide range of functional group tolerance and good to excellent yields of the products under mild reaction conditions.
Introduction
Quinazolinone derivatives as an important class of fused heterocyclic compounds and their valuable intermediates in synthetic organic chemistry are present a large family of products with potential biological and pharmaceutical activities.1 These exhibit various medicinal properties such as anti-tumor,2 anti-cancer,3 antibacterial,4 anticonvulsant,5 anti-inflammatory,6 antifungal,7 antihypertension,8 anti-diabetes,9 analgesic,10 herbicidal, diuretic as well as plant growth regulation11 and some of them have been shown to act as potent HIV-1 reverse transcriptase inhibitors.12 In view of their widely increased application value, a number of classical methods for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones have been reported in the literature.13–15 These methods include: (i) condensation of o-aminobenzamide with aldehydes or ketones using Lewis or Brønsted acids16 (ii) reductive cyclization of o-nitrobenzamide/or o-azido-benzamide with aldehydes or ketones using SnCl2/or metallic samarium in the presence of iodine or SmI216a,17 (iii) condensation of isatoic anhydride, primary amines or ammonium salts with aldehydes or ketones in the presence of p-toluenesulfonic acid (PTSA),16b citric acid,18 silica sulfuric acid (SSA),19 silica-bonded N-propylsulfamic acid,20 silica-bonded S-sulfonic acid,21 MCM-41-SO3H,22 ethylenediamine diacetate,16g Ga(OTf)3,23 KAl(SO4)2·12H2O,24 Al(H2PO4)3,25 montmorillonite K-10,26 heteropolyacids (HPAs),27 powdered diethylaminoethyl cellulose as the biomass-derived support for phosphotungstic acid,28 SrCl3·6H2O,29 molecular iodine,30 magnetic Fe3O4, nano-indium oxide, Al/Al2O3 nanoparticles,31–33 or with the aid of ultrasound irradiation catalyzed by dodecylbenzenesulfonic acid34 and microwaves in the presence of Amberlyst-1535 or Cu–CNTs.36 2,3-Dihydroquinazolin-4(1H)-one derivatives were previously prepared by multi-step reactions.37 In comparison, multi-component reactions (MCRs) not only produced 2,3-dihydroquinazolin-4(1H)-one derivatives in a single step but also diversity could be achieved simply by varying the reacting components. Moreover, today the development of an efficient, rapid, simple, low cost, easy work-up and environmentally benign protocol with energy conservation using a recyclable catalyst and a green solvent under mild reaction conditions for the synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives is desirable and in demand.38In recent years, HAP has attracted considerable interest in many areas. HAP as the main component of hard tissues of vertebrates such as bones and teeth has properties such as ion-exchange ability, adsorption capacity, acid–base properties and efficient catalytic activity in organic reactions. The small size of HAP NPs as one of the important biocompatible and bioactive materials with higher surface areas and lower particle sizes can provide greater catalytic activity in organic synthesis. However, few common applications as the catalyst or catalyst support have emerged so far.39
In the present study, we would like to report a new application of HAP NPs, as an inexpensive, non-toxic, non-inflammatory, green heterogeneous catalyst for three-component one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones in aqueous media.
Results and discussion
Synthesis and characterization of HAP NPs
Following our interest in development of efficient and environmentally benign heterogeneous catalysts in organic transformation,40 in this research, initially, HAP NPs were simply synthesized according to the method reported previously (Scheme 1).41The nanocatalyst was characterized by the following techniques: FT-IR spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM-EDS) and transmission electron microscopy (TEM).
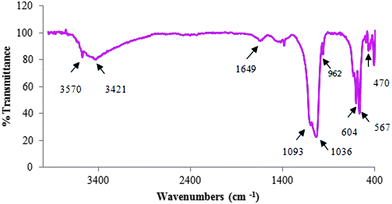
The FT-IR spectrum of HAP NPs is shown in Fig. 1. Broad bands centered at 3421 and 1649 cm−1 are due to stretching and bending vibrations of molecularly adsorbed water. Also, the stretching vibration at around 3570 cm−1 confirms the presence of hydroxyl groups. The absorption bands at 1093 (νs,as), 1036 (νs,as), 604 (δ) and 567 (δ) cm−1 are attributed to asymmetric stretching and bending vibration frequencies of the PO43− ion, respectively. The symmetric stretching vibrations of the PO43− ion were also found at around 962 and 470 cm−1. The above results of FT-IR spectroscopy suggest that the HAP NPs were synthesized successfully.
Fig. 2a shows the XRD pattern of HAP NPs. In the pattern, well defined Bragg peaks were obtained at specific 2θ angles indicating that nanoparticles were ordered. The diffraction peaks, particularly in the planes (002), (211), (300), (202), (310), (222), (213) and (004) well-matched the hexagonal phase hydroxyapatite with space group P63/m (JCPDS: 74-0565). The average crystallite size of HAP NPs was calculated to be 16 nm using the Debye–Scherrer equation d = KL/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos(θ).42
cos(θ).42
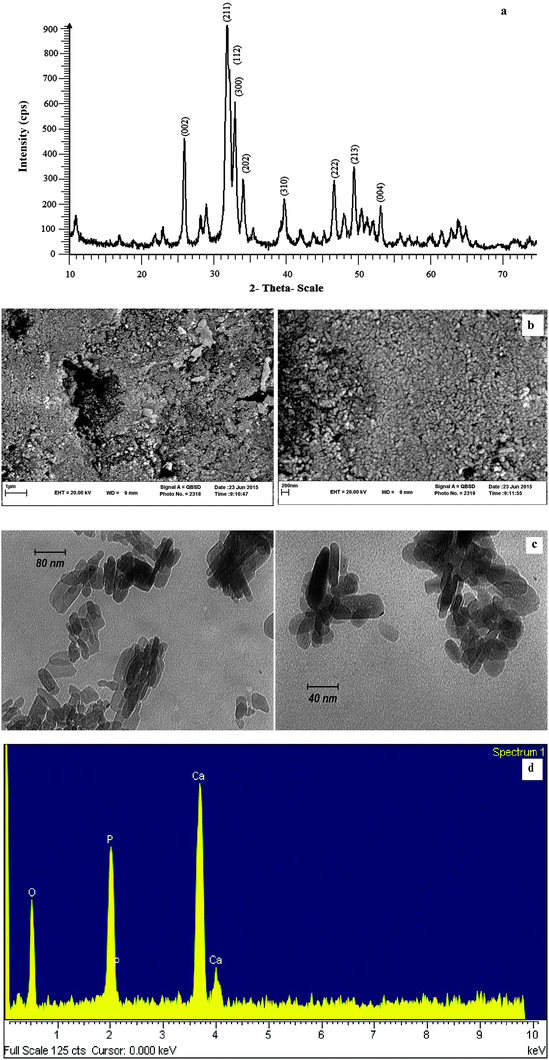 | ||
| Fig. 2 (a) XRD pattern of HAP NPs, (b) SEM images of HAP NPs, (c) TEM images of HAP NPs, and (d) The EDS spectrum of HAP NPs. | ||
The structure and morphology of the HAP NPs were further confirmed by scanning electron microscopy (SEM) (Fig. 2b). It can be seen that the synthesized HAP NPs are nearly nanorod particles. As the shape of the synthesized HAP NPs is not clearly defined in the SEM images (Fig. 2b), the transmission electron microscopy (TEM) images were recorded to determine the exact size and shape of HAP NPs (Fig. 2c). The TEM analysis revealed the synthesized HAP NPs with the nanorod shape and particle size of around 10 to 80 nm.
The energy dispersive spectroscopy (EDS) confirms the presence of Ca, P and O elements in the structure of HAP NPs (Fig. 2d).
Synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives in the presence of HAP NPs
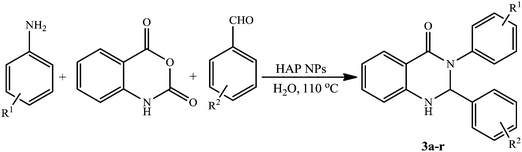
As a result of our great interest in preparation of heterocyclic compounds by applying heterogeneous catalysts,40a,b herein we wish to report an efficient procedure to synthesize 2,3-dihydroquinazolin-4(1H)-one derivatives through a three-component one-pot condensation of isatoic anhydride, aromatic aldehydes and primary amines/or ammonium salts using HAP NPs as the catalyst in aqueous media (Scheme 2).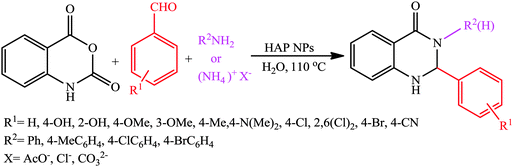 | ||
| Scheme 2 Synthesis of 2,3-disubstituted-2,3-dihydroquinazolin-4(1H)-one derivatives in the presence of HAP NPs in H2O. | ||
Then, the reaction of isatoic anhydride, benzaldehyde and aniline was chosen as a model reaction. Subsequently, to study the effects of solvent, temperature, molar ratio of reactants and the amount of catalyst on the reaction rate and yield, the model reaction was carried out under different conditions. The results are summarized in Table 1. By applying a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of isatoic anhydride
1 molar ratio of isatoic anhydride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aniline
aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde in the absence of the catalyst, the yield was poor even for a longer time (Table 1, entry 1). Among all the solvents screened, such as EtOH, H2O/EtOH (1
benzaldehyde in the absence of the catalyst, the yield was poor even for a longer time (Table 1, entry 1). Among all the solvents screened, such as EtOH, H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), acetonitrile, DMF, 1,4-dioxane, EtOAc, dichloromethane and H2O, water is the best (Table 1, entries 2–9). The reaction temperature also played an important role in this reaction. Therefore, we tried to optimize the reaction temperature in the model reaction. As could be seen in Table 1, at 110 °C, the reaction proceeded quickly in the highest yield (Table 1, entries 10 and 11). It should be noted that 0.05 g of catalyst was efficient enough to catalyze the reaction, and increasing the amount of catalyst did not improve the yield significantly (Table 1, entry 12). The catalytic effect of HAP NPs efficiently decreased under solvent free conditions (Table 1, entry 14). Additional amounts of aniline and benzaldehyde did not have any influence on the reaction rate (Table 1, entries 15 and 16).
1), acetonitrile, DMF, 1,4-dioxane, EtOAc, dichloromethane and H2O, water is the best (Table 1, entries 2–9). The reaction temperature also played an important role in this reaction. Therefore, we tried to optimize the reaction temperature in the model reaction. As could be seen in Table 1, at 110 °C, the reaction proceeded quickly in the highest yield (Table 1, entries 10 and 11). It should be noted that 0.05 g of catalyst was efficient enough to catalyze the reaction, and increasing the amount of catalyst did not improve the yield significantly (Table 1, entry 12). The catalytic effect of HAP NPs efficiently decreased under solvent free conditions (Table 1, entry 14). Additional amounts of aniline and benzaldehyde did not have any influence on the reaction rate (Table 1, entries 15 and 16).
| Entry | Molar ratios of isatoic anhydride![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aniline aniline![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) benzaldehyde benzaldehyde |
Catalyst (g) | Solvent | Temperature (°C) | Time (h) | Isolated yield (%) |
|---|---|---|---|---|---|---|
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | H2O | 110 | 24 | 20 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | EtOH | 80 | 3 | 85 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | H2O/EtOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
80 | 3 | 80 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | CH3CN | 80 | 5 | 40 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | DMF | 120 | 6 | 50 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | 1,4 Dioxane | 101 | 6 | 20 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | EtOAc | 77 | 5 | 60 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | CH2Cl2 | 40 | 6 | 10 |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | H2O | 100 | 3 | 85 |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | H2O | 110 | 1 | 95 |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | H2O | 120 | 1 | 95 |
| 12 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.1 | H2O | 110 | 1 | 95 |
| 13 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.025 | H2O | 110 | 4 | 80 |
| 14 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | — | 110 | 6 | 60 |
| 15 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.05 | H2O | 110 | 1 | 95 |
| 16 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.05 | H2O | 110 | 1 | 95 |
Encouraged by the initial success in the production of 2,3-diphenyl-2,3-dihydroquinazolin-4(1H)-one via the multicomponent reaction strategy, to investigate the general scope and versatility of this method in the preparation of substituted 2,3-dihydroquinazolin-4(1H)-one derivatives, isatoic anhydride and different substituted amines and aldehydes were examined under optimized conditions. Condensation of aniline with commercially available aromatic aldehydes having electron-donating and electron withdrawing substituents produced 3a–l in high yields (Table 2, entries 1–12). According to the results, aldehydes bearing electron-donating groups produced the desired products more quickly than the aldehydes with electron-withdrawing groups. Though meta- and para substituted aromatic aldehydes reacted quickly, ortho-substituted aromatic aldehydes give products in longer reaction times (Table 2, entries 9 and 10). Subsequently, the substrate scope of amines was examined for this three-component reaction. From Table 2, it can be observed that in the condensation reaction of substituted anilines with aromatic aldehydes the nature of substituents on the aromatic ring of amines had no noticeable effect on the reaction rate as the corresponding products were obtained in high yields and in short reaction times (Table 2, entries 13–15). Excitingly, the results shown in Table 2 confirm that the reaction was compatible successfully with a broad range of substituents (both electron-donating and electron-withdrawing groups) in the amines or the aldehydes.
| Entry | R1 | R2 | Product | Time (min) | Isolated yield (%) |
|---|---|---|---|---|---|
| 1 | H | H | 3a | 60 | 95 |
| 2 | H | 4-OH | 3b | 30 | 90 |
| 3 | H | 2-OH | 3c | 30 | 90 |
| 4 | H | 4-OMe | 3d | 30 | 95 |
| 5 | H | 3-OMe | 3e | 30 | 90 |
| 6 | H | 4-Me | 3f | 30 | 95 |
| 7 | H | N(Me)2 | 3g | 30 | 87 |
| 8 | H | 4-Cl | 3h | 60 | 85 |
| 9 | H | 2,6-(Cl)2 | 3i | 180 | 85 |
| 10 | H | 5-Br-2-OH | 3j | 60 | 90 |
| 11 | H | 4-Br | 3k | 60 | 90 |
| 12 | H | 4-CN | 3l | 60 | 90 |
| 13 | 4-Me | H | 3m | 30 | 85 |
| 14 | 4-Cl | H | 3n | 30 | 90 |
| 15 | 4-Br | H | 3o | 30 | 90 |
| 16 | 4-Me | 4-Me | 3p | 40 | 80 |
| 17 | 4-Me | 4-OMe | 3q | 45 | 75 |
| 18 | 4-Br | 4-Me | 3r | 60 | 85 |
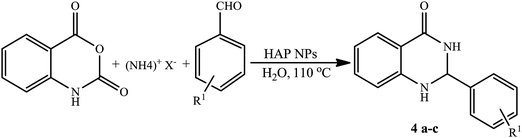
As some ammonium salts are the source of ammonia in the synthesis of nitrogen containing heterocyclic compounds, 2,3-dihydroquinazolin-4(1H)-ones derivatives were prepared under the optimal reaction conditions with different ammonium sources such as acetate, chloride and carbonate (Table 3). Accordingly, the corresponding 2,3-dihydroquinazolin-4(1H)-ones (4a–c) were synthesized efficiently in the presence of HAP NPs. In comparison, under the same reaction conditions ammonium acetate reacted more quickly with isatoic anhydride and aldehydes than ammonium chloride and ammonium carbonate.
All known products were characterized by comparing their physical data and 1H and 13C NMR spectra with those of authentic samples reported in the literature. Also, most of the known products were characterized by FT-IR spectroscopy, mass spectrometry and elemental analysis. The data for novel compounds (Table 2, 3j and 3r) are reported in this paper.
In the FT-IR spectra of compounds 3a–r, the strong and sharp absorption bands due to NH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups were observed at around 3379–3282 cm−1 and 1655–1608 cm−1, respectively. Moreover, absorption bands at 3312–3303 and 3192–3187 cm−1 in the FT-IR spectra of compounds 4a–c can be attributed to stretching vibrations of NH and NH–CO, respectively. Also, in the 1HNMR spectra, the NH proton of the dihydroquinazolin ring appeared as a broad signal at 8.36–7.03 ppm. The 13C NMR spectra showed a signal at 171–162 ppm assigned to the C
O groups were observed at around 3379–3282 cm−1 and 1655–1608 cm−1, respectively. Moreover, absorption bands at 3312–3303 and 3192–3187 cm−1 in the FT-IR spectra of compounds 4a–c can be attributed to stretching vibrations of NH and NH–CO, respectively. Also, in the 1HNMR spectra, the NH proton of the dihydroquinazolin ring appeared as a broad signal at 8.36–7.03 ppm. The 13C NMR spectra showed a signal at 171–162 ppm assigned to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group that confirmed the synthesis of 2,3-dihydroquinazolin-4(1H)-ones.
O group that confirmed the synthesis of 2,3-dihydroquinazolin-4(1H)-ones.
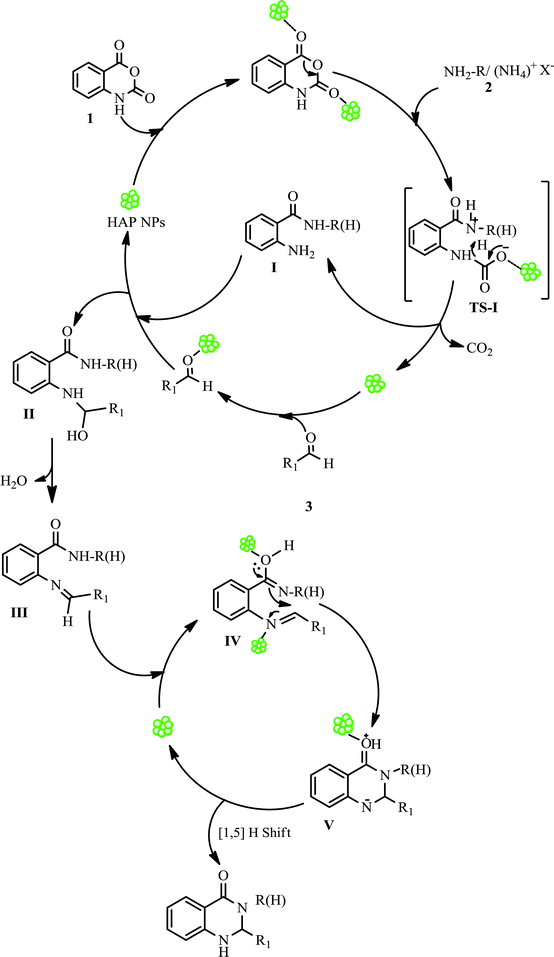
On the basis of our experimental results and by referring to the literature,23,31,32 the following plausible mechanism was proposed to account the condensation of isatoic anhydride and aromatic aldehydes with primary amines/or ammonium salts catalysed by HAP NPs (Scheme 3). The question is how this catalyst operates? Evidently, HAP NPs function as a good Lewis acid, which in the initial step attracts and activates isatoic anhydride (1) by coordination with carbonyl groups and provides active sites on which the reaction occurs successively leading to the desired product. The nucleophilic attack of a primary amine/ammonium salt (2) to the carbonyl group followed by extrusion of CO2 results in the formation of intermediate I. HAP NPs also promote the condensation of I with aldehyde (3) which produces an imine intermediate III. An intramolecular nucleophilic attack of the amide nitrogen to imine, which is catalysed by HAP NPs, produces cyclized intermediate V which upon 1,5-proton transfer leads to the formation of 2,3-dihydroquinazolin-4(1H)-ones. Finally, HAP NPs re-enter the catalytic cycle. Nevertheless, at this time there is no experimental evidence for the formation of the above mentioned intermediates in this manner, and further studies to elucidate the details of the mechanism are ongoing.
To investigate the reusability of HAP NPs, after initial experimentation, the catalyst was recovered by centrifugation, washed with hot ethanol, dried under vacuum at 50 °C and subjected to the second run (Fig. 3). The results of this experiment and five subsequent experiments were almost consistent in yields (95, 95, 95, 92, 90, and 90%). Although slightly more time was required to complete the reaction in the sixth run, the yields were comparable to those seen earlier.
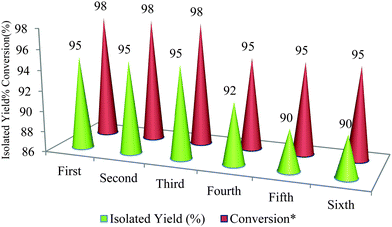 | ||
| Fig. 3 Synthesis of 2,3-diphenyl-2,3-dihydroquinazolin-4(1H)-one in the presence of reused HAP NPs. * The data refer to conversion of benzaldehyde. | ||
Finally, the efficiency of the present protocol was compared with different heterogeneous catalysts reported previously in the literature. The results are shown in Table 4. It can be seen from Table 4 that the green nano catalyst gives better yields in shorter reaction times than the other heterogeneous catalysts.
| Entry | Catalyst | Solvent | Temperature (°C) | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| a Powdered diethylaminoethyl cellulose as the biomass-derived support for phosphotungstic acid. | ||||||
| 1 | Ga(OTf)3 | EtOH | 70 | 60 | 79 | 23 |
| 2 | KAl(SO4)2·12H2O | H2O | Reflux | 60 | 65 | 24 |
| 3 | Nano-indium oxide | EtOH/H2O (1/2) | 80 | 320 | 87 | 32 |
| 4a | PTA@DEAEC | EtOH | Reflux | 360 | 79 | 28 |
| 5 | Silica sulfuric acid | EtOH | Reflux | 390 | 80 | 19 |
| 6 | Nano Fe3O4 | H2O | Reflux | 120 | 80 | 31 |
| 7 | SrCl3·6H2O | EtOH/H2O (1/3) | Reflux | 90 | 94 | 29 |
| 8 | Hydroxyapatite nanoparticles (HAP) | H2O | 110 | 60 | 95 | Present study |
Conclusion
In summary, we have described for the first time, a successful strategy for the efficient and convenient preparation of substituted 2,3-dihydroquinazolin-4(1H)-one derivatives using HAP NPs as a catalyst in water by the multi-component one-pot condensation of isatoic anhydride with amines/or ammonium salts and aldehydes. The novelty and synthetic valuability of this methodology developed the mild reaction conditions, avoiding the use of organic solvents, easy experimental procedure, ease of product isolation, and recovery of the catalyst for at least six runs without any significant impact on the yield of the products. These conditions may be ideally suited for an effective synthesis on a larger scale.Experimental
General
The purity determinations of the products were accomplished by thin layer chromatography (preparative TLC was carried out using a Merck GF 254 silica gel on a glass support). The melting points of products were determined using an Electrothermal Type 9100 melting point apparatus. The FT-IR spectra were recorded on an Avatar 370 FT-IR Therma Nicolet spectrometer. The NMR spectra were recorded on Brucker Avance 300, 400 and 500 MHz instruments in DMSO-d6 and CDCl3. Elemental analyses were performed using a Thermo Finnegan Flash EA 1112 Series instrument. Mass spectra were recorded using a CH7A Varian MAT Bremen instrument at 70 eV; in m/z (rel%). Transmission electron microscopy (TEM) was performed using a Leo 912 AB (120 kV) microscope (Zeiss, Germany). Elemental compositions were determined using a Leo 1450 VP scanning electron microscope equipped with an SC7620 energy dispersive spectrometer (SEM-EDS) presenting a 133 eV resolution at 20 kV. The crystal structure of the catalyst was analyzed by X-ray diffraction (XRD) on a Bruker D8 ADVANCE diffractometer using a Cu target (λ = 1.54 Å). All yields refer to isolated products after purification by recrystallization or thin layer chromatography.Preparation of HAP NPs
To a solution of Ca(NO3)2·4H2O (50 mL, 1.08 M, at pH adjusted to 10 with NH4OH) in a three-necked 500 mL round-bottomed flask equipped with a condenser, argon gas inlet tube and dropping funnel, solution of (NH4)2HPO4 (50 mL, 0.65 M at pH adjusted to 10 with NH4OH) was added at 90 °C with stirring. After 5 h the suspension was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and washed repeatedly with CO2-free distilled water (3 × 20 mL). HAP NPs were dried at 50 °C under vacuum for 12 h.41
000 rpm for 10 min and washed repeatedly with CO2-free distilled water (3 × 20 mL). HAP NPs were dried at 50 °C under vacuum for 12 h.41
Typical procedure for synthesis of 2,3-diphenyl-2,3-dihydroquinazolin-4(1H)-one
HAP NPs (0.05 g) were added to a mixture of isatoic anhydride (1 mmol, 0.163 g), benzaldehyde (1 mmol, 0.106 g) and aniline (1 mmol, 0.093 g) in H2O (5 mL). The mixture was stirred for 1 h at 110 °C. The progress of the reaction was monitored by TLC (EtOAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether, 1
petroleum ether, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). After completion of the reaction, the nanocatalyst was separated by centrifugation. The mixture was extracted by ethyl acetate (3 × 3 mL). The organic layer was dried over anhydrous sodium sulfate. Ethyl acetate was evaporated under reduced pressure to give the crude product. The pure product was obtained by recrystallization from ethanol obtaining 0.285 g of white solid (95% yield).
2). After completion of the reaction, the nanocatalyst was separated by centrifugation. The mixture was extracted by ethyl acetate (3 × 3 mL). The organic layer was dried over anhydrous sodium sulfate. Ethyl acetate was evaporated under reduced pressure to give the crude product. The pure product was obtained by recrystallization from ethanol obtaining 0.285 g of white solid (95% yield).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1609, 1510, 1484, 1439, 1391, 1300, 1245, 1157, 1107, 1028, 882, 820, 784, 753, 695, 656, 602, 549, 515; 1H NMR (300 MHz, DMSO-d6, ppm) δ 2.25 (s, 3H, CH3), 3.68 (s, 3H, OCH3), 6.16 (d, J = 2.7 Hz, 1H, CH), 6.86–6.70 (m, 4H, Ph), 7.29–7.11 (m, 7H, Ph), 7.50 (1H, br s, NH), 7.70 (d, J = 11.4 Hz, 1H, Ph); 13C NMR (75 MHz, DMSO-d6, ppm) δ 20.6, 55.4, 72.4, 110.1, 115.3, 122.5, 123.5, 123.7, 125.3, 127.5, 128.0, 128.8, 129.6, 132.5, 136.8, 137.6, 141.3, 144.2, 146.8, 158.7, 171.3; MS, m/z (%): 344 [3, M+], 236 [100, M+ − 108], 91 [45, M+ − 239].
O), 1609, 1510, 1484, 1439, 1391, 1300, 1245, 1157, 1107, 1028, 882, 820, 784, 753, 695, 656, 602, 549, 515; 1H NMR (300 MHz, DMSO-d6, ppm) δ 2.25 (s, 3H, CH3), 3.68 (s, 3H, OCH3), 6.16 (d, J = 2.7 Hz, 1H, CH), 6.86–6.70 (m, 4H, Ph), 7.29–7.11 (m, 7H, Ph), 7.50 (1H, br s, NH), 7.70 (d, J = 11.4 Hz, 1H, Ph); 13C NMR (75 MHz, DMSO-d6, ppm) δ 20.6, 55.4, 72.4, 110.1, 115.3, 122.5, 123.5, 123.7, 125.3, 127.5, 128.0, 128.8, 129.6, 132.5, 136.8, 137.6, 141.3, 144.2, 146.8, 158.7, 171.3; MS, m/z (%): 344 [3, M+], 236 [100, M+ − 108], 91 [45, M+ − 239].
Acknowledgements
The authors gratefully acknowledge the partial support of this study by Ferdowsi University of Mashhad Research Council (Grant no. p/3/32111).Notes and references
- D. A. Erlanson, R. S. McDowell and T. O. Brien, J. Med. Chem., 2004, 47, 3463–3482 CrossRef CAS PubMed.
- H. Wu, X. Xie and G. Liu, J. Comb. Chem., 2010, 12, 346–355 CrossRef CAS PubMed.
- G. M. Chinigo, M. Paige, S. Grindrod, E. Hamel, S. Dakshanamurthy, M. Chruszcz, W. Minor and M. L. Brown, J. Med. Chem., 2008, 51, 4620–4631 CrossRef CAS PubMed.
- (a) R. J. Alaimo and H. E. Russell, J. Med. Chem., 1972, 15, 335–336 CrossRef CAS PubMed; (b) Q. Li, L. A. Mitscher and L. L. Shen, Med. Res. Rev., 2000, 20, 231–293 CrossRef CAS PubMed.
- V. K. Srivastava and A. Kumar, Eur. J. Med. Chem., 2002, 37, 873–882 CrossRef.
- (a) D. Rambabu, S. Kiran Kumar, B. Yogi Sreenivas, S. Sandra, A. Kandale, P. Misra, M. V. Basaveswara Rao and M. Pal, Tetrahedron Lett., 2013, 54, 495–501 CrossRef CAS; (b) M. Sharma, K. Chauhan, R. Shivahare, P. Vishwakarma, M. K. Suthar, A. Sharma, S. Gupta, J. K. Saxena, J. Lai, P. Chandra, B. Kumar and P. M. S. Chauhan, J. Med. Chem., 2013, 56, 4374–4392 CrossRef CAS PubMed.
- R. J. Cox and D. O'Hagan, J. Chem. Soc., Perkin Trans. 1, 1991, 2537–2540 RSC.
- V. J. Ram, B. K. Tripathi and A. K. Srivastava, Bioorg. Med. Chem., 2003, 11, 2439–2444 CrossRef CAS PubMed.
- J. Rudolph, W. P. Esler, S. O'Connor, P. D. G. Coish, P. L. Wickens, M. Brands, D. E. Bierer, B. T. Bloomquist, G. Bondar, L. Chen, C. Y. Chuang, T. H. Claus, Z. Fathi, W. Fu, U. R. Khire, J. A. Kristie, X. G. Liu, D. B. Lowe, A. C. Mcclure, M. Michels, A. A. Ortiz, P. D. Ramsden, R. W. Schoenleber, T. E. Shelekhin, A. Vakalopoulos, W. F. Tang, L. Wang, L. Yi, S. J. Gardell, J. N. Livingston, L. J. Sweet and W. H. Bullock, J. Med. Chem., 2007, 50, 5202–5216 CrossRef CAS PubMed.
- O. I. El-Sabbagh, S. M. Ibrahim, M. M. Baraka and H. Kothayer, Arch. Pharm., 2010, 343, 274–281 CAS.
- E. Hamel, C. M. Lin, J. Plowman, H. Wang, K. Lee and K. D. Paull, Biochem. Pharmacol., 1996, 51, 53–59 CrossRef CAS PubMed.
- V. Dolle, E. Fan, C. H. Nguyen, A. M. Aubertin, A. Kirn, M. L. Andreola, G. Jamieson, L. Tarragolitvak and E. Bisagni, J. Med. Chem., 1995, 38, 4679–4686 CrossRef CAS PubMed.
- S. D. Sharma and V. Kaur, Synthesis, 1989, 677–680 CrossRef CAS.
- (a) D. Q. Shi, L. C. Rong, J. X. Wang, X. S. Wang, S. J. Tu and H. W. Hu, Chem. J. Chin. Univ., 2004, 25, 2051–2054 CAS; (b) D. Q. Shi, J. X. Wang, L. C. Rong, Q. Y. Zhuang, S. J. Tu and H. W. Hu, J. Chem. Res., 2003, 10, 671–673 CrossRef; (c) D. Q. Shi, C. L. Shi, J. X. Wang, L. C. Rong, Q. Y. Zhuang and X. S. Wang, J. Heterocycl. Chem., 2005, 40, 173–183 CrossRef; (d) G. P. Cai, X. L. Xu, Z. F. Li, P. Lu and W. P. Weber, J. Heterocycl. Chem., 2002, 39, 1271–1272 CrossRef CAS.
- H. Asakawa and M. Matano, Chem. Pharm. Bull., 1979, 27, 1287–1298 CrossRef CAS PubMed.
- (a) C. L. Yoo, J. C. Fettinger and M. Kurth, J. Org. Chem., 2005, 70, 6941–6943 CrossRef CAS PubMed; (b) J. Chen, W. Su, H. Wu, M. Liu and C. Jin, Green Chem., 2007, 9, 972–975 RSC; (c) X. Cheng, S. Vellalath, R. Goddard and B. List, J. Am. Chem. Soc., 2008, 130, 15786–15787 CrossRef CAS PubMed; (d) M. Sharma, S. Pandey, K. Chauhan, D. Sharma, B. Kumar and P. M. S. Chauhan, J. Org. Chem., 2012, 77, 929–937 CrossRef CAS PubMed; (e) M. Prakash and V. Kesavan, Org. Lett., 2012, 14, 1896–1899 CrossRef CAS PubMed; (f) M. Narasimhulu and Y. R. Lee, Tetrahedron, 2011, 67, 9627–9634 CrossRef CAS; (g) K. V. Sashidhara, G. R. Palnati, R. P. Dodda, S. R. Avula and P. Swami, Synlett, 2013, 1795–1800 CrossRef CAS.
- (a) W. K. Su and B. B. Yang, Aust. J. Chem., 2002, 55, 695–697 CrossRef CAS; (b) W. K. Su and B. B. Yang, J. Chem. Res., Synop., 2002, 604–605 CrossRef CAS.
- A. Ghorbani-Choghamarani and T. Taghipour, Lett. Org. Chem., 2011, 8, 470–476 CrossRef CAS.
- M. Dabiri, P. Salehi, M. Baghbanzadeh, M. A. Zolfigol, M. Agheb and S. Heydari, Catal. Commun., 2008, 9, 785–788 CrossRef CAS.
- K. Niknam, N. Jafarpour and E. Niknam, Chin. Chem. Lett., 2011, 22, 69–72 CrossRef CAS.
- K. Niknam, M. R. Mohammadizadeh and S. Mirzaee, Chin. J. Chem., 2011, 29, 1417–1422 CrossRef CAS.
- S. Rostamizadeh, A. M. Amani, G. H. Mahdavinia, H. Sepehrian and S. Ebrahimi, Synthesis, 2010, 1356–1360 CrossRef CAS.
- J. X. Chen, D. Z. Wu, F. He, M. C. Liu, H. Y. Wu, J. C. Ding and W. K. Su, Tetrahedron Lett., 2008, 49, 3814–3818 CrossRef CAS.
- M. Dabiri, P. Salehi, S. Otokesh, M. Baghbanzadeh, G. Kozehgary and A. A. Mohammadi, Tetrahedron Lett., 2005, 46, 6123–6126 CrossRef CAS.
- H. R. Shaterian, A. R. Oveisi and M. Honarmand, Synth. Commun., 2010, 40, 1231–1242 CrossRef CAS.
- P. Salehi, M. Dabiri, M. Baghbanzadeh and M. Bahramnejad, Synth. Commun., 2006, 36, 2287–2292 CrossRef CAS.
- S. Allameh, M. M. Heravi, M. M. Hashemi and F. F. Bamoharram, Chin. Chem. Lett., 2011, 22, 131–134 CrossRef CAS.
- S. Li, Q. Zhang and Y. Peng, Monatsh. Chem., 2015, 146, 1859–1864 CrossRef CAS.
- M. Wang, T. T. Zhang, Y. Liang and J. J. Gao, Chin. Chem. Lett., 2011, 22, 1423–1426 CrossRef CAS.
- S. Rostamizadeh, A. M. Amani, R. Aryan, H. R. Ghaieni and N. Shadjou, Synth. Commun., 2008, 38, 3567–3576 CrossRef CAS.
- Z. H. Zhang, H. Y. Lü, S. H. Yang and J. W. Gao, J. Comb. Chem., 2010, 12, 643–646 CrossRef CAS PubMed.
- S. Santra, M. Rahman, A. Roy, A. Majee and A. Hajra, Catal. Commun., 2014, 49, 52–57 CrossRef CAS.
- M. Z. Kassaee, S. Rostamizadeh, N. Shadjou, E. Motamedi and M. Esmaeelzadeh, J. Heterocycl. Chem., 2010, 47, 1421–1424 CrossRef CAS.
- B. H. Chen, J. T. Li and G. F. Chen, Ultrason. Sonochem., 2015, 23, 59–65 CrossRef CAS PubMed.
- M. P. Surpur, P. R. Singh, S. B. Patil and S. D. Samant, Synth. Commun., 2007, 37, 1965–1970 CrossRef CAS.
- J. Safari and S. Gandomi-Ravandi, J. Mol. Catal. A: Chem., 2013, 371, 135–140 CrossRef CAS.
- (a) J. A. Moore, G. J. Sutherland, R. Sowerby, E. G. Kelly, S. Palermo and W. Webster, J. Org. Chem., 1969, 34, 887–892 CrossRef CAS; (b) J. M. Khurana and G. Kukreja, J. Heterocycl. Chem., 2003, 40, 677–679 CrossRef CAS; (c) Y. S. Sadanandam, R. M. Reddy and A. Bhaskar Rao, Eur. J. Med. Chem., 1987, 22, 169–173 CrossRef CAS.
- J. T. Li, Y. Yin and M. X. Sun, Ultrason. Sonochem., 2010, 17, 363–366 CrossRef CAS PubMed.
- (a) M. Zahouily, Y. Abrouki, B. Bahlaouan, A. Rayadh and S. Sebti, Catal. Commun., 2003, 4, 521–524 CrossRef CAS; (b) Y. Zhang, Y. Zhao and Ch. Xia, J. Mol. Catal. A: Chem., 2009, 306, 107–112 CrossRef CAS.
- (a) N. Razavi and B. Akhlaghinia, RSC Adv., 2015, 5, 12372–12381 RSC; (b) S. S. E. Ghodsinia and B. Akhlaghinia, RSC Adv., 2015, 5, 49849–49860 RSC; (c) Z. Zarei and B. Akhlaghinia, Chem. Pap., 2015, 69, 1421–1437 CrossRef CAS; (d) M. Zarghani and B. Akhlaghinia, Appl. Organomet. Chem., 2015, 29, 683–689 CrossRef CAS; (e) M. Zarghani and B. Akhlaghinia, RSC Adv., 2015, 5, 87769–87780 RSC.
- E. Boanini, M. Gazzano, K. Rubini and A. Bigi, Adv. Mater., 2007, 19, 2499–24502 CrossRef CAS.
- R. Jenkins and R. L. Snyder, Chemical Analysis: Introducti on to X-ray Powder Diffractometry, John Wiley & Sons, Inc., New York, 1996, vol. 138, pp. 89–91 Search PubMed.
- J. Zhang, D. Ren, Y. Ma, W. Wang and H. Wu, Tetrahedron, 2014, 70, 5274–5282 CrossRef CAS.
- R. Noel, N. Gupta, V. Pons, A. Goudet, M. Garcia-Castillo, A. Michau, J. Martinez, D. Buisson, L. Johannes, D. Gillet, J. Barbier and J. Cintrat, J. Med. Chem., 2013, 56, 3404–3413 CrossRef CAS PubMed.
- L. Wang, L. Hu, J. Shao, J. Yu and L. Zhang, J. Fluorine Chem., 2008, 129, 1139–1145 CrossRef CAS.
- Z. Karimi-Jaberi and R. Arjmandi, Monatsh. Chem., 2011, 142, 631–635 CrossRef CAS.
- K. Niknam, M. R. Mohammadizadeh and S. Mirzaee, Chin. J. Chem., 2011, 29, 1417–1422 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5nj02123e |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |