Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Sputtering synthesis and optical investigation of octadecanethiol-protected fluorescent Au nanoparticles†
Yohei
Ishida
,
Taiki
Sumi
and
Tetsu
Yonezawa
*
Division of Material Science and Engineering, Faculty of Engineering, Hokkaido University, Kita 13, Nishi 8, Kita-ku, Sapporo, Hokkaido 060-8628, Japan. E-mail: tetsu@eng.hokudai.ac.jp
First published on 29th May 2015
Abstract
Herein we report for the first time the synthesis of octadecanethiol-capped gold nanoparticles (Au NPs) by sputtering of Au over a liquid matrix (silicone oil). Au NPs prepared in silicone oil showed plasmon absorption; however, those prepared in the presence of 1-octadecanethiol did not show plasmon absorption but fluoresced in the near IR region.
Metal nanoparticles (NPs) are attracting considerable interest due to a large number of potential applications resulting from both high surface-to-bulk ratio and quantum confinement of electrons localized inside NPs.1–4 Various preparation methods for metal NPs have been investigated but, recently, sputtering deposition over liquid matrices has been intensively studied in order to generate stable colloidal metal NPs.5,6 This synthetic method takes advantage of the extremely low vapor pressure of liquid matrices so that the preparation of NPs can be carried out at low pressure or under high vacuum conditions. In a sputtering process, ionized argon at high voltage attacks the target metal and ejects the target atoms or atom clusters into a vacuum chamber. Ejected atoms and clusters coalesce into NPs in the gas phase or at the interface of the liquid matrix. In the past, several liquid matrices have been examined including ionic liquids,5,7 propane-1,2,3-triol,8 pentaerythritol ethoxylate,9 polyethylene glycol (PEG),10–12 6-mercaptohexyl-trimethylammoniumbromide,6 and pentaerythritol tetrakis(3-mercaptopropionate).9 All these liquid matrices were able to produce Au NPs with approximately 10 nm diameter. The last two matrices listed above, however, produced rather small NPs with diameters of approximately 1–2 nm. This phenomenon was attributed to the coordination of mercapto groups in the liquid matrix molecules to the Au NP surface that prevented the aggregation and growth of Au NPs. The Au NPs obtained in such “thiolate matrices” fluoresced due to their very small particle sizes hence plasmon absorption was negligible. Very recently, we reported the synthesis of fluorescent Au and Ag NPs by sputtering onto thiolate-containing PEG. In this procedure, we successfully controlled the diameter of NPs by changing the concentration of thiolate ligands.11,12 This approach can be expanded to other combinations of liquid matrices and thiolate ligands, and will broaden the spectrum of NP chemistry using this sputtering technique.
We herein present a novel set of fluorescent Au NPs synthesized by sputtering onto liquid matrices (silicone oil: Si-oil) containing alkylthiol (1-octadecanethiol: ODT). This method aims at the stabilization of Au NPs by ODT both inside and at the interface of the Si-oil. It is expected that the concentration of ODT directly controls the diameter of Au NPs to single nanometer sizes. Alkylthiol is usually very volatile and not suitable for matrix sputtering, but ODT can be used for this purpose due to its high boiling point.
Three different matrix compositions were studied in this work: (a) Si-oil, (b) 3.6 × 10−3 M ODT in Si-oil, and (c) molten ODT (3.0 M). Extinction spectra of Au NP dispersions were measured in a quartz cell with 1 mm optical path just after sputtering (Fig. 1). Samples (a) and (b) were measured without dilution, and sample (c) was measured after a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 dilution with acetonitrile (1 vol. part ODT: 5 vol. parts acetonitrile). A broad absorption peak at around 520 nm was observed for sample (a). This peak is usually observable for Au NPs and corresponds to the localized plasmon absorption of Au. On the other hand, samples (b) and (c) did not show any plasmon absorption peaks in their absorption spectra. The results for (b) and (c) may be due to the difference in the sizes of these NPs. Surface plasmon absorption generally arises from a vibration of free electrons on the surface of NPs and so shows an absorption corresponding to these vibration frequencies. Plasmon absorption thus requires a certain particle size.13,14 When the number of atoms in each particle decreases, the energy band gap becomes wider according to the quantum size effect and a particle becomes non-metallic. Thus, plasmon absorption is not observable in particles less than 2 nm in diameter for Au.15 Judging from the absence of plasmon absorption in Fig. 1, very small particle diameters are expected for samples (b) and (c).
5 dilution with acetonitrile (1 vol. part ODT: 5 vol. parts acetonitrile). A broad absorption peak at around 520 nm was observed for sample (a). This peak is usually observable for Au NPs and corresponds to the localized plasmon absorption of Au. On the other hand, samples (b) and (c) did not show any plasmon absorption peaks in their absorption spectra. The results for (b) and (c) may be due to the difference in the sizes of these NPs. Surface plasmon absorption generally arises from a vibration of free electrons on the surface of NPs and so shows an absorption corresponding to these vibration frequencies. Plasmon absorption thus requires a certain particle size.13,14 When the number of atoms in each particle decreases, the energy band gap becomes wider according to the quantum size effect and a particle becomes non-metallic. Thus, plasmon absorption is not observable in particles less than 2 nm in diameter for Au.15 Judging from the absence of plasmon absorption in Fig. 1, very small particle diameters are expected for samples (b) and (c).
Transmission electron microscopy (TEM) was used to determine the particle diameters. Fig. 2 shows representative TEM images and size-distribution histograms. The average sizes of the Au NPs were determined to be 4.9 ± 1.4 nm, 1.9 ± 0.4 nm, and 1.3 ± 0.3 nm for samples (a)–(c), respectively. As we determined from the extinction spectra in Fig. 1, the diameter of sample (a) was significantly larger than that of (b) and (c). Moreover, the average NP diameter for samples (b) and (c) was quite different. This could have originated from the suppression of the coalescence of NPs on or in the matrix. The concentration of ODT in sample (b) was 3.6 × 10−3 M, and the concentration of molten ODT for sample (c) was 3.0 M. Thus, the difference in ODT concentration in these two samples affected the collision probability between ODT and the Au NPs, which may be the reason for such a difference in their sizes.
From these TEM images, we can recognize the inter-particle interactions in the current system. ODT-capped Au NPs formed in this study were not soluble in common organic solvents such as chloroform, hexane, and dimethylsulfoxide. In contrast, ODT-capped Au NPs prepared by the chemical reduction method were quite soluble in these solvents.16 The chain length of ODT is approximately 2 nm,17 thus the distance between adjacent Au NPs should be larger than 4 nm when the Au NPs exist as mono–nanoparticles without subsequent aggregation. From Fig. 2c, the average inter-particle distance was calculated to be 2.1 nm (see the histogram of edge-to-edge inter-particle distances shown in Fig. S1, ESI†). Thus, it is obvious that the long hydrophobic alkyl chains in ODT were entangled between 2 or more nanoparticles. The insolubility of these Au NPs likely resulted from this phenomenon. This entanglement of the surface ligands may be attributed to the very high concentration of ODT (3.0 M) and their long hydrophobic alkyl chains.
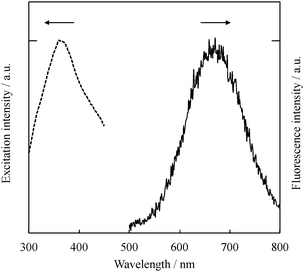
The absence of plasmon absorption and the very small particle sizes of samples (b) and (c) led us to study the fluorescence properties of Au NPs stabilized by ODT. The excitation wavelength was set at 300 nm. Sample (a), which shows plasmon absorption in Fig. 1, did not fluoresce. On the other hand, samples (b) and (c) fluoresced in the near IR region. Fig. 3 shows a fluorescence spectrum of sample (c) (also see sample pictures under sunlight and UV-irradiation in Fig. S2, ESI†). The spectral shapes of samples (b) and (c) were similar but the fluorescence maxima (λFl) were slightly different from each other. The λFl were 677 and 664 nm for samples (b) and (c), respectively. As we previously reported, in the matrix sputtering synthesis of Au NPs, the fluorescence maxima tend to shift to the red as the average diameter of the nanoparticles increases.11,12 This phenomenon is attributed to the quantum size effect,18 in which a band gap of NPs depends on the number of atoms making up a particle.
The excitation spectrum of sample (c) was observed at the fluorescence maximum (664 nm) as shown by the dashed line in Fig. 3. The excitation maximum was observed at 356 nm; therefore, the Stokes shift for these Au NPs was determined to be 1.62 eV. This large Stokes shift was reported in our previous matrix sputtering synthesis work. The main reason for this shift was considered to be the stabilization of the d band and/or the destabilization of the sp-conduction band in the Au NPs, which usually results in a blue shift in the absorption (excitation) spectrum.6
The fluorescence quantum yields of samples (b) and (c) were measured by the absolute method. Both samples showed a 1.0% quantum yield. These values were similar to those of gold nanoparticles and nanoclusters prepared by various other synthetic methods including sputtering and chemical reduction methods.19,20
In conclusion, we have reported the synthesis of octadecanethiol-capped fluorescent Au NPs by sputtering over silicone oil. The photophysical characteristics were fully investigated by absorption and fluorescence measurements combined with TEM. The sputtering method presented here can be expanded to a wide variety of liquid matrix–thiolate ligand combinations. Hence, it is expected to be a novel synthetic candidate for functional metal NPs with precisely controlled diameters and photophysical characteristics.
Experimental section
The liquid matrix used in this work was a silicone oil obtained from Shinetsu (Si-oil, KF-96-50cs, kinematic viscosity of 50 mm2 s−1 at 25 °C) that is often used in synthetic baths. 1-Octadecanethiol (ODT, Wako) was used as the alkylthiol stabilizer for Au NPs. An Au target (99.9%) was supplied by Tanaka Precious Metals (Japan). The experimental procedure is described below. First, in order to remove any volatile substances, Si-oil and ODT were dried under vacuum at 100 °C for 2 h. Three different capturing matrix compositions were used in this work: (a) 1.0 g of Si-oil, (b) 1.0 g of Si-oil containing 10 mg of ODT (corresponding to 3.6 × 10−3 M ODT in Si-oil), and (c) 1.0 g of molten ODT (corresponding to 3.0 M).For the sputtering syntheses, solutions (a)–(c) were added separately into glass Petri dishes having a diameter of 30 mm and were horizontally positioned against the sputtering target. Au was sputtered at a current of 30 mA under Ar and the pressure was kept at 20 Pa. The sputtering voltage was ca. 200 V. The distance between the surface of PEG and the surface of the gold target was 25 mm. Sputtering was carried out for 30 min at 50 °C under stirring at 100 rpm. Due to the temperature inside the sputtering chamber, ODT completely dissolved in Si-oil at the concentration prepared for sample (b) and was completely liquefied in sample (c). The experimental details for the characterization and observation for the resulting NPs are described in the ESI.†
Acknowledgements
This work was partially supported by Hokkaido University and Grant-in-Aid for Scientific Research (A) (to TY 24241041), and by Building of Consortia for the Development of Human Resources in Science and Technology (to YI). This work was also partially supported by Hokkaido University, microstructural characterization platform as a program of Nanotechnology Platform of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.Notes and references
- N. Toshima and T. Yonezawa, New J. Chem., 1998, 22, 1179 RSC.
- D. V. Talapin, J.-S. Lee, M. V. Kovalenko and E. V. Shevchenko, Chem. Rev., 2010, 110, 389 CrossRef CAS PubMed.
- R. L. Whetten and R. C. Price, Science, 2007, 318, 407 CrossRef CAS PubMed.
- E. V. Shevchenko, D. V. Talapin, N. A. Kotov, S. O'Brien and C. B. Murray, Nature, 2006, 439, 55 CrossRef CAS PubMed.
- T. Torimoto, K.-I. Okazaki, T. Kiyama, K. Hirahara, N. Tanaka and S. Kuwabata, Appl. Phys. Lett., 2006, 89, 243117 CrossRef PubMed.
- Y. Shishino, T. Yonezawa, K. Kawai and H. Nishihara, Chem. Commun., 2010, 46, 7211 RSC.
- H. P. S. Castro, H. Wender, M. A. R. C. Alencar, S. R. Teixeira, J. Dupont and J. M. Hickmann, J. Appl. Phys., 2013, 114, 183104 CrossRef PubMed.
- J. Siegel, O. Kvítek, P. Ulbrich, Z. Kolská, P. Slepička and V. Švorčík, Mater. Lett., 2012, 89, 47 CrossRef CAS PubMed.
- Y. Shishino, T. Yonezawa, S. Udagawa, K. Hase and H. Nishihara, Angew. Chem., Int. Ed., 2010, 50, 703 CrossRef PubMed.
- Y. Hatakeyama, T. Morita, S. Takahashi, K. Onishi and K. Nishikawa, J. Phys. Chem. C, 2011, 115, 3279 CAS.
- T. Sumi, S. Motono, Y. Ishida, N. Shirahata and T. Yonezawa, Langmuir, 2015, 31, 4323 CrossRef CAS PubMed.
- Y. Ishida, R. Nakabayashi and T. Yonezawa, New J. Chem., 2015, 39, 4227 RSC.
- N. Schaeffer, B. Tan, C. Dickinson, M. J. Rosseinsky, A. Laromaine, D. W. McComb, M. M. Stevens, Y. Wang, L. Petit, C. Barentin, D. G. Spiller, A. I. Cooper and R. Lévy, Chem. Commun., 2008, 3986 RSC.
- S. H. Yau, O. Varnavski and T. Goodson, Acc. Chem. Res., 2013, 46, 1506 CrossRef CAS PubMed.
- C. Kumara, X. Zuo, D. A. Cullen and A. Dass, ACS Nano, 2014, 8, 6431 CrossRef CAS PubMed.
- A. Badia, L. Cuccia, L. Demers, F. Morin and R. B. Lennox, J. Am. Chem. Soc., 1997, 119, 2682 CrossRef CAS.
- N. A. Ray, R. P. Van Duyne and P. C. Stair, J. Phys. Chem. C, 2012, 116, 7748 CAS.
- J. A. McLean, K. A. Stumpo and D. H. Russell, J. Am. Chem. Soc., 2005, 127, 5304 CrossRef CAS PubMed.
- P. Yu, X. Wen, Y.-R. Toh and J. Tang, J. Phys. Chem. C, 2012, 116, 6567 CAS.
- T. Chen, S. Xu, T. Zhao, L. Zhu, D. Wei, Y. Li, H. Zhang and C. Zhao, ACS Appl. Mater. Interfaces, 2012, 4, 5766 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures. See DOI: 10.1039/c5nj01011j |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |