Theoretical studies on the desulfurization of benzothiophene (thianaphthene) and thienothiophene (thiophthene) by carbon–sulfur bond cleavage: binuclear iron carbonyl intermediates†
Abstract
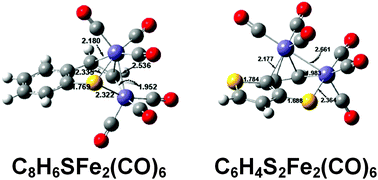
Thiophene is known experimentally to be desulfurized by Fe3(CO)12 under mild conditions to give the tricarbonyl ferrole (η4,η2-C4H4)Fe2(CO)6. A similar reaction of benzothiophene (thianaphthene) with Fe3(CO)12 gives a (C8H6S)Fe2(CO)6 complex in which an iron carbonyl moiety has inserted into the thiophene ring to give a thiaferranaphthalene ligand. Density functional theory shows this experimental structure to be the lowest energy structure. Furthermore, the lowest energy structures of the diiron pentacarbonyl (C8H6S)Fe2(CO)5 are simply derived from this (C8H6S)Fe2(CO)6 by loss of a CO group retaining the thiaferranaphthalene ligand. However, a higher energy isomeric (η6,η2-C8H6S)Fe2(CO)5 structure retains the original benzothiophene ligand with the C6 ring bonded to an Fe(CO)2 moiety as a hexahapto ligand and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond of the C4S ring bonded to an Fe(CO)3 moiety as a dihapto ligand with an Fe→Fe dative bond between the iron atoms. Similar insertion of an iron atom into a thiophene ring to give a thiaferrabenzene ring is predicted to occur in the lowest energy (C6H4S2)Fe2(CO)6 structure derived from either the anti or syn isomers of thienothiophene. However, the bonding of the exocyclic iron atom to the resulting thiaferrabenzothiophene ligand involves atoms in both rings in contrast to the (C8H6S)Fe2(CO)6 complex where the benzene ring is not involved in the ligand–iron bonding.
C double bond of the C4S ring bonded to an Fe(CO)3 moiety as a dihapto ligand with an Fe→Fe dative bond between the iron atoms. Similar insertion of an iron atom into a thiophene ring to give a thiaferrabenzene ring is predicted to occur in the lowest energy (C6H4S2)Fe2(CO)6 structure derived from either the anti or syn isomers of thienothiophene. However, the bonding of the exocyclic iron atom to the resulting thiaferrabenzothiophene ligand involves atoms in both rings in contrast to the (C8H6S)Fe2(CO)6 complex where the benzene ring is not involved in the ligand–iron bonding.

 Please wait while we load your content...
Please wait while we load your content...