Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Asymmetric induction by retgersite, nickel sulfate hexahydrate, in conjunction with asymmetric autocatalysis
Arimasa
Matsumoto
,
Hirokazu
Ozawa
,
Ayako
Inumaru
and
Kenso
Soai
*
Department of Applied Chemistry, Research Institute for Science and Technology, Tokyo University of Science, Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: soai@rs.kagu.tus.ac.jp
First published on 16th July 2015
Abstract
Retgersite, nickel sulfate hexahydrate, forms a chiral crystal with space group P41212 or P43212. A chiral crystal of nickel sulfate hexahydrate was found to act as a chiral initiator of asymmetric autocatalysis, affording highly enantiomerically enriched pyrimidyl alkanols with the corresponding absolute configurations.
The origin of biological homochirality is of great interest to the scientific community and several possible origins of homochirality have been proposed.1 Chiral inorganic crystals are considered to be one possible origin of homochirality because the inorganic minerals are considered to have existed widely on Earth before the emergence of life.2,3 Metal sulfates are one of the most abundant minerals on Earth, and the sulfate and metal ions are symmetric achiral ions.
Retgersite, nickel sulfate hexahydrate, is a naturally occurring mineral and exhibits enantiomorphism. Nickel sulfate forms various hydrate salts such as hexahydrate and heptahydrate depending on the crystallization conditions. The relatively stable α-form of hexahydrate has a chiral structure. The hexahydrate crystal 1, crystallized at 31.5–53.3 °C,4 has a bright blue-green colour and has absorption bands in the visible light region; the optical properties of the crystal have been studied, including the chiral optical properties such as ORD and CD.5 The absolute structure, analyzed by X-ray and ORD spectra, of 1 was first reported in 1987 and the detailed chiral optical properties of the crystal were studied recently by Asahi et al.6 The chiral optical properties of the chiral crystal of achiral nickel sulfate have thus attracted broad interest. However, to the best of our knowledge, usage of the chirality of NiSO4·6H2O in chemical reactions has not been realized. In this paper, we report asymmetric induction using chiral crystals of nickel sulfate hexahydrate in conjunction with asymmetric autocatalysis (Scheme 1).
Asymmetric autocatalysis is the reaction in which the product acts as an asymmetric catalyst for the reaction to form a product with the same structure and absolute configuration. We have been studying the asymmetric autocatalytic reaction of the pyrimidyl alkanols with amplification of enantiomeric excess.7–9 By using asymmetric autocatalysts, we have reported that various inorganic10 and organic11 crystals act as chiral triggers for the asymmetric autocatalysis. Herein, we report that the chiral nickel sulfate hexahydrate crystal triggers asymmetric autocatalysis.
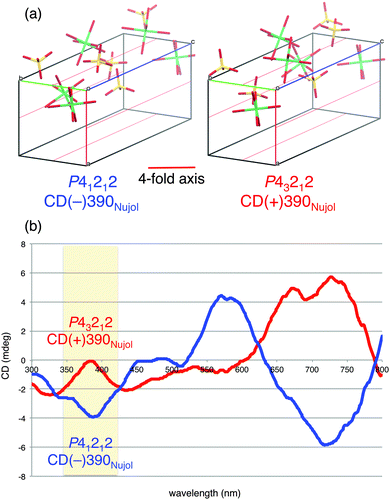
The chiral single crystal of nickel sulfate hexahydrate was obtained by recrystallization. NiSO4·6H2O 1 (25 g) was dissolved in distilled water (33 mL) at 70 °C and was kept for 12 h and water was allowed to evaporate for 2–3 d at above 31.5 °C in a Petri dish to give a crystal of NiSO4·6H2O 1 of ca. 2 cm size (Fig. 1). The solid state CD spectrum was measured with the powdered crystal of 1 in Nujol. Although the base line of the obtained CD spectrum was not flat due to the anisotropy of the crystal powders, a distinguishable signal was observed around the 390 nm region (Fig. 2b). The chirality of the crystal was determined by the CD spectrum in the 390 nm region and confirmed by the X-ray single crystal diffraction analysis using the Flack parameter (Fig. 2a).12,13 The P41212 crystal exhibits the negative Cotton effect at 390 nm, and the P43212 crystal exhibits the opposite positive Cotton effect at 390 nm.5,6
Using these chiral crystals of NiSO4·6H2O 1 as chiral initiators, asymmetric autocatalysis of diisopropylzinc (i-Pr2Zn) and pyrimidine-5-carbaldehyde 2 was performed.14 Thus, the chiral crystal of NiSO4·6H2O 1 was ground with agate and mortar, then it was mixed immediately with pyrimidine-5-carbaldehyde 2 and used in the reaction before the progress of the dehydration of the crystal. By adding i-Pr2Zn slowly, addition reaction was performed in combination with asymmetric autocatalysis with amplification of enantiomeric excess (ee). After purification, the enantiomeric excess of the formed pyrimidyl alkanol 3 was determined by HPLC using a chiral column.
The results are summarized in Table 1. As shown in entry 1, (S)-pyrimidyl alkanol 3 was obtained in the presence of a chiral crystal of NiSO4·6H2O with the positive Cotton effect at 390 nm [CD(+)390Nujol]. On the other hand, (R)-3 was obtained in the presence of a chiral crystal of NiSO4·6H2O with the negative Cotton effect [CD(−)390Nujol] (entry 2). Although the yields and ees were not so high probably due to the heterogeneous conditions with the existence of crystallization water, the sense of enantioselectivity exhibits good reproducibility for the several different crystal samples. It should be noted that the ee of the formed alkanol 3 was amplified to be nearly enantiomerically pure (>99.5% ee) during the subsequent asymmetric autocatalysis (entries 1 and 2).
| Entry | Crystal 1 | Pyrimidyl alkanol 3 | Amplification of ee by asymmetric autocatalysisc | |||
|---|---|---|---|---|---|---|
| CD390 nmNujol | Yield (%) | eed (%) | Yield (%) | eed (%) | Configuration | |
a Reaction conditions: molar ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-Pr2Zn = 16 i-Pr2Zn = 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 in toluene at 0 °C, and additional 2 3 in toluene at 0 °C, and additional 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-Pr2Zn = 2 i-Pr2Zn = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 8 4 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 were added stepwise.
b Reaction conditions: molar ratio, 1 16 were added stepwise.
b Reaction conditions: molar ratio, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-Pr2Zn = 32 i-Pr2Zn = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 in toluene at 0 °C, and additional 2 9 in toluene at 0 °C, and additional 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i-Pr2Zn = 2 i-Pr2Zn = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and 8 12 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 were added stepwise.
c Asymmetric autocatalytic reaction with the obtained alkanol after removing NiSO4·6H2O.
d The ee value was determined using HPLC on a chiral stationary phase. HPLC conditions: CHIRALPAK IB (4.6 mm ∅ × 250 mm), 5% 2-propanol in hexane, 1.0 mL min−1, 254 nm, r.t., retention time (min) 10.3 for (S)-3, 13.9 for (R)-3.
e After additional 2 to 5 rounds of asymmetric autocatalytic reaction. 48 were added stepwise.
c Asymmetric autocatalytic reaction with the obtained alkanol after removing NiSO4·6H2O.
d The ee value was determined using HPLC on a chiral stationary phase. HPLC conditions: CHIRALPAK IB (4.6 mm ∅ × 250 mm), 5% 2-propanol in hexane, 1.0 mL min−1, 254 nm, r.t., retention time (min) 10.3 for (S)-3, 13.9 for (R)-3.
e After additional 2 to 5 rounds of asymmetric autocatalytic reaction.
|
||||||
| 1a | (+) | 27 | 3 | 77 | 50 (>99.5)e | S |
| 2a | (−) | 28 | 3 | 72 | 37 (>99.5)e | R |
| 3a | (+) | 81 | 9 | 72 | 28 | S |
| 4a | (+) | 27 | 2 | 78 | 76 | S |
| 5a | (+) | 27 | 5 | 74 | 95 | S |
| 6a | (−) | 37 | 6 | 80 | 91 | R |
| 7b | (+) | 14 | 4 | 85 | 53 | S |
| 8b | (−) | 30 | 4 | 73 | 68 | R |
We have demonstrated, by using asymmetric autocatalysis of pyrimidyl alkanols, that the chiral natural mineral of retgersite NiSO4·6H2O induces the chirality of the chiral organic compound with high ee. These results suggest that common inorganic sulfate minerals on Earth may be considered to be the origin of chirality of the chiral organic compounds.
Acknowledgements
This work has been financially supported by the Grant-in-Aid for Scientific Research from the Promotion of Science (JSPS KAKENHI grant numbers 26810026 & 15H03781) and the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016.Notes and references
- (a) I. Weissbuch, L. Addadi, Z. Berkovitch-Yellin, E. Gati, M. Lahav and L. Leiserowitz, Nature, 1984, 310, 161 CrossRef CAS PubMed; (b) M. Bolli, R. Micura and A. Eschenmoser, Chem. Biol., 1997, 4, 309 CrossRef CAS; (c) B. L. Feringa and R. A. van Delden, Angew. Chem., Int. Ed., 1999, 38, 3418 CrossRef; (d) M. M. Green, J.-W. Park, T. Sato, A. Teramoto, S. Lifson, R. L. B. Selinger and J. V. Selinger, Angew. Chem., Int. Ed., 1999, 38, 3139 CAS; (e) J. M. Ribó, J. Crusats, F. Sagués, J. Claret and R. Rubires, Science, 2001, 292, 2063 CrossRef PubMed; (f) D. K. Kondepudi and K. Asakura, Acc. Chem. Res., 2001, 34, 946 CrossRef CAS PubMed; (g) K. Mislow, Collect. Czech. Chem. Commun., 2003, 68, 849 CrossRef CAS; (h) K. Soai, I. Sato, T. Shibata, S. Komiya, M. Hayashi, Y. Matsueda, H. Imamura, T. Hayase, H. Morioka, H. Tabira, J. Yamamoto and Y. Kowata, Tetrahedron: Asymmetry, 2003, 14, 185 CrossRef CAS; (i) S. Pizzarello and A. L. Weber, Science, 2004, 303, 1151 CrossRef CAS PubMed; (j) C. Viedma, Phys. Rev. Lett., 2005, 94, 65504 CrossRef; (k) Y. Hayashi, M. Matsuzawa, J. Yamaguchi, S. Yonehara, Y. Matsumoto, M. Shoji, D. Hashizume and H. Koshino, Angew. Chem., Int. Ed., 2006, 45, 4593 CrossRef CAS PubMed; (l) M. Klussmann, H. Iwamura, S. P. Mathew, D. H. Wells, U. Pandya, A. Armstrong and D. G. Blackmond, Nature, 2006, 441, 621 CrossRef CAS PubMed; (m) V. A. Soloshonok, H. Ueki, M. Yasumoto, S. Mekala, J. S. Hirschi and D. A. Singleton, J. Am. Chem. Soc., 2007, 129, 12112 CrossRef CAS PubMed; (n) T. Satyanarayana, S. Abraham and H. B. Kagan, Angew. Chem., Int. Ed., 2009, 48, 456 CrossRef CAS PubMed; (o) R. Breslow and Z.-L. Cheng, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5723 CrossRef CAS PubMed; (p) I. Weissbuch and M. Lahav, Chem. Rev., 2011, 111, 3236 CrossRef CAS PubMed; (q) F. E. Held, A. Fingerhut and S. B. Tsogoeva, Tetrahedron: Asymmetry, 2012, 23, 1663 CrossRef CAS PubMed; (r) Y. Saito and H. Hyuga, Rev. Mod. Phys., 2013, 85, 603 CrossRef CAS; (s) G. Valero, J. M. Ribó and A. Moyano, Chem. – Eur. J., 2014, 20, 17395 CrossRef CAS PubMed; (t) S. Olsson, P. M. Björemark, T. Kokoli, J. Sundberg, A. Lennartson, C. J. McKenzie and M. Håkansson, Chem. – Eur. J., 2015, 21, 5211 CrossRef CAS PubMed.
- (a) D. K. Kondepudi, R. J. Kaufman and N. Singh, Science, 1990, 250, 975 CAS; (b) J. M. McBride and R. L. Carter, Angew. Chem., Int. Ed., 1991, 30, 293 CrossRef PubMed.
- (a) R. M. Hazen, T. R. Filley and G. A. Goodfriend, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 5487 CrossRef CAS PubMed; (b) R. M. Hazen, in Progress in Biological Chirality, ed. G. Pályi, C. Zicchi and L. Caglioti, Elsevier, Oxford, 2004, pp. 137–151 Search PubMed; (c) R. M. Hazen and D. A. Sverjensky, Cold Spring Harbor Perspect. Biol., 2010, 2, a002162 CrossRef PubMed.
- V. L. Manomenova, E. B. Rudneva, A. É. Voloshin, L. V. Soboleva, A. B. Vasil and B. V. Mchedlishvili, Crystallogr. Rep., 2005, 50, 877 CrossRef CAS.
- (a) L. R. Ingersoll, P. Rudnick, F. G. Slack and N. Underwood, Phys. Rev., 1940, 57, 114 CrossRef; (b) K. Stadnicka, A. M. Glazer and M. Koralewski, Acta Crystallogr., Sect. B: Struct. Sci., 1987, 43, 319 CrossRef; (c) T. Harada, T. Sato and R. Kuroda, Chem. Phys. Lett., 2008, 456, 268 CrossRef CAS PubMed.
- Symposium abstract: T. Taniguchi, K. Ishikawa, M. Tanaka, T. Asahi, Chirality 2013, P-016, Shanghai, July 7–10, 2013.
- (a) K. Soai, T. Shibata, H. Morioka and K. Choji, Nature, 1995, 378, 767 CrossRef CAS PubMed; (b) I. Sato, H. Urabe, S. Ishiguro, T. Shibata and K. Soai, Angew. Chem., Int. Ed., 2003, 42, 315 CrossRef CAS PubMed; (c) T. Kawasaki, Y. Matsumura, T. Tsutsumi, K. Suzuki, M. Ito and K. Soai, Science, 2009, 324, 492 CrossRef CAS PubMed; (d) A. Matsumoto, S. Oji, S. Takano, K. Tada, T. Kawasaki and K. Soai, Org. Biomol. Chem., 2013, 11, 2928 RSC; (e) T. Kawasaki, M. Nakaoda, Y. Takahashi, Y. Kanto, N. Kuruhara, K. Hosoi, I. Sato, A. Matsumoto and K. Soai, Angew. Chem., Int. Ed., 2014, 53, 11199 CrossRef CAS PubMed.
- (a) K. Soai and T. Kawasaki, Top. Curr. Chem., 2008, 284, 1 CrossRef CAS; (b) T. Kawasaki and K. Soai, Bull. Chem. Soc. Jpn., 2011, 84, 879 CrossRef CAS; (c) K. Soai and T. Kawasaki, Asymmetric Autocatalysis – Discovery and State of The Art, in The Soai Reaction and Related Topic, ed. G. Pályi, C. Zicchi and L. Caglioti, Academia Nationale di Scienze Lettere e Arti Modena, Modena, Edizioni Artestampa, 2012, pp. 9–34 Search PubMed; (d) T. Kawasaki and K. Soai, Isr. J. Chem., 2012, 52, 582 CrossRef CAS PubMed; (e) T. Kawasaki, I. Sato, H. Mineki, A. Matsumoto and K. Soai, Yuki Gosei Kagaku Kyokaishi, 2013, 71, 109 CrossRef CAS; (f) K. Soai and T. Kawasaki, Top. Organomet. Chem., 2013, 44, 261 CrossRef CAS; (g) K. Soai, T. Kawasaki and A. Matsumoto, Chem. Rec., 2014, 14, 70 CrossRef CAS PubMed; (h) K. Soai, T. Kawasaki and A. Matsumoto, Acc. Chem. Res., 2014, 47, 3643 CrossRef CAS PubMed.
- (a) D. G. Blackmond, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5732 CrossRef CAS PubMed; (b) J. Podlech and T. Gehring, Angew. Chem., Int. Ed., 2005, 44, 5776 CrossRef CAS PubMed; (c) J. M. Brown, I. Gridnev and J. Klankermayer, Top. Curr. Chem., 2008, 284, 35 CrossRef CAS; (d) L. Caglioti and G. Pályi, Chim. Oggi, 2008, 26, 41 CAS; (e) T. Gehring, M. Busch, M. Schlageter and D. Weingand, Chirality, 2010, 22, E173 CrossRef CAS PubMed; (f) B. Barabás, J. Toth and G. Pályi, J. Math. Chem., 2010, 48, 457 Search PubMed; (g) G. Lente, Symmetry, 2010, 2, 767 CrossRef CAS PubMed; (h) J.-K. Micheau, C. Coudret and T. Buhse, in The Soai Reaction and Related Topic, ed. G. Pályi, C. Zucchi and L. Caglioti, Accad. Nazl. Sci. Lett. Arti, Editioni Artestampa, Modena, 2012, p. 169 Search PubMed; (i) T. Gehring, M. Quaranta, B. Odell, D. G. Blackmond and J. M. Brown, Angew. Chem., Int. Ed., 2012, 51, 9539 CrossRef CAS PubMed; (j) A. J. Bissette and S. P. Fletcher, Angew. Chem., Int. Ed., 2013, 52, 12800 CrossRef CAS PubMed; (k) K. Micskei, G. Rábai, E. Gál, L. Caglioti and G. Pályi, J. Phys. Chem. B, 2008, 112, 9196 CrossRef CAS PubMed; (l) I. D. Gridnev and A. K. Vorobiev, ACS Catal., 2012, 2, 2137 CrossRef CAS; (m) G. Ercolani and L. Schiaffino, J. Org. Chem., 2011, 76, 2619 CrossRef CAS PubMed; (n) B. Barabás, C. Zucchi, M. Maioli, K. Micskei and G. Pályi, J. Mol. Model., 2015, 21, 33, DOI:10.1007/s00894-015-2576-6.
- (a) K. Soai, S. Osanai, K. Kadowaki, S. Yonekubo, T. Shibata and I. Sato, J. Am. Chem. Soc., 1999, 121, 11235 CrossRef CAS; (b) I. Sato, K. Kadowaki and K. Soai, Angew. Chem., Int. Ed., 2000, 39, 1510 CrossRef CAS; (c) H. Shindo, Y. Shirota, K. Niki, T. Kawasaki, K. Suzuki, Y. Araki, A. Matsumoto and K. Soai, Angew. Chem., Int. Ed., 2013, 52, 9135 CrossRef CAS PubMed.
- (a) T. Kawasaki, K. Jo, H. Igarashi, I. Sato, M. Nagano, H. Koshima and K. Soai, Angew. Chem., Int. Ed., 2005, 44, 2774 CrossRef CAS PubMed; (b) T. Kawasaki, K. Suzuki, Y. Hakoda and K. Soai, Angew. Chem., Int. Ed., 2008, 47, 496 CrossRef CAS PubMed; (c) T. Kawasaki, Y. Hakoda, H. Mineki, K. Suzuki and K. Soai, J. Am. Chem. Soc., 2010, 132, 2874 CrossRef CAS PubMed; (d) D. J. Carter, A. L. Rohl, A. Shtukenberg, S. D. Bian, C.-H. Hu, L. Baylon, B. Kahr, H. Mineki, K. Abe, T. Kawasaki and K. Soai, Cryst. Growth Des., 2012, 12, 2138 CrossRef CAS; (e) H. Mineki, T. Hanasaki, A. Matsumoto, T. Kawasaki and K. Soai, Chem. Commun., 2012, 48, 10538 RSC; (f) T. Kawasaki, M. Uchida, Y. Kaimori, T. Sasagawa, A. Matsumoto and K. Soai, Chem. Lett., 2013, 42, 711 CrossRef CAS; (g) A. Matsumoto, T. Ide, Y. Kaimori, S. Fujiwara and K. Soai, Chem. Lett., 2015, 44, 688 CrossRef CAS.
- S. Parsons and H. D. Flack, Acta Crystallogr., Sect. A: Found. Crystallogr., 2004, 60, s61 Search PubMed.
- The Flack x determined using the quotients [(I+) − (I−)]/[(I+) + (I−)] method is 0.025(5) for P41212 and 0.044(7) for P43212.
- Typical experimental procedure (Table 1, entries 1–6): a crystal of nickel sulfate hexahydrate 1 was ground using a pestle and mortar (particle size estimated from microscope images to be 66–140 μm). Toluene solution of i-Pr2Zn (0.15 mL, 0.15 mmol) was added dropwise at 0 °C with stirring to a mixture of powder-like crystal 1 (0.21 g, 0.80 mmol) and aldehyde 2 (9.4 mg, 0.050 mmol) in the presence of toluene (0.50 mL). After stirring overnight at 0 °C, toluene (1.0 mL) was added to the mixture. Then toluene solution
of i-Pr2Zn (0.20 mL, 0.20 mmol) and toluene (0.5 mL) solution of aldehyde 2 (19 mg, 0.10 mmol) were added dropwise alternately over a period of 1 h. After stirring for 4 h at 0 °C, toluene (1.5 mL) was added, then toluene solution of i-Pr2Zn (0.80 mL, 0.80 mmol) and toluene (1.0 mL) solution of 2 (75 mg, 0.40 mmol) were added dropwise alternately over a period of 2 h and the mixture was stirred overnight. The reaction was quenched with a mixed solution (14 mL) of saturated aq. ammonium chloride and aq. ammonia (sat. NH4Cl
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30% NH4OH = 2
30% NH4OH = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v)). The resulting mixture was extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate. Purification of the residue by silica gel column chromatography gave 5-pyrimidyl alkanol 3. Further asymmetric autocatalysis using the obtained alkanol 3 was performed to amplify the enantiomeric excess.
1 (v/v)). The resulting mixture was extracted with ethyl acetate. The combined organic layers were dried over anhydrous sodium sulfate. Purification of the residue by silica gel column chromatography gave 5-pyrimidyl alkanol 3. Further asymmetric autocatalysis using the obtained alkanol 3 was performed to amplify the enantiomeric excess.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |