Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A polydiacetylene–nested porphyrin conjugate for dye-sensitized solar cells†
Nuttapol
Pootrakulchote
a,
Chawanwit
Reanprayoon
b,
Jacek
Gasiorowski
c,
Niyazi Serdar
Sariciftci
c and
Patchanita
Thamyongkit
*d
aDepartment of Chemical Technology, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand
bProgram in Petrochemistry and Polymer Science, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand
cLinz Institute for Organic Solar Cells (LIOS), Institute of Physical Chemistry, Johannes Kepler University Linz, Linz 4040, Austria
dDepartment of Chemistry, Faculty of Science, Chulalongkorn University, Bangkok 10330, Thailand. E-mail: patchanita.v@chula.ac.th; Fax: +662-254-1309; Tel: +662-218-7587
First published on 17th September 2015
Abstract
A polydiacetylene (PDA)–nested zinc-porphyrin derivative was prepared and investigated for its potential applicability in dye-sensitized solar cells (DSSCs). Absorption enhancement at 525–625 nm was observed as a proof of successful PDA formation. Cyclic voltammetry analysis suggested the appropriate positions of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) energy levels of the target material for DSSCs. Optimum DSSCs based on the PDA–nested zinc-porphyrin exhibited a short-circuit photocurrent density (Jsc), an open-circuit voltage (Voc) and a fill factor (FF) of 4.2 mA cm−2, 0.7 V and 0.78, respectively, with an overall power conversion efficiency (PCE) of 2.3%. The photovoltage decay analysis also indicated that the electron recombination lifetime of the cells was prolonged as a result of the presence of the PDA-containing C25 alkyl chains in the porphyrin dye system.
Introduction
Porphyrins are attractive materials due to their adjustable photophysical and electrochemical properties via modification of peripheral substituents and central metals,1 as well as thermal- and photostability. Up to date, several porphyrinic derivatives have been proposed for being used as key constituents in (opto)electronic devices, such as photovoltaic cells,2 organic light-emitting diodes (OLEDs),3 and organic field-effect transistors (OFETs).4 Recently, they have proven to be promising candidates for dye-sensitized and bulk-heterojunction solar cells (DSSCs5 and BHJ-SCs,6 respectively).Our recent work reported the synthesis and use of polydiacetylene (PDA)–nested zinc-porphyrin for the BHJ-SCs, showing that the presence of a PDA network significantly enhanced the absorption of the porphyrin at the region of 530–600 nm and suggesting the potential use as an electron donating material in the BHJ-SCs.7 The objective of this work is to explore the possibility of using a novel PDA–nested zinc-porphyrin derivative as a dye in the DSSCs. The porphyrin monomer contains three meso-C25 alkyl chains having diacetylene (DA) units, which can be photopolymerized to give a porphyrin embedded PDA web, and one carboxyl anchoring group at the remaining meso position of the porphyrin. According to previous studies,7,8 this system is designed with three main advantages: (i) π-stacking of the porphyrin macrocycle can facilitate the local orientation of the DA units for the desirable photopolymerization, (ii) the long chain of the DA-containing C25 alkyl chains can serve as solubilizing groups in organic solvents, and (iii) the formation of the PDA network can enhance the absorption at 500–650 nm, where the porphyrin has low absorptivity. To the best of our knowledge, PDA was previously studied as a hole transport material in solid state DSSCs by Wang et al.,9 but a porphyrin–PDA conjugate has not been investigated for use in the DSSCs. In this work, we report the novel PDA–nested zinc-porphyrin conjugate exhibiting suitable photophysical and electrochemical properties for DSSC applications, and giving favorable device performance. The results from these studies will become a useful guideline for the development of other porphyrin-based and PDA-based materials for optoelectronic applications.
Experimental
Materials and methods
All chemicals were of analytical grade, purchased from commercial suppliers and used as received without further purification. 1H-NMR and 13C-NMR spectra were obtained in deuterated chloroform (CDCl3) using an NMR spectrometer operated at 400 megahertz (MHz) for 1H and 100 MHz for 13C nuclei. Chemical shifts (δ) are reported in parts per million (ppm) relative to the residual CHCl3 peak (7.26 ppm for 1H-NMR and 77.0 ppm for 13C-NMR). Coupling constants (J) are reported in Hz. Mass spectra were obtained by matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) using dithranol as a matrix. Absorption and emission spectra of dye solutions were measured in toluene at room temperature. Absorption data of a film of porphyrin–PDA conjugate were collected from the film prepared by submerging a TiO2 film into a 0.3 mM monomer solution in a mixed solvent of tetrahydrofuran (THF) and ethanol (v/v, 1/4) at room temperature for 16 h, followed by exposing the resulting dry film under ambient light for at least 1 h or under a 254 nm ultraviolet radiation for 15 min.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOH
EtOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TEA (96
TEA (96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)]. Compound 4 (0.101 g) was obtained as a purple solid which was used in the Zn-metallation step without further purification. λabs/nm 419, 514, 548, 593, 648; λem (λex = 419 nm)/nm 651, 712; MALDI-TOF-MS m/z obsd 1817.311 (M+, 100), calcd 1815.622 (M = C123H159N7O5).
1)]. Compound 4 (0.101 g) was obtained as a purple solid which was used in the Zn-metallation step without further purification. λabs/nm 419, 514, 548, 593, 648; λem (λex = 419 nm)/nm 651, 712; MALDI-TOF-MS m/z obsd 1817.311 (M+, 100), calcd 1815.622 (M = C123H159N7O5).
Following a previously published procedure,13 a solution of compound 4 (0.101 g, 0.055 mmol) in chloroform (3 mL) was reacted with a solution of zinc acetate dihydrate (0.063 g, 0.29 mmol) in methanol (1 mL) at room temperature for 3 h. The resulting mixture was extracted with CH2Cl2 and H2O. The organic phase was dried over anhydrous magnesium sulfate and concentrated to dryness. The resulting crude was purified by washing with hexane and then methanol to afford compound Zn-4 as a purple solid (0.081 g, 25% from 1). mp > 230 °C (from methanol); λabs/nm 425, 557, 598; λem (λex = 425 nm)/nm 605, 651; 1H NMR δ 0.89 (9 H, t, J = 7.3 Hz, CH3), 1.10–1.80 (102 H, m, CH2), 2.00–2.50 (12 H, m, CH2), 3.00–3.50 (6 H, m, CH2), 4.50–4.60 (1 H, m, NH), 5.20–5.50 (2 H, m, NH), 5.90–6.30 (4 H, m, CH), 7.50–7.75 (12 H, m, CH), 8.05–8.30 (6 H, m, CH), 8.80–8.90 (2 H, m, CH); 13C NMR δ 14.1, 19.2, 22.7, 25.5, 26.8, 26.9, 27.0, 28.3, 28.4, 28.7, 28.8, 28.9, 29.0, 29.1, 29.2, 29.3, 29.5, 29.6, 30.1, 31.9, 35.4, 35.9, 36.5, 36.7, 39.6, 40.2, 40.6, 62.7, 65.3, 127.1, 137.4, 158.5, 166.6, 171.9; MALDI-TOF-MS m/z obsd 1373.194 [(M-C37H63)+, 45], 1881.103 [M+, 55] calcd 1879.017 [M+], (M = ZnC123H157N7O5).
To characterize the DSSCs, a 450 W xenon light source (Oriel, USA) was used. The current density–voltage (J–V) characteristics were obtained by applying external potential bias to the cell and measuring the generated photocurrent using a Keithley model 2400 digital source meter (Keithley, USA). The devices were masked to attain an illuminated active area of 0.159 cm2. Loss of light due to reflection from the photoanode glass was reduced by applying a self-adhesive fluorinated polymer anti-reflecting film (ARKTOP, Asahi glass). Up to four devices were fabricated for each experimental variable change to give accurate statistics. Detailed methods for the TiO2 film preparation, the device fabrication and the current–voltage measurements are described elsewhere.14
A modulated light intensity data acquisition system was used to control the incident photon-to-current conversion efficiency (IPCE) measurement. The modulation frequency was about 1 Hz. Light from a 300 W Xenon lamp (ILC Technology, USA) was focused through a computer controlled Gemini-180 double monochromator (JobinYvon Ltd, UK) onto the photovoltaic cell. White light bias was used to bring the total light intensity on the device closer to operating conditions.
In the transient photoelectrical decay experiments, different steady-state light levels were provided by a homemade white light-emitting diode array tuning the driving voltage. A red light-emitting diode array controlled with a fast solid-state switch was used to generate a perturbation pulse of 50 ms duration. The pulsed red- and steady-state white-light were both incident on the working electrode side of the test cell. The intensity of the red light pulse was carefully controlled by the driving potential of the red diode array to keep the modulated photovoltage below 10 mV. In a transient photovoltage decay measurement, the cells were maintained at open circuit voltage under the white light and the transient photovoltage decay following the red light pulse was monitored. Normally, the decay follows closely a monoexponential form, thus, a recombination rate constant can be extracted from a slope of a semilogarithmic plot.15
Results and discussion
Synthesis
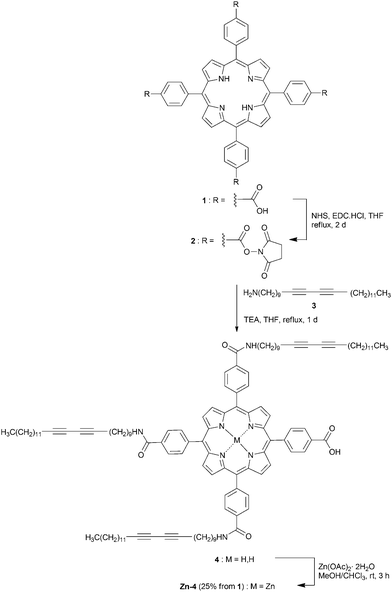
The target compound was obtained from a series of three reactions as shown in Scheme 1. A reaction of the tetracarboxylic acid 110 with NHS and EDC·HCl yielded ester 2 which immediately reacted with diacetylene 3,11 due to its hygroscopicity, to afford compound 4. The formation of compound 4 was confirmed by the presence of its molecular peak at m/z 1817.311 on a MALDI-TOF mass spectrum corresponding to its molecular mass, and characteristic patterns of free base porphyrin on its absorption and emission spectra.A subsequent Zn-metallation of compound 4 with Zn(OAc)2·2H2O gave Zn-4 in 25% overall yield. The completion of the metallation step was indicated by the absence of an emission peak at 712 nm and a 1H-NMR peak of the inner protons at about δ −2 ppm in the spectra of compound Zn-4. The solubility of Zn-4 was found to be more than 20 mg mL−1 in common organic solvents, e.g. CH2Cl2, CHCl3, and THF, which is sufficient for a routine wet process in DSSC fabrication.
Photophysical and electrochemical properties
The photophysical properties, namely absorption as a function of a wavelength of an incident light, were determined for the monomer solution and the polymer film. The resulting curves are presented in Fig. 1. The UV-vis absorption spectra of a Zn-4 solution in toluene exhibited a characteristic pattern of Zn-porphyrin having a B-band at 425 nm, and Q-bands at 557 and 598 nm (black solid line). A poly-Zn-4 film, prepared by the 254 nm ultraviolet radiation of a Zn-4-coated TiO2 film at room temperature for 15 min, gave the similar absorption pattern with slightly broader bands and higher intensity of the first Q-band (red dashed line). The broadening of the absorption bands can be explained by the possible macrocycle aggregation. Although the film was relatively thin on the TiO2 surface, the enhancement of the Q-band, possibly resulted from the co-absorption of the PDA formed in the film, can be seen. These results are in a good agreement with our previous report.7The electrochemical properties of the poly-Zn-4 film on the ITO/glass glass substrate were studied by cyclic voltammetry. Results revealed that the poly-Zn-4 film can be both electrochemically oxidized and reduced (Fig. 2). In the oxidation domain (Fig. 2a, black solid line), two quasireversible anodic peaks of poly-Zn-4 were observed at the peak potentials of +0.7 V and +1.3 V, related to two successive one-electron oxidation processes. Due to the dissolvation of the film into the electrolyte solution during the measurement, the positive scanning was not performed beyond the potential of +1.5 V. Compared with 5-(4-carboxyphenyl)-10,15,20-(triphenyl)porphinatozinc(II) (ZnTPP-COOH; Fig. 2a, red dashed line), it was observed that the first oxidation of poly-Zn-4 occurred in the similar potential (+0.7 V vs. +0.8 V), while the second oxidation of poly-Zn-4 occurred at 0.2 V higher potential (+1.3 V vs. +1.1 V). In the reduction process of poly-Zn-4 (Fig. 2b), the dissolvation of the film was observed beyond the potential of −1.8 V, and therefore the measurement was carried out from 0.0 V to −1.8 V (Fig. 2b, black solid line). In this region, poly-Zn-4 gave one irreversible peak at −1.5 V, which is corresponding to the first cathodic potential of ZnTPP-COOH (−1.5 V; Fig. 2b, red dashed line). In the case of ZnTPP-COOH, the second cathodic signal was observed at −1.8 V with the indefinable anodic signal from −0.4 and −0.8 V. This is likely to be resulted from the possible formation of unknown products from the reduction process(es) of ZnTPP-COOH, which is attributed to the molecular cleavage, as this feature was more conspicuous at higher cycle numbers. In comparison with the results obtained from ZnTPP-COOH, these small shifts of redox potentials and the film instability observed from poly-Zn-4 film indicate a significant effect of the presence of the PDA-containing alkyl chains on the electrochemical characteristics of the porphyrin ring.
Following previous studies,16 a highest occupied molecular orbital (HOMO) energy level of a dye can be represented by its E1/2 value of the first oxidation or E1/2(ox1), while a lowest unoccupied molecular orbital (LUMO) energy level can be estimated from an excited state oxidation potential (E0–0*) by the following equation:
| E0–0* = E1/2(ox1) − E0–0 |
Photovoltaic characteristics. The poly-Zn-4-based solar cells were assembled using a double layer TiO2 film (8 + 5 μm) in conjugation with an acetonitrile-based electrolyte solution. A detailed description is given in the Device fabrication section. Fig. 3 shows the photovoltaic performance of the devices under standard AM 1.5 G 1000 W m−2 illumination. From the current–voltage (J–V) curve shown in Fig. 3a, the best poly-Zn-4-based solar cell exhibited, respectively, a short-circuit photocurrent density (Jsc), an open-circuit voltage (Voc) and a fill factor (FF) of 4.2 mA cm−2, 0.7 V and 0.78, respectively, which yielded an overall power conversion efficiency (PCE) value of 2.3%. It is important to note that the PCE was increased by 35% of the original value (from 1.7% to 2.3%) when the device was illuminated under the standard AM 1.5 G 1000 W m−2 light intensity for ∼40 minutes before the J–V measurement was performed. According to the previous study,17 the substitution of the DA-containing alkyl chains in the meso-phenyl groups of the porphyrin ring resulted in retardation of the charge recombination processes and enhanced the interfacial charge separation. Moreover, compared with the previous study of ZnTPP-COOH-based DSSCs,18 the PDA-containing alkyl chains in poly-Zn-4 resulted in a remarkable increase in PCE value (1.8% vs. 2.3%). This phenomenon could be attributed to the solid-state photo-induced PDA formation in the poly-Zn-4 film, leading to the increase in the number of π-conjugation network and, as a result, raising the light harvesting efficiency of the dye. However, a relatively low value of PCE might have resulted from the porphyrin aggregation on the TiO2 surface as indicated by the broadened absorption bands of the poly-Zn-4 film on TiO2. A similar observation has also been found in the case of ZnTPP-COOH.18 The aggregation can cause the quenching processes of the excited states to the adjacent porphyrin units, subsequently increasing unfavorable charge recombination processes and reducing the electron injection efficiency.19
The corresponding IPCE spectrum of the poly-Zn-4-based solar cell is shown in Fig. 3b. The pattern of the IPCE spectrum is consistent with the absorption spectra of the Zn-4 solution and the poly-Zn-4 film with the IPCE values at 430, 570 and 610 nm of 48.9% and 37.6% and 34.8%, respectively. In addition, the presence of the PDA network in the case of poly-Zn-4 significantly increased the ratio of the long-wavelength peaks to the short-wavelength peak in the IPCE spectrum (0.77 and 0.71 for the peaks at 570 and 610 nm, respectively) compared to that of ZnTPP-COOH (approximately 0.3 and 0.2, excerpted from ref. 18). This suggested an enhancement in the light harvesting efficiency in the visible region of poly-Zn-4 bound to the TiO2 surface resulting from the PDA-containing C25 alkyl units.
To further investigate the electron recombination between rate poly-Zn-4 and TiO2 nanoparticles, the transient photovoltage decay measurement was performed. The decay of the recombination lifetime exhibits an exponential dependence with respect to the photovoltage under open-circuit conditions as shown in Fig. 4. It appeared that the electron lifetime became shorter as more charges were injected from the excited states of poly-Zn-4 into the conduction band of TiO2 nanoparticles, leading to the faster reaction of electrons and I2- species in the electrolyte when the solar cell was exposed to the light at stronger intensity. The electron lifetime of the poly-Zn-4-based solar cell at 0.60 V was approximately 40 ms, which is twice longer than the value of 21.2 ms that was reported for ZnTPP-COOH.18 We attribute this effect to the substitution of PDA-containing C25 alkyl chains in the meso-phenyl groups of the porphyrin ring. Furthermore, the chemical capacitance (Cμ) of the poly-Zn-4-based solar cell increased exponentially with an increasing bias potential under open-circuit conditions. It is also suggested that the chemical capacitance dependence on Voc follows an exponential law as Cμ∝ αqVoc/kBT with α = 0.34.
 | ||
| Fig. 4 Plots of electron recombination lifetime (blue square) and chemical capacitance (red triangle) vs. open-circuit voltage of the poly-Zn-4-based solar cells. | ||
Conclusions
The target diacetylene–porphyrin monomer was successfully prepared and showed satisfactory solubility characteristics. The formation of the polydiacetylene–nested zinc-porphyrin was indicated by the slight enhancement of the absorption in the Q-band region relative to the monomer. The results from cyclic voltammetry showed that the polydiacetylene–nested zinc-porphyrin exhibited the suitable levels of the highest occupied molecular orbital and the lowest unoccupied molecular orbital for use in dye-sensitized solar cells. Device studies suggested that the incorporation of the polydiacetylene network and the porphyrinic system was advantageous for the device performance with respect to the previously reported system based on 5-(4-carboxyphenyl)-10,15,20-(triphenyl)porphinatozinc(II).Acknowledgements
This research was supported by National Research University Project of Thailand, Office of the Higher Education Commission (Project No: WCU-035-EN-57), and Graduate School of Chulalongkorn University (The 90th Anniversary of Chulalongkorn University Fund, Ratchadaphiseksomphot Endowment Fund). Device fabrication and the photovoltaic measurements were performed at École Polytechnique Fédérale de Lausanne (EPFL), Switzerland.References
- K. M. Kadish, E. V. Caemelbecke and G. Royal, in The Porphyrin Handbook, ed K. M. Kadish, K. M. Smith and R. Guilard, Academic Press, New York, 2000, vol. 8, ch. 55 Search PubMed.
- Selected publications: (a) K. Kalyanasundaram, N. Vlachopoulos, V. Krishnan, A. Monnier and M. Grätzel, J. Phys. Chem., 1987, 91, 2342 CrossRef CAS; (b) A. Kay and M. Grätzel, J. Phys. Chem., 1993, 97, 6272 CrossRef CAS; (c) D. Wohrle, B. Tennigkeit, J. Elbe, L. Kreienhoop and G. Schurpfeil, Mol. Cryst. Liq. Cryst., 1993, 230, 221 CrossRef PubMed; (d) S. Cherian and C. C. Wamser, J. Phys. Chem. B, 2000, 104, 3624 CrossRef CAS; (e) F. Odobel, E. Blart, M. Lagree, M. Villieras, H. Boujtita, N. El Murr, S. Caramori and C. A. Bignozzi, J. Mater. Chem., 2003, 13, 502 RSC; (f) W. M. Campbell, A. K. Burrell, D. L. Officer and K. W. Jolly, Coord. Chem. Rev., 2004, 248, 1363 CrossRef CAS PubMed; (g) H. Imahori, J. Phys. Chem. B, 2004, 108, 6130 CrossRef CAS PubMed; (h) T. Hasobe, P. V. Kamat, M. A. Absalom, Y. Kashiwagi, J. Sly, M. J. Crossley, K. Hosomizu, H. Imahori and S. Fukuzumi, J. Phys. Chem. B, 2004, 108, 12865 CrossRef CAS; (i) Q. Wang, W. M. Campbell, E. E. Bonfantani, K. W. Jolley, D. L. Officer, P. J. Walsh, K. Gordon, R. Humphry-Baker, M. K. Nazeeruddin and M. Grätzel, J. Phys. Chem. B, 2005, 109, 15397 CrossRef CAS PubMed; (j) T. Hasobe, S. Fukuzumi and P. V. Kamat, J. Phys. Chem. B, 2006, 110, 25477 CrossRef CAS PubMed; (k) W. M. Campbell, K. W. Jolly, P. Wagner, K. Wagner, P. J. Walsh, K. C. Gordon, L. S. Mende, M. K. Nazeerruddin, Q. Wang, M. Grätzel and D. L. Officer, J. Phys. Chem. C, 2007, 111, 11760 CrossRef CAS; (l) H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009, 42, 1809 CrossRef CAS PubMed; (m) R. M. Ma, P. Guo, H. J. Cui, X. X. Zhang, M. K. Nazeerruddin and M. Grätzel, J. Phys. Chem. A, 2009, 113, 10119 CrossRef CAS PubMed.
- Selected publications: (a) M. A. Baldo, D. F. O’Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151 CrossRef CAS; (b) R. C. Kwong, S. Sibley, T. Dubovoy, M. Baldo, S. R. Forrest and M. E. Thompson, Chem. Mater., 1999, 11, 3709 CrossRef CAS; (c) T. F. Guo, S. C. Chang, Y. Yang, R. C. Kwong and W. E. Thompson, Org. Electrochem., 2000, 1, 15 CrossRef CAS; (d) E. M. Gross, N. R. Armstrong and R. M. Wightman, J. Electrochem. Soc., 2002, 149, E137 CrossRef CAS PubMed; (e) V. A. Montes, C. Perez-Bolivar, N. Agarwal, J. Shinar and P. Anzenbacher, J. Am. Chem. Soc., 2006, 128, 12436 CrossRef CAS PubMed; (f) X. Wang, H. Wang, Y. Yang, Y. He, L. Zhang, Y. Li and X. Li, Macromolecules, 2010, 43, 709 CrossRef CAS.
- Selected publications: (a) Y. Y. Noh, K. Yase and S. Nagamatsu, Appl. Phys. Lett., 2003, 83, 1243 CrossRef CAS PubMed; (b) Y. Y. Noh, J. J. Kim, Y. Yoshida and K. Yase, Adv. Mater., 2003, 15, 699 CrossRef CAS PubMed; (c) P. Checcoli, G. Conte, S. Salvatori, R. Paolesse, A. Bolognesi, A. Berliocchi, F. Brunetti, A. D’Amico, A. Di Carlo and P. Lugli, Synth. Met., 2003, 138, 261 CrossRef CAS; (d) P. B. Shea, J. Kanicki and N. Ono, J. Appl. Phys., 2005, 98, 014503 CrossRef PubMed; (e) C.-M. Che, H.-F. Xiang, S. S.-Y. Chui, Z.-X. Xu, V. A. L. Roy, J. J. Yan, W.-F. Fu, P. T. Lai and I. D. Williams, Chem. – Asian J., 2008, 3, 1092 CrossRef CAS PubMed; (f) X. B. Huang, C. L. Zhu, S. M. Zhang, W. W. Li, Y. L. Guo, X. W. Zhan, Y. Q. Liu and Z. Z. Bo, Macromolecules, 2008, 41, 6895 CrossRef CAS; (g) P. Ma, Y. Chen, X. Cai, H. Wang, Y. Zhang, Y. Gao and J. Jiang, Synth. Met., 2010, 160, 510 CrossRef CAS PubMed.
- (a) A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, S. M. Zakeeruddin and M. Grätzel, Science, 2011, 334, 629 CrossRef CAS PubMed; (b) S. Mathew, A. Yella, P. Gao, R. Humphry-Baker, B. F. E. Curchod, N. Ashari-Astani, I. Tavernelli, U. Rothlisberger, M. K. Nazeeruddin and M. Grätzel, Nat. Chem., 2014, 6, 242 CrossRef CAS PubMed.
- (a) Mitsubishi Chemical Holdings Corporation, http://www.m-kagaku.co.jp/english/aboutmcc/RC/special/feature1.html, accessed April 17, 2012; (b) R. F. Service, Science, 2011, 332, 293 CrossRef CAS PubMed.
- C. Reanprayoon, J. Gasiorowski, M. Sukwattanasinitt, N. S. Sariciftci and P. Thamyongkit, RSC Adv., 2014, 4, 3045 RSC.
- M. Shirakawa, N. Fujita and S. Shinkai, J. Am. Chem. Soc., 2006, 127, 4164 CrossRef PubMed.
- Y. P. Wang, K. Yang, X. Y. Wang, R. Nagarajan, L. A. Samuelson and J. Kumar, Org. Electron., 2006, 7, 546 CrossRef CAS PubMed.
- A. D. Adler, F. R. Longo, J. D. Finarelli, J. Goldmacher, J. Assour and L. Korsakoff, J. Org. Chem., 1967, 32, 476 CrossRef CAS.
- N. M. Howarth, W. E. Lindsell, E. Murray and P. N. Preston, Tetrahedron, 2005, 61, 8875 CrossRef CAS PubMed.
- S. J. Kew and E. A. H. Hall, J. Mater. Chem., 2006, 16, 2039 RSC.
- J. Jiao, P. Thamyongkit, I. Schmidt, J. S. Lindsey and D. F. Bocian, J. Phys. Chem. C, 2007, 111, 12693 CAS.
- P. Wang, M. Zakeeruddin, P. Comte, R. Charvet, R. Humphry-Baker and M. Grätzel, J. Phys. Chem. B, 2003, 107, 14336 CrossRef CAS.
- M. Wang, X. Li, H. Lin, P. Pechy, S. M. Zakeeruddin and M. Grätzel, Dalton Trans., 2009, 10015 RSC.
- (a) D. P. Hagberg, J.-H. Yum, H. Lee, F. D. Angelis, T. Marinado, K. M. Karlsson, R. Humphry-Baker, L. Sun, A. Hagfeldt, M. Grätzel and M. K. Nazeeruddin, J. Am. Chem. Soc., 2008, 130, 6259 CrossRef CAS PubMed; (b) J. K. Park, H. R. Lee, J. Chen, H. Shinokubo, A. Osuka and D. Kim, J. Phys. Chem. C, 2008, 112, 16691 CrossRef CAS.
- S. M. Feldt, E. A. Gibson, E. Gabrielsson, L. Sun, G. Boschloo and A. Hagfeldt, J. Am. Chem. Soc., 2010, 132, 16714 CrossRef CAS PubMed.
- H. He, A. Gurung and L. Si, Chem. Commun., 2012, 48, 5910 RSC.
- M. R. Viseu, G. Hungerford and M. I. C. Ferreira, J. Phys. Chem. B, 2002, 106, 1853 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Spectral data, including the 1H-NMR spectrum, 13C-NMR spectrum and mass spectra of new compounds. See DOI: 10.1039/c5nj01583a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |