Open Access Article
Open Access ArticleHigh photoconductive combustion synthesized TiO2 derived nanobelts for photocatalytic water purification under solar irradiation
Neerugatti KrishnaRao
Eswar
a,
Praveen Chandrashekarapura
Ramamurthy
ab and
Giridhar
Madras
 *c
*c
aCentre for Nanoscience and Engineering, Indian Institute of Science, Bangalore-560012, India
bDepartment of Materials Engineering, Indian Institute of Science, Bangalore-560012, India
cDepartment of Chemical Engineering, Indian Institute of Science, Bangalore-560012, India. E-mail: giridhar@chemeng.iisc.ernet.in; Fax: +91 80 23600683; Tel: +91 80 22932321
First published on 29th May 2015
Abstract
Drinking water scarcity is a major issue that needs to be addressed seriously. Water needs to be purified from organic pollutants and bacterial contamination. In this study, sunlight driven photocatalysis for the degradation of dyes and bacterial inactivation has been conducted over TiO2 nanoparticles (CST) and TiO2 nanobelts (CSTNB). TiO2 nanoparticles were synthesized by a solution combustion process using ascorbic acid as a fuel. Acid etched TiO2 nanobelts (CSTNB) were synthesized using combustion synthesized TiO2 as a novel precursor. The mechanism of formation of TiO2 nanobelts was hypothesized. The antibacterial activity of combustion synthesized TiO2 and acid etched TiO2 nanobelts were evaluated against Escherichia coli and compared against commercial TiO2. Various characterization studies like X-ray diffraction analysis, BET surface area analysis, diffused reflectance measurements were performed. Microscopic structures and high resolution images were analyzed using scanning electron microscopy, transmission electron microscopy. The extent of photo-stability and reusability of the catalyst was evaluated by conducting repeated cycles of photo degradation experiments and was compared to the commercial grade TiO2. The reactive radical species responsible for high photocatalytic and antibacterial activity has been determined by performing multiple scavenger reactions. The excellent charge transfer mechanism, high generation of hydroxyl and hole radicals resulted in enhanced photocatalytic activity of the acid etched TiO2 nanobelts compared to commercial TiO2 and nanobelts made from commercial TiO2.
1. Introduction
Recent human activities have drastically increased environmental pollution, which exhibits a negative impact on clean water availability. The release of toxic wastes into rivers and streams not only increases the demand for clean water but also for sustainable technology for water purification. Water-borne epidemics still occur in many parts of the world.1 Various filtration and disinfection technologies are available to provide clean water; however, they also have several disadvantages. Conventional water disinfection systems such as chlorination etc. produce hazardous disinfection byproducts. The microbes also tend to develop resistance towards these disinfection methods. Water purification systems such as adsorption and filtration do not result in the destruction of these pollutants. Therefore, studies are focused on advanced oxidation processes as an alternative water treatment technique to purify polluted water. The advanced oxidation process is basically generation of highly reactive radical species that subsequently results in the degradation of organic pollutants.2Due to the rapid growth of nanotechnology, several nanomaterials are engineered for these advanced oxidation processes including microbial disinfection.3 Among them, TiO2 is used widely because of its abundance and non-toxicity. TiO2 has been widely tested for its activity against a variety of pathogens4 and towards organic contaminants.5,6 In addition to TiO2, titanium phosphates have also been used for catalytic degradation of dyes and organic compounds.7,8 Various methods such as aerosol,9 hydrothermal,10,11 inert-gas condensation12 and sol–gel13 have been used to synthesize TiO2. However, all these techniques have their limitations. TiO2 synthesized using the aerosol method tends to aggregate because of high temperature, while TiO2 synthesized using the sol–gel technique requires utilization of multiple solvents with many laborious steps. TiO2 synthesized by the inert gas condensation technique requires high vacuum which makes the technique much costlier. The synthesis by the hydrothermal method requires several post synthesis processing steps that make the technique difficult.
The synthesis of TiO2 using a solution combustion method is easier, faster and efficient compared to other methods14,15 offering nanoparticles with lower band gap and high activity towards degradation of organic contaminants and killing pathogens.16 Though there are various techniques to synthesize TiO2, its properties depend on crystal structure, size, dimension and morphology.17 Photocatalysis of organic contaminants using natural sunlight8,18,19 rather than artificially powered lights have more advantage in reducing the cost, process and also utilizing both visible and ultraviolet regions of the light spectrum. Many recent reports on extending the absorption of light towards the visible region using the catalysts have shown promising results.20–25 If there is no significant visible light absorption, increasing the photoactivity by just controlling the morphological features can also be given equal importance. Considering various morphologies, the nanobelt structure of TiO2 is of great interest for photocatalysis.26,27 These nanobelts are synthesized by reacting TiO2 with a high alkaline solution at low temperature and high pressure. These nanobelt structures are utilized as substrates for making heterostructures combined with other oxides in order to engineer its bandgap and increase the activity.28,29 Besides photocatalysis, these nanobelt structures of TiO2 have been used for developing sensors and antibacterial applications.30,31
In this report, we have prepared TiO2 by a solution combustion method using ascorbic acid as a fuel. To the best of our knowledge, this is a novel study to report combustion synthesis using ascorbic acid as a reducer and synthesis of TiO2 nanobelts using combustion synthesized TiO2 as a precursor. The photocatalytic activities of the two compounds synthesized have been evaluated by degrading dyes and evaluating their antibacterial activity. The degradation and antibacterial properties of combustion synthesized TiO2 and combustion synthesized TiO2 derived nanobelts have been compared with commercial TiO2 (Degussa-P25) and nanobelts synthesized from commercial TiO2.
2. Experimental
2.1. Materials
Titanium iso-propoxide (>97% purity) was purchased from Sigma-Aldrich (USA). L-Ascorbic acid-LR, methylene blue, methyl orange were purchased from SD fine chemicals Ltd. (India). Commercial TiO2 was obtained from Evonik. Sodium hydroxide, sulphuric acid (98%), hydrochloric acid (35%) and nitric acid (69%) were purchased from Merck (India). Luria nutrient broth and nutrient agar were purchased from Hi Media (India). Double distilled Millipore water was used for all the experiments.2.2. Catalyst synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 nitric acid (by volume) was used to dissolve the white precipitate to obtain a transparent solution of titanyl nitrate. A known concentration of titanyl nitrate was mixed with a stoichiometric amount of L-ascorbic acid to maintain an equal molar ratio of oxidizer to fuel. The combustion reaction is as follows:
2 nitric acid (by volume) was used to dissolve the white precipitate to obtain a transparent solution of titanyl nitrate. A known concentration of titanyl nitrate was mixed with a stoichiometric amount of L-ascorbic acid to maintain an equal molar ratio of oxidizer to fuel. The combustion reaction is as follows: | (1) |
2.3. Catalyst characterization
X-ray diffraction spectra were obtained using a Rigaku diffractometer using Cu-Kα radiation with a scan rate of 1° min−1 in the 10°–80° scan range. Scanning electron microscopic images were captured using ULTRA55 FESEM, Carl Zeiss. Catalyst particles were dispersed in absolute ethanol and sonicated for 10 min. These dispersed samples were drop-casted on silica wafers which were stuck to carbon tape on a SEM aluminium stub. Prepared samples were kept under vacuum for 12 h; samples were gold sputtered using Quorum sputtering before imaging. Transmission electron microscopic pictures were acquired using Tecnai T20 operated at 180 kV. Samples for TEM analysis were prepared by dispersing particles in iso-propanol and ultra-sonicated for 10 min before drop casting on copper grids.The grids were maintained under vacuum for 24 h before subjecting them for imaging. Band gap measurements were done using a solid state UV-visible spectrophotometer (Perkin Elmer, Lambda 35). Absorbance measurements for photocatalysis experiments were analyzed using a UV-visible spectrophotometer (Shimadzu-UV 1700). Catalyst particles were regenerated at 120 °C for 2 h for BET surface analysis using Nova-1000 Quantachrome. The photoconductivity experiments were carried out using ITO coated electrode substrates and a class 3A solar simulator. The solar simulator consists of a 450 W xenon lamp with an Oriel Sol 1.5 air filter. The catalyst samples were drop casted between ITO electrodes.
2.4. Photocatalysis
3. Result and discussion
3.1. Characterization
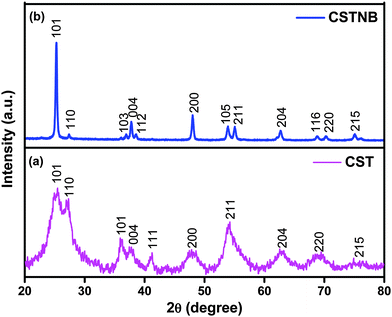
The powder X-ray diffraction pattern of combustion synthesized TiO2 and acid etched TiO2 nanobelts is shown in Fig. 1(a) and (b). The rutile phase at a 2θ value of 27° in the pattern was observed besides a predominant anatase phase (JCPDS No: 00-004-0477) at a 2θ value of 25.4° with (101) as 〈hkl〉 parameters. It is known that low temperature combustion synthesis of TiO2 results in the formation of pure anatase phase.14,15 However, the synthesized product with ascorbic acid as a fuel has shown the possibility of formation of a rutile phase that has been noticed at a 2θ value of 36.0° and 54.2° (JCPDS No: 00-001-1292). It has been reported that TiO2 synthesized using titanyl nitrate and ascorbic acid in 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio resulted in a pure anatase phase.33 However, if the stoichiometric ratio becomes 2
3 molar ratio resulted in a pure anatase phase.33 However, if the stoichiometric ratio becomes 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the rutile phase co-exists with anatase.
1, the rutile phase co-exists with anatase.
The X-ray diffraction pattern of acid etched TiO2 nanobelts shows a huge peak that corresponds to anatase (JCPDS No: 00-004-0477) and also a small adjacent peak at 27.6° corresponding to rutile (JCPDS No: 00-001-1292).
UV-vis diffuse reflectance spectra of combustion synthesized TiO2 and acid etched TiO2 nanobelts are shown in Fig. 2. The combustion synthesized TiO2 shows significant absorption at 400–500 nm. The band gap of combustion synthesized TiO2 is lower compared to the other two catalysts because the combustion synthesis involves generation of adventitious carbon to the TiO2 and hence that red shifts the absorption spectra of light.14 The band gap was calculated using the Kubelka–Munk equation and was found to be 2.98 eV, 3.13 eV and 3.20 eV for combustion synthesized TiO2, acid etched TiO2 nanobelts and commercial grade TiO2 Degussa P-25 respectively. Since the catalysts show a strong absorption in the UV region, utilization of natural solar energy is also feasible rather than experimenting photocatalysis under an artificial UV source.
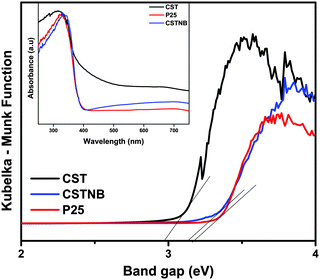 | ||
| Fig. 2 Diffused reflectance spectra of combustion synthesized TiO2 (CST), acid etched TiO2 nanobelts (CTSNB) and Degussa P-25, inset shows the absorbance spectra. | ||
The mechanism of formation of partially etched TiO2 nanobelts can be proposed as follows. When the TiO2 precursor is reacted with high caustic concentration, Ti–O–Ti bonds would break and again reassemble through edges.34 Later, the Ti–O–Ti bond becomes Ti–O–Na bonds. Many such units assemble together and form dimers. These dimers condense with each other and form belt like structures.35,36 During an ion exchange reaction between the layered sheets of sodium titanates and hydrochloric acid, sodium titanate is converted into hydrogen titanate. Corrugated or etched structures of these belts were obtained by reacting hydrogen titanate under acid hydrothermal conditions. The protionic species in sulphuric acid reacts with the hydrogen titanate belt structure to form Ti4+ ions in the reaction mixture. These ions tend to get hydrolyzed on the surface of existing belt structures through heterogeneous nucleation resulting in the formation of a large number of etched surfaces.29 Since combustion synthesized TiO2 is used as a precursor for the first time, we hypothesize that the raw material has been partially etched causing some of the belts to get etched completely forming bits and pieces of TiO2 islands leaving behind etched surfaces as shown in Fig. 3.
The scanning electron microscopic images show pristine hydrogen titanate belts with many inherent voids. Fig. 4(a) and (b) shows a wide distribution of nanobelts and the particle islands on top of them. The combustion synthesized TiO2 nanoparticles are 10–15 nm in size and spherical in shape. The size of the TiO2 nanobelts was found to be in the range of 100–300 nm. The surface area for combustion synthesized TiO2 and titanate nanobelts were found to be 120 m2 g−1 and 35 m2 g−1 respectively. Though a number of voids have formed in the belt structure, there was no significant increase in the surface area of the nanobelts. However, after the acid etching process, the surface area of TiO2 nanobelts increased to 72 m2 g−1. After acid etching, several TiO2 islands and separate particles formed on the existing belt structures and on the partially etched structures; this could have contributed to the increase in surface area.29
 | ||
| Fig. 4 Scanning electron microscopic images of (a) and (b) pristine nanobelts and (c) and (d) acid etched TiO2 nanobelts. | ||
The transmission electron microscopic image of acid etched TiO2 nanobelts (Fig. 5b) shows the presence of oxidation induced void formation. The d-spacing of the rutile phase was not clearly distinguishable whereas it was found to be 0.35 nm for combustion synthesized TiO2 and acid etched TiO2 nanobelts.
The particle size of combustion synthesized TiO2 is very small and highly nanocrystalline compared to that of P25. It also consists of a lot of adventitious carbon. From the X-ray diffraction pattern, the combustion synthesized TiO2, using ascorbic acid as a fuel, consists almost equal composition of anatase and rutile. Similarly, the surface area of combustion synthesized TiO2 is very high compared to that of P25 because of its high porous nature. Therefore, the structural and morphological properties of the nanobelts vary significantly with respect to change in precursor TiO2.37 Due to the above reasons, combustion synthesized TiO2 developed many defects which became further easier to form unique multiple etched TiO2 islands during acid hydrothermal treatment. Hence, it can be proposed that utilizing highly porous combustion synthesized TiO2 instead of commercial P25 results in the development of high photoactive TiO2 nanobelts.
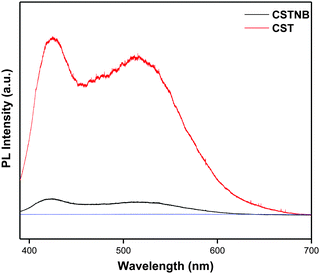
The photoluminescence spectra for combustion synthesized TiO2 and acid etched nanobelts are shown in Fig. 6. The photoluminescence spectra were recorded by exciting the sample at 325 nm. The combustion synthesized TiO2 exhibited high PL intensity compared to the TiO2 nanobelts. Basically, the photoluminescence emission of anatase TiO2 might be due to different reasons such as surface states,38 presence of oxygen vacancies39 and self-trapped excitons.40 However, the combustion synthesized TiO2 exhibits both anatase and rutile phases. The recorded PL spectra could arise from both anatase and rutile properties of TiO2. The emission at 425 nm can correspond to intrinsic states of TiO2 which is close to 412 nm41 and not surface states. The peak at 525 nm can be correlated to oxygen vacancies.39,42 Oxygen vacancies tend to be helpful in the photocatalytic dye degradation process. However, oxygen vacancies on the (110) rutile phase of TiO2 have been shown to exhibit an inhibitive effect on the photocatalytic effect. The unpaired electrons at the oxygen vacancy sites tend to combine with the photo-excited holes at the surface.43 Hence, combustion synthesized TiO2 has many oxygen vacancies but also it consists of a significant rutile phase which could probably lead to a high intensity of photoluminescence. However, the photoluminescence intensity was less for acid etched TiO2 nanobelts suggesting that a higher recombination occurred for combustion synthesized TiO2 and it is prevented in acid etched TiO2 nanobelts, which is also supported by photo-conductivity analysis.
The intensity of electron counts with respect to binding energy was plotted for carbon, oxygen and titanium elements using X-ray photoelectron spectroscopy analysis. Fig. 7(a) and (b) show the XPS spectra for Ti-2p of TiO2 nanobelts and combustion synthesized TiO2 respectively. The peak at 458.7 eV and 457.7 eV (2p3/2) corresponds to the Ti4+ and Ti3+ state of TiO2 respectively. The Ti-2p XPS spectra of combustion synthesized TiO2, as shown in Fig. 7(b), exhibits Ti in +3 state at 458.2 eV, and in +4 state at 459.2 eV.44
Fig. 7(c) and (d) shows the XPS peaks of O 1s of combustion synthesized TiO2 and TiO2 nanobelts. Both the spectra consist of peaks corresponding to lattice oxygen near 530 eV.45,46 Besides lattice oxygen, oxygen vacancies and chemisorbed or dissociated oxygen species can also be observed in the O 1s profile of metal oxides.47,48 The surface hydroxyl groups in combustion synthesized TiO214 would very much likely contribute to the excellent photoactivity despite the fact that most of the photo-excited holes are recombining at the surface. Similarly, chemisorbed or dissociated oxygen species in TiO2 nanobelts could also contribute in direct participation of photocatalysis by generating superoxide radical species, which can also be seen from scavenger experiments. Hence, it shows significant photoactivity similar to commercial TiO2.
Conductivity studies were carried out to measure the photocurrent generated during the experiment. The photocurrent was measured at 0 V and by radiating the light from a solar simulator using a 2420 Keithley source meter. Fig. 8 shows the photocurrent measured with respect to the on and off switch cycles of light irradiation. The measured photocurrent was steady throughout the on and off cycles of light irradiation. The photocurrent response of TiO2 nanobelts was higher compared to commercial TiO2, indicating that the TiO2 nanobelts utilize the illuminated light to the maximum extent. Even after switching off the light irradiation, the TiO2 nanobelt sample exhibited residual current that could be due to trapped charges having extended life time.49
3.2. Catalysis
The photocatalytic performance of these materials was evaluated by degrading organic dye molecules under direct sunlight irradiation. Fig. 9(a) and (b) show the degradation profiles of anionic (methyl orange) and cationic (methylene blue) dyes with these catalysts and this is compared to standard commercial grade TiO2, Degussa P-25. The blank experiments were conducted in the absence of sunlight, where the catalysts were experimented for their adsorption abilities. The as prepared combustion synthesized TiO2 adsorbed around 15% of the initial concentration of methylene blue and methyl orange, respectively. The acid etched TiO2 nanobelts and Degussa P-25 showed negligible dye adsorption. The photocatalytic efficiency of the as prepared combustion synthesized TiO2 is similar to that of commercial TiO2 for both the organic dyes. The acid etched TiO2 nanobelts showed a much higher photocatalytic activity for both the dyes. The degradation of the dyes by the catalyst was analyzed by plotting C/C0 with time. | (2) |
| Catalyst | Rate constant, kapp (×10−4 min−1) | ||
|---|---|---|---|
| Methylene blue | Methyl orange | E. coli | |
| a Unit of k for E. coli degradation is (CFU ml−1)(1 − n)/min. | |||
| Degussa P-25 | 388.8 ± 7.3 | 175.1 ± 1.4 | 641.7 ± 4.3 |
| Combustion synthesized TiO2 | 388 ± 3.1 | 181.7 ± 7.5 | 776.5 ± 34.3 |
| Acid etched TiO2 nanobelts | 1257 ± 3.0 | 421.1 ± 4.7 | 1175 ± 24.4 |
From the kinetic plot, as shown in Fig. 9(c) and (d), the rate constants obtained for acid etched TiO2 nanobelts for both methylene blue ((1257 ± 3.0) × 10−4 min−1) and methyl orange ((421.1 ± 4.7) × 10−4 min−1) degradation were significantly higher compared to the rate constants of combustion synthesized TiO2 and commercial TiO2 indicating its superior photocatalytic activity.
The catalysts were also used to degrade methyl orange under an illuminated UV source. The degradation profile and kinetics of methyl orange photocatalysis are shown in Fig. 9(e) and (f). The rate of methyl orange degradation was slightly higher than the degradation rate under solar irradiation. This could be due to maximum spectral irradiance between 350 and 400 nm of light spectra where the band gap of catalysts exists and also only 2–5% of sunlight comprises UV light. Similarly, the photon energy of UV radiation from artificial lamps is higher than that of solar radiation. However, it has to be emphasized clearly that solar irradiation is abundant, sustainable, inexpensive and renewable. Hence, utilization of sunlight for catalysis is much favored. Similarly the photocatalytic activity of nanobelts derived from commercial Degussa P-25 was determined. The synthesized P25 nanobelts were also acid corroded. Both the P25 nanobelts and acid corroded P25 nanobelts were used to degrade methyl orange under solar irradiation and compared with acid etched combustion synthesized TiO2 derived nanobelts. Fig. 9(g) shows the degradation profile of methyl orange using P25 nanobelts under solar radiation. It can be found that the photocatalytic degradation of the dyes by acid etched TiO2 nanobelts was very higher compared to combustion synthesized TiO2 and commercial TiO2 nanoparticles and nanobelts. Efficient charge separation because of island – belt heterostructures and majority active phase exposure could be possible reasons for the enhanced photoactivity exhibited by acid etched TiO2 nanobelts.
The nanobelts synthesized from P25 show a lower photocatalytic activity than pristine P25. This is because the surface area of P25 particles is 52 m2 g−1 but it is 32 m2 g−1 for P25 nanobelts. Furthermore, P25 particles form a thorough and good homogeneous dispersion in a dye solution compared to nanobelt structures. Therefore, better dispersion and higher surface area of P25 nanoparticles compared to P25 nanobelts contribute to its better photocatalytic activity.
 | (3) |
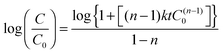 | (4) |
| TiIV − OH− + eCB− → TiIII − OH− (Surface trap) | (5) |
| TiIV + eCB− → TiIII (Deep trap) | (6) |
Facets and phase of the nano structure play a very important role in determining the property of a material. The faceted TiO2 having an anatase phase exhibits superior photocatalytic activity.60 There are many reports showing that the combination of anatase and rutile phases of TiO2 exhibits superior catalytic activity compared to their individual phases. The exposure of the (101) plane of the anatase phase and the anatase to rutile ratio in the acid etched TiO2 nanobelts is higher than the combustion synthesized TiO2. This could be one of the reasons for the higher photocatalytic activity shown by acid etched TiO2 nanobelts because combinations of anatase and rutile phases of TiO2 have been proved to show a synergistic effect.61 The presence of a rutile phase helps in transporting electrons to the anatase conduction band and leaving the holes behind reducing the carrier recombination. The rutile phase also extends the wavelength of light absorption62,63 towards the visible region, which is observed in the case of combustion synthesized TiO2 whose band gap is lower than that of other catalysts. The interface between small TiO2 islands and the acid etched TiO2 nanobelts, majority exposure of the active planes of the anatase phase, the aspect ratio of nano belt structures and combination of anatase and rutile, efficient charge transfer and maximum utilization of photons are responsible for the enhanced photocatalytic activity for dye degradation and antibacterial activity exhibited by acid etched TiO2 nanobelts.
4. Conclusions
The results from these studies demonstrate the utilization of natural sunlight as a source to degrade organic contaminants and inactivate harmful bacteria in the presence of combustion synthesized TiO2 and acid etched TiO2 nanobelts. TiO2 nanoparticles were synthesized by a facile solution combustion technique using ascorbic acid as a fuel. Large surface area, red shift of diffused reflectance spectra and co-existence of both anatase and rutile phases have contributed to the high photocatalytic activity similar to that of commercial TiO2. TiO2 nanobelts were synthesized using combustion synthesized TiO2 as a novel precursor. Unlike Degussa P-25, combustion synthesized TiO2 as a precursor resulted in nanobelts with oxidation induced voids. Similarly, acid treatment of nanobelts resulted in the formation of partially etched belts and many small TiO2 particle islands. The mechanism of formation of nanobelts was proposed. Acid etched TiO2 nanobelts have shown enhanced photocatalytic activity than pristine combustion synthesized TiO2, commercial Degussa P-25 and nanobelts synthesized from commercial TiO2 for dye and bacterial degradation. The rate constants and order of the photocatalytic reactions were determined. Both dye and bacterial degradation by these catalysts have been ascertained to follow first order rate kinetics. Majority exposure of active planes of the anatase phase, combination of anatase and rutile phases, the aspect ratio of the nanobelt structure and effective charge transport between particle-island and belts have contributed to the enhanced photocatalytic activity. Evaluation of various reactive radical species responsible for high photoactivity was done using scavenger experiments. Formation of a large volume of holes and hydroxyl radical by the acid etched TiO2 nanobelts upon irradiation using sunlight has caused both organic dyes and bacteria to degrade rapidly. This study suggests that morphological tuning of nanobelts by utilizing various precursors of TiO2 results in superior catalytic activity, and this can also be utilized as an effective substrate for active enhancement of light absorption by developing hybrid photocatalysts.Acknowledgements
The authors thank Department of science and technology for financial support, Prof. Jayant Modak, Dept. of Chemical Engineering, IISc, for allowing us to perform microbial experiments, CeNSE and AFMM for characterization facilities. The authors thank Ms Disha Jain and Ms Leelavathi for assisting in XPS and TEM characterization.References
- A. Dufour, M. Snozzi, W. Koster, J. Bartram, E. Ronchi and L. Fewtrell, Microbial safety of drinking water: Improving approaches and methods, World Health Organization, 2003 Search PubMed.
- M. Pera-Titus, V. García-Molina, M. A. Baños, J. Giménez and S. Esplugas, Appl. Catal., B, 2004, 47, 219–256 CrossRef CAS PubMed.
- J. Y. Kim, C. Lee, M. Cho and J. Yoon, Water Res., 2008, 42, 356–362 CrossRef CAS PubMed.
- K. P. Kühn, I. F. Chaberny, K. Massholder, M. Stickler, V. W. Benz, H.-G. Sonntag and L. Erdinger, Chemosphere, 2003, 53, 71–77 CrossRef.
- H. Lachheb, E. Puzenat, A. Houas, M. Ksibi, E. Elaloui, C. Guillard and J.-M. Herrmann, Appl. Catal., B, 2002, 39, 75–90 CrossRef CAS.
- N. Miranda-García, M. I. Maldonado, J. Coronado and S. Malato, Catal. Today, 2010, 151, 107–113 CrossRef PubMed.
- D. Das and K. Parida, Appl. Catal., A, 2007, 324, 1–8 CrossRef CAS PubMed.
- D. Das, N. Biswal, S. Martha and K. Parida, J. Mol. Catal. A: Chem., 2011, 349, 36–41 CrossRef CAS PubMed.
- M. K. Akhtar, S. E. Pratsinis and S. V. Mastrangelo, J. Am. Ceram. Soc., 1992, 75, 3408–3416 CrossRef CAS PubMed.
- S. T. Aruna, Solution Combustion Synthesis- An Overview, in Combustion Synthesis: Novel routes to Novel Materials, Bentham Science Publishers Ltd, 2010, pp. 206–221 Search PubMed.
- J. Yang, S. Mei and J. M. Ferreira, J. Am. Ceram. Soc., 2000, 83, 1361–1368 CrossRef CAS PubMed.
- J. Rubio, J. Oteo, M. Villegas and P. Duran, J. Mater. Sci., 1997, 32, 643–652 CrossRef CAS.
- L. Campbell, B. Na and E. Ko, Chem. Mater., 1992, 4, 1329–1333 CrossRef CAS.
- K. Nagaveni, M. Hegde, N. Ravishankar, G. Subbanna and G. Madras, Langmuir, 2004, 20, 2900–2907 CrossRef CAS.
- G. Sivalingam, K. Nagaveni, M. Hegde and G. Madras, Appl. Catal., B, 2003, 45, 23–38 CrossRef CAS.
- S. Sontakke, J. Modak and G. Madras, Chem. Eng. J., 2010, 165, 225–233 CrossRef CAS PubMed.
- C. Melendres, A. Narayanasamy, V. Maroni and R. Siegel, J. Mater. Res., 1989, 4, 1246–1250 CrossRef CAS.
- R. Akbarzadeh, S. B. Umbarkar, R. S. Sonawane, S. Takle and M. K. Dongare, Appl. Catal., A, 2010, 374, 103–109 CrossRef CAS PubMed.
- A. Nezamzadeh-Ejhieh and S. Hushmandrad, Appl. Catal., A, 2010, 388, 149–159 CrossRef CAS PubMed.
- M. Xing, J. Zhang and F. Chen, Appl. Catal., B, 2009, 89, 563–569 CrossRef CAS PubMed.
- J. Schneider, M. Matsuoka, M. Takeuchi, J. Zhang, Y. Horiuchi, M. Anpo and D. W. Bahnemann, Chem. Rev., 2014, 114, 9919–9986 CrossRef CAS PubMed.
- M. Xing, X. Li and J. Zhang, Sci. Rep., 2015, 5, 8591 CrossRef PubMed.
- B. Qiu, M. Xing and J. Zhang, J. Am. Chem. Soc., 2014, 136, 5852–5855 CrossRef CAS PubMed.
- L. G. Devi, S. G. Kumar, B. N. Murthy and N. Kottam, Catal. Commun., 2009, 10, 794–798 CrossRef CAS PubMed.
- L. G. Devi, N. Kottam, B. N. Murthy and S. G. Kumar, J. Mol. Catal. A: Chem., 2010, 328, 44–52 CrossRef CAS PubMed.
- N. Wu, J. Wang, D. N. Tafen, H. Wang, J.-G. Zheng, J. P. Lewis, X. Liu, S. S. Leonard and A. Manivannan, J. Am. Chem. Soc., 2010, 132, 6679–6685 CrossRef CAS PubMed.
- J. Wang, D. N. Tafen, J. P. Lewis, Z. Hong, A. Manivannan, M. Zhi, M. Li and N. Wu, J. Am. Chem. Soc., 2009, 131, 12290–12297 CrossRef CAS PubMed.
- M. Li, Y. Jiang, R. Ding, D. Song, H. Yu and Z. Chen, J. Electron. Mater., 2013, 42, 1290–1296 CrossRef CAS PubMed.
- W. Zhou, G. Du, P. Hu, G. Li, D. Wang, H. Liu, J. Wang, R. I. Boughton, D. Liu and H. Jiang, J. Mater. Chem., 2011, 21, 7937–7945 RSC.
- Y. Wang, G. Du, H. Liu, D. Liu, S. Qin, N. Wang, C. Hu, X. Tao, J. Jiao and J. Wang, Adv. Funct. Mater., 2008, 18, 1131–1137 CrossRef CAS PubMed.
- W. Zeng, T. Liu and Z. Wang, J. Mater. Chem., 2012, 22, 3544–3548 RSC.
- W. Zhou, H. Liu, J. Wang, D. Liu, G. Du and J. Cui, ACS Appl. Mater. Interfaces, 2010, 2, 2385–2392 CAS.
- A. D. Mani, P. M. K. Reddy, M. Srinivaas, P. Ghosal, N. Xanthopoulos and C. Subrahmanyam, Mater. Res. Bull., 2015, 61, 391–399 CrossRef CAS PubMed.
- T. Kasuga, M. Hiramatsu, A. Hoson, T. Sekino and K. Niihara, Langmuir, 1998, 14, 3160–3163 CrossRef CAS.
- B. Yao, Y. Chan, X. Zhang, W. Zhang, Z. Yang and N. Wang, Appl. Phys. Lett., 2003, 82, 281–283 CrossRef CAS PubMed.
- Z.-Y. Yuan, J.-F. Colomer and B.-L. Su, Chem. Phys. Lett., 2002, 363, 362–366 CrossRef CAS.
- V. Bellat, R. Chassagnon, O. Heintz, L. Saviot, D. Vandroux and N. Millot, Dalton Trans., 2015, 44, 1150–1160 RSC.
- L. Forss and M. Schubnell, Appl. Phys. B: Photophys. Laser Chem., 1993, 56, 363–366 CrossRef.
- N. Serpone, D. Lawless and R. Khairutdinov, J. Phys. Chem., 1995, 99, 16646–16654 CrossRef CAS.
- H. Tang, H. Berger, P. Schmid, F. Levy and G. Burri, Solid State Commun., 1993, 87, 847–850 CrossRef CAS.
- L. Saraf, S. Patil, S. Ogale, S. Sainkar and S. Kshirsager, Int. J. Mod. Phys. B, 1998, 12, 2635–2647 CrossRef CAS.
- G. Redmond, D. Fitzmaurice and M. Graetzel, J. Phys. Chem., 1993, 97, 6951–6954 CrossRef CAS.
- Z.-T. Wang, N. A. Deskins, M. A. Henderson and I. Lyubinetsky, Phys. Rev. Lett., 2012, 109, 266103 CrossRef.
- L.-B. Xiong, J.-L. Li, B. Yang and Y. Yu, J. Nanomater., 2012, 2012, 9 Search PubMed.
- Y.-G. Zhang, L.-L. Ma, J.-L. Li and Y. Yu, Environ. Sci. Technol., 2007, 41, 6264–6269 CrossRef CAS.
- B. Santara, P. Giri, K. Imakita and M. Fujii, J. Phys. Chem. C, 2013, 117, 23402–23411 CAS.
- X.-G. Han, H.-Z. He, Q. Kuang, X. Zhou, X.-H. Zhang, T. Xu, Z.-X. Xie and L.-S. Zheng, J. Phys. Chem. C, 2008, 113, 584–589 Search PubMed.
- J. Zheng, Q. Jiang and J. Lian, Appl. Surf. Sci., 2011, 257, 5083–5087 CrossRef CAS PubMed.
- J. Zhuang, S. Weng, W. Dai, P. Liu and Q. Liu, J. Phys. Chem. C, 2012, 116, 25354–25361 CAS.
- J. Kiwi and V. Nadtochenko, Langmuir, 2005, 21, 4631–4641 CrossRef CAS.
- H. A. Foster, I. B. Ditta, S. Varghese and A. Steele, Appl. Microbiol. Biotechnol., 2011, 90, 1847–1868 CrossRef CAS PubMed.
- P.-C. Maness, S. Smolinski, D. M. Blake, Z. Huang, E. J. Wolfrum and W. A. Jacoby, Appl. Environ. Microbiol., 1999, 65, 4094–4098 CAS.
- G. Gogniat and S. Dukan, Appl. Environ. Microbiol., 2007, 73, 7740–7743 CrossRef CAS PubMed.
- S. Sontakke, C. Mohan, J. Modak and G. Madras, Chem. Eng. J., 2012, 189, 101–107 CrossRef PubMed.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- A. L. Linsebigler, G. Lu and J. T. Yates Jr, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- A. Leelavathi, G. Madras and N. Ravishankar, Phys. Chem. Chem. Phys., 2013, 15, 10795–10802 RSC.
- V. Subramanian, E. Wolf and P. V. Kamat, J. Phys. Chem. B, 2001, 105, 11439–11446 CrossRef CAS.
- R. Liu, P. Hu and S. Chen, Appl. Surf. Sci., 2012, 258, 9805–9809 CrossRef CAS PubMed.
- E. Grabowska, M. Diak, M. Marchelek and A. Zaleska, Appl. Catal., B, 2014, 156, 213–235 CrossRef PubMed.
- T. Ohno, K. Sarukawa, K. Tokieda and M. Matsumura, J. Catal., 2001, 203, 82–86 CrossRef CAS.
- G. Li and K. A. Gray, Chem. Phys., 2007, 339, 173–187 CrossRef CAS PubMed.
- D. C. Hurum, A. G. Agrios, K. A. Gray, T. Rajh and M. C. Thurnauer, J. Phys. Chem. B, 2003, 107, 4545–4549 CrossRef CAS.
- H. Zhang, R. Zong and Y. Zhu, J. Phys. Chem. C, 2009, 113, 4605–4611 CAS.
- S. Yan, Z. Li and Z. Zou, Langmuir, 2010, 26, 3894–3901 CrossRef CAS PubMed.
- D. Wang, Y. Duan, Q. Luo, X. Li, J. An, L. Bao and L. Shi, J. Mater. Chem., 2012, 22, 4847–4854 RSC.
- W. Li, D. Li, S. Meng, W. Chen, X. Fu and Y. Shao, Environ. Sci. Technol., 2011, 45, 2987–2993 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2015 |